Introduction
Cocaine is one of the most powerful addictive drugs
widely abused, mostly in Western countries. This drug is an
alcaloid, naturally occurring in the Erythroxylon plants of
the Erythroxylaceae family. In certain countries like Argentina,
Bolivia, Columbia and Peru, certain species of the genus
Erythroxylon are used as cash crops. Cocaine possesses two
pharmacological actions: one as a local anesthetic and the other as
a psychostimulant. The anesthetic property has been experienced by
the people of different countries for several centuries by chewing
the leaves of Erythroxylon plants to treat headaches, tooth
aches and body pains. However, since repeated consumption of
cocaine causes a state called ‘drug-dependence’ its possession or
use or distribution is illegal in most countries.
Cocaine easily reaches various domains of the brain
and vital organs outside of the central nervous system (CNS), such
as liver, heart, and kidney, and triggers different levels of
cellular toxicity. Current estimates indicate that approximately
1.4 million Americans regularly use cocaine (1). To date, there is no specific
pharmacological medication approved by the Food and Drug
Administration to treat cocaine toxicity (2). Several drugs that are originally
designed for treatment of diseases have been tested in this
context, but none has proved satisfactory in terms of outcome. For
example, modafinil, antabuse and bromocriptine show numerous
undesirable side-effects on healthy cells (3–6). The
profound limitations of these drugs clearly highlight the need to
investigate other compounds as potential therapeutic agents for the
treatment of cocaine addiction.
Compounds that are relatively devoid of undesirable
side-effects are highly desirable for use as therapeutic agents,
and are of importance in pharmaceutical applications, since they
address issues concerning the quality of life in general. It is
widely believed that most herbal products have mild to no
side-effects, and these products are often used as alternative
medicine for several types of diseases and disorders. For
centuries, herbal products have been used to treat different types
of ailments. Recently, the use of herbal products has significantly
increased, due to the fact that synthetic drugs have a high cost, a
short shelf-life and severe side-effects. It is not surprising that
>40% Americans currently use complementary medicine for its
various health benefits (7). One
of the herbal products used worldwide comes from the milk thistle
(MT).
MT, or Silybum marianum (Linn.) Gaertneri, is
a plant species of the Asteraceae/Compositae family. The fruits of
MT have been used for a long time as a remedy for liver and
gallbladder disorders (8). Results
of preclinical studies have highlighted the benefits of using MT or
its purified compounds without any adverse reactions (9–11).
Silymarin was identified as the active constituent of MT that is
responsible for these effects (8).
In the USA, MT is commonly used as a dietary supplement. Given its
protective effects on liver, we hypothesized that MT may play an
important role against cocaine-induced toxicity in different cell
types.
In the present study, we evaluated cocaine toxicity
in different tissue cell lines, namely astroglial, liver, and
kidney cells. Then, we investigated the protective role of MT
against cocaine-induced toxicity. We specifically used MT seed
powder, since MT seeds are recognized for their medicinal value.
Silymarin, the active ingredient of MT, represents 80% of the seed
powder. The short-term effects of cocaine and MT were also
evaluated on living brine shrimp larvae, which have a relatively
simple nervous system; these experiments further provided the
opportunity to directly monitor the behavior of living shrimp
larvae in the presence of cocaine.
Materials and methods
Materials
RPMI-1640 (cat. no. 15-040-CV, Mediatech, Inc.
Manassas, VA, USA), fetal bovine serum (FBS),
penicillin/streptomycin sulfate, amphotericin B, phosphate-buffered
saline (PBS), and L-glutamine were purchased from Mediatech
(Herndon, VA, USA). Cocaine hydrochloride (ecgonine methyl ester
benzoate), crystal violet, 2,2-diphenyl-1-picrylhydrazyl,
L-glutaraldehyde, 0.5 M ethylene diamine tetraacetic acid (EDTA)
solution, 5,5-dithiobis-2-nitrobenzoic acid (DTNB), glutathione
(GSH) reductase (cat. no., G-6004), N-acetyl-L-cysteine (NAC; ≥99%
purity), nicotinamide adenosine dinucleotide phosphate (NADPH),
5-sulfosalicylic acid, and trypan blue were supplied by
Sigma-Aldrich (St. Louis, MO, USA). The remaining chemicals used in
this study were of analytical grade.
