Introduction
Hepatocellular carcinoma (HCC), accounting for
70–85% of the total liver cancer burden, is one of the most common
malignancies and is the third leading cause of cancer mortality
worldwide, with an estimated >500,000 new cases per year
(1,2). At present, curative therapies,
including resection, liver transplantation and ablation, provide
effective treatment for only a small number of patients presenting
with early stage HCC in the clinic. The majority of patients with
intermediate-advanced HCC are only eligible for the mainstream
palliative treatments, including transarterial chemoembolization
and systemic therapy with molecular targeted drugs (3). However, therapies against liver
cancer to date have not been completely effective. In this context,
the development of new, effective therapeutic approaches for liver
cancer remains one of the most challenging goals in cancer
research.
Numerous traditional Chinese plants have been
identified to possess biological activities with potential
therapeutic applications. Saikosaponin-d (SSD), a saponin
derivative extracted from several species of Bupleurum
(Umbelliferae), has been traditionally used in the treatment
of infectious diseases due to its anti-inflammatory, antipyretic
and analgesic effects (4,5). Previous studies have demonstrated
that SSD also has hepatoprotective, antifibrotic (6,7) and
immunomodulatory (8,9) activities. Furthermore, traditional
use and scientific studies have suggested that SSD is a potential
candidate as an anticancer agent (10,11),
which has been demonstrated to have anti-proliferative and
apoptotic effects on various cancer cells, including human leukemia
cancer, non-small cell lung cancer (12) and hepatic cancer (13). Our previous study demonstrated that
SSD inhibits the proliferation and induces the apoptosis of HCC
SMMC-7721 cells by downregulating cyclooxygenase (COX)-2 at the
mRNA and protein level and inhibiting the production of
prostaglandin E2 (PGE2) (14).
However, the specific mechanism underlying how SSD controls COX-2
expression remains to be elucidated.
COX-2, a key inducible enzyme in prostanoid
biosynthesis, is overexpressed in solid malignancies, including
colon, prostate, breast and HCC (15). A significant negative correlation
between the overexpression of COX-2 and the survival rates of
patients in various types of cancer has been reported in
retrospective studies (16–18).
Inhibiting the activity or expression of COX-2 has shown promise
for tumor therapy in animal models and cancer patients (19,20).
In HCC patients, the protein expression of COX-2 correlates well
with the differentiation grades, suggesting that abnormal COX-2
expression has an important effect in hepatocarcinogenesis
(21). It is well established that
non-steroidal anti-inflammatory drugs (NSAIDs) have anti-tumor
effects by acting on COX-2 (22).
SSD has a similar pharmacological activity to NSAIDs, and it has
been documented that SSD inhibits HCC cell proliferation by
modulating COX-2 expression (14).
However, how SSD regulates the expression of COX-2 remains to be
elucidated.
Materials and methods
Cell culture and reagents
The liver cancer cell lines SMMC-7721 and HepG2,
obtained from the Transform Medical Center of Xi’an Jiaotong
University (Xi’an, China), were cultured in RPMI-1640 medium
(Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (HyClone, Logan, UT, USA) and 1%
penicillin/streptomycin, and incubated at 37°C in a humidified 5%
CO2 atmosphere. The Janus kinase 2 (JAK2) selective
inhibitor AG-490, hypoxia simulator cobalt chloride
(CoCl2), mammalian target of rapamycin (mTOR) and SSD
were obtained from Sigma (Poole, UK). AG-490, rapamycin and SSD
were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St.
Louis, MO, USA), and interleukin-6 (IL-6) was dissolved in acetic
acid (Sigma-Aldrich). For all experiments, final concentrations of
the tested regents were prepared on the day of assessment by
diluting the stock with RPMI-1640 medium and the final
concentration of DMSO was <0.1%, which was not considered to be
harmful to the cells.
Cell proliferation assay
The effect of SSD on cell proliferation was examined
using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. SMMC-7721 cells were plated in 96-well plates
at a density of 5×103 cells per well and were allowed to
grow to 70% confluence. After 24 h, the cells were randomly
separated into four groups and were treated with SSD at 2.5, 5.0,
10 and 15 μg/ml, respectively. After 0, 24, 48 and 72 h, 20 μl of
MTT test solution, which was freshly prepared, was added to each
well. After 4 h incubation, the supernatant was discarded and 150
μl DMSO was added to dissolve the crystal. All analyses were
performed in triplicate. The absorbance was measured on an ELISA
reader (Thermo Fisher Scientific, Waltham, MA, USA) at a test
wavelength of 490 nm. Proliferation inhibition rate (%) = (control
well A490 - experiment well A490) / control well A490 × 100%.
