Introduction
It has been well-documented that fetuses and adults
have different strategies for cutaneous wound repair. In adults,
wound healing is characterized by intense inflammation and scar
formation; whereas, in fetuses in the first two trimesters (at
early- and mid-gestation), there is a diminished inflammatory
response, decreased angiogenesis, and the absence of contraction or
scar formation during the response to tissue injury (1–4).
Previous research has concentrated on understanding the mechanisms
underlying these different repair strategies, with the aim of
providing clinical benefits for adult patients. Wound healing
involves the recruitment of numerous cell types to the wound area
in a temporally- and spatially-defined manner (5). Re-epithelialization largely coincides
with the recruitment of dermal fibroblasts, and it is likely that
the interaction between epidermal keratinocytes (KCs) and dermal
fibroblasts is important during the rebuilding of tissue integrity
(6). Healing of extensive wounds
often results in excessive scarring, disgorging, and functional
impairment of the affected area (7). This is particularly significant in
the healing of large burns, and early re-epithelialization or
coverage of the wounded area with autologous skin grafts limits the
excessive deposition of connective tissue. The co-culture of KCs
with fibroblasts has previously been demonstrated to stimulate
paracrine loops of cytokine activation between the two cell types,
which is a phenomenon that may also occur in vivo to
regulate cellular function (8).
Fetal KCs are important skin cells, and numerous
studies have indicated that they may be involved in fetal skin
wound healing (9–11). However, the underlying mechanisms
remain to be elucidated. Therefore, the aim of the present study
was to investigate the effects of human fetal KCs, specifically the
role of their secreted growth factors, on fetal and adult human
dermal fibroblasts, in association with crucial parameters of
tissue repair. The effects of human fetal epidermal KCs on the
initial proliferative and migratory responses of dermal fibroblasts
to injury were determined, as well as the signaling pathways
involved.
Materials and methods
Cell culture
Fetal KCs and fibroblasts were obtained from
full-thickness fetal skin specimens of miscarried fetuses
(gestational age 20–23 weeks; three males and two females). Adult
fibroblasts were isolated from the dermis of adult donors (aged
20–30 years; three males and two females), undergoing surgical
debridement. The experiments of the present study were conducted
according to the Ethical Rules for Human Experimentation, as stated
in the 1975 Declaration of Helsinki, and were approved by the
Ethics Committee of the Shengjing Hospital of China Medical
University (Shengyang, China). Primary cultures of KCs and
fibroblasts were prepared as described by previous methods
(12–14). Briefly, the full-thickness skin
samples were incubated at 4°C overnight in Dispase II (Roche
Diagnostics, Indianapolis, IN, USA), and the dermal components were
isolated by collagenase (1 mg/ml; Sigma-Aldrich, St Louis, MO, USA)
digestion. The dermal components were then minced into 2 mm pieces
using scissors, and the isolated fibroblasts were cultured in
Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; Invitrogen Life Technologies). Following 0.25% trypsin
digestion, the cultures of the released primary KCs from the fetal
epidermis were initiated using tissue culture flasks coated with
collagen (BD Biosciences, Franklin Lakes, NJ, USA) in Epilife™
growth medium (Life Technologies Ltd., Paisley, UK) supplemented
with 1% human keratinocyte growth supplement (Life Technologies).
At passage three, the KCs were co-cultured with fetal and adult
fibroblasts, and used for further analysis and
characterization.
Co-culture of human fetal and adult
dermal fibroblasts with fetal epidermal KCs
The adult and fetal fibroblasts were co-cultured
with fetal KCs in Transwell® chambers with 3 μm pore
filters (Costar®; Corning Incorporated, Corning, NY,
USA) for 4 days. The fibroblasts were also cultured alone in DMEM
supplemented with 10% FBS, this culture served as the control.
Cell proliferation assay
At days 2, 3 and 4 after the initiation of the
co-culture, the proliferation of the dermal fibroblasts was
measured using a tetrazolium reagent WST-1 Cell Counting kit
(Beyotime Institute of Biotechnology, Haimen, China). The
fibroblasts were incubated with WST-1 for 4 h at 37°C. The staining
intensity in the medium was determined by measuring the absorbance
at a wavelength of 450 nm, using a Safire2 microplate
reader (Tecan AG, Männedorf, Switzerland), and the data were
expressed as ratios of the control value. The data were compiled
from three independent experiments, each performed in
duplicate.
Immunofluorescence assay
Immunostaining of the KCs and fibroblasts was
performed according to a standard protocol as previously described
(12). The primary antibodies used
for immunostaining were mouse monoclonal cytokeratin 14 (CK14;
ab9220; 1:200) and mouse monoclonal vimentin (VIM; ab8978; 1:300)
(Abcam, Cambridge, MA, USA). All of the samples were imaged using
an Olympus FK-40 fluorescence microscope (Olympus Corporation,
Tokyo, Japan).
