Introduction
Nasopharyngeal carcinoma (NPC) is common in southern
regions of China. Radiotherapy is the main therapeutic strategy for
the treatment of patients with NPC, however >35% of patients
relapse following radiotherapy. Resistance to radiation remains a
major obstacle for the successful treatment of NPC (1). Therefore, the development of a novel
radiosensitizer that enhances NPC sensitivity to ionizing radiation
is urgently required. An increasing number of studies have
demonstrated that the bioreductive agent emodin
(1,3,8-trihydroxy-6-methylanthraquinone) is able to reverse
multidrug resistance or enhance the cytotoxicity of
chemotherapeutic drugs. In our previous study, it was revealed that
emodin possesses radiosensitizing effects in NPC cells in
vitro and in vivo (2,3). In
order to obtain a higher number of possible therapeutic agents,
1,8-dihydroxy-3-acetyl-6-methyl-9,10 anthraquinone (DAMA) was
synthesized from emodin (4). In
the present study it was investigated whether the DAMA compound was
able to increase the radiosensitivity of the CNE-1 NPC cell line
in vitro. Confocal microscopy and electron microscopy were
employed to characterize the mechanism of action associated with
mitochondrial dysfunction and the level of intercellular
Ca2+. In particular, the study aimed to determine
whether DAMA-treated cancer cells exhibit a profile consistent with
an oncosis-like (non-apoptotic) mechanism.
Materials and methods
Cell culture
Human nasopharyngeal carcinoma CNE-1 cells were
obtained from the Shanghai Institutes for Biological Sciences of
the Chinese Academy of Sciences (Shanghai, China). The CNE-1 human
NPC cell line, was grown in culture medium RPMI-1640 (Gibco-BRL,
Grand Island, NY, USA) containing 10% (v/v) heat-inactivated
newborn bovine serum (NBS; Gibco-BRL), 100 μg/ml streptomycin
(Lukang Pharmaceutical, Shandong, China) and 100 IU/ml penicillin
(Lukang Pharmaceutical) at 37°C in a humidified atmosphere with 5%
CO2. All of the cultures were examined routinely and
found to be free of contamination by mycoplasma or fungi.
Reagents and chemicals
DAMA (98% purity) was synthesized and the chemical
properties were consistent with the literature reported (4). It was fully dissolved as a stock
solution in 100% ethyl alcohol at 1 g/l. For the cell treatments,
the drugs were further diluted in culture medium to the required
concentrations with a final ethyl alcohol concentration of
<1.6%. MTT was obtained from Sigma (St. Louis, MO, USA) and
dimethylsulfoxide (DMSO) was obtained from Bodi Chemical (Tianjin,
China). TRIzol reagent was obtained from Invitrogen Life
Technologies (Carlsbad, CA, USA). RevertAid first strand cDNA
synthesis kit was purchased from MBI Fermentas (Hanover, MD, USA).
All of the polymerase chain reaction (PCR) reagents were purchased
from Takara Biotech (Dalian, China).
MTT assay
The non cytotoxic dose (growth rate >90%) of DAMA
for CNE-1 cells was 10 μg/ml and thus the non cytotoxic dose of
DAMA was selected as the final experimental concentration.
The MTT assay was performed as described previously with minor
modifications (11). Briefly, the
CNE-1 cells were harvested with trypsin and resuspended to a final
density of 1×105 cells/ml. Aliquots of 100 μl from each
cell suspension were distributed evenly into Costar 96-well cell
culture plates (Gibco-BRL). Following incubation of the cells for
24 h, the designated wells were treated with different
concentrations of DAMA. Following incubation for 48 h, 20 μl MTT
solution (5 mg/ml) was added into each well and incubated at 37°C
in a 5% CO2 atmosphere for 4 h. Next, the solution was
removed from the wells and the formazan crystals were solubilized
in 200 μl DMSO in every well. The reduction of MTT was quantified
by the absorbance at a wavelength of 490 nm using a Multiskan MK3
(Thermo Fisher Scientific, Waltham, MA, USA). Three wells were
measured in each group. The percentage of inhibition was calculated
as follows: % inhibition = [1−(mean A of sample/mean A of control)]
× 100.
