Introduction
Endometriosis manifests in ~5–20% of females with
pelvic pain, 20–50% of infertile females and 6–10% of females of a
reproductive age (1). This
gynecological disease is defined as the presence of endometrial
tissue outside of the uterine cavity, including in the peritoneum,
ovaries, fallopian tubes, pleura, lungs or, rarely, the brain
(1). The majority of endometriosis
cases are diagnosed through direct inspection of the abdominal
cavity by laparoscopy or laparotomy (2,3).
However, these diagnostic approaches are invasive and may not
always be feasible. As a result, a number of non-invasive
diagnostic tools have emerged, which facilitate the diagnosis of
minimal-mild and moderate-severe endometriosis with high
sensitivity and clinically accurate specificity. Serum cancer
antigen 125 (CA125) has been most widely utilized for the
prediction of endometriosis (4);
however, a number of reports have suggested that CA125 is not a
reliable diagnostic tool for patients (5,6).
Aside from CA125, five other plasma biomarkers including
interleukin-6 (IL-6), IL-8, tumor necrosis factor-α,
high-sensitivity C-reactive protein and CA19-9 have also been
considered as non-invasive diagnostic biomarkers of endometriosis
(7). In addition, a number of
other proteins, including anti-α-enolase-autoAb,
anti-serine/threonine protein kinase-autoAb and
anti-syntaxin5-autoAb, have also been detected in the serum of
patients with endometriosis (8–10).
However, the majority of these proteins lack clinical significance
as tools for endometriosis diagnosis. Currently, novel approaches,
such as microarrays and proteomics, are emerging as preferred
techniques in the study of endometriosis (11–13).
A variety of samples, including serum, peritoneal fluid, eutopic
and ectopic endometrial tissue, and endometrial fluid have been
analyzed using these strategies, which has subsequently facilitated
the progression of endometriosis diagnostic research (14–18).
In the present study, plasma samples obtained from females with
(n=15) and without (n=15) endometriosis were analyzed by proteomic
techniques, and then the observed candidate proteins were validated
in the plasma of females with endometriosis and mice with
surgically induced endometriosis.
Subjects and methods
Recruited female subjects
This study was approved by the Institutional Review
Board at Pusan National University Hospital (PNUH IRB 2010144;
Yangsan, Gyeongnam, South Korea) and all experiments involving
animals were approved by the Animal Care and Use Committee of Pusan
National University (PNU-2012-0120). Females with (n=15;
endometriosis) and without (n=15; control) histologically confirmed
endometriosis were enrolled. All the recruited subjects signed an
informed consent form prior to their participation in the study.
Females in the control group (aged, 25–48) were undergoing
laparoscopy or laparotomy for gynecological problems other than
endometriosis, i.e., ovarian cysts (mature cystic teratoma,
functional cysts and hemorrhagic cysts). The endometriosis group
consisted of females (aged, 27–40) who were diagnosed with
endometriosis following laparoscopy or laparotomy. These patients
complained of abdominal/pelvic pain, dysmenorrhea and/or
subfertility. The patients underwent ultrasonography, pelvic CT
and/or pelvic MRI in an outpatient clinic. Two patients were
classified with minimal to mild disease (stages I and II) and 13
patients with moderate to severe disease (stages III and IV). Blood
samples were collected during the proliferative phase from
recruited patients. Blood was centrifuged at 400 × g for 15 min at
4°C and then the supernatant was collected and stored at −80°C
until assayed.
Murine model of intraperitoneal
endometriosis
Seven-week-old female BALB/C mice (n=16) were
obtained from Koa Tech, Ltd. (Pyeongtaek, Gyeonggi, South Korea).
The mice were housed in cages in environmentally controlled rooms
(ambient temperature, 22±2°C; relative humidity, 50±10%) under a
12/12 h light-dark cycle (lights on 8:00 am-8:00 pm). Food and
water were available ad libitum.
The endometriosis mouse model was established
according to the methods previously described (19). Briefly, donor mice were
subcutaneously injected with β-estradiol in corn oil (100 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA). One week later, donor mice were
anesthetized by intraperitoneal injection of Zoletil (weight, 15
mg/kg; Virbac, Carros, France) and 2% xylazine (Rompun; 15 mg/kg
body weight; Bayer, Leverkusen, Germany). The entire uterus was
then removed and transferred to a petri dish containing saline.