Cell cycle analysis
A cell cycle analysis was conducted according to
standard protocols (15). Briefly,
the cells were trypsinized with 0.05% trypsin-EDTA (Sigma-Aldrich)
and fixed with 70% ethanol (Sigma-Aldrich). The cells were then
incubated with RNase-A (50 μg/ml; Sigma-Aldrich) and propidium
iodide (25 μg/ml; Sigma-Aldrich) in phosphate-buffered saline (PBS)
containing 0.1% Triton X-100 at 106/ml (Sigma-Aldrich),
for 25 min in the dark. Analysis of the cells in the different
phases of the cell cycle was performed using flow cytometry. A
minimum of 10,000 cell events per assay were acquired using a
FACSCalibur™ flow cytometer (BD Biosciences).
Transwell migration assay
Fibroblast migration was measured using a
Transwell® migration assay (8 μm pore size; Corning
Costar), as previously described by Liu et al (14). The cells were randomly selected
from eight regions per well and counted.
Western blotting
Fibroblasts were washed three times with ice-cold
PBS prior to extraction of protein. Western blot analysis was
performed according to standard protocols, as previously described
(14). The primary antibodies used
in the present study were: Rabbit polyclonal mouse double minute 2
homolog (MDM2; 1:1,000; ab58530; Abcam), rabbit polyclonal cyclin
B1 (1:1,000; ab7957; Abcam), rabbit polyclonal AKT (1:1,000; #9272;
Cell Signaling Technology, Danvers, MA, USA), mouse polyclonal
extracellular signal-regulated kinase (ERK; 1:1,000; #9102; Cell
Signaling Technology), mouse monoclonal matrix metalloproteinase
(MMP)-2 (1:1,000; #4022; Cell Signaling Technology), rabbit
polyclonal C-X-C chemokine receptor 4 (CXCR4; ab2074; 1:1,000;
Abcam), mouse polyclonal phospho-AKT (1:1,000; sc-42940; Santa Cruz
Biotechnology Inc., Dallas, TX, USA), rabbit polyclonal MMP-9
(1:1,000; ab38898; Abcam), rabbit polyclonal phospho-cyclin
dependent kinase (CDK)1 (1:1,000; #9111s; Cell Signaling
Technology), rabbit polyclonal CDK1 (1:1,000; sc-53219; Santa Cruz
Biotechnology), rabbit polyclonal phospho-ERK (1:1,000; #9106s;
Cell Signaling Technology), mouse polyclonal phospho-p38
mitogen-activated protein kinase (MAPK; 1:1,000; sc-7973; Santa
Cruz Biotechnology Inc.), and rabbit polyclonal GAPDH (1:2,000;
ab37168; Abcam).
Statistical analysis
Statistical analyses were performed using SPSS
version 19.0 software (SPSS Inc., Chicago, IL, USA). The data
represent the mean ± standard deviation. Comparisons between the
groups were analyzed using Student’s t test, and P<0.05 was
considered to indicate a statistically significant difference. The
data presented in the figures are representative of three
independent experiments
Results
Identification of fetal epidermal
KCs
The primary fetal KCs formed radiation-shaped
colonies at 6–7 days post-culture (Fig. 1A). The majority of the cells
acquired a typical “paving stone” shape and reached near-confluence
at 14 days post-culture (Fig. 1B).
In addition, specific green fluorescence was observed in the cells,
indicating the expression of the typical KC surface marker CK14
(Fig. 1C).
Identification of fetal and adult dermal
fibroblasts
At 3–7 days after the dermal tissue parts were
plated, numerous triangular and spindle cells were shown to
dissociate from the tissues (Fig.
1D). By day 10, the majority of the cells had acquired a long
spindle shape and had reached near-confluence (Fig. 1E). At passage three, the
fibroblasts exhibited a spindle shape and, upon reaching
confluence, formed a ‘whirlpool-like’ pattern. Fetal and adult
fibroblasts were negative for CK14, but positively expressed VIM
(Fig. 1F), as determined by
immunohistochemistry. These results confirm the purity of the
isolated fibroblasts.
