Introduction
Liver transplantation is considered to be the most
effective treatment for end-stage liver disease. However, a variety
of postoperative complications severely affect patient survival,
one of which is acute lung injury (ALI). It has been indicated that
the incidence of ALI in liver transplantation patients is
34.2~77.8% (1). Among patients
with ALI, those who develop acute respiratory distress syndrome
(ARDS) have a mortality rate of 76.5%. Thus, the treatment of
complications in the lungs is crucial to the recovery of patients
undergoing liver transplantation. Strategies used to protect the
lungs in this operation, however, remain limited, thus it is
important to determine novel protective methods.
During liver transplantation, the inferior vena cava
(IVC) and portal vein (PV) are interrupted, which causes
hypotension and intestinal congestion, resulting in
ischemia-reperfusion injury. Reactive oxygen species (ROS),
endotoxins and cytokines enter into the circulation and damage
remote organs and systems, of which the lungs are considered to be
the most vulnerable (2).
Therefore, therapeutic strategies alleviating ALI induced by liver
transplantation are required to improve patient prognosis.
Oxidative stress and inflammation are considered as
the main causes of ALI (3), and
previous studies have provided evidence that oxidative stress or
inflammatory damage serve an important function in this
pathological process (4,5). Thus, antioxidative therapies for lung
protection require investigation, particularly in liver
transplantation (6). NADPH oxidase
is one of the key enzymes in ROS production; its inhibition may be
effective in reducing the levels of ROS, thus further reducing
oxidative stress and inflammation (7). Therapeutic strategies targeting NADPH
oxidase have not been widely studied, and few have been utilized
clinically.
Propofol, an intravenous anesthetic with a hydroxyl
group attached to its benzene ring (phenol) (8), is widely used clinically. The
antioxidant and anti-inflammatory properties of propofol, which are
considered to be due to its phenolic hydroxyl group, have become a
focus of recent research. However, whether propofol can effectively
attenuate ALI induced by liver transplantation remains unclear.
Thus, in the current study, a rat OALT model was used to explore
the protective effects of propofol on ALI induced by liver
transplantation compared with positive controls, including: NAC [a
non-specific antioxidant (14)]
and AP [a specific NADPH oxidase inhibitor (15)]. The mechanisms of the protective
effect of propofol were analyzed, and suggested to be associated
with the reduction in oxidative stress and inflammatory reaction
mediated by the inhibition of NADPH oxidase.
Materials and methods
Animals
The National Institutes of Health criteria for the
care and use of laboratory animals in research was followed. The
study was approved by the Laboratory Animal Care Committee of Sun
Yat-Sen University (Guangzhou, China). Adult specific pathogen-free
male Sprague-Dawley rats (body weight, 180–220 g) were purchased
from the Laboratory Animal Center of Sun Yat-Sen University.
Rat OALT model establishment
Rats were fasted for 8 h with free access to
drinking water prior to surgery, and were subsequently injected
with 1mg/kg atropine (Nanjing Keygen Biotech Co., Ltd, Nanjing,
China) 15 min prior to surgery in order to prevent secretion, which
may lead to asphyxia in rats. An open face guard was used to
administer 2% ether anesthetic via inhalation (Nanjing Keygen
Biotech Co., Ltd). The constructed OALT model was similar to that
described previously (9,10). Subsequent to entering the abdominal
cavity, the falciform ligament of the liver was resected and
ligated, and the left vena phrenica along the esophagus was
severed. The liver was exposed until the superior vena cava (SVC)
was completely freed. Silk thread was use to raise the freed SVC
slightly, in order to be easily blocked using vascular clamps at a
later stage. The liver was then placed back in its original
position and the IVC was dissected until the upper region of the
left renal vein was completely separated. The first hepatic portal
was dissected and the PV was separated from the convergence of the
inferior mesenteric and splenic veins. The hepatic artery and
biliary tract were dissected and separated together due to their
anatomical relationship. The hepatic portal veins were ligated.