Western blot analysis
Tumor cells were plated in 100 mm cell culture
dishes (Nunc A/S, Roskilde, Denmark) with ~300×104 cells
per dish. When cells grew to 60–70% confluence, they were randomly
separated into different groups to be treated with either
CoCl2, CoCl2 + rapamycin, CoCl2 +
AG490 or CoCl2 + SSD. After 24 h, whole cell protein
extracts were prepared by lysing cells with
radioimmunoprecipitation assay lysis buffer supplemented with a
protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany)
and phosphate inhibitor PhosStop (Roche Diagnostics). Protein
concentration was quantified using the Bradford method. For western
blotting, total cell lysates (~100 μg per lane) were subjected to
SDS-PAGE. The protein was then transferred onto polyvinylidene
difluoride membranes (Millipore Corp., Billerica, MA, USA) using
semi-dry transfer instruments (Bio-Rad Laboratories, Hercules, CA,
USA) at 15 V for 30 min. The membranes were incubated with blocking
buffer (0.05% Tween 20 with 5% nonfat milk) for 1 h at room
temperature followed by anti-COX-2, anti-HIF-1α or
anti-phospho-STAT3 primary rabbit anti-human monoclonal antibody
(Cell Signaling Technology, Inc., Danvers, MA, USA; 1:1,000)
dilution buffer overnight at 4°C. Following washing three times
with washing buffer (blocking buffer without 5% nonfat milk) for 10
min each time, the membranes were incubated with horseradish
peroxidase-labeled goat anti-rabbit secondary antibody (polyclonal;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 1:5,000) for 1
h at 37°C. The membranes were washed again and detection was
performed using an enhanced chemiluminescence western blotting
detection system (Pierce Biotechnology, Inc., Rockford, IL,
USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total cellular RNA was isolated from each of the
experimental groups using TRIzol solution (Invitrogen Life
Technologies). RT was performed on RNA samples, followed by PCR
amplification. For RT, 1.0 μg of the RNA sample was added to 20 μl
of RT reaction mixture (Fermentas, Waltham, MA, USA). The reaction
was performed by treating the samples at 65°C for 5 min, at 42°C
for 60 min and at 70°C for 5 min. PCR was conducted using the
following primers specific for each of the target genes: HIF-1α,
sense 5′-CATTAGAAAGCAGTTCCGCAAGC-3′ and antisense
5′-CAGTGGTAGTGGTGGCATTAGC-3′; COX-2, sense
5′-AGTATCACAGGCTTCCATTGACCAG-3′ and antisense
5′-CCACAGCATCGATGTCACCATAG-3′; β-actin, sense
5′-ATCGTGCGTGACATTAAGGAGAAG-3′ and antisense
5′-AGGAAGGAAGGCTGGAAGAGTG-3′. The PCR was initiated in a thermal
cycle programmed at 94°C for 5 min, 94°C for 30 sec, 58°C for 30
sec, 72°C for 60 sec, and amplified for 30 cycles with HIF-1α and
β-actin, and 35 cycles with COX-2. The amplified products were
visualized on 1.5% agarose gels.
Immunocytochemical staining
Immunocytochemical staining was performed on the
coverslips obtained from the experimental groups. The antibodies
against HIF-1α and COX-2 were purchased from Beijing Biosynthesis
Biotechnology Co., Ltd (Beijing, China) and used according to the
manufacturer’s instructions. Briefly, the coverslips were incubated
for 20 min in 3% H2O2. Following washing with
phosphate-buffer saline, the coverslips were incubated with the
appropriately diluted first antibody (1:400) at 4°C overnight in a
humid chamber, followed by treatment with biotinylated
immunoglobulin for 12 min after washing, and then with
streptavidin/horseradish peroxidase for 12 min at 37°C. The color
reaction was developed using diaminobenzidine working solution
(Tiangen Biotech Co., Ltd., Beijing, China) for 3–5 min and
counterstained with hematoxylin.