Isolated uterine horns were reduced to small fragments with
scissors, which were then resuspended in saline. Half of the
preparation was injected into the pertitoneum of two anesthetized
recipient mice with a syringe (day 0). Then, the incision was
closed with running 4-0 prolene muscle and skin sutures. Following
surgery, recipient mice (n=8; endometriosis) were injected with
β-estradiol in corn oil (5 mg/kg; Sigma-Aldrich) every week,
whereas control mice (n=4) were treated with corn oil only.
Two weeks later, all mice were sacrificed by
CO2 asphyxiation. Blood samples were collected into
tubes coated with heparin and the plasma was obtained following
centrifugation at 400 × g for 15 min. Aliquots of plasma samples
were frozen (−80°C) until analyzed. In addition, the uterine horns
and endometriotic lesions were collected and fixed in formalin for
three days for the following histological evaluation. Subsequent to
fixation, mice endometrial lesions and uterine horns were imbedded
in paraffin wax and sectioned into 5-μm thick slices. The sections
were stained with hematoxylin and eosin (Sigma-Aldrich). Stained
sections were observed by light microscopy (CX31, Olympus, Tokyo,
Japan).
Protein identification and 2-dimentional
electrophoresis (2-DE)
Albumin and IgGs were removed from the human plasma
using a Multiple Affinity Removal System Spin Cartridge HAS/IgG
(Agilent Technologies, Wilmington, DE, USA). Following this, the
samples were concentrated using a Spin Concentrator (Agilent
Technologies) according to the manufacturer’s instructions. The
total protein concentrations of the samples were determined using a
bicinchoninic acid assay kit (Pierce Biotechnology, Inc., Rockford,
IL, USA). Electrophoretic separation of the proteins was performed
as previously described (20).
Briefly, 100 μg proteins diluted in isoelectric focusing buffer was
loaded onto pH 3–10 NL Immobiline DryStrip gels (18 cm; GE
Healthcare, Piscataway, NJ, USA). The strips were then equilibrated
for 15 min in an equilibration buffer [50 mM Tris-HCl (pH 8.8), 6 M
urea, 2% sodium dodecyl sulfate (SDS), 30% glycerol and 0.002%
(w/v) bromophenol blue] containing 1% dithiothreitol or 135 mM
iodoacetamide, separately. The equilibrated strips were inserted
into 12% SDS-polyacrylamide gel electrophoresis (PAGE) gels (18 cm)
in an Ettan DALT 2-D gel system (GE Healthcare) and the gels were
stained using a PlusOne Silver Staining kit (GE Healthcare). The
spots were analyzed by ProteomWeaver software 2.2 (Definiens AG,
Munich, Germany). Spots exhibiting differences in staining
intensity of at least 2-fold were selected for electrospray
ionization-quadrupole-time of flight/mass spectrometer
(ESI-Q-TOF/MS) analysis. The details of ESI-Q-TOF/MS analysis have
been described in a previous study (21).
Western blotting
Each human or mouse (10 or 80 μg) sample was
separated by 8 or 10% SDS-PAGE and then transferred onto
nitrocellulose membranes using a Trans-blot® SD Semi-dry Transfer
Cell (Bio-Rad, Hercules, CA, USA) for 30 min. The nitrocellulose
membrane (Whatman, Dassel, Germany) was immediately blocked with a
5% non-fat milk solution at room temperature for 1 h. The membrane
was then incubated overnight at 4°C with the appropriate primary
antibodies: Anti-α-2macroglobin (1:1000; Abcam, Cambridge, MA,
USA), anti-apolipoprotein E (1:1000; Abcam), anti-apolipoprotein L1
(1:1000; Abcam), anti-C4A (1:5000; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), anti-haptoglobin (HP) protein (1:250000;
Abcam) and anti-leucine-rich α-2-glycoprotein precursor (LRG;
1:1000; Abcam). Following this, membranes were washed three times
with 1X Tris-buffered saline with Tween-20 and incubated (for 20
min at room temperature) with the following secondary antibodies:
Anti-rabbit IgG-horseradish peroxidase (HRP) (1:5000 or 1:1000,
Thermo Fisher Scientific, Inc., Rockford, USA) and anti-mouse
IgG-HRP (1:5000; Abcam). The proteins on the membrane were
visualized using an enhanced chemiluminescence detection kit
(Surmodics, Eden Prairie, MN, USA). Bands were quantified using
Image J 1.43 software (http://rsb.info.nih.gov/ij/download.html) and protein
levels were normalized to those of β-actin on the same
membrane.