Vascular clamps were used on the hepatic artery, SVC, IVC and at
the convergence of the inferior mesenteric and splenic veins. The
PV was punctured with a 24-gauge needle and fixed in place with a
vascular clamp in preparation for reperfusion. Ringer’s lactate
solution (Jetway Biotech Co., Ltd, Guangzhou, China), precooled to
4°C, was injected during reperfusion at 2.5 ml/min and a 1-mm
incision was made on the wall of the IVC as an outflow tract. The
needle was then extracted and the openings of the PV and the IVC
were closed using 8-0 sutures and the PV, SVC, IVC and hepatic
artery were unclamped. The anhepatic phase lasted for 20±1 min.
Animal groups
The experimental animals were randomly divided into
seven groups using a random number table, which considered the
weights of the animals, as follows (n=8 in each group): The sham
(S), model with saline (M), fat milk (FM), low propofol dose (LP),
high propofol dose (HP), NAC (NC) and apocynin (AP) groups. Groups
S and M were injected with 2 ml/day physiological saline (Jetway
Biotech Co., Ltd) for three consecutive days. Groups FM, LP, HP, NC
and AP were injected with fat milk [2 ml/day; intraperitoneal
(i.p.) injection; Mitsubishi Pharma Co., Ltd, Guangzhou, China],
propofol (50 mg/kg; i.p.; 1% Diprivan; CG411; AstraZeneca,
Caponago, Italy), at previously determinend doses (11); propofol (100 mg/kg; i.p.), NAC (150
mg/kg; i.p; Sigma-Aldrich, St. Louis, MO, USA) (12); or apocynin (5 mg/kg; i.p.;
Sigma-Aldrich) (13),
respectively, for three consecutive days. All drugs were diluted in
2 ml physiological saline. On the fourth day, the rats in group S
were subject to celiotomy and vascular separation under anesthesia.
The rats in the other groups underwent the OALT operation. Lung
tissues were collected 8 h subsequent to liver transplantation
(10).
The rat body weights and the anhepatic phase
duration times in the seven groups are displayed in Table I, with no significant differences
observed between groups (P>0.05).
 | Table IRat body weight and anhepatic phase
duration time. |
Table I
Rat body weight and anhepatic phase
duration time.
| Group | Weight (g) | Anhepatic phase
(min) |
|---|
| S | 198.3±10.3 | - |
| M | 202.6±9.7 | 19.7±0.5 |
| FM | 213.4±12.3 | 20.0±0.9 |
| LP | 195.1±15.4 | 19.9±1.1 |
| HP | 208.3±18.0 | 19.3±0.6 |
| NC | 199.2±13.3 | 20.5±0.3 |
| AP | 215.8±19.2 | 19.4±0.5 |
Disposal of specimens
The animals were anesthetized via intraperitoneal
injection of 10% chloral hydrate (3.5 ml/kg; Jetway Biotech Co.,
Ltd) 8 h subsequent to OALT; subsequently, 2ml air was injected
into the tail vein in order to sacrifice the rats. The thorax was
opened and all lung tissues were removed. The middle lobe of the
right lung was weighed on an electronic scale and the inferior lobe
was fixed in 10% buffered formalin (Nanjing Keygen Biotech Co.,
Ltd) and embedded in paraffin (Nanjing Keygen Biotech Co., Ltd) for
histological evaluation. The remaining lung tissue was promptly
transferred into liquid nitrogen (Nanjing Keygen Biotech Co., Ltd)
for storage until it was required for p47phox and gp91phox
expression assays and the measurement of hydrogen peroxide
(H2O2), malondialdehyde (MDA) and superoxide
dismutase (SOD) activity.
Lung histology
The lung tissues were sectioned (~4 mm) and stained
with hematoxylin and eosin (H&E; Nanjing Keygen Biotech Co.,
Ltd). The sample groups were analyzed blindly and the pathology was
scored as described by Franco-Gou et al (16). The graded edema of the alveolar
mesenchyme, intra-alveolar cell infiltration and alveolar
hemorrhage were also scored.