Clonogenic cell survival assay
The CNE-1 cells were trypsinized and counted. The
appropriate number of cells were plated in 60-mm dishes and allowed
to attach for 24 h. Following treatment with 5 mg/l and 10 mg/l of
DAMA for 24 h, the cells were irradiated (2, 4, 6, 8 Gy) and
incubated for 8–10 days. The colonies were stained with crystal
violet (Sigma Chemical Co., St. Louis, MO, USA), and colonies of
≥50 cells were counted. Clonogenic fractions of irradiated cells
were normalised to the plating efficiency (PE) of the unirradiated
controls. The PE was calculated as follows: PE = (colonies
counted/cells inoculated) × 100. The number of colonies that arise
following treatment of cells, expressed in terms of PE, was termed
the surviving fraction (SF). The surviving fraction was calculated
as follows: no. of colonies formed following treatment/(no. of
cells seeded × PE).
Quantitative (q)PCR
Total RNA was extracted by TRIzol (Invitrogen Life
Technologies) and reverse transcribed by Superscript III
(Invitrogen Life Technologies). The qPCR experiments were performed
using the Roche SYBR Green PCR Master mix with the ABI 7500
Real-Time PCR system (Applied Biosystems, Foster City, CA, USA).
All of the experimental procedures were performed according to the
manufacturer’s instructions. qPCR primer sequences are
comprehensively listed in Table I.
The remaining procedures were performed identically to mRNA
quantification.
 | Table IPrimer sequences of oncosis-related
genes. |
Table I
Primer sequences of oncosis-related
genes.
| Gene | Strand | Primer seq
(5′-3′) | Product size
(bp) |
|---|
| ATP6 | Sense |
GTGATTATAGGCTTTCGCTCT | 160 |
| Antisense |
CAGTAATGTTAGCGGTTAGGC | |
| ATP8 | Sense |
ACTCCTTACACTATTCCTCATCAC | 147 |
| Antisense |
GGCAATGAATGAAGCGAACAG | |
| β-actin | Sense |
AACTCCATCATGAAGTGTGA | 247 |
| Antisense |
ACTCCTGCTTGCTGATCCAC | |
| CYPD | Sense |
GTTATTGAGACAGCAGATAGAG | 185 |
| Antisense |
AATCCTTGCCATCCTTGAG | |
| CHMP6 | Sense |
TGGACAGGACGGAGAACC | 149 |
| Antisense |
CACCTCTTCAATGGACATCAC | |
Mitochondrial membrane potential
assays
Rhodamine 123 [2-(6-Amino-3-imino-3H-xanthen-9-yl)
benzoic acid methyl ester, hydrochloride] is also used in
biochemistry to inhibit mitochondrial function. It appears to bind
to the mitochondrial membranes and inhibit transport processes.
Mitochondrial energization induces quenching of Rhodamine 123
fluorescence and the rate of fluorescence decay is proportional to
the mitochondrial membrane potential. Each group of cells were
harvested and centrifuged at 400 × g for 5 min, and the cell pellet
was resuspended in 0.5 ml Rh123 solution (10 μg/ml) for 10 min. The
cells were then washed and resuspended in phosphate-buffered saline
for confocal microscope analysis using a Nikon A1 confocal
microscope (Nikon, Tokyo, Japan).
Determination of intracellular ATP
concentration
The level of available intracellular ATP in CNE-1
cell lines was measured as an indirect parameter of the activity of
ATPase. A commercially available ATP determination kit (Beyotime
Institute of Biotechnology, Shanghai, China) based on luciferase
activity was used according to the manufacturer’s instructions.
Determination of intracellular
Ca2+ concentration by confocal laser fluorescent
microscopy
Fluo 3 AM is a long wavelength calcium probe that is
practically non-fluorescent in its free ligand form, but its
fluorescence increases 50–100 times when it forms complexes with
calcium. Therefore, it has been widely used with confocal laser
fluorescent microscopy for the determination of the calcium loaded
into cells by incubation. Each group of cells were harvested and
centrifuged at 400 × g for 5 min. Aliquots of the cell suspension
were incubated with 10 mM fura-3AM with pluronic (2%) in medium.