Cell lines and animals
The CNS-derived C6 astroglial cell line CCL-107, the
liver cell line CRL-1439, and the MDCK kidney cell line CCL-34 were
obtained from the American Type Culture Collection (Rockville, MD,
USA), and were maintained separately as monolayer cultures.
Astroglial and liver cells were cultured as described earlier
(12), while MDCK cells were grown
in Dulbecco’s modified Eagle’s medium (DMEM) with 4,500 mg/l high
glucose (cat. no. D-6429, Sigma-Aldrich, St. Louis, MO, USA). All
media were supplemented with 2 mM L-glutamine, 10% (v/v) FBS, 100
U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin
B. Cells were grown in an incubator with humidified atmosphere
containing 5% CO2, at 37°C. The brine shrimp cysts
(Artemia salina) were obtained from a local pet store
(Carol’s Critters, Tallahassee, FL, USA). The cysts were seeded for
rehydration on the surface of artificial seawater, prepared with
1.9% salt mixture (Instant Ocean, Aquarium Systems, Inc., Mentor,
OH, USA) in deionized water in a tank under constant illumination
at room temperature (22–28°C) for 48 h.
Treatments
Cocaine treatment was carried out in polystyrene,
flat-bottom 96-well microtiter plates. Astroglial and liver cells
were seeded at a starting density of 2×104 cells/well in
a final volume of 195 μl RPMI-1640 supplemented with 10% FBS. The
cells were allowed to adhere overnight in the incubator. Kidney
cells have the ability to form tight junctions, and were thus
seeded at an initial density of 2×103 cells/well in a
final volume of 97.5 μl of DMEM supplemented with 10% FBS. However,
prior to cocaine treatment of kidney cells, the DMEM was replaced
with RPMI-1640 supplemented with 10% FBS so that media in all
cultures remained the same. Cocaine treatment of astroglial and
liver cells was performed on the day following cell seeding, while
treatment of kidney cells started four days after seeding, in order
to facilitate the formation of tight junctions. On the day of
treatment, 1 M stock solution of cocaine was prepared in PBS
immediately prior to the assay. From the stock solution, various
working dilutions (80–160 mM) were prepared in PBS, and added to
the cells in order to achieve final concentrations of 2, 3 and 4 mM
cocaine. Addition of the cocaine solution was performed in a
minimum volume (5 μl/well for astroglial and liver cells; 2.5
μl/well for kidney cells), to avoid changes in the pH of the
medium. Untreated cells received an equal volume of PBS (5 or 2.5
μl/well) and served as the vehicle control. Cells with medium only
(200 or 100 μl/well) served as an additional negative control.
Treatments lasted 1 h and were performed at a 37°C, 5%
CO2 incubator. The duration of cocaine treatment (1 h)
was selected based on our earlier study (13).
Viability, morphology and
vacuolation
At the end of the 1-h incubation, cell viability was
evaluated by a crystal violet dye uptake assay as in (14). For gross evaluation of
morphological changes and vacuolation, the crystal violet
(0.1%)-stained cells were observed under an inverted phase contrast
IX-70 Olympus microscope (Olympus, Melville, NY, USA) with a ×40
objective, and pictures were acquired as described elsewhere
(12). The neutral red dye uptake
(0.05%) method was adopted to quantify the vacuoles in the cells,
as described in (15).
Antioxidant activity of MT
A known amount of MT dry powder (Paradise Herbs and
Essentials, Inc., Huntington Beach, CA, USA) from capsules (crude
extract from seeds, containing 80% silymarin) was dissolved in
dimethyl sulfoxide (DMSO), to obtain a stock solution of 25 mg/ml.
The antioxidant activity assay was carried out in eppendorf tubes
without cells at different concentrations of MT (25, 50, 75 and 100
μg/ml), using ethanol as the solvent. NAC, a known antioxidant, was
used as a positive control (1 mM, 0.163 mg/ml). The percentage of
scavenging activity was calculated based on a method described
earlier (16).
Evaluation of MT cytotoxicity in
astroglial cells
Experiments were performed in 96-well culture plates
for astroglial cells, in a total volume of 195 μl RPMI-1640 medium
supplemented with 10% FBS. From the MT stock solution, various
working stocks (0.1 to 8 mg/ml) were prepared in the medium prior
to treatment and added to the cells in a minimum volume of 5
μl/well, to achieve final concentrations of 25 to 200 μg/ml.