Statistical analysis
The results were analyzed for statistical
significance using Student’s t-test between the incubation
conditions of normoxia and hypoxia under multiple exposure
conditions using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
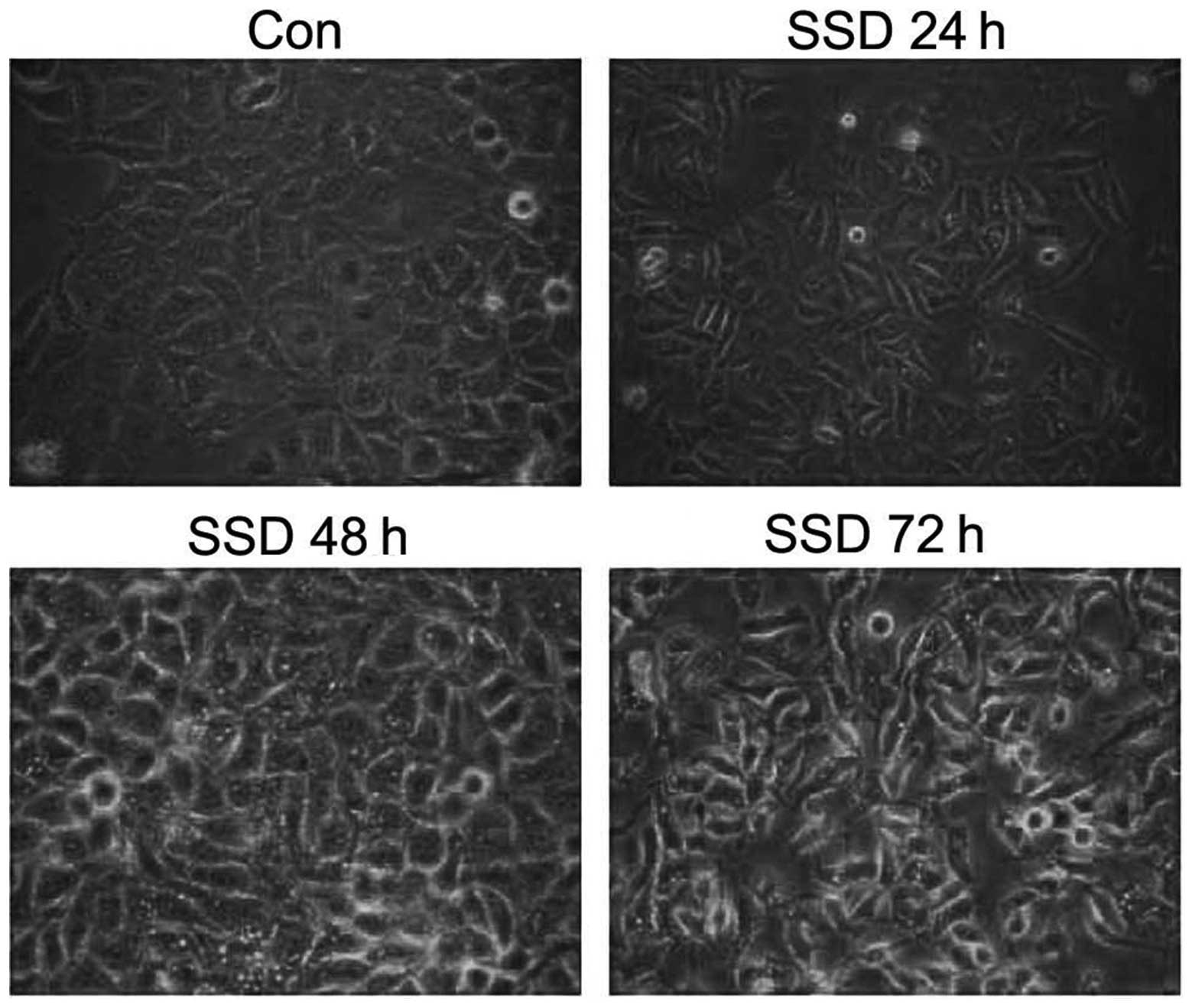
SSD inhibits SMMC-7721 cell proliferation
and alters its cell morphology
MTT assay was used to detect the effect of SSD on
SMMC-7721 cell proliferation. SMMC-7721 cells were treated with SSD
at various concentrations (2.5, 5, 10 and 15 μg/ml) for 0, 24, 48
and 72 h. The results demonstrated that the growth inhibitory
effect of SSD on SMMC-7721 cells was in a time- and dose-dependent
manner (Fig. 1). Following
treatment with 10 μg/ml SSD, SMMC-7721 cell proliferation activity
was significantly reduced. Morphologically, the cells detached from
the bottle and became round. In addition, a transparent vacuolar
structure and pyknosis of the nucleus was observed (Fig. 2). This phenomenon was most clear at
72 h.