Following loading with fura-3AM at 37°C for 30 min, the cells were
washed twice for 3 min each time, then centrifuged at 400 × g for 5
min to remove extracellular fluorophores and resuspended in 1.2 ml
of the same medium without albumin for confocal microscope
analysis.
Statistical analysis
All experiments were performed at least three times.
Statistical analysis was performed using one-way analysis of
variance to compare the effect among the control and treated cells.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Modulation of radiation resistance by
DAMA
The colony numbers and the surviving fraction of
CNE-1 cells treated with 10 μg/ml DAMA combined with radiation are
demonstrated in Table II and
Fig. 1, respectively. The survival
curve and the sensitization enhancement effect of DAMA are
demonstrated in Fig. 2. There were
evident effects on the radiosensitivity of CNE-1 cells exposed to
DAMA at non-cytotoxic concentrations (10 μg/ml). There was a dose
modifying factor (DMF) of 1.46.
 | Table IIParameters of cell survival curve. |
Table II
Parameters of cell survival curve.
| Group | D0 | DMF |
|---|
| Control group | 5.63 | |
| DAMA | 3.86 | 1.46 |
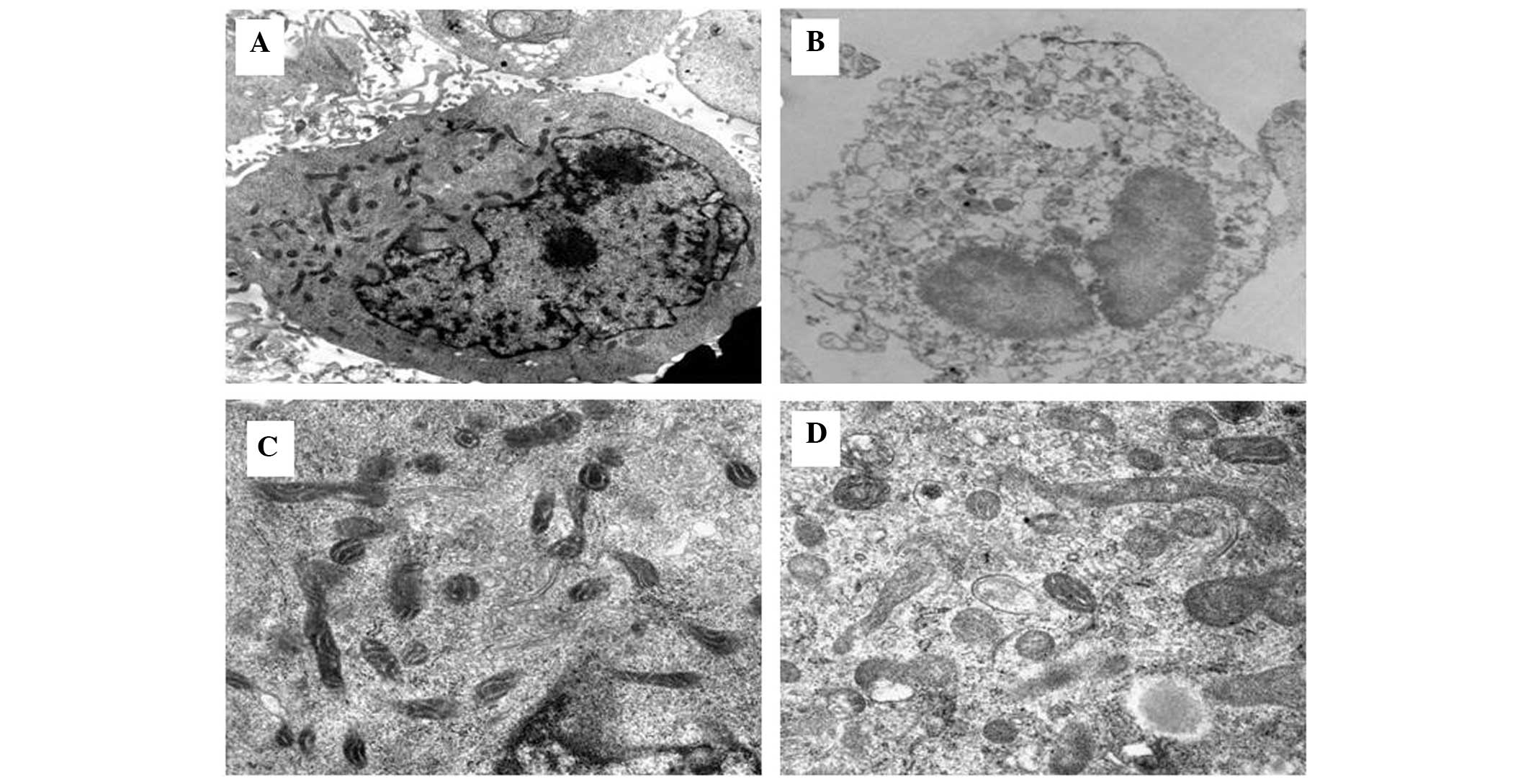
Cell ultrastructure
Electron microscopy (Hitachi 7650 electron
microscope; Hitachi, Tokyo, Japan) confirmed that the control CNE-1
cells had an intact cellular morphology with ultrastructurally
normal nuclei and organelles (Fig.