Lung water content
The wet weight of the superior lobe of the right
lung was measured, and then the samples were placed in an oven for
24 h at 80°C in order for them to dry out; once water was
evaporated the tissue reached a constant weight. The water content
of the lung was calculated as follows: Water content = (lung wet
weight − lung dry weight)/lung wet weight × 100 (11).
Western blot analysis
Lung tissues were finely homogenized, suspended in
ice-cold lysis buffer (1.5 ml/g tissue; Nanjing Keygen Biotech Co.,
Ltd) and then centrifuged (12,000 × g for 10 min at 4 °C). The
supernatants were collected for analysis. Following measurement of
the protein concentration of each sample, 50 μg of the sample was
solubilized in sodium dodecyl sulfate (SDS) loading buffer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) by boiling. The samples were
loaded onto a 10% polyacrylamide gel (Invitrogen Life Technologies,
Carlsbad, CA, USA) and SDS-PAGE (Bio-rad Laboratories, Inc.) was
conducted. They were then transferred to a polyvinylidene
difluoride (PVDF; Bio-rad Laboratories, Inc.) membrane. The PVDF
membrane was subsequently incubated with monoclonal mouse
anti-human p47-phox (sc-17845) and gp91-phox (sc-74514) antibodies
(1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) as well as
monoclonal mouse Immunoglobulin G1 anti-β-actin (1:8,000; A5441;
Sigma-Aldrich), followed by the corresponding horseradish
peroxidase-conjugated secondary antibodies (1:2,00; Santa Cruz
Biotechnology, Inc.). Protein-antibody complexes were detected with
an enhanced chemiluminescence system (KGP1125; Nanjing Keygen
Biotech). Protein band sizes were estimated using AlphaView
2.2.14407 software (ProteinSimple, Santa Clara, CA, USA). The
density measurement was correlated to the protein expression and
normalized to β-actin.
Detection of H2O2,
MDA, SOD, tumor necrosis factor-α (TNF-α) and interleukin-6
(IL-6)
H2O2, MDA and SOD levels were
measured using the corresponding kits (H202
kit, MDA kit and SOD assay kit; all purchased from Nanjing Keygen
Biotech. Co., Ltd) according to the manufacturer’s instruction. The
concentrations of TNF-α and IL-6 were measured using respective
ELISA kits (Nanjing Keygen Biotech. Co., Ltd.).
Statistical analysis
All data are presented as the mean ± standard error
and analyzed using SPSS software, version 12.0 (SPSS, Inc.,
Chicago, IL, USA). The differences between groups were analyzed
using one-way ANOVA. P<0.05 was considered to indicate a
statistically significant difference.
Results
Propofol protects against OALT-induced
changes in lung morphology
Alterations in lung morphology following OALT were
estimated using H&E staining. Greater lung damage was observed
in group M than group S (blood vessel liberation only). Clear
inflammatory cell infiltration and alveolar exudates were observed
and the pulmonary interstitium exhibited hyperemia and severe
hemorrhage (Fig. 1A and B)
(16). Propofol, at low (50 mg/kg)
and high (100 mg/kg) doses, significantly protected against lung
damage, producing similar results as the two established
antioxidants, NAC and AP. In addition, the high-dose propofol was
more efficacious than the low-dose, and as a solvent control of
propofol, fat milk did not exhibit any protective effects on lung
tissue (group FM).
 | Figure 1Pathological alterations in lung
tissue following OALT. (A) Histological pathological changes in
lung tissue. Magnification, ×100. (B) Lung pathological scores. (C)
Alterations in water content of the lung tissues following OALT.