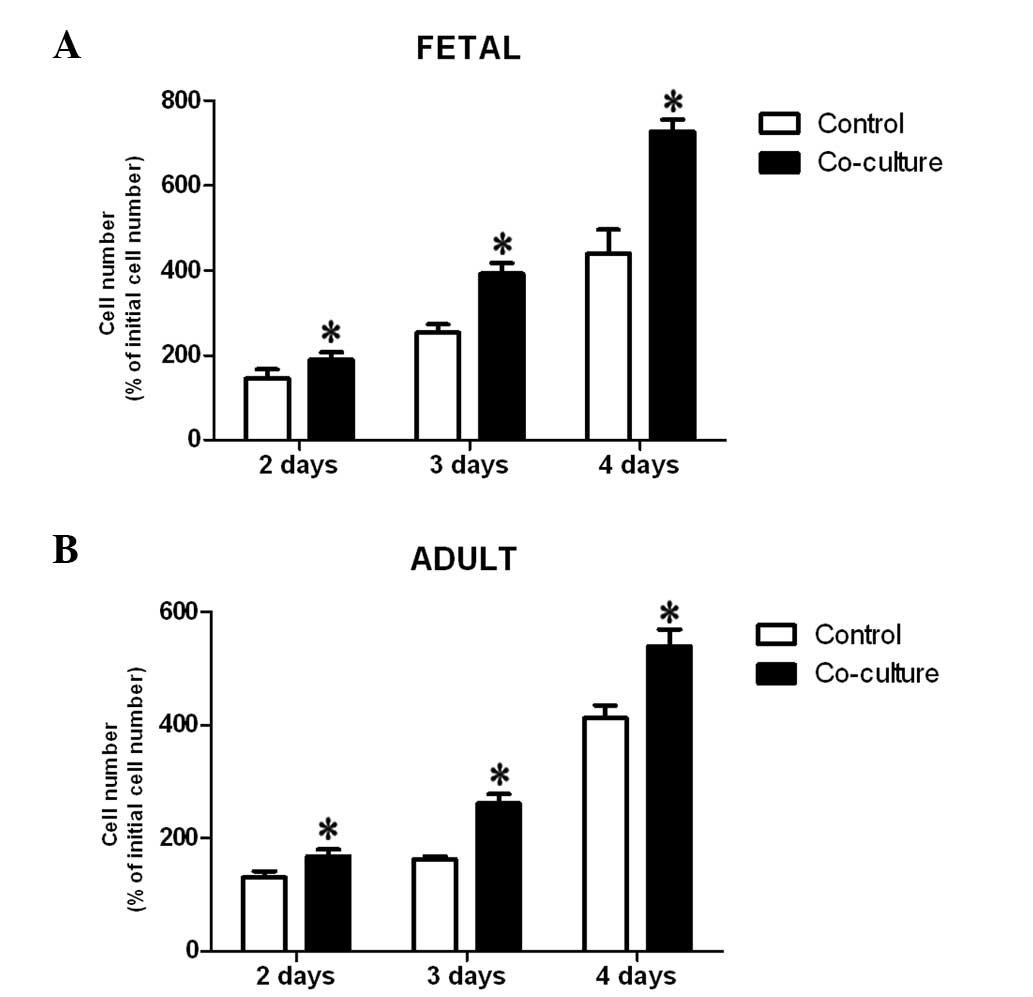
Fetal KCs promote the proliferation of
fetal and adult dermal fibroblasts
To determine whether fetal epidermal KCs exhibited
any biological effects on the proliferation of fibroblasts, a WST-1
analysis was conducted. Quantitative analysis showed that
co-culture of fetal and adult dermal fibroblasts with fetal
epidermal KCs resulted in a marked acceleration of fibroblast
proliferation at days 2, 3 and 4 of culture, as compared with the
control cells (Fig. 2A and B). A
cell cycle analysis of the co-cultured fetal and adult dermal
fibroblasts was also performed. The distribution of S and
G2/M phase fibroblasts was higher in the fibroblasts
co-cultured with the fetal KCs, as compared with the control
fibroblasts (Table I).