Untreated cells received an equal volume of DMSO (0.8% final) and
served as the vehicle control. Cells in medium only (200 μl/well)
served as an additional negative control. Treatment lasted 1 h and
was performed at a 37°C, 5% CO2 incubator. Cell
viability was assessed as previously described (14).
Pretreatment of astroglial cells with
MT
Experiments were performed in 96-well culture plates
for astroglial cells, in a total volume of 190 μl RPMI-1640 medium
supplemented with 10% FBS. The cells were pretreated with 200 μg/ml
MT (5 μl/well, final DMSO, 0.8%) for 30 min, followed by
co-treatment with cocaine (5 μl/well, 2–4 mM final) for 1 h. Cells
containing 0.8% DMSO in the medium, and cells with medium only
served as the negative controls. At the end of the incubation, cell
viability was measured as described earlier (14), and the GSH level was assessed as
described below.
Total GSH level in astroglial cells
Cellular glutathione levels were assayed according
to the method described in Smith et al (17). Briefly, following treatment with
three different concentrations of cocaine (2–4 mM) in RPMI-1640
medium for 1 h in 96-well microtiter plates, the cells were
deproteinized with 2% 5-sulfosalicylic acid (10 μl/well) for 30 min
at 37°C, followed by addition of 90 μl of reaction mixture,
containing 0.416 mM sodium EDTA, 0.416 mM NADPH, 0.835 mM DTNB,
glutathione reductase (0.825 units) and 0.083 mM sodium phosphate
buffer, pH 7.5. The cells were incubated with the mixture for 30
min at 37°C. The absorbance (Abs) was measured at 412 nm on a
MQX200; microplate reader (Bio-Tek Instruments Inc., Winooski, VT,
USA).
In vivo brine shrimp lethality assay
The brine shrimp larvae lethality assay was
performed as described by Meyer et al (18) and with modification suggested by
McLaughlin (19). The assay was
carried out in triplicate vials with ten larvae each, and with
fixed doses of MT (200 μg/ml), or various doses of cocaine (2, 3
and 4 mM). Untreated shrimp larvae in artificial seawater, or DMSO
(0.8%)-exposed larvae in artificial seawater served as controls.
The treatment lasted 1 h at room temperature, under constant
light.
Statistical analysis
The experimental results were expressed as the mean
± standard error of the mean (SEM). Data were analyzed by a one-way
analysis of variance (ANOVA), followed by Dunnett’s multiple
comparison tests, using the GraphPad Prism software, version 5.00
(GraphPad Software, San Diego, CA, USA). The values of the lethal
concentration needed to kill 50% of the cells (LC50) and
of the median effective dose (ED50), were determined
from the graphs based on a method described earlier (20).
Results
Dose-dependent toxicity of cocaine
The toxic effect of cocaine was evaluated in
vitro following 1 h of exposure of C6 astroglial, liver, and
kidney cells to 2, 3, and 4 mM of the drug. The astroglial cells
were more sensitive to cocaine (Fig.
1A) than liver and kidney cells. The percentage of viability of
astroglial cells significantly decreased (P<0.05, n=3) with the
increasing cocaine dose, an observation consistent with an earlier
study on these cells (14).
Compared to the control (100% viability), the average astroglial
cell viability (± SEM) at 2, 3, and 4 mM of cocaine was 88±1.1,
51±2.2, and 7±1%, respectively. The cocaine LC50 was 3.0
mM in these cells. Notably, cocaine treatment did not markedly
alter the viability of liver and kidney cells (Fig. 1B and C). The cocaine
LC50 in these cell lines was >4.0 mM.
We then used living brine shrimp larvae as a simple
in vivo model to evaluate the effects of treatment with
different concentrations of cocaine (2, 3 and 4 mM) for 1 h. All
larvae in the control vials were alive and active, swimming in all
directions. Treatment with cocaine for 1 h did not cause
significant shrimp death, nor did it affect their activity. All
larvae were active and resembled the controls (P>0.05). The
ED50 was >4 mM (Fig.
1D).