3A). By contrast, the CNE-1 cells treated with 10 μg/ml DAMA
and radiation for 48 h exhibited cytoplasmic swelling with a marked
disruption of cytoarchitecture, including numerous small and large
cytoplasmic vacuoles as well as swollen and internally disorganized
mitochondria. The most prominent nuclear changes were dilation of
the nuclei, irregular clumping of chromatin and the appearance of
cleared chromatin-free nuclear domains (Fig. 3B–D).
Alteration of the mitochondrial
transmembrane potential
The fluorescence intensity was 40.63±2.36 in the
control group. In the 2 Gy radiation group and DAMA group, the
values were 36.73±2.62 and 26.56±1.29, respectively. Their
mitochondrial transmembrane potentials were significantly decreased
(P<0.05) compared with that of the control group. Notably, the
fluorescence intensity of the radiosensitization group D was
19.14±3.84, thus the mitochondrial transmembrane potential was
evidently decreased. The difference compared with the control group
was statistically significant (P<0.01; Fig. 4).
mRNA expression of oncosis-related genes
in CNE-1 cells treated by DAMA
As revealed in Fig.
5, the mRNA expression of the oncosis-related genes, ATP
synthase protein 8 (ATP8), chromatin modifying protein 6 (CHMP6)
and cyclophilin D (CYPD), increased significantly in the 8 Gy
radiation group and 2 Gy radiation combined with DAMA group. A
significant decrease in the ATP synthase F0 subunit 6 (ATP6) mRNA
expression was observed.