Data are presented as the mean ± standard deviation (n=8 in each
group). *P<0.05 vs. group S, #P<0.05
vs. group M, §P<0.05 vs. group LP. S, sham; M, saline
control; FM, fat milk control; LP, low-dose propofol; HP, high-dose
propofol; NC, N-acegysteine positive control; AP, apocynin positive
control; OALT, orthotopic autologous liver transplantation. |
Propofol reduces lung water content
The water content of the middle lobe of the right
lung was measured in order to estimate the extent of pulmonary
edema following OALT, as this measure is indicative of pathological
damage. Propofol reduced the water content of lungs significantly,
producing similar results to NAC and AP (Fig. 1C). The results suggested that ALI
induced by liver transplantation was severe, but propofol was able
to reverse this damage and attenuate pulmonary edema.
NADPH oxidase protein expression in lung
tissue
NADPH oxidase, a key multiprotein system involved in
the generation of ROS, consists of cytochrome b558 (gp91phox and
p22phox) on the membrane and cytosolic soluble proteins (p67phox,
p47phox, p40phox and Rac1/Rac2). The current study focused on the
alterations in p47phox and gp91phox levels in the lungs subsequent
to OALT, as these proteins are fundamental for the activity of the
NADPH oxidase system. AP (an inhibitor of NAPDH oxidase) and NAC (a
ROS scavenger) were used as positive controls. As demonstrated in
Fig. 2, propofol, NAC and AP
pretreatment significantly reduced the expression of p47phox and
gp91phox induced by OALT, compared with the saline control group,
with levels similar to those of the sham group. The results
suggested that NADPH oxidase activity is enhanced in lung tissues
following liver transplantation. Thus, as propofol produces similar
antioxidant effects to NAC and AP, this is thought to be mediated
by the inhibition of NADPH oxidase activity.
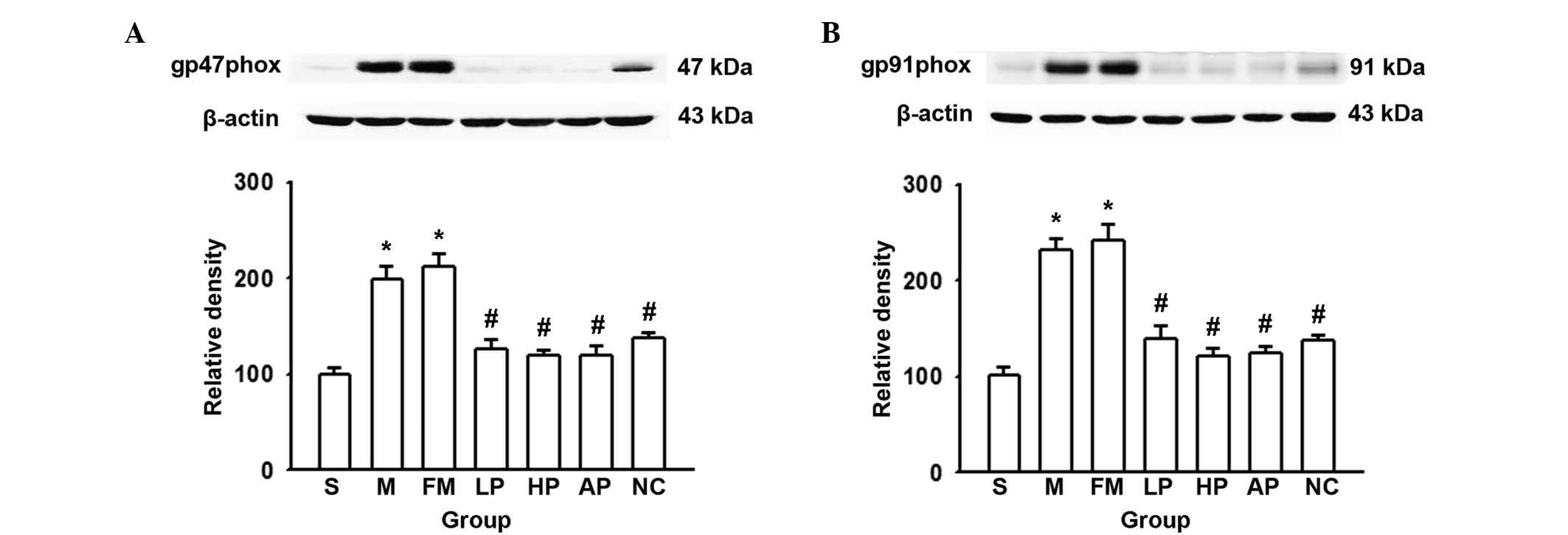 | Figure 2Expression levels of the NADPH
oxidase subunits p47phox and gp91phox in lung tissues following
OALT. (A) Relative density of p47phox to β-actin expression. (B)
Relative density of gp91phox to β-actin expression. Data are
presented as the mean ± standard deviation (n=8 in each group).