HIF-1α is necessary for COX-2 expression
in HCC cells
Hypoxia commonly occurs in solid tumors and HIF-1α
is critical in the hypoxia adaptation process. Several studies have
investigated the importance of COX-2 in tumorigenesis and analysis
has identified COX-2 as a direct target for HIF-1α in colorectal
tumor cells (12,13). Furthermore, COX-2 upregulation
represents a pivotal cellular adaptive response to hypoxia with
implications for colorectal tumor cell survival and angiogenesis.
Therefore, the present study aimed to determine whether COX-2
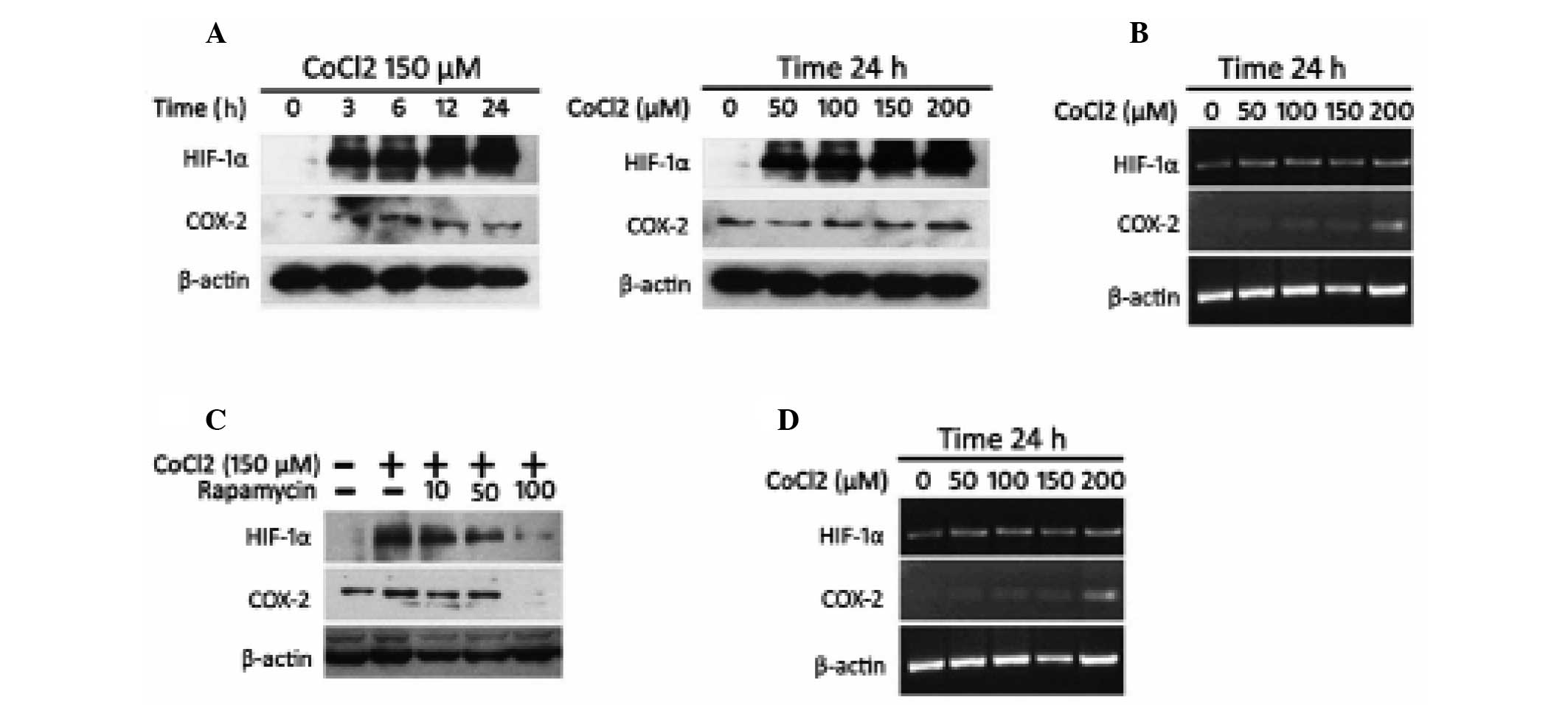
expression was controlled by HIF-1α in HCC. CoCl2 was
able to inhibit the degradation of HIF-1α under normoxic conditions
and is used to simulate hypoxia in experiments (23). SMMC-7721 cells were subjected to
CoCl2-stimulated hypoxia and protein extracts were
prepared over time at several concentrations of CoCl2.