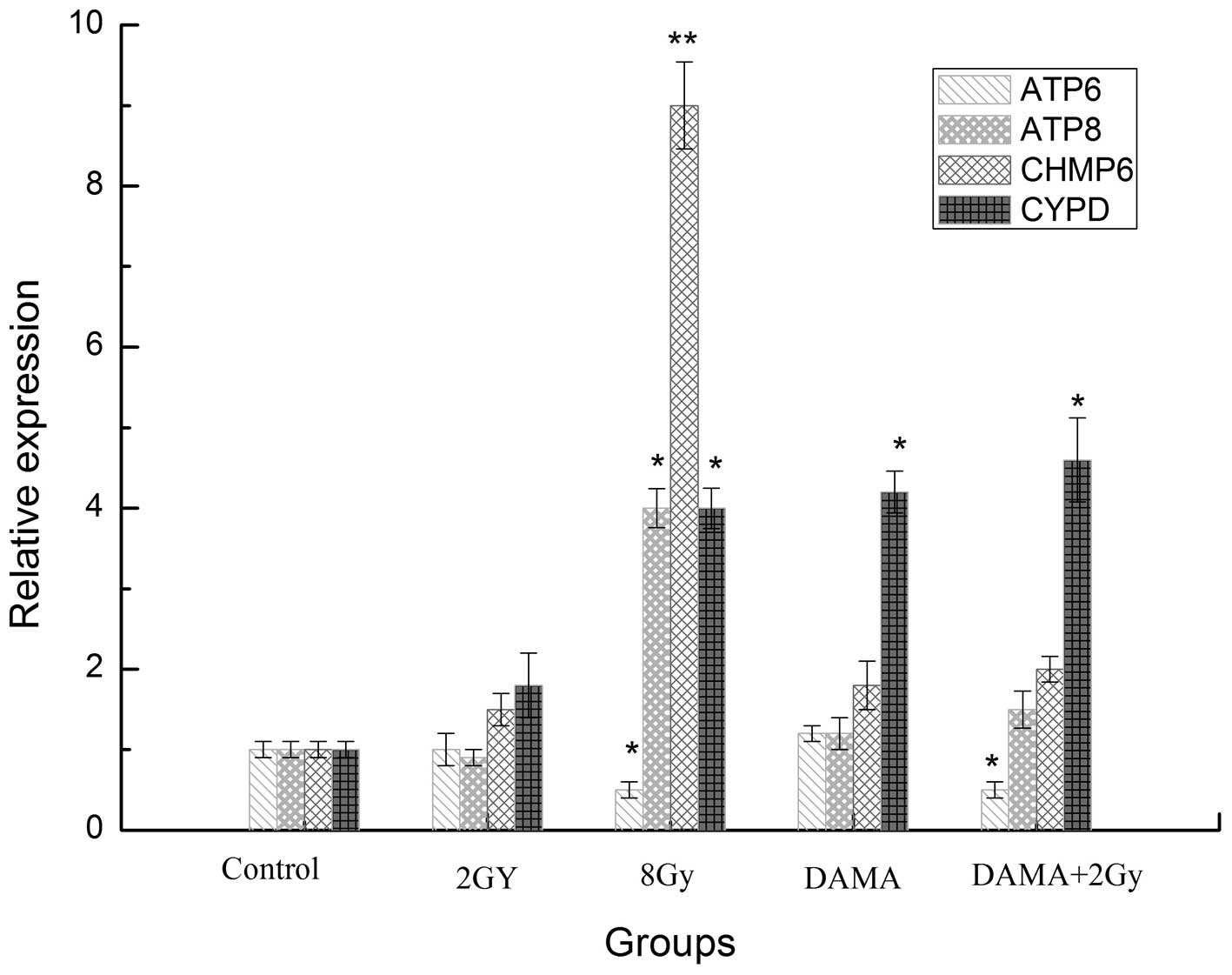 | Figure 5ATP6, ATP8, CHMP6, CYPD mRNA
expression of CNE-1 cells treated by DAMA. DAMA,
1,8-dihydroxy-3-acetyl-6-methyl-9,10 anthraquinone; CHMP6,
chromatin modifying protein 6; CYPD, cyclophilin D; ATP6, ATP
synthase F0 subunit 6; ATP8, ATP synthase protein 8.
*P<0.05, **P<0.01, compared with the
control. |
Intracellular concentration of ATP and
Ca2+ in each group
The fluorescence intensity was 1164.17±68.69 in the
control group following Fluo-3 AM staining. The fluorescence
intensity was 1391.83±33.35 and 1406.0±48.02 in the 2 Gy group and
10 μg/ml DAMA group, respectively, which was significantly
different compared with the control group (P<0.05). The
fluorescence intensity was 1940.08±55.74 in the 2 Gy combined with
10 μg/ml DAMA group, which was significantly increased compared
with the 2 Gy group and 10 μg/ml DAMA group (P<0.05) as well as
the control group (P<0.01). The results demonstrated that there
was evident Ca2+ overloading in the cells. Following
treatment with DAMA, or DAMA combined with 2 Gy, ATP levels
decreased to 75.3 and 45.7%, respectively (Table III). It has been demonstrated
that the intracellular ATP level is an important factor in deciding
the form of cell death when cells were exposed to a lethal
stimulation (5). Lieberthal et
al (6) demonstrated that the
proximal tubule cells subjected to severe ATP depletion die by
necrosis, whereas moderate ATP depletion results in apoptosis. Our
results suggested that depletion of ATP may convert the cellular
morphology from apoptosis- to oncosis-typical characteristics,
suggesting that intracellular ATP levels regulate the mode of cell
death.