*P<0.05 vs. group S, #P<0.05 vs. group
M, §P<0.05 vs. group AP. S, sham; M, saline control;
FM, fat milk control; LP, low-dose propofol; HP, high-dose
propofol; NC, N-acegysteine positive control; AP, apocynin positive
control; OALT, orthotopic autologous liver transplantation. |
Alterations in levels of oxidative stress
and inflammation in lung tissue following liver
transplantation
NADPH oxidase produces large quantities of ROS,
which are involved in the processes of oxidative stress,
inflammation, cell signal transduction, cell proliferation and
apoptosis (17,18). Previous studies have implicated
oxidative stress and the inflammatory reaction to serve important
functions in the development of ALI (19,20).
Thus, alterations to oxidative and inflammatory damage in ALI
induced by liver transplantation were focused upon in the present
study. The concentrations of H2O2, MDA and
SOD in the lungs subsequent to OALT were determined, which reflects
the balance of oxidative stress (21,22).
Results indicated a significant increase in the levels of
H2O2 and MDA subsequent to OALT, and
propofol, NAC and AP pretreatment reduced this increase. The
high-dose propofol produced a significantly reduced level of
H2O2 and MDA compared with the low dose
(Fig. 3A and B). However, the
opposite effect was observed in the levels of SOD, with the lowest
levels observed in the saline and fat milk groups, and an increase
following propofol, AP and NAC pretreatment (Fig. 3C). The results suggested that
propofol effectively reduced ROS production and alleviated
oxidative damage to protect against the lung damage induced by
OALT, in a similar manner to the positive controls NAC and AP.
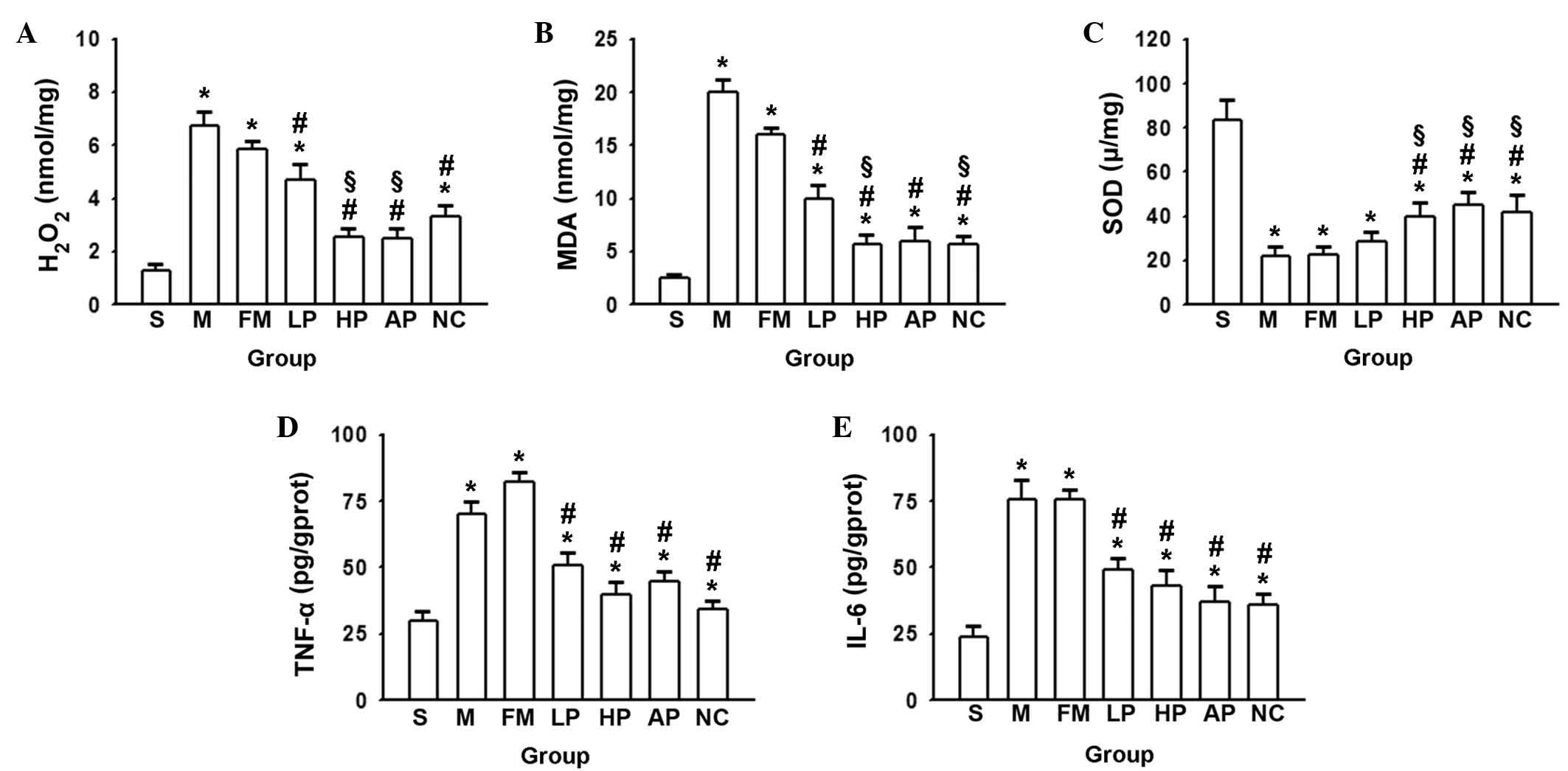 | Figure 3Concentration of (A)
H2O2, (B) MDA, (C) SOD, (D) TNF-α and (E)
IL-6 following OALT. Data are presented as the mean ± standard
deviation (n=8 in each group). *P<0.05 vs. group S,
#P<0.05 vs. group M, §P<0.05 vs. group
LP. H2O2, hydrogen peroxide; MDA,
malondialdehyde; SOD, superoxide dismutase; TNF-α, tumor necrosis