Western blotting revealed that HIF-1α and COX-2 protein levels were
rapidly induced by CoCl2 in a time- and dose-dependent
manner (Fig. 3A). The gene
expression of HIF-1α and COX-2 was also detected. Although HIF-1α
mRNA levels did not alter with different concentrations of
CoCl2, COX-2 mRNA levels increased under
CoCl2-stimulated hypoxic conditions in a dose-dependent
manner in RT-PCR analysis (Fig.
3B).
To further examine the effect of HIF-1α on COX-2
induction, SMMC-7721 cells were treated with rapamycin, a reagent
which could inhibit the synthesis of HIF-1α. Western blotting and
RT-PCR analysis demonstrated that rapamycin eliminated COX-2
upregulation at the protein and mRNA levels under
CoCl2-stimulated hypoxia conditions (Fig. 3C and D). This suggested that HIF-1α
was an upstream regulator of COX-2 expression in HCC.
Inhibition of STAT3 phosphorylation
reduces the expression of HIF-1α and COX-2
The activated form of STAT3 (p-STAT3) is highly
expressed in several malignancies, which has been demonstrated to
induce HIF-1α protein synthesis in human breast tumor MCF-7 cells
(24). However, the association
between COX-2, HIF-1α and p-STAT3 in HCC cells remains to be
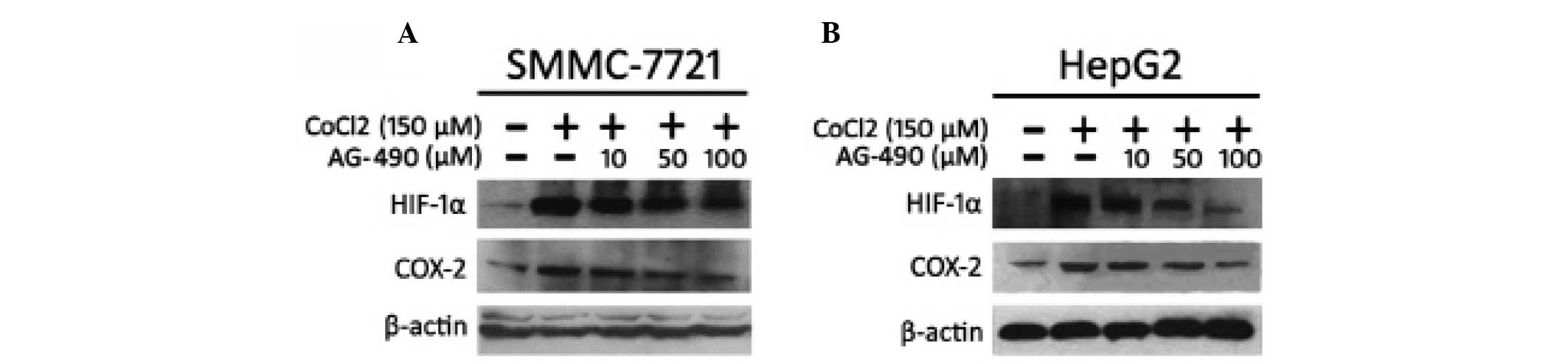
elucidated. In order to determine the association between p-STAT3
and HIF-1α/COX-2, SMMC-7721 cells were treated with AG-490 for 30
min prior to the addition of CoCl2. AG-490 is the
selective inhibitor of JAK2, which can inhibit the activation of
STAT3 (25). The results
demonstrated that AG-490 resulted in the downregulation of HIF-1α
and COX-2 at the protein level under the hypoxic conditions
simulated by CoCl2 (Fig.
4A). In order to confirm this effect, this was repeated on
HepG2 cells and the result was the same as that observed in
SMMC-7721 cells (Fig. 4B).
Effects of SSD on the protein expression
of p-STAT3, HIF-1α and COX-2
In order to determine the mechanism of SSD targeting
in HCC cells, the expression of COX-2, HIF-1α and p-STAT3 was
determined by immunocytochemistry following SSD treatment. The
results suggested that p-STAT3 staining in SMMC-7721 tumor cells
demonstrated nuclear localization, with HIF-1α located in the
cytoplasm and particularly in the nucleus, and COX-2 expressed in
the cytoplasm and nuclear membrane. SSD not only significantly
reduced the expression of HIF-1α and COX-2 induced by
CoCl2, but also deceased the expression of p-STAT3 and
COX-2 induced by IL-6 (Fig. 5).
These results were partially verified by western blotting, which
indicated that SSD inhibited the protein expression of COX-2,
HIF-1α and p-STAT3 (Fig. 6).