 | Table IIIFluorescence intensity of
Ca2+ in CNE-1 cells. |
Table III
Fluorescence intensity of
Ca2+ in CNE-1 cells.
| Group | Relative ATP level
(%) | Ca2+
fluorescence intensity (mean ± SD) |
|---|
| Control | 100.0 | 1164.17±68.69 |
| DAMA | 75.3±10.3 | 1391.83±33.35a |
| 2 Gy | 55.6±6.4 | 1406.00±48.02a |
| DAMA + 2 Gy | 45.7±5.3 | 1940.08±55.74b |
Discussion
The cell death pathway is divided into apoptosis and
oncosis according to the American Society of Toxicology Pathology
(7). Oncosis is a pathway of cell
death that is different from apoptosis, and which has become the
focus of a number of morphological studies in recent years
(8–10). In the present study, it was
identified that DAMA significantly enhanced NPC cell sensitivity to
radiation therapy. The ultrastructure of the control cells was
observed by transmission electron microscopy, and it was noted that
the mitochondria were circular or ovoid, the mitochondria cristae
were clearly visible and there was no swelling or cavities.
Following treatment with radiotherapy combined with DAMA, the NPC
cells exhibited mitochondrial cytoplasmic swelling with a marked
disruption of cytoarchitecture, including numerous small and large
cytoplasmic vacuoles as well as swollen and internally disorganized
mitochondria. Therefore, it appears that induced mitochondria
oncosis may be an important radiosensitization mechanism of
DAMA.
As is well established, mitochondria have an
important role in the cell death pathway (11,12).
Mitochondria are the main cellular organelle that produce ATP and
store energy. Previous studies suggest that apoptosis and oncosis
may be triggered by the same types of stimulation and depend on the
stimulus intensity and duration time. When the stimulus intensity
is weak, the activity duration is relatively short and apoptosis is
prioritized over oncosis. When the stimulus intensity is larger,
the activity duration time is longer and the cell damage is
relatively severe, and oncosis is prioritized over apoptosis. For
example, a low dose of cisplatin results in apoptosis whereas a
high dose induces necrosis (13).
It therefore may be concluded that the ATP levels affect the type
of cell death that is triggered (12). Numerous studies suggest that
apoptosis is associated with the consumption of ATP that initiates
cell death, while oncosis is correlated with a lack of ATP during
passive cell death. Mallat and Tedgui (14) reported that when the intracellular
ATP level is high, apoptosis is induced, while when the
intracellular ATP levels are lacking, oncosis is triggered. ATP is
produced by ATP synthase, which is a protein complex located within
the mitochondrial membrane that binds ADP and a phosphate group
together to produce ATP. ATP6 is a subunit of the F0 complex of
transmembrane F-type ATP synthase. ATP8 is a protein that, in
humans, is encoded by the MT-ATP8 gene and acts as a functional
subunit of mitochondrial ATP synthase. This subunit appears to be
an integral component of the stator stalk in mitochondrial ATP
synthase, which is composed of spherical F1 and F0 subbases.
Mitochondrial ATP synthase, or complex V, generates >95% of
cellular ATP. Complex V is a multisubunit complex consisting of two
functional domains, F1 and F0, connected by a stalk. The F0 domain
is embedded in the mitochondrial inner membrane and consists of
eight subunits. Two of the F0 subunits, subunit a (or subunit 6)
and subunit A6L (or subunit 8) are encoded by the mtDNA ATP6 and
ATP8 genes, respectively (15).