Statistical analysis
All results were expressed as the mean ± standard
error of the mean. A comparison between the two groups was
performed using Student’s t-test and Tukey’s HSD Kramer comparison
test. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using JMP 7.0.1 (SAS Institute Inc., Cary, NC, USA).
Results
Proteomic analysis of plasma proteins in
females with endometriosis
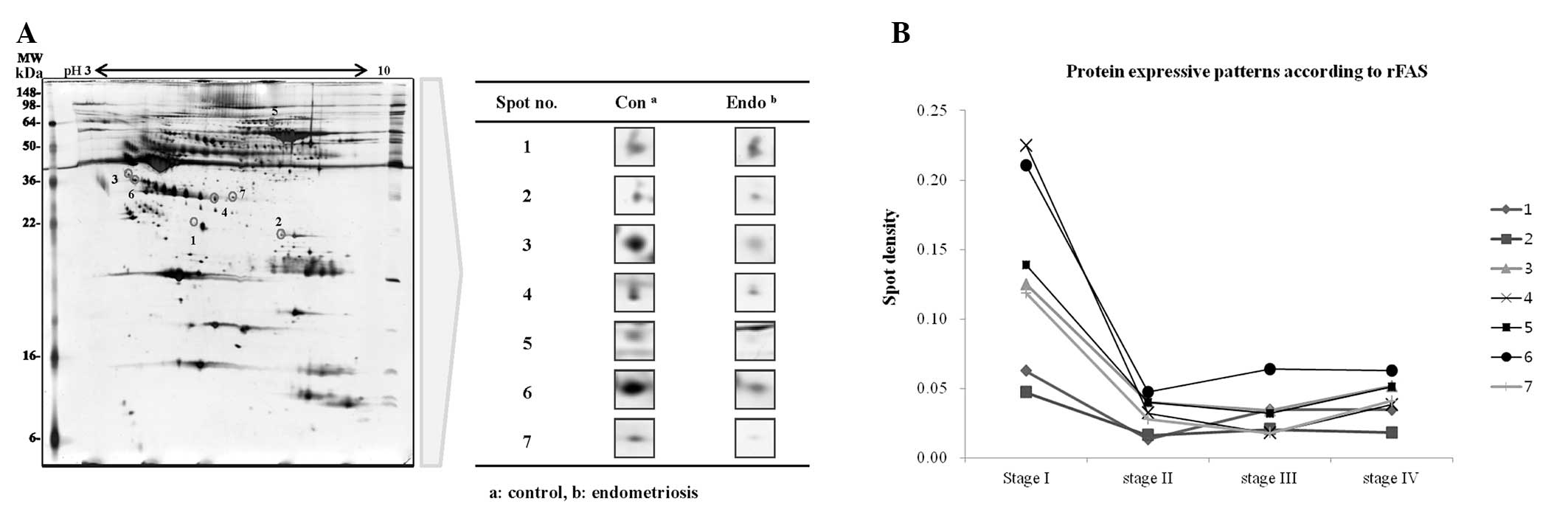
Plasma samples from endometriosis and control
patients were pooled into two groups and the differences in spot
density within 2-DE maps were determined using ProtemoWeaver
software (Definiens AG). A total of 220 protein spots in the
endometriosis group (n=15) and 203 spots in the control group
(n=15) were observed, of which 25 of these spots were identified to
have differences in spot density of at least 2-fold. Twenty spots
in the endometriosis samples had lower densities, whereas five
spots were detected only in the control. Seven spots were then
selected after the densities comparison followed the clinical stage
(stage I=1, stage II=1, stage III=3, stage IV=10) of endometriosis.