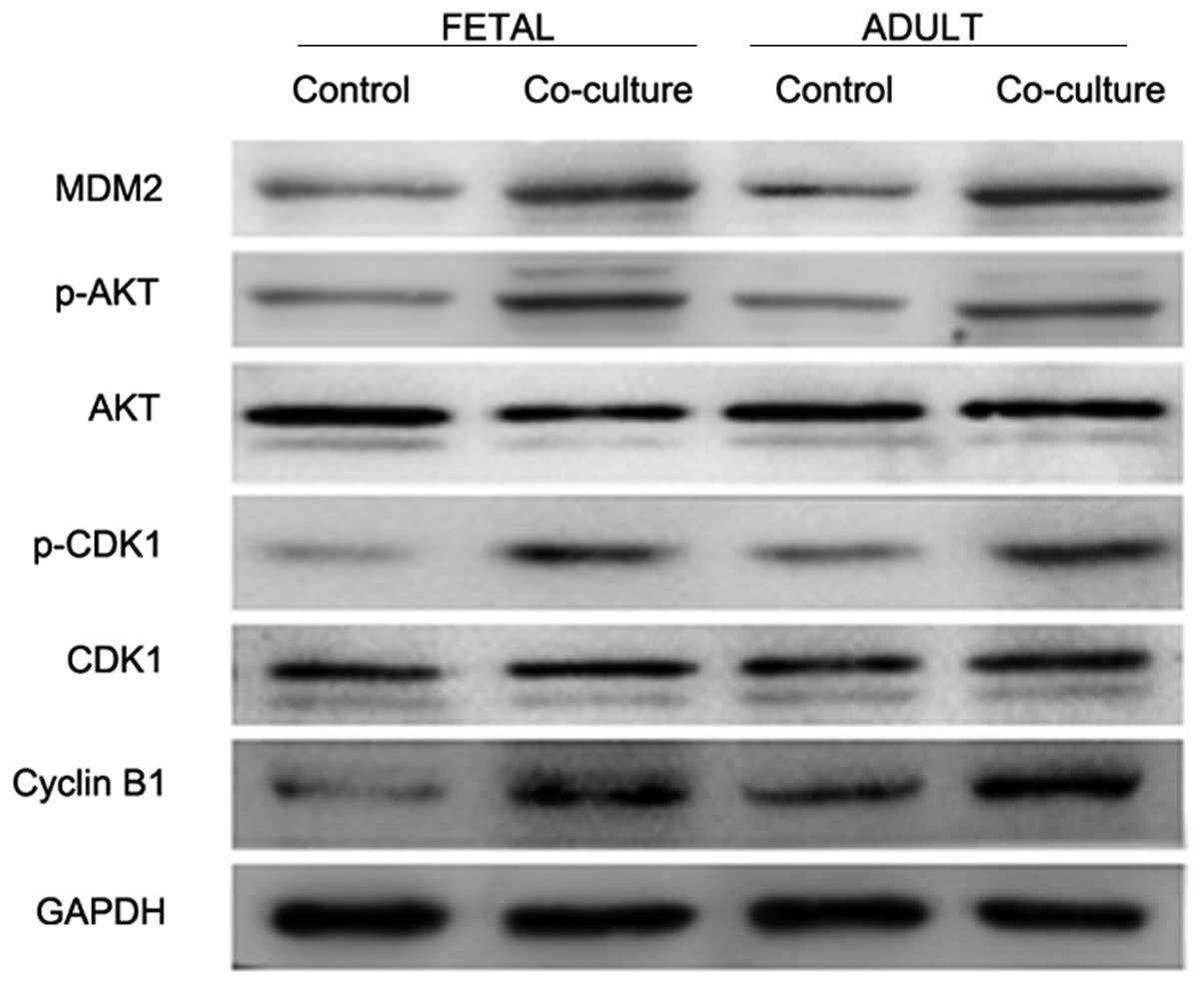
Furthermore, western blotting was performed in order to determine
the expression levels of cell survival and proliferation
regulators. The protein expression levels of MDM2, phospho-AKT,
phospho-CDK1 and cyclin B1 were upregulated in the co-cultured
fibroblasts, as compared with the control cells (Fig. 3).
 | Table ICell cycle analysis of the co-culture
of human skin fibroblasts with fetal KCs. |
Table I
Cell cycle analysis of the co-culture
of human skin fibroblasts with fetal KCs.