factor-α; IL-6, interleukin 6; S, sham; M, saline control; FM, fat
milk control; LP, low-dose propofol; HP, high-dose propofol; NC,
N-acegysteine positive control; AP, apocynin positive control;
OALT, orthotopic autologous liver transplantation. |
To analyze another aspect, the levels of two
cytokines (TNF-α and IL-6) that are important in ALI were measured.
Fig. 3D and E illustrates that the
levels of TNF-α and IL-6 were reduced by propofol pretreatment
prior to OALT compared with levels following saline treatment,
similar to the levels observed following NAC and AP pretreatment.
This suggests that propofol attenuated OALT-induced inflammatory
factors in order to protect the lung tissue against inflammatory
damage.
Discussion
ALI is a major complication of OALT that
significantly affects prognosis, resulting in an increased
mortality rate (23). Thus, the
influence of liver transplantation on remote lung damage was
investigated in the present study, with an aim to develop a novel
strategy to protect the lungs during this procedure. In the current
study, a rat OALT model was used to observe alterations in
pathological lung injuries. The rat model closely mimicked the
aspects of the liver transplantation procedure, including blockade
of the SVC, IVC and PV, cold liver protection, fluid perfusion,
liver ischemia-reperfusion injury and passive congestion of the
intestine. All of the above contributed to the investigation of the
effects of OALT procedures on lung damage, but did not take into
account the complex situation of liver rejection. The data
demonstrated that ALI induced by liver transplantation was serious,
and the ROS induced by NADPH oxidase participated in this process
through activating secundum oxidative stress and inflammatory
reaction. AP and NAC (inhibitors of NADPH oxidase) preconditioning
diminished this damage effectively, and the observation that
propofol produced similar antioxidative effects to these suggests
that the protective mechanism of propofol involves NADPH oxidase
inhibition.