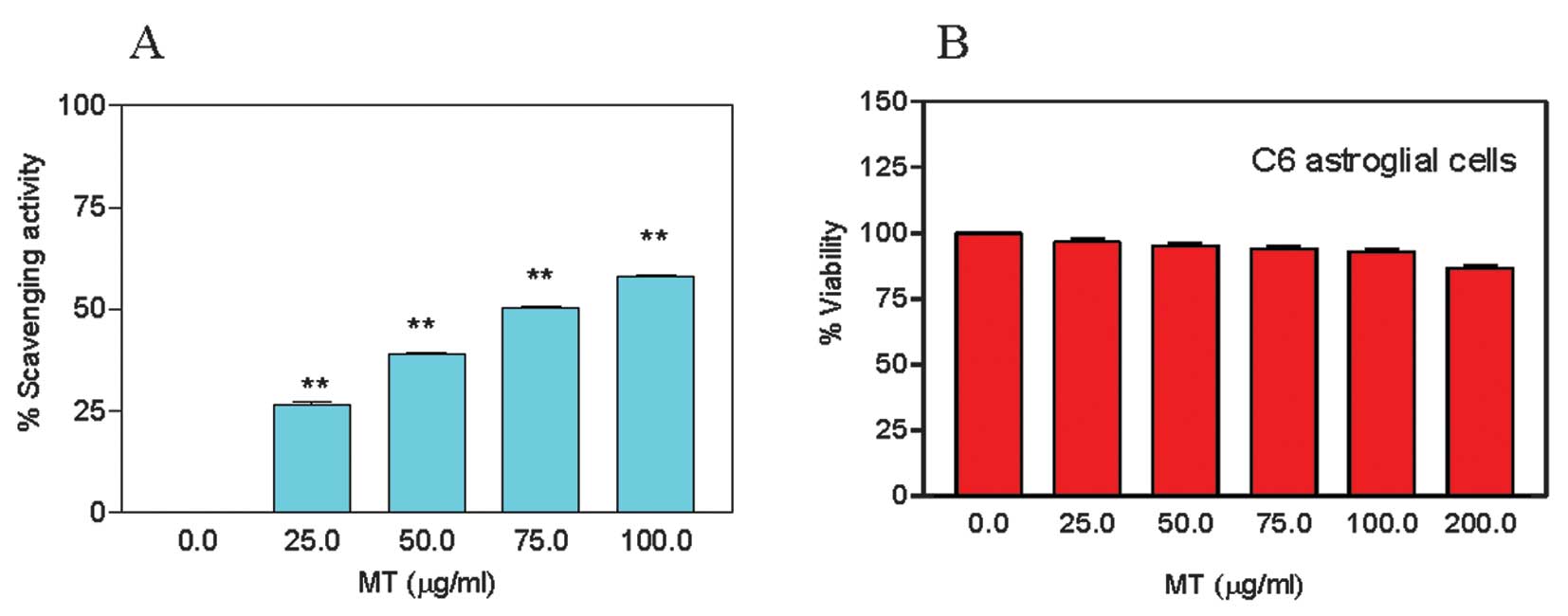
Antioxidant activity of MT
Prior to evaluating the potential role of MT in
alleviating the cocaine-induced toxicity, we determined its
antioxidant activity. It has been previously reported that,
although most plant extracts exhibit antioxidant activity,
different extraction procedures can affect this activity, with
variations ranging from 53 to 173 μg/ml (21,22).
MT exhibited a dose-dependant increase in the free radical
scavenging activity (Fig. 2A).
Compared to the control, the average activity (± SEM) at 25, 50,
75, and 100 μg/ml was 26.5±0.6, 38.9±0.4, 50.4±0.3, and 58.1±0.5%,
respectively. The MT ED50 free radical scavenging
activity was evaluated at ~75 μg/ml. NAC (7.66 μg/ml, MW, 163.19),
used as a positive control in this study, had an ED50 of
0.047 mM. These results demonstrate that MT has a relatively high
antioxidant activity.
MT is not cytotoxic
We sought to determine the highest non-toxic dose of
MT required to alleviate cocaine toxicity in astroglial cells. For
this purpose, we treated the cells with 25, 50, 75, 100, and 200
μg/ml of MT for 1 h. MT did not cause significant death in
astroglial cells (P>0.05) at any concentration (Fig. 2B). The estimated LC50
was >200 μg/ml, therefore, we used this concentration in the
following experiments. Treatment with 200 μg/ml of MT for 1 h did
not cause death in the larvae either, as determined by the brine
shrimp assay (data not shown).