One study identified that mithochondrial ATP6 expression for
maintaining stability had an important role in the mitochondrial
genome. A number of studies have suggested that when cells are
damaged, cellular stress increases ATP6 gene expression, which has
a role in stabilizing the mitochondrial genome and protecting cells
(11,16). The extent of ATP8 downregulation
and ATP6 upregulation is positively correlated with the degree of
cellular damage. Regardless of whether ATP6 expression is
downregulated or ATP8 is upregulated, ATP synthase becomes
dysfunctional, which reduces the subsequent ATP levels, leading to
cell oncosis.
CYPD, a mitochondrial peptidyl prolyl
cis-trans isomerase that regulates mitochondrial
permeability transition, has been demonstrated to control oncosis
activated by diverse stimuli (17). CYPD is considered to regulate the
opening of the permeability transition pore via its interactions
with adenine nucleotide translocator (ANT). These interactions are
inhibited by cyclosporine A, which supports the hypothesis that
cyclophilin D has a role in pore opening (18). CHMP6 decreased the cell
mitochondrial membrane potential and was involved in the regulation
of cell oncosis. Cell death induced by CHMP6 was shown to be a
caspase-independent mechanism, as anti-apoptotic gene Bcl-xl and
caspase family inhibitors were observed to only exhibit a weak
effect on the induction of cell death (19). The mitochondrial membrane
permeability transition (MPT) is a Ca2+-dependent
increase in mitochondrial membrane permeability that leads to
mitochondrial swelling, and rupture of the outer mitochondrial
membrane. The MPT is hypothesized to occur following opening of the
permeability transition pore, which putatively consists of the
voltage-dependent anion channel, the ANT, cyclophilin D and other
molecule(s) (20). When cells
become damaged, the expression of CYPD is enhanced, as it has
protective effects against Ca2+-overload and oxidative
stress-induced cell death (21).
The present study found that following treatment with DAMA and
radiation, the CNE-1 cells over-expressed CHMP6 and CYPD, and began
to swell and accumulate a large number of vacuoles. In addition,
the organelles were observed to swell and the membrane integrity
was damaged. Low dose irradiation only marginally enhanced ATP8
expression and downregulated ATP6. By contrast DAMA combined with
irradiation treatment was able to markedly enhance the ATP8 levels
and weaken the ATP6 expression. The present study suggests that
DAMA exhibits radiosensitiozation for human nasopharyngeal
carcinoma cells via a novel form of oncosis-like cell death,
leading to ATPase synthase disorder and Ca2+ overload,
activation of CHMP and CYPD expression, which ultimately results in
mitochondrial permeability transition pore opening, membrane
potential dissipation, mitochondrial and cytoplasmic swelling, ATP
depletion and the early rupture of the plasma membrane.
In conclusion, the mechanism of oncosis may be a
complex process involving numerous proteins and multiple genes.
Additional investigations are required to clarify the role of this
pathway in the pathogenesis and treatment of human NPC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 30660047 and
81060270) and the Guangxi Natural Science Foundation (grant no.
0728112). The authors would like to thank the proteomics department
the Medical Scientific Research Centre of Guangxi Medical
University for their technical support.
References
|
1
|
Jia WH, Huang QH, Liao J, Ye W, Shugart
YY, Liu Q, Chen LZ, Li YH, Lin X, Wen FL, et al: Trends in
incidence and mortality of nasopharyngeal carcinoma over a 20–25
year period (1978/1983–2002) in Sihui and Cangwu counties in
southern China. BMC Cancer. 6:1782006.PubMed/NCBI
|
|
2
|
Liu Y, Hou H, Li DR, et al: Enhancement
effect of emodin on radiosensitivity of human nasopharyngeal
carcinoma transplanted in nude mice. Chinese Pharmaceutical
Journal. 45:1331–1334. 2010.
|
|
3
|
Liu Y, Hou H, Li DR, et al: Correlation of
enhancement radiosensitization of emodin isolated from Guangxi P.
multiflorum Thunb on hypoxic nasopharyngeal cancer cells with
expression of DNA repair genes. Chinese Pharmacological Bulletin.