| Cell cycle phase
distribution (%) |
|---|
|
|
|---|
|
G0/G1 | S | G2/M |
|---|
| Fetal
fibroblasts |
| Control | 63.5 | 19.2 | 17.3 |
| Co-culture with
fetal KCs | 40.2 | 32.4 | 27.4 |
| Adult
fibroblasts |
| Control | 72.0 | 15.9 | 12.1 |
| Co-culture with
fetal KCs | 48.7 | 25.1 | 26.2 |
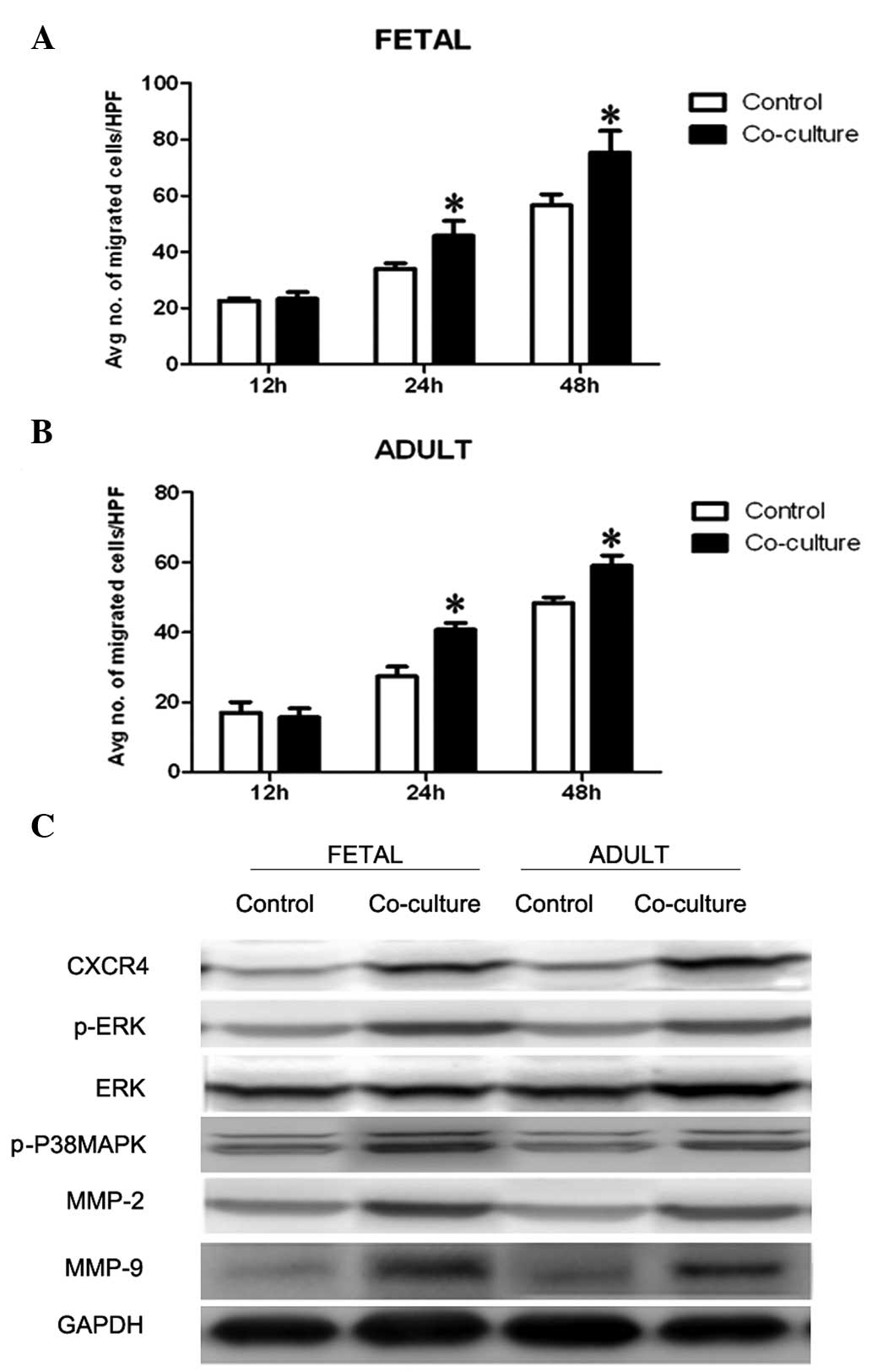
Fetal KCs promote the migration of fetal
and adult dermal fibroblasts
The migratory ability of fibroblasts was measured
using Transwell® assays. The fibroblasts migrated to the
bottom chamber and were stained at 4 days after co-culture
initiation. Quantitative analysis confirmed that the migration of
the fetal and adult dermal fibroblasts to the bottom chamber was
greater in the presence of fetal KCs, as compared with the control
fibroblasts (Fig. 4A).
Furthermore, western blotting demonstrated that fibroblasts
co-cultured with fetal KCs had increased expression levels of the
cell migration regulators CXCR4, phospho-ERK, and phospho38MAPK,
MMP-2 and MMP-9 (Fig. 4B).
Discussion
Numerous studies have investigated the dynamics of
epidermal KCs and dermal fibroblasts in co-culture, along with the
influence of KCs on fibroblast proliferation and migration, two
crucial steps in the wound healing process (10). However, in numerous studies, the
KCs and fibroblasts used were derived from various species at
different ages, and were sometimes tested in heterologous assay
systems (16–20). In addition, previous research has
not always focused on fetal skin healing, but on other functions of
KCs in wound healing. A previous study demonstrated that KCs from
the juvenile human foreskin were not able to stimulate the
proliferation and migration of dermal fibroblasts from the juvenile
human foreskin (16). Conversely,
human KCs were shown to stimulate the proliferation and migration
of WS1 human fibroblasts in a three-dimensional extracellular
matrix (ECM) (17). Furthermore,
conditioned medium from cultured human KCs was shown to stimulate
DNA synthesis in various human cell types (18,19),
and it has been shown that human KCs have a positive effect on
adult skin wound healing. In a previous study it was demonstrated
that KCs from newborn mice have an overall anti-fibrotic influence
on fetal and postnatal fibroblasts in co-culture conditions
(10). This result is in
concordance with the findings of the present study.