Several studies have illustrated that oxidative
stress and the inflammatory reaction are key in the pathogenesis of
ALI (24,25). During liver transplantation, the
IVC and PV require interruption, which results in hypotension and
intestinal congestion, leading to ischemia-reperfusion injury, in
which ROS, endotoxins and cytokines enter into the blood
circulation and damage remote organs and systems. ROS directly
damage lung parenchymal cells via lipid peroxidation, in addition
to the basement membrane of capillaries and pulmonary interstitial
cells, thereby resulting in severe pulmonary edema (26). Previous studies have indicated that
a reduction in ROS production may alleviate liver or lung
ischemia-reperfusion injury (27),
as a sudden influx of ROS can overwhelm innate protective measures
and lead to organ injury (28).
ROS are formed through various key enzymes, including xanthine
oxidase, NADPH oxidase and nitric oxide synthase (29–31),
of which NADPH oxidase-dependent ROS formation is considered to be
the most important. Thus, in the current study, the main focus was
upon this pathway, to explore the effects of liver transplantation
on remote lung damage. NADPH oxidase is present in neutrophils,
macrophages and on the membranes of endothelial cells, particularly
in pulmonary vasculature where it is prevalent (31,32).
Previous studies have demonstrated that the increase in NADPH
oxidase is the main mechanism of ischemia-reperfusion injury, and
its inhibition with diphenyliodonium (31) and apocynin (15) for example, have markedly reduced
ROS formation and tissue damage in previous in vivo studies.
Apocynin is a direct inhibitor of NADPH oxidase, and NAC may act as
a ROS scavenger by promoting the synthesis of glutathione (33). The observation that two NADPH
oxidase inhibitors with different mechanisms yielded similar
results suggested that ROS production mediated by NAPDH oxidase
serves an important role in ALI induced by OALT. Thus, antioxidant
preconditioning reduced the resulting lung damage.
Propofol contains a phenol hydroxyl group that
confers antioxidant activity. Takao et al (34) suggested that a high dose of
propofol mitigates the physiological, biochemical and histological
deterioration of ALI during endotoxemia. Propofol has also been
reported to exert significant protective activity against
ischemia-reperfusion-induced cardiac injury, partly through the
reduction in ROS and H2O2 generation
(35,36). In the current study, propofol was
demonstrated to produce a similar effect to that of NAC and AP on
lung tissue; reducing expression of NADPH oxidase (Fig. 2) and levels of
H2O2 and MDA, but increasing the level of SOD
(Fig. 3). Propofol was also
observed to act as an antioxidant, inhibiting NAPDH oxidase to
protect lung tissue from oxidative stress. The results of the
current study indicate that propofol, as an antioxidant, can be
applied and used clinically.
Excessive inflammation is considered to be one of
the underlying mechanisms of the pathogenesis of ALI/ARDS, in which
TNF-α and IL-6 are the major cytokines involved (37). Oxidative stress and the
inflammatory reaction interact in a complex way to act in the
pathogenesis of lung damage. Studies have demonstrated that ROS are
fundamental to oxidative stress and the inflammatory reaction
(38,39). The current study demonstrated that
NAC and AP application inhibited NAPDH oxidase in the earlier phase
and reduced or eliminated ROS production. This was beneficial for
reducing oxidative stress and the inflammatory reaction in the
lungs, mediated by OALT. Propofol preconditioning was observed to
produce similar effects to NAC or AP, thus propofol was
hypothesized to also reduce the inflammatory reaction initiated by
ROS through the inhibition of NAPDH oxidase (Fig. 3D and E). An additional study
demonstrated that propofol also possessed anti-inflammatory
properties, however, it was unknown whether it was able to effect
oxidative stress through its anti-inflammatory action (40). Further investigations to further
elucidate this are required.