Discussion
HCC is one of the most common types of malignancy
worldwide, the incidence and mortality rate of HCC are extremely
high in Asia and have also increased rapidly in the United States
(1,2). Due to the occult onset of HCC, the
majority of HCC patients are at an advanced stage when diagnosed
and to date there remains no completely effective therapy for HCC
(3). Numerous active compounds
extracted from traditional plants, including SSD, have been
demonstrated to have anti-tumor activities (10,11).
SSD could not only inhibit growth and differentiation of human
leukemia cells (26) and glioma
cells (27), but was also be able
to increase the radiosensitivity of HCC SMMC-7721 cells by
adjusting the G0/G1 and G2/M
checkpoints of the cell cycle (28). The present study demonstrated that
SSD inhibited COX-2 expression through the STAT3/HIF-1α signaling
pathway, which may be the specific antitumor mechanism of SSD.
COX, the key enzyme for prostanoid biosynthesis, has
two isoforms: COX-1 and COX-2. COX-1 is constitutively expressed in
several tissues and cell types, whereas COX-2 is an inducible
enzyme expressed only in response to certain stimuli. COX-2 is
overexpressed in a subset of malignant tumors and accumulating
evidence suggests that COX-2 may be important in tumorigenesis
through multiple mechanisms (15).
Previous studies have confirmed that COX-2 is not only
overexpressed in HCC, but also correlates well with the
differentiation grades of HCC (21). Our previous study (14) found that SSD inhibited COX-2
expression in HCC SMMC-7721 cells, which confirmed the hypothesis
that SSD, with similar pharmacological activities as NASIDs, could
inhibit SMMC-7721 proliferation through the COX-2 pathway.
The association between tumors and microenvironments
has attracted more attention (29). The hypoxic microenvironment, one of
the basic features in solid tumors, characterized by deficiency in
oxygen and nutrients, leads to epigenetic and genetic adaptation of
clones and an increase in invasiveness and metastasis (30). These hypoxic adaptations, including
increasing vascularization, activation of proto-oncogenes,
increasing glucose transportation and inducing glycolytic enzymes
and various apoptotic-related genes make the tumors more difficult
to treat and confers increased resistance to chemotherapy and
radiotherapy (31). It is believed
that the HIF-1 complex, composed of a heterodimer pair of HIF-1α
and HIF-1β is important in mediating these adaptations. At present,
HIF-1α has emerged as an important transcription factor in cancer
biology and is expressed in the early stages of several types of
human malignant tumor, including HCC (32). Csiki et al (33) demonstrated that COX-2 is
upregulated in hypoxic lung cancer cells in an HIF-1-dependent
manner. Another study provided the first evidence, to the best of
our knowledge, demonstrating that HIF-1 directly binds a specific
hormone response element located at the COX-2 promoter (34). Dai et al (35) demonstrated a positive correlation
between HIF-1α and COX-2 in HCC. The present study found that
hypoxia, imitated by CoCl2, could induce the expression
of HIF-1α accompanied by the protein level of COX-2 in SMMC-7721
cells. Rapamycin, a selective inhibitor of mTOR, could inhibit the
expression of HIF-1α and the COX-2 protein, and SSD had a similar
effect. The results of the present study were consistent with
previous studies, demonstrating that HIF-1α was obligatory for
COX-2 expression in HCC cells and is possibly an important upstream
factor for COX-2.
The level of HIF-1α can be regulated not only by
hypoxia through a ubiquitin-proteasome pathway, but can also be
modulated by several other pathways. The JAK/STAT3 pathway appears
to be important in modulating HIF-1α expression. Activated STAT3
can increase HIF-1α protein levels by inhibiting HIF-1α degradation
and accelerating its de novo synthesis in ischemic rat
kidneys and hypoxic human renal carcinoma cells (36). STAT3 knockout eliminates estrogen
receptor-α-induced HIF-1α and subsequent vascular endothelial
growth factor (VEGF) production (37). In human breast cancer MCF-7 cells,
STAT3 regulates HIF-1α, and targeting STAT3 with siRNA knockdown
inhibits CoCl2-mediated HIF-1α nuclear accumulation and
recruitment on the VEGF promoter (38). The present study demonstrated that
activated STAT3 was involved in the expression of HIF-1α in HCC
cells, and p-STAT3 was able to increase HIF-1α protein levels but
not mRNA levels. This suggested that activation of STAT3 modulated
HIF-1α expression through transcriptional or posttranscriptional
mechanisms in HCC. The results of the present study suggested that
p-STAT3 may be the upstream regulator of HIF-1α and COX-2 and that
there was a p-STAT3/HIF-1α/COX-2 signal transduction pathway in HCC
SMMC-7721 cells. In the present study, SSD inhibited SMMC-7721
growth accompanied by a reduction in the expression of p-STAT3,
HIF-1α and COX-2. This indicated that SSD may suppress HCC
SMMC-7721 proliferation by inhibiting the expression of COX-2
through the p-STAT3/HIF-1α signaling pathway.