In the present study, the co-culture of fetal and
adult dermal fibroblasts with human fetal leg epidermal KCs
significantly increased the proliferation of the two types of
fibroblasts. Cutaneous wound repair is dependent on the
proliferation of dermal fibroblasts, and these data suggest that
mid-gestational leg KCs may stimulate fibroblast proliferation by
upregulation of genes associated with the regulation of DNA
synthesis. The results of the present study are concordant with
previous observations of enhanced proliferation of fibroblasts
(16). The protein expression
levels of phospho-AKT and MDM2 in the co-cultured fibroblasts were
markedly greater in the presence of fetal KCs. AKT and MDM2 are
established key regulators of cell survival and proliferation.
Activation of the phosphoinositide 3-kinase (PI3K)-AKT pathway has
been shown to have diverse roles in fibroblasts, and the PI3K-AKT
signaling pathway is known to activate a survival signal in cells,
allowing them to proliferate and counteract apoptotic stimuli.
Activation of the AKT signaling pathway modulates cell
proliferation by transcriptional regulation of numerous genes
(21,22). PI3K is partly involved in
facilitating cell viability by β1 integrin interaction with the
ECM, in response to mechanical forces in skin fibroblasts.
Furthermore, the PI3K-AKT pathway stimulates collagen synthesis by
actively promoting cell spreading and activation of the
platelet-derived growth factor (23). The MDM2 protein facilitates
G1-to-S phase transition by activating E2F1 and may
enhance cell survival by suppressing the function of wild-type p53.
Previous studies have shown that MDM2 activity is regulated by the
NF-κB family of transcription factors (24–27).
To examine whether fetal KCs contributed to positive
effects on the cell cycle, analysis of the cells in the different
phases of the cell cycle was performed using flow cytometry. The
expression levels of cell cycle regulatory components CDK1 and
cyclin B1 were also evaluated. The results demonstrated that in the
co-culture group, there were more fibroblasts in the S and
G2/M phases, as compared with the control group. The
co-cultured fibroblasts also exhibited markedly increased
expression levels of phospho-CDK1 and cyclin B1. CDK1 is known to
have a critical role in cell cycle regulation, through controlling
the progression of G1 to S, and G2 to M phase
(28). CDKs and their
corresponding cyclins form cyclin-CDK complexes that regulate cell
cycle progression (29).
Specifically, cyclin B1 is a regulatory protein involved in mitosis
that forms a complex with CDK1 to form the maturation-promoting
factor. Cyclin B1-CDK1 is involved in the early events of mitosis,
such as chromosome condensation, nuclear envelope breakdown and
spindle assembly.
The migratory ability of fibroblasts is a key factor
in determining the efficiency of skin wound healing. Therefore, the
present study investigated the influence of fetal KCs on the
migratory ability of fibroblasts. One of the crucial limitations of
wound healing is attributed to poor migration from adjacent healthy
sites to the injured regions. Therefore, increasing the migratory
ability of fibroblasts is one of the most promising approaches to
improve the efficiency of skin wound healing (30,31).
The present study showed that co-culture with fetal KCs induced a
significant increase in fibroblast cell migration. Previous studies
have shown that CXCR4 and its receptor AKT, along with ERK and p38
MAPK are key mediators of fibroblast migration (32–39).
It has previously been reported that MMP-2 and MMP-9
are also regulators of fibroblast migration (40). MMPs are members of the
zinc-dependent endopeptidase family and have an important role in
ECM turnover. The present study demonstrated that co-culture with
fetal KCs also increased the protein expression levels of MMP-2 and
MMP-9.
In conclusion, the present study demonstrated that
fetal KCs may promote the proliferation and migration of fetal and
adult fibroblasts. The authors of the present study are currently
investigating the effects of fetal KCs on other important
parameters of skin wound healing, such as cell migration,
contraction, and ECM accumulation and organization; and aim to
determine whether keratinocyte growth factor, or other cytokines
have a role in this important physiological process. The
identification of the cytokine components that may contribute to
complete tissue regeneration, which is a characteristic of wound
healing in fetuses, is of clinical importance for treating adult
wounds.
Acknowledgements
This study was supported by the National Basic
Science and Development Program (973 Program grant no.
2012CB518103), the National Natural Science Foundation (grant no.
81370883), the Liaoning Province Science (grant no. 2012225080) and
the Technology Plan and the Shenyang Science and Technology Program
(grant no. F11-262-9-01).