MT pretreatment protects from cocaine
toxicity, morphological alterations and vacuolation
The astroglial cells were pretreated with 200 μg/ml
of MT for 30 min, followed by co-treatment with 2, 3, or 4 mM
cocaine for 1 h. The viability of MT-pretreated, cocaine co-treated
cells was significantly increased (P<0.01) compared to the cells
treated with cocaine alone (Fig.
3A). The average cell viability (± SEM) following MT
pretreatment was increased to 86±1.7 and 74±3.7%, respectively,
compared to non MT-pretreated cells, treated with 3 and 4 mM of
cocaine (50±2.6 and 8±1.1%, respectively). These results clearly
indicated that MT provides important cell protection against
cocaine toxicity.
Furthermore, microscopic observations of crystal
violet (0.1%)-stained cells revealed that, in comparison to the
shape of untreated control cells (Fig.
3B-i), there was no significant morphological difference in the
MT-pretreated control cells (Fig.
3B-ii) or in the MT-pretreated and cocaine co-treated cells
(Fig. 3B-iv). Morphological
observations also indicated that in the absence of MT pretreatment,
cocaine elicited the formation of vacuoles in the cytoplasm
(Fig. 3B-iii and C). However,
pretreatment with 200 μg/ml MT not only prevented vacuole formation
(Fig. 3B-iv), but also maintained
the morphological features of the cells (Fig. 3B-i). These results suggest that
astroglial cells are highly sensitive to cocaine, while MT
pretreatment allows to maintain cell morphology in cocaine-treated
cells.
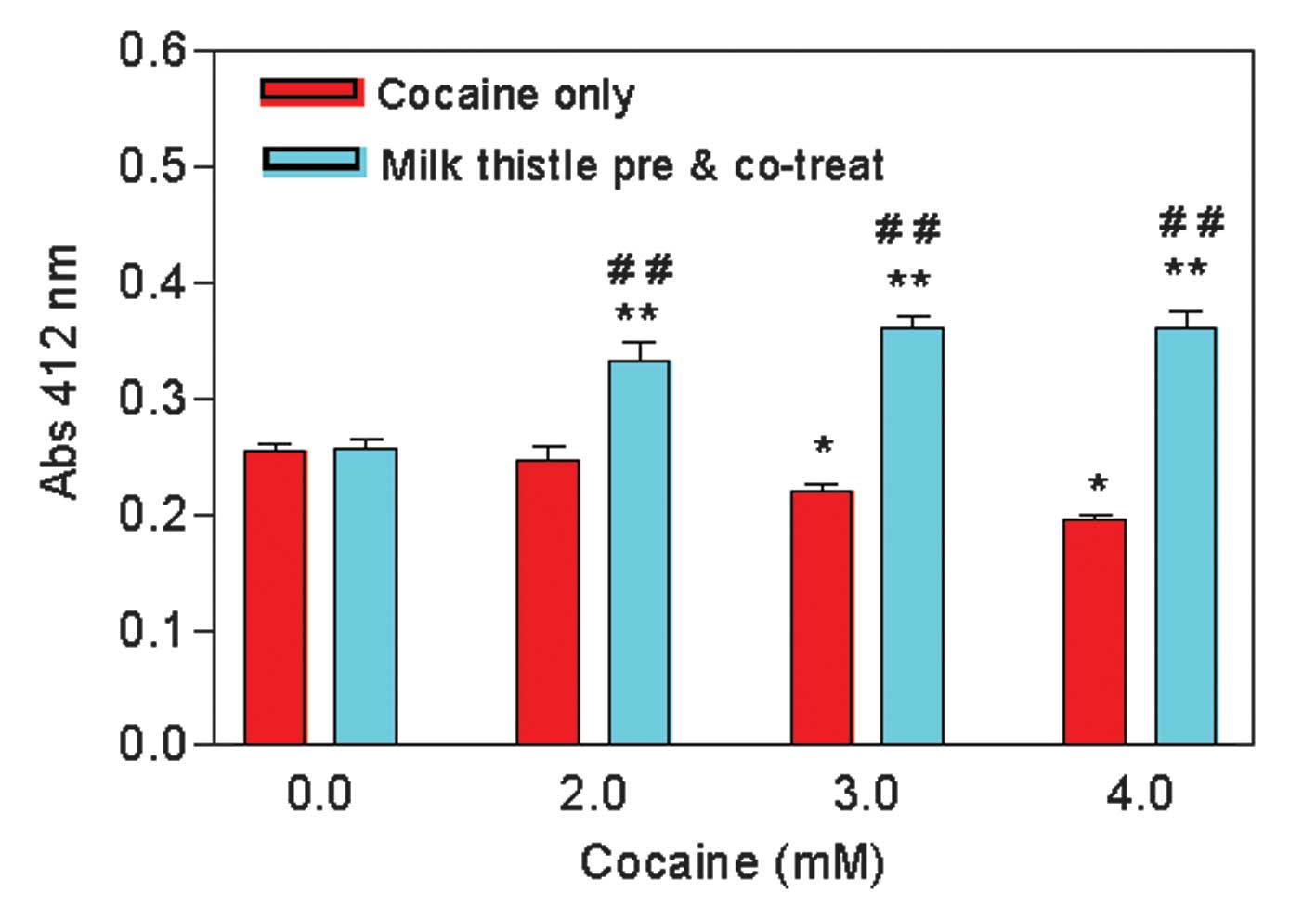
MT pretreatment increases the
intracellular GSH level
We evaluated the role of MT on the cellular GSH
level. Since we used the glutathione reductase enzyme in the assay,
our glutathione measurements represent the total glutathione level,
i.e., oxidized and reduced. The GSH level remained the same in
MT-pretreated and control cells, treated with 0 mM of cocaine
(Fig. 4). In the absence of
pretreatment with MT, the GSH level was significantly decreased
(P<0.01) to 97.3±3.8, 87.1±1.8, and 76.8±1.5%, at 2, 3, and 4 mM
of cocaine, respectively, compared to the level of the control
(Fig. 4). Pretreatment of cells
with MT markedly (>25%) increased the GSH level at all doses
compared to the cells treated with cocaine alone (Fig. 4). These data indicate that MT
pretreatment increases the total GSH level in cocaine-treated
cells.
Discussion
The cocaine concentrations used in this study were
similar to those applied in other in vitro experiments,
reported earlier (12,23–25),
where the LC50 or EC50 values were in the millimolar