(Fig. 1). Finally, they were
identified as Hp, LRG, C4A protein, apolipoprotein E precursor
(Apo-E), apolipoprotein L1 precursor (ApoL-1) and
alpha-2-macroglobulin precursor (α-2M) by ESI-Q-TOF/MS (Table I).
 | Table IList of plasma protein spots
identified by ESI-Q-TOF/MS. |
Table I
List of plasma protein spots
identified by ESI-Q-TOF/MS.
| Spot no. | Accession no. | Protein name | Score | MW
(kDa/pI) | Expressiona | Function |
|---|
| 1 | gi|114039 | APOE | 201 | 36.2/5.65 | Down | Lipoprotein particle
mediator |
| 2 | gi|34782950 | C4A protein | 115 | 32.1/8.52 | Down | Innate immune
response |
| 3 | gi|16418467 | LRG | 236 | 38.2/6.45 | Down | Signal
transduction |
| 4 | gi|3337390 | Hp | 673 | 38.7/6.14 | Down | Cellular
homeostasis |
| 5 | gi|177870 | α-2M | 110 | 32.1/8.52 | Down | Protein
inhibitor |
| 6 | gi|16418467 | LRG | 139 | 38.2/6.45 | Down | Signal
transduction |
| 7 | gi|12232634 | APOL1 | 41 | 42.4/6.37 | Down | Innate immune
response |
Validation of biomarkers by western
blotting
Western blotting was performed on a total of 13
samples including from females with endometriosis (early stage=2,
advanced stage=7) and the control group (n=4). Quantification of
protein densities was conducted by Image J software (Fig. 2).
As demonstrated in Fig.
2B, the relative densities of Hp, ApoL-1 and LRG in the
endometriosis group were decreased compared with those in the the
control group. In particular, quantitation of the Hp expression was
reduced 3.05-fold compared with that of the control (P<0.05). By
contrast, there was no change in Apo-E expression in females with
endometriosis compared with those without. C4A and α-2M protein
expression in females with endometriosis generally increased
compared with that in the control group. LRG expression gradually
decreased from the early to advanced stages.
Fig. 2C
demonstrates the results of early (stages I and II) and advanced
(stages III and IV) stage groups when compared with the control, in
patients with and without endometriosis. The relative densities of
Hp were reduced in early and advanced stages compared with the
control (P<0.05). The expression of ApoL-1 generally decreased
in the early and advanced stage groups compared with the control
group. However, the expression of ApoL-1 in the advanced stage
group increased compared with the early stage. The relative density
of Apo-E was not observed to be altered in each group. The levels
of α-2M and C4A increased in the early and advanced stages compared
with the controls.
Verification of biomarkers of
endometriosis in mouse plasma
Histological examination revealed that uterine