In conclusion, the present study provided primary
evidence that HIF-1α promoted COX-2 expression under hypoxic
conditions in HCC cells and HIF-1α was induced by activated STAT3.
SSD may suppress HCC SMMC-7721 cell proliferation by inhibiting the
expression of COX-2 through the p-STAT3/HIF-1α signaling
pathway.
Acknowledgements
The authors would like to thank the staff of the
Endemic Laboratory, Xi’an Jiaotong University for their technical
assistance in these studies. This study was supported by the
Chinese National Natural Science Foundation (grant no.
30771895).
References
|
1
|
Montalto G, Cervello M, Giannitrapani L,
Dantona F, Terranova A and Castagnetta LA: Epidemiology, risk
factors, and natural history of hepatocellular carcinoma. Ann NY
Acad Sci. 963:3–20. 2002.PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu CN, Yuan ZG, Zhang XL, et al:
Saikosaponin a and its epimer saikosaponin d exhibit
anti-inflammatory activity by suppressing activation of NF-κB
signaling pathway. Int Immunopharmacol. 14:121–126. 2012.PubMed/NCBI
|
|
5
|
Hattori T, Nishimura H, Kase Y and Takeda
S: Saireito and saikosaponin D prevent urinary protein excretion
via glucocorticoid receptor in adrenalectomized WKY rats with
heterologous-phase anti-GBM nephritis. Nephron Physiol. 109:19–27.
2008. View Article : Google Scholar
|
|
6
|
Fan J, Li X, Li P, et al: Saikosaponin-d
attenuates the development of liver fibrosis by preventing
hepatocyte injury. Biochem Cell Biol. 85:189–195. 2007.PubMed/NCBI
|
|
7
|
Dang S, Wang B, Cheng Y, Song P, Liu Z and
Li Z: Inhibitory effects of saikosaponin-d on CCl4-induced hepatic
fibrogenesis in rats. World J Gastroenterol. 13:557–563. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kato M, Pu M, Isobe K, et al:
Characterization of the immunoregulatory action of saikosaponin-d.
Cell Immunol. 159:15–25. 1994. View Article : Google Scholar
|
|
9
|
Wong VK, Zhou H, Cheung SS, Li T and Liu
L: Mechanistic study of saikosaponin-d (Ssd) on suppression of
murine T lymphocyte activation. J Cell Biochem. 107:303–315. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Man S, Gao W, Zhang Y, Huang L and Liu C:
Chemical study and medical application of saponins as anti-cancer
agents. Fitoterapia. 81:703–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bachran C, Bachran S, Sutherland M,
Bachran D and Fuchs H: Saponins in tumor therapy. Mini Rev Med
Chem. 8:575–584. 2008. View Article : Google Scholar
|
|
12
|
Hsu Y, Kuo P and Lin C: The proliferative
inhibition and apoptotic mechanism of Saikosaponin D in human
non-small cell lung cancer A549 cells. Life Sci. 75:1231–1242.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu Y, Kuo P, Chiang L and Lin C:
Involvement of p53, nuclear factor kappaB and Fas/Fas ligand in
induction of apoptosis and cell cycle arrest by saikosaponin d in
human hepatoma cell lines. Cancer Lett. 213:213–221. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He SX, Luo JY, Zhao G, et al: Effect of
saikosaponins-d on cyclooxygenase-2 expression of human
hepatocellular carcinoma cell line smmc-7721. Zhonghua Gan Zang
Bing Za Zhi. 14:712–714. 2006.(In Chinese).