25:348–352. 2009.
|
|
4
|
Liang Y, Hou HX, Li DR, Qin CM, Chen DL
and Wu HH: Synthesis of 1,8-dihydroxy-3-acetyl-6-methyl-9,10
anthraquinone and its inhibition effect on ovarian carcinoma cells
SKOV3. Chinese Journal of New Drugs. 2012.1038–1041. 2012.(In
Chinese).
|
|
5
|
Miyoshi N, Watanabe E, Osawa T, et al: ATP
depletion alters the mode of cell death induced by benzyl
isothiocyanate. Biochim Biophys Acta. 1782:566–573. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lieberthal W, Menza SA and Levine JS:
Graded ATP depletion can cause necrosis or apoptosis of cultured
mouse proximal tubular cells. Am J Physiol. 274:F315–F327.
1998.PubMed/NCBI
|
|
7
|
Levin S: Apoptosis, necrosis, or oncosis:
what is your diagnosis? A report from the Cell Death Nomenclature
Committee of the Society of Toxicologic Pathologists. Toxicol Sci.
41:155–156. 1998. View Article : Google Scholar
|
|
8
|
Zhou X, Sun WJ, Wang WM, et al: Artesunate
inhibits the growth of gastric cancer cells through the mechanism
of promoting oncosis both in vitro and in vivo. Anticancer Drugs.
24:920–927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma LS, Jiang CY, Cui M, et al: Fluopsin C
induces oncosis of human breast adenocarcinoma cells. Acta
Pharmacol Sin. 34:1093–1100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun L, Zhao Y, Yuan H, et al: Solamargine,
a steroidal alkaloid glycoside, induces oncosis in human K562
leukemia and squamous cell carcinoma KB cells. Cancer Chemother
Pharmacol. 67:813–821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaudhry MA and Omaruddin RA:
Mitochondrial gene expression in directly irradiated and
nonirradiated bystander cells. Cancer Biother Radiopharm.
26:657–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gu ML, Wang YJ, Shi L, Zhang YB and Chu
JY: Comparison on mitochondrial ATP6, ATP8 and Cyt b genes between
Chinese Tibetans in three different zones: detecting the signature
of natural selection on mitochondrial genome. Yi Chuan. 31:147–152.
2009.(In Chinese).
|
|
13
|
Lieberthal W, Triaca V and Levine J:
Mechanisms of death induced by cisplatin in proximal tubular
epithelial cells: apoptosis vs. necrosis Am J Physiol.
270:F700–F708. 1996.PubMed/NCBI
|
|
14
|
Mallat Z and Tedgui A: Apoptosis in the
vasculature: mechanisms and functional importance. Br J Pharmacol.
130:947–962. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Contamine V and Picard M: Maintenance and
integrity of the mitochondrial genome: a plethora of nuclear genes
in the budding yeast. Microbiol Mol Biol Rev. 64:281–315. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arnold RS, Sun CQ, Richards JC, et al:
Mitochondrial DNA mutation stimulates prostate cancer growth in
bone stromal environment. Prostate. 69:1–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakagawa T, Shimizu S, Watanabe T, et al:
Cyclophilin D-dependent mitochondrial permeability transition
regulates some necrotic but not apoptotic cell death. Nature.
434:652–658. 2005. View Article : Google Scholar
|
|
18
|
Baines CP, Kaiser RA, Purcell NH, et al:
Loss of cyclophilin D reveals a critical role for mitochondrial
permeability transition in cell death. Nature. 434:658–662. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu D, Tian L, Peng Z, et al:
Overexpression of CHMP6 induces cellular oncosis and apoptosis in
HeLa cells. Biosci Biotechnol Biochem. 73:494–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsujimoto Y and Shimizu S: Role of the
mitochondrial membrane permeability transition in cell death.
Apoptosis. 12:835–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Javadov S and Kuznetsov A: Mitochondrial
permeability transition and cell death: the role of cyclophilin d.
Front Physiol. 4:762013. View Article : Google Scholar : PubMed/NCBI
|