tissue samples at 14 days following transplantation into the
peritoneal cavity had developed into endometriotic lesions with a
typical histological appearance (Fig.
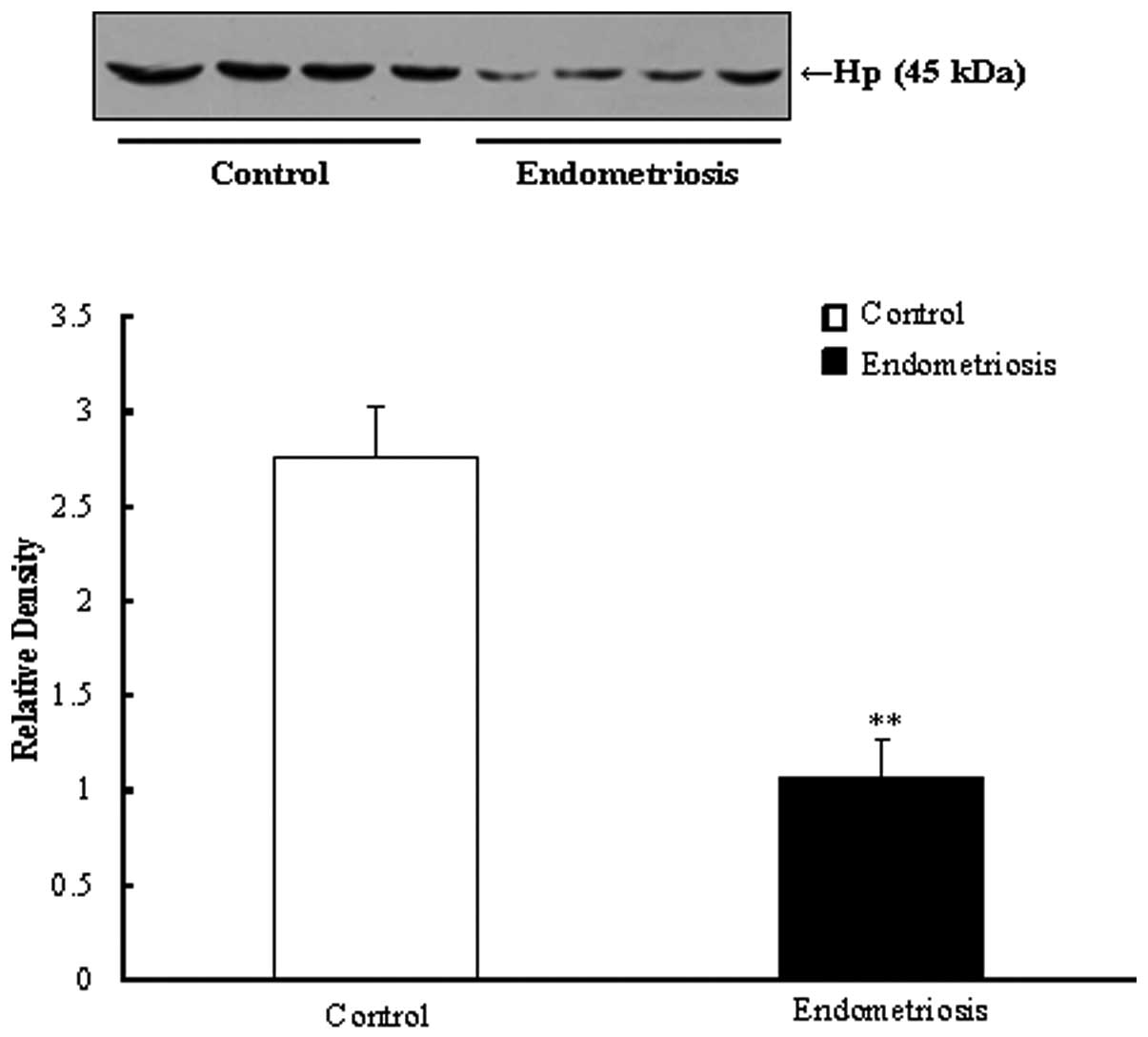
3). As illustrated in Fig. 4,
the plasma Hp level was significantly downregulated in the
surgically induced mouse model, as compared with the control mice
(P<0.01).
Discussion
Endometriosis is defined as the presence of
proliferating, functional, endometriotic-like tissue outside of the
uterine cavity (22). A definite
diagnosis of the disease relies on inspection of endometriotic
lesions with histological confirmation by laparoscopy or
laparotomy. Additionally, transvaginal ultrasound or specific
biomarkers, such as CA-125, have been utilized as diagnostic
strategies. However, these methods are not diagnostically useful,
due to their relatively low sensitivity and specificity in the
early stages of the disease (23,24).
Therefore, in the present study, the disease was investigated on a
molecular level by analyzing the plasma of females with and without
endometriosis. The six identified proteins included α-2M, Hp,
ApoL-1, LRG precursor, Apo-E precursor and C4A protein. These
proteins have a variety of functions, involving cellular
homeostasis, immune response, apoptosis regulation and signal
transduction. In the present study, only Hp was identified by
western blotting analysis. The expression of this protein was
significantly lower level in plasma samples from patients with
endometriosis compared with controls. Downregulation of Hp in
plasma is disease-specific and since Hp is an acute-phase protein
and is usually induced in the periphery as a marker of
inflammation, it may be a distinguishing factor of inflammatory
disease (25). Cocciolo et
al (26) demonstrated that the
Hp β-chain is significantly downregulated and oxidatively modified
in Alzheimer’s disease. Oxidative post-synthetic modifications lead
to Hp β-chain dysfunction and may be correlated with disease
pathology. Reactive oxygen species (ROS)-induced protein
modifications consequently alter protein function and antigenecity
and are therefore implicated in immunological deleterious reactions
associated with inflammatory and/or autoimmune injury (27,28).
This oxidative stress mechanism may have an important role in the
development and progression of endometriosis (29). However, the correlation between
decreased expression and oxidation of the Hp protein, and oxidative
stress in endometriosis has not been reported, therefore, further
studies are required to further elucidate this.