|
|
15
|
Ristimaki A: Cyclooxygenase 2: from
inflammation to carcinogenesis. Novartis Found Symp. 256:215–269.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bin W, He W, Feng Z, et al: Prognostic
relevance of cyclooxygenase-2 (COX-2) expression in Chinese
patients with prostate cancer. Acta Histochem. 113:131–136. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsubayashi H, Infante JR, Winter JM, et
al: Tumor COX-2 expression and prognosis of patients with
resectable pancreatic cancer. Cancer Biol Ther. 6:1569–1575. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mascaux C, Martin B, Paesmans M, et al:
Has Cox-2 a prognostic role in non-small-cell lung cancer? A
systematic review of the literature with meta-analysis of the
survival results. Br J Cancer. 95:139–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harris RE: Cyclooxygenase-2 (COX-2)
blockade in the chemoprevention of cancers of the colon, breast,
prostate, and lung. Inflammopharmacology. 17:55–67. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harris RE, Beebe-Donk J and Alshafie GA:
Cancer chemoprevention by cyclooxygenase 2 (COX-2) blockade:
results of case control studies. Subcell Biochem. 42:193–212. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bae SH, Jung ES, Park YM, et al:
Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma
and growth inhibition of hepatoma cell lines by a COX-2 inhibitor,
NS-398. Clin Cancer Res. 7:1410–1418. 2001.PubMed/NCBI
|
|
22
|
Cha YI and DuBois RN: NSAIDs and cancer
prevention: Targets downstream of COX-2. Annu Rev Med. 58:239–252.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan Y, Hilliard G, Ferguson T and
Millhorn DE: Cobalt inhibits the interaction between
hypoxia-inducible factor-alpha and von Hippel-Lindau protein by
direct binding to hypoxia-inducible factor-alpha. J Biol Chem.
278:15911–15916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Q, Briggs J, Park S, et al: Targeting
Stat3 blocks both HIF-1 and VEGF expression induced by multiple
oncogenic growth signaling pathways. Oncogene. 24(36): 5552–5560.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu JH, Kim KH and Kim H: Suppression of
IL-1beta expression by the Jak 2 inhibitor AG490 in
cerulein-stimulated pancreatic acinar cells. Biochem Pharmacol.
72:1555–1562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bu S, Xu J and Sun J: Effect of
saikosaponin-d on up-regulating GR mRNA expression and inhibiting
cell growth in human leukemia cells. Zhongguo Zhong Xi Yi Jie He Za
Zhi. 20:350–352. 2000.(In Chinese).
|
|
27
|
Tsai YJ, Chen I, Horng LY and Wu RT:
Induction of differentiation in rat C6 glioma cells with
Saikosaponins. Phytother Res. 16:117–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang B, Dai Z, Wang X, et al:
Saikosaponin-d increases the radiosensitivity of smmc-7721
hepatocellular carcinoma cells by adjusting the g0/g1 and g2/m
checkpoints of the cell cycle. BMC Complement Altern Med.
13:2632013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartkowiak K, Riethdorf S and Pantel K:
The interrelating dynamics of hypoxic tumor microenvironments and
cancer cell phenotypes in cancer metastasis. Cancer Microenviron.
5:59–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng S, Chen X, Yin X and Zhang B:
Prognostic significance of HIF-1α expression in hepatocellular
carcinoma: a meta-analysis. PLoS One. 8:e657532013.
|
|
33
|
Csiki I, Yanagisawa K, Haruki N, et al:
Thioredoxin-1 modulates transcription of cyclooxygenase-2 via
hypoxia-inducible factor-1alpha in non-small cell lung cancer.
Cancer Res. 66:143–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaidi A, Qualtrough D, Williams AC and
Paraskeva C: Direct transcriptional up-regulation of
cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes
colorectal tumor cell survival and enhances HIF-1 transcriptional
activity during hypoxia. Cancer Res. 66:6683–6691. 2006. View Article : Google Scholar
|
|
35
|
Dai C, Gao Q, Qiu S, et al:
Hypoxia-inducible factor-1 alpha, in association with inflammation,
angiogenesis and MYC, is a critical prognostic factor in patients
with HCC after surgery. BMC Cancer. 9:4182009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jung JE, Lee HG, Cho IH, et al: STAT3 is a
potential modulator of HIF-1-mediated VEGF expression in human
renal carcinoma cells. FASEB J. 19:1296–1298. 2005.PubMed/NCBI
|
|
37
|
Wang M, Tan J, Coffey A, Fehrenbacher J,
Weil BR and Meldrum DR: Signal transducer and activator of
transcription 3-stimulated hypoxia inducible factor-1 alpha
mediates estrogen receptor-alpha-induced mesenchymal stem cell
vascular endothelial growth factor production. J Thorac Cardiovasc
Surg. 138:163–171. 2009. View Article : Google Scholar
|
|
38
|
Cascio S, D’Andrea A, Ferla R, et al:
miR-20b modulates VEGF expression by targeting HIF-1 alpha and
STAT3 in MCF-7 breast cancer cells. J Cell Physiol. 224:242–249.
2010.PubMed/NCBI
|