range (>3 mM). Under our experimental conditions, the cocaine
LC50 in C6 astroglial cells was ~3 mM at 1 h exposure.
This value is closer to that of a recent report (13). It is notable that cocaine was not
prominently toxic to liver and kidney cells or to living shrimp
larvae (Fig. 1B–D).
Natural products have few to no side-effects and may
thus represent a good alternative to current treatments for cocaine
toxicity. In order to investigate whether plant-derived natural
products can alleviate cocaine toxicity, we evaluated the seed
extract of MT (containing 80% silymarin) on astroglial cells, the
most abundant neuronal support cell in the adult brain (26,27).
Death of astrocytes due to cocaine toxicity can lead to neuronal
dysfunction. This problem can be largely avoided at the initial
stages of cocaine addiction if astrocytes are protected from
cocaine toxicity with compounds that are devoid of
side-effects.
Our study demonstrated that MT has an antioxidant
activity (Fig. 2A) and is
non-toxic to C6 astroglial cells (Fig.
2B). When the cells were pretreated with MT extract for 30 min,
followed by co-treatment with cocaine for 1 h, MT not only
significantly sustained cell viability (Fig. 3A), but also contributed to the
maintenance of cell morphological features (Fig. 3B), since MT-pretereated cells were
similar to the control cells without vacuoles (Fig. 3C). Similar observations were
recently reported for NAC pretreatment (13). In addition, we showed that the
increased cell viability of cocaine-treated cells is accompanied by
an increase in the GSH level. In order to investigate whether the
increased cell viability is due to increased GSH levels with MT
pretreatment, we measured the total GSH level in MT-pretreated
cells.
The results of this experiment revealed that cocaine
treatment causes a significant reduction in the total GSH level of
the cells; however, pretreatment of cells with MT, followed by
co-treatment with cocaine, significantly increased the GSH level
(Fig. 4). Similar results were
reported in C6 cells for cocaine and NAC treatment (13). The decrease in the GSH level in
cocaine-treated cells and the increase in GSH due to pretreatment
with either NAC (13) or MT
(Fig. 4) suggest that compounds
that induce intracellular GSH synthesis could be therapeutically
effective against cocaine toxicity. The dose-dependent increase in
the GSH level at 2, 3 and 4 mM cocaine-co-treated cells suggests
that a different pathway may be responsible for the increase in the
GSH level. Further studies will be needed to identify this
mechanism.