In the present study a surgically-induced
endometriosis mouse model was successfully established. The
induction of endometriosis-like lesions in mice was performed by
sygeneic and autologous transplantation of uterine tissue into the
peritoneal cavity, as spontaneous development of endometriosis is
dependent on menstruation. In this model, endometriosis-like
lesions demonstrated a histopathology similar to that observed in
human endometriotic lesions. Therefore, our mouse model reliably
represented the histomorphology of human endometriosis. Hp
identified in human plasma was also downregulated in this
surgically-induced endometriosis murine model. These data imply
that Hp may be a disease-specific protein in endometriosis.
In conclusion, six differentially expressed proteins
were identified in the plasma of females with endometriosis.
However, only Hp was identified to be significantly decreased in
the plasma of endometriosis patients and in the surgically-induced
murine model. Therefore, Hp may be used as a potential biomarker
for endometriosis diagnostic strategies for the future. However,
further studies are required to evaluate its clinical utility.
Acknowledgements
This study was supported by a grant of the Korea
Health Technology R&D Project, Ministry of Health &
Welfare, South Korea (no. A101367) and the Next-Generation BioGreen
21 Program (no. PJ00819105), Rural Development Administration,
South of Korea.
References
|
1
|
Sasson IE and Taylor HS: Stem cells and
the pathogenesis of endometriosis. Ann NY Acad Sci. 1127:106–115.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ozkan S, Murk W and Arici A: Endometriosis
and infertility: epidemiology and evidence-based treatments. Ann NY
Acad Sci. 1127:92–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bulun SE: Endometriosis. N Engl J Med.
360:268–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Audebert A: Endometriosis coaching.
Gynecol Obstet Fertil. 34:329–336. 2006.(In French).
|
|
5
|
Bast RC Jr, Xu FJ, Yu YH, Barnhill S,
Zhang Z and Mills GB: CA 125: the past and the future. Int J Biol
Marker. 13:179–187. 1998.PubMed/NCBI
|
|
6
|
Chen FP, Soong YK, Lee N and Lo SK: The
use of serum CA-125 as a marker for endometriosis in patients with
dysmenorrhea for monitoring therapy and for recurrence of
endometriosis. Acta Obstet Gynecol Scand. 77:665–670. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mihalyi A, Gevaert O, Kyama CM, Simsa P,
Pochet N, De Smet F, De Moor B, Meuleman C, Billen J, Blanckaert N,
Vodolazkaia A, Fulop V and D’Hooghe TM: Non-invasive diagnosis of
endometriosis based on a combined analysis of six plasma
biomarkers. Hum Reprod. 25:654–664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nabeta M, Abe Y, Kagawa L, Haraguchi R,
Kito K, Ueda N, Sugita A, Yokoyama M, Kusanagi Y and Ito M:
Identification of anti-α-enolase autoantibody as a novel serum
marker for endometriosis. Proteomics Clin Appl. 3:1201–1210.
2009.
|
|
9
|
Nabeta M, Abe Y, Haraguchi R, Kito K,
Kusanagi Y and Ito M: Serum anti-PDIK1L autoantibody as a novel
marker for endometriosis. Fertil Steril. 94:2552–2557. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nabeta M, Abe Y, Takaoka Y, Kusanagi Y and
Ito M: Identification of anti-syntaxin 5 autoantibody as a novel
serum marker of endometriosis. J Reprod Immunol. 91:48–55. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eyster KM, Boles AL, Brannian JD and
Hansen KA: DNA microarray analysis of gene expression markers of
endometriosis. Fertil Steril. 77:38–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arimoto T, Katagiri T, Oda K, Tsunoda T,
Yasugi T, Osuga Y, Yoshikawa H, Nishii O, Yano T, Taketani Y and
Nakamura Y: Genome-wide cDNA microarray analysis of gene-expression
profiles involved in ovarian endometriosis. Int J Oncol.