Abbreviations:
|
KCs
|
keratinocytes
|
|
ECM
|
extracellular matrix
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MMP
|
matrix metalloproteinase
|
References
|
1
|
Wulff BC, Yu L, Parent AE and Wilgus TA:
Novel differences in the expression of inflammation-associated
genes between mid- and late-gestational dermal fibroblasts. Wound
Repair Regen. 21:103–112. 2013. View Article : Google Scholar :
|
|
2
|
Namazi MR, Fallahzadeh MK and Schwartz RA:
Strategies for prevention of scars: what can we learn from fetal
skin? Int J Dermatol. 50:85–93. 2011. View Article : Google Scholar
|
|
3
|
Pouyani T, Papp S and Schaffer L:
Tissue-engineered fetal dermal matrices. In Vitro Cell Dev Biol
Anim. 48:493–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng J, Yu H, Deng S and Shen G: MicroRNA
profiling in mid- and late-gestational fetal skin: implication for
scarless wound healing. Tohoku J Exp Med. 221:203–209. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowinski D, Höijer P, Engstrand T, et al:
Keratinocytes inhibit expression of connective tissue growth factor
in fibroblasts in vitro by an interleukin-1alpha-dependent
mechanism. J Invest Dermatol. 119:449–455. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fusenig NE, Limat A, Stark HJ and
Breitkreutz D: Modulation of the differentiated phenotype of
keratinocytes of the hair follicle and from epidermis. J Dermatol
Sci. 7:S142–S151. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haverstock BD: Hypertrophic scars and
keloids. Clin Podiatr Med Surg. 18:147–159. 2001.PubMed/NCBI
|
|
8
|
Maas-Szabowski N, Stark HJ and Fusenig NE:
Keratinocyte growth regulation in defined organotypic cultures
through IL-1-induced keratinocyte growth factor expression in
resting fibroblasts. J Invest Dermatol. 114:1075–1084. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naik-Mathuria B, Gay AN, Yu L, et al:
Fetal wound healing using a genetically modified murine model: the
contribution of P-selectin. J Pediatr Surg. 43:675–682. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Colwell AS, Yun R, Krummel TM, Longaker MT
and Lorenz HP: Keratinocytes modulate fetal and postnatal
fibroblast transforming growth factor-beta and Smad expression in
co-culture. Plast Reconstr Surg. 119:1440–1445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gangnuss S, Cowin AJ, Daehn IS, et al:
Regulation of MAPK activation, AP-1 transcription factor expression
and keratinocyte differentiation in wounded fetal skin. J Invest
Dermatol. 122:791–804. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hahn JM, Glaser K, McFarland KL, et al:
Keloid-derived keratinocytes exhibit an abnormal gene expression
profile consistent with a distinct causal role in keloid pathology.
Wound Repair Regen. 21:530–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeh J, Green LM, Jiang TX, et al:
Accelerated closure of skin wounds in mice deficient in the
homeobox gene Msx2. Wound Repair Regen. 17:639–648. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Wang Z, Wang R, et al: Direct
comparison of the potency of human mesenchymal stem cells derived
from amnion tissue, bone marrow and adipose tissue at inducing
dermal fibroblast responses to cutaneous wounds. Int J Mol Med.
31:407–415. 2013.
|
|
15
|
Zhang L, Aerziguli T and Guzalnur A:
Establishment and characterization of a new carcinoma cell line
from uterine cervix of Uyghur women. Zhonghua Bing Li Xue Za Zhi.
41:248–253. 2012.(In Chinese). PubMed/NCBI
|
|
16
|
Wang Z, Wang Y, Farhangfar F, Zimmer M and
Zhang Y: Enhanced keratinocyte proliferation and migration in
co-culture with fibroblasts. PLoS One. 7:e409512012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laeeq S and Faust R: Modeling the
cholesteatoma microenvironment: coculture of HaCaT keratinocytes
with WS1 fibroblasts induces MMP-2 activation, invasive phenotype,
and proteolysis of the extracellular matrix. Laryngoscope.
117:313–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bernerd F: Human skin reconstructed in
vitro as a model to study the keratinocyte, the fibroblast and
their interactions: photodamage and repair processes. J Soc Biol.