NAC, an antioxidant compound, has been recognized as
a relevant therapeutic agent for several oxidant-related CNS
diseases (28). This compound was
also proposed as a promising pharmacological agent for the
treatment of cocaine dependence (29). Its administration to addicted
individuals resulted in reduced cocaine-craving behavior (29,30).
Since NAC hydrolysis in the body provides cysteine for
intracellular GSH biosynthesis, it is possible that the observed
benefit of NAC in cocaine addicts is related to the increased GSH
level. No studies to date have provided evidence for an association
between the increased GSH level and reduced cocaine-craving
behavior in addicted individuals. If this is the case, then the
role of additional natural products that support GSH synthesis in
CNS cells and are devoid of serious side-effects merits further
investigation. We argue that studies on natural products such as MT
will provide alternative medicine for drug-induced psychiatric
illnesses at a low price, and may reduce cocaine-related mental
disorders such as depression, aggressiveness, and paranoia
(31), possibly through an
increase in the GSH level. Additional research is needed to explore
this hypothesis.
Acknowledgements
This study was supported by the NIH grants NCRR/RCMI
G12 RR03020 and NIMHD 8G12 MD 007582-28 (National Institutes of
Health, Bethesda, MD, USA). Authors thank Dr Janet P. Barber for
critical reading of the manuscript.
Abbreviations:
|
BBB
|
blood brain barrier
|
|
CNS
|
central nervous system
|
|
DTNB
|
5,5-dithiobis-2-nitrobenzoic acid
|
|
ED50
|
effective concentration needed to show
50% of a defined response
|
|
EDTA
|
ethylene diamine tetraacetic acid
|
|
FBS
|
fetal bovine serum
|
|
GSH
|
glutathione (total, i.e., oxidized and
reduced)
|
|
LC50
|
lethal concentration needed to kill
50%of the cells
|
|
MT
|
milk thistle
|
|
NAC
|
N-acetyl-L-cysteine
|
|
NADPH
|
nicotinamide adenosine dinucleotide
phosphate
|
|
OD
|
optical density
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
National Institute on Drug Abuse.
Nationwide Trends. Drug Facts (NIDA Research Report Series). NIDA
DRUGPUBS; Bethesda, MD: 2011
|
|
2
|
Simpson DD, Joe GW, Fletcher BW, et al: A
national evaluation of treatment outcomes for cocaine dependence.
Arch Gen Psych. 56:507–514. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weil C: The safety of bromocriptine in
long-term use: a review of the literature. Curr Med Res Opin.
10:25–51. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boukriche Y, Weisser I, Aubert P, et al:
MRI findings in a case of late onset disulfiraminduced
neurotoxicity. J Neurol. 247:714–715. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madras BK, Xie Z, Lin Z, et al: Modafinil
occupies dopamine and norepinephrine transporters in vivo and
modulates the transporters and trace amine activity in vitro. J
Pharmacol Exp Ther. 319:561–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Leeuw van Weenen JE, Parlevliet ET,
Maechler P, et al: The dopamine receptor D2 agonist bromocriptine
inhibits glucose-stimulated insulin secretion by direct activation
of the alpha2-adrenergic receptors in beta cells. Biochem
Pharmacol. 79:1827–1836. 2010.
|
|
7
|
Eisenberg DM, Davis RB, Ettner SL, et al:
Trends in alternative medicine use in the United States, 1990–1997
results of a follow-up national survey. JAMA. 280:1569–1575.
1998.
|
|
8
|
Post-White J, Ladas EJ and Kelly KM:
Advances in the use of milk thistle (Silybum marianum).
Integr Cancer Ther. 6:104–109. 2007. View Article : Google Scholar
|
|
9
|
Hernández R and Nazar E: Effect of
silymarin in intrahepatic cholestasis of pregnancy (preliminary
communication). Rev Chil Obstet Ginecol. 47:22–29. 1982.(In
Spanish).
|
|
10
|
Flora K, Hahn M, Rosen H and Benner K:
Milk thistle (Silybum marianum) for the therapy of liver
disease. Am J Gastroenterol. 93:139–143. 1998.
|
|
11
|
Schröder FH, Roobol MJ, Boevé ER, et al:
Randomized, double-blind, placebo-controlled crossover study in men
with prostate cancer and rising PSA: effectiveness of a dietary
supplement. Eur Urol. 48:922–930. 2005.PubMed/NCBI
|
|
12
|
Badisa RB, Darling-Reed SF and Goodman CB:
Cocaine induces alterations in mitochondrial membrane potential and
dual cell cycle arrest in rat c6 astroglioma cells. Neurochem Res.