22:551–560. 2003.PubMed/NCBI
|
|
13
|
Kao LC, Germeyer A, Tulac S, Lobo S, Yang
JP, Taylor RN, Osteen K, Lessey BA and Giudice LC: Expression
profiling of endometrium from women with endometriosis reveals
candidate genes for disease-based implantation failure and
infertility. Endocrinology. 144:2870–2881. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta S, Agarwal A, Sekhon L, Krajcir N,
Cocuzza M and Falcone T: Serum and peritoneal abnormalities in
endometriosis: potential use as diagnostic markers. Minerva
Ginecol. 58:527–551. 2006.PubMed/NCBI
|
|
15
|
Zhang H, Niu Y, Feng J, Guo H, Ye X and
Cui H: Use of proteomic analysis of endometriosis to identify
different protein expression in patients with endometriosis versus
normal controls. Fertil Steril. 86:274–282. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrero S, Gillott DJ, Remorgida V,
Anserini P, Leung KY, Ragni N and Grudzinskas JG: Proteomic
analysis of peritoneal fluid in women with endometriosis. J
Proteome Res. 6:3402–3411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fowler PA, Tattum J, Bhattacharya S,
Klonisch T, Hombach-Klonisch S, Gazvani R, Lea RG, Miller I,
Simpson WG and Cash P: An investigation of the effects of
endometriosis on the proteome of human eutopic endometrium: a
heterogeneous tissue with a complex disease. Proteomics. 7:130–142.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ametzazurra A, Matorras R, García-Velasco
JA, Prieto B, Simón L, Martínez A and Nagore D: Endometrial fluid
is a specific and non-invasive biological sample for protein
biomarker identification in endometriosis. Hum Reprod. 24:954–965.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Somigliana E, Viganò P, Rossi G, Carinelli
S, Vignali M and Panina-Bordignon P: Endometrial ability to implant
in ectopic sites can be prevented by interleukin-12 in a murine
model of endometriosis. Hum Reprod. 14:2944–2950. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang T, Lee HG, Hwang JH, Oh JJ, Lim JN,
Kang HS, Joo JK and Lee KS: Myoglobin: a promising exogenous
reference marker using in proteomics analysis. Food Sci Biotechnol.
22:393–398. 2013. View Article : Google Scholar
|
|
21
|
Hwang JH, Oh JJ, Wang T, Jin YC, Lee JS,
Choi JR, Lee KS, Joo JK and Lee HG: Identification of biomarkers
for endometriosis in eutopic endometrial cells from patients with
endometriosis using a proteomics approach. Mol Med Rep. 8:183–188.
2013.PubMed/NCBI
|
|
22
|
Galle PC: Clinical presentation and
diagnosis of endometriosis. Obstet Gynecol Clin North Am. 16:29–42.
1989.PubMed/NCBI
|
|
23
|
Mol BW, Bayram N, Lijmer JG, Wiegerinck
MA, Bongers MY, van der Veen F and Bossuyt PM: The performance of
CA-125 measurement in the detection of endometriosis: a
meta-analysis. Fertil Steril. 70:1101–1108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moore J, Copley S, Morris J, Lindsell D,
Golding S and Kennedy S: A systematic review of the accuracy of
ultrasound in the diagnosis of endometriosis. Ultrasound Obstet
Gynecol. 20:630–634. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Slobodianik NH, Feliu MS, Perris P,
Barbeito S, Strasnoy I, Franchello A and Ferraro M: Inflammatory
biomarker profile in children with cystic fibrosis: preliminary
study. Proc Nutr Soc. 69:354–356. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cocciolo A, Di Domenico F, Coccia R,
Fiorini A, Cai J, Pierce WM, Mecocci P, Butterfield DA and Perluigi
M: Decreased expression and increased oxidation of plasma
haptoglobin in Alzheimer disease: Insights from redox proteomics.
Free Radic Biol Med. 53:1868–1876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iborra A, Palacio JR and Martinez P:
Oxidative stress and autoimmune response in the infertile woman.
Chem Immunol Allergy. 88:150–162. 2005.PubMed/NCBI
|
|
28
|
Sohal RS: Role of oxidative stress and
protein oxidation in the aging process. Free Radic Biol Med.
33:37–44. 2002.PubMed/NCBI
|
|
29
|
Carvalho LF, Samadder AN, Agarwal A,
Fernandes LF and Abrão MS: Oxidative stress biomarkers in patients
with endometriosis: systematic review. Arch Gynecol Obstet.
286:1033–1040. 2012. View Article : Google Scholar : PubMed/NCBI
|