199:313–320. 2005.(In French). View Article : Google Scholar
|
|
19
|
Kratz G, Haegerstrand A and Dalsgaard CJ:
Conditioned medium from cultured human keratinocytes has growth
stimulatory properties on different human cell types. J Invest
Dermatol. 97:1039–1043. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brunet A, Bonni A, Zigmond MJ, et al: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li N, Bu X, Tian X, et al: Fatty acid
synthase regulates proliferation and migration of colorectal cancer
cells via HER2-PI3K/Akt signaling pathway. Nutr Cancer. 64:864–870.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Srivastava VK, Gara RK, Bhatt ML, Sahu DP
and Mishra DP: Centchroman inhibits proliferation of head and neck
cancer cells through the modulation of PI3K/mTOR pathway. Biochem
Biophys Res Commun. 404:40–45. 2011. View Article : Google Scholar
|
|
23
|
Ivarsson M, McWhirter A, Borg TK and Rubin
K: Type I collagen synthesis in cultured human fibroblasts:
regulation by cell spreading, platelet-derived growth factor and
interactions with collagen fibers. Matrix Biol. 16:409–425. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu M, Breyssens H, Salter V, et al:
Restoring p53 function in human melanoma cells by inhibiting MDM2
and cyclin B1/CDK1-phosphorylated nuclear iASPP. Cancer Cell.
23:618–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Polager S and Ginsberg D: p53 and E2f:
partners in life and death. Nat Rev Cancer. 9:738–748. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hipfner DR and Cohen SM: Connecting
proliferation and apoptosis in development and disease. Nat Rev Mol
Cell Biol. 5:805–815. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwakuma T and Agarwal N: MDM2 binding
protein, a novel metastasis suppressor. Cancer Metastasis Rev.
31:633–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nowack MK, Harashima H, Dissmeyer N, et
al: Genetic framework of cyclin-dependent kinase function in
Arabidopsis. Dev Cell. 22:1030–1040. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dissmeyer N, Weimer AK, Pusch S, et al:
Control of cell proliferation, organ growth, and DNA damage
response operate independently of dephosphorylation of the
Arabidopsis Cdk1 homolog CDKA;1. Plant Cell. 21:3641–3654. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Makino T, Jinnin M, Muchemwa FC, et al:
Basic fibroblast growth factor stimulates the proliferation of
human dermal fibroblasts via the ERK1/2 and JNK pathways. Br J
Dermatol. 162:717–723. 2010. View Article : Google Scholar
|
|
31
|
Liu Y, Liang C, Liu X, et al: AGEs
increased migration and inflammatory responses of adventitial
fibroblasts via RAGE, MAPK and NF-kappaB pathways. Atherosclerosis.
208:34–42. 2010. View Article : Google Scholar
|
|
32
|
Tarnowski M, Grymula K, Liu R, et al:
Macrophage migration inhibitory factor is secreted by
rhabdomyosarcoma cells, modulates tumor metastasis by binding to
CXCR4 and CXCR7 receptors and inhibits recruitment of
cancer-associated fibroblasts. Mol Cancer Res. 8:1328–1343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang D, Shao S, Shuai H, et al: SDF-1α
reduces fibronectin expression in rat mesangial cells induced by
TGF-β1 and high glucose through PI3K/Akt pathway. Exp Cell Res.
319:1796–1803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Blanc A, Pandey NR and Srivastava AK:
Synchronous activation of ERK 1/2, p38mapk and PKB/Akt signaling by
H2O2 in vascular smooth muscle cells:
potential involvement in vascular disease (review). Int J Mol Med.
11:229–234. 2003.PubMed/NCBI
|
|
35
|
Song J, Xu H, Lu Q, et al: Madecassoside
suppresses migration of fibroblasts from keloids: involvement of
p38 kinase and PI3K signaling pathways. Burns. 38:677–684. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park G, Yoon BS, Moon JH, et al: Green tea
polyphenol epigallocatechin-3-gallate suppresses collagen
production and proliferation in keloid fibroblasts via inhibition
of the STAT3-signaling pathway. J Invest Dermatol. 128:2429–2441.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Zhu DL, Shen WL and Gao PJ:
Increased migration of vascular adventitial fibroblasts from
spontaneously hypertensive rats. Hypertens Res. 29:95–103. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jeffery TK, Upton PD, Trembath RC and
Morrell NW: BMP4 inhibits proliferation and promotes myocyte
differentiation of lung fibroblasts via Smad1 and JNK pathways. Am
J Physiol Lung Cell Mol Physiol. 288:L370–L378. 2005. View Article : Google Scholar
|
|
39
|
Stawowy P, Goetze S, Margeta C, Fleck E
and Graf K: LPS regulate ERK1/2-dependent signaling in cardiac
fibroblasts via PKC-mediated MKP-1 induction. Biochem Biophys Res
Commun. 303:74–80. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yijing L, Liu H, Yuan C, et al: The
effects of qindan-capsule-containing serum on the TGF-β1/ERK
signaling pathway, matrix metalloproteinase synthesis and cell
function in adventitial fibroblasts. Pharm Biol. 51:712–721. 2013.
View Article : Google Scholar : PubMed/NCBI
|