35:288–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Badisa RB, Goodman CB and Fitch-Pye CA:
Attenuating effect of N-acetyl-L-cysteine against acute cocaine
toxicity in rat C6 astroglial cells. Int J Mol Med. 32:497–502.
2013.PubMed/NCBI
|
|
14
|
Badisa RB, Tzakou O, Couladis M and
Pilarinou E: Cytotoxic activities of some Greek Labiatae herbs.
Phytother Res. 17:472–476. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papini E, Bugnoli M, De Bernard M, et al:
Bafilomycin A1 inhibits Helicobacter pylori-induced
vacuolization of HeLa cells. Mol Microbiol. 7:323–327. 1993.
|
|
16
|
Patel RM and Patel NJ: In vitro
antioxidant activity of coumarin compounds by DPPH, super oxide and
nitric oxide free radical scavenging methods. J Adv Pharm Educ Res.
1:52–68. 2011.
|
|
17
|
Smith IK, Vierheller TL and Thorne CA:
Assay of glutathione reductase in crude tissue homogenates using
5,5′-dithiobis(2-nitrobenzoic acid). Anal Biochem. 175:408–413.
1988.PubMed/NCBI
|
|
18
|
Meyer BN, Ferrigni NR, Putnam JE, et al:
Brine shrimp: A convenient general bioassay for active plant
constituents. Planta Med. 45:31–34. 1982. View Article : Google Scholar
|
|
19
|
McLaughlin JL: The brine shrimp lethality
bioassay. Methods in Plant Biochemistry. Hostettmann K: 6. Academic
Press; London: pp. 8–10. 1991
|
|
20
|
Ipsen J and Feigl P: Bancroft’s
Introduction to Biostatistics. Harper and Row; New York, NY: pp.
2331970
|
|
21
|
Falleh H, Ksouri R, Chaieb K, et al:
Phenolic composition of Cynara cardunculus L. organs and
their biological activities. CR Biol. 331:372–379. 2008.
|
|
22
|
Kukic J, Popovic V, Petrovic S, et al:
Antioxidant and antimicrobial activity of Cynara cardunculus
extracts. Food Chem. 107:861–868. 2008. View Article : Google Scholar
|
|
23
|
Cunha-Oliveira T, Rego AC, Cardoso SM, et
al: Mitochondrial dysfunction and caspase activation in rat
cortical neurons treated with cocaine or amphetamine. Brain Res.
1089:44–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cunha-Oliveira T, Rego AC, Morgadinho MT,
et al: Differential cytotoxic responses of PC12 cells chronically
exposed to psychostimulants or to hydrogen peroxide. Toxicology.
217:54–62. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Badisa RB and Goodman CB: Effect of
chronic cocaine to rat C6 astroglial cells. Int J Mol Med.
30:687–692. 2012.
|
|
26
|
Chiu SY and Kriegler S:
Neurotransmitter-mediated signaling between axons and glial cells.
Glia. 11:191–200. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Araque A: Astrocyte-neuron signaling in
the brain-implications for disease. Curr Opin Investig Drugs.
7:619–624. 2006.PubMed/NCBI
|
|
28
|
Deigner HP, Haberkorn U and Kinscherf R:
Apoptosis modulators in the therapy of neurodegenerative diseases.
Expert Opin Investig Drugs. 9:747–764. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
LaRowe SD, Mardikian P, Malcolm R, et al:
Safety and tolerability of N-acetylcysteine in cocaine dependent
individuals. Am J Addict. 15:105–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baker DA, McFarland K, Lake RW, et al:
N-acetyl cysteine-induced blockade of cocaine-induced
reinstatement. Ann NY Acad Sci. 1003:349–351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Satel SL, Southwick SM and Gawin FH:
Clinical features of cocaine-induced paranoia. Am J Psychiatry.
148:495–498. 1991. View Article : Google Scholar : PubMed/NCBI
|