In conclusion, the current study demonstrated that
propofol, a common clinically used anesthetic, protects against
lung damage via the inhibition of oxidative stress and the
inflammatory reaction, particularly via NADPH oxidase inhibition.
This may be used as a novel strategy for organ protection during
liver transplantation. Further investigation into the optimal
protective dose and administration time of propofol should be
conducted in future studies.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81401628 and
30972858); the Natural Science Foundation of Guangdong Province,
China (grant no. S2012010008930), and the Medical Research
Foundation of Guandong Province (grant no. B2014141).
References
|
1
|
Hong SK, Hwang S, Lee SG, et al: Pulmonary
complications following adult liver transplantation. Transplantat
Proc. 38:2979–2981. 2006. View Article : Google Scholar
|
|
2
|
Hirsch J, Niemann CU, Hansen KC, et al:
Alterations in the proteome of pulmonary alveolar type II cells in
the rat after hepatic ischemia-reperfusion. Crit Care Med.
36:1846–1854. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bellingan GJ: The pulmonary physician in
critical care * 6: The pathogenesis of ALI/ARDS. Thorax.
57:540–546. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang A, Liu C, Song Y, et al: NF-κB
induced the donor liver cold preservation related acute lung injury
in rat liver transplantation model. PloS One. 6:e249602011.
View Article : Google Scholar
|
|
5
|
Hei Z, Chi X, Cheng N, Luo G and Li S:
Upregulation of TLR2/4 expression in mononuclear cells in
postoperative systemic inflammatory response syndrome after liver
transplantation. Mediators Inflamm. 2010:5195892010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Altintas ND, Atilla P, Iskit AB and Topeli
A: Long-term simvastatin attenuates lung injury and oxidative
stress in murine acute lung injury models induced by oleic Acid and
endotoxin. Respir Care. 56:1156–1163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramonaite R, Skieceviciene J, Kiudelis G,
et al: Influence of NADPH oxidase on inflammatory response in
primary intestinal epithelial cells in patients with ulcerative
colitis. BMC Gastroenterol. 13:1592013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murphy PG, Myers DS, Davies MJ, Webster NR
and Jones JG: The antioxidant potential of propofol
(2,6-diisopropylphenol). Br J Anaesth. 68:613–618. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin C, Zhang PJ, Wu XM, et al: Impact of
hypoxic preconditioning on apoptosis and its possible mechanism in
orthotopic liver autotransplantation in rats. Hepatobiliary
Pancreat Dis Int. 8:40–45. 2009.PubMed/NCBI
|
|
10
|
Chi X, Zhang A, Luo G, et al: Knockdown of
myeloid differentiation protein-2 reduces acute lung injury
following orthotopic autologous liver transplantation in a rat
model. Pulm Pharmacol Ther. 26:380–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu KX, Chen SQ, Huang WQ, Li YS, Irwin MG
and Xia Z: Propofol pretreatment reduces ceramide production and
attenuates intestinal mucosal apoptosis induced by intestinal
ischemia/reperfusion in rats. Anesth Analg. 107:1884–1891. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin X, Wang L, Wu HS, et al:
N-acetylcysteine inhibits activation of toll-like receptor 2 and 4
gene expression in the liver and lung after partial hepatic
ischemia-reperfusion injury in mice. Hepatobiliary Pancreat Dis
Int. 6:284–289. 2007.PubMed/NCBI
|
|
13
|
Sonta T, Inoguchi T, Tsubouchi H, et al:
Evidence for contribution of vascular NAD(P)H oxidase to increased
oxidative stress in animal models of diabetes and obesity. Free
Radic Biol Med. 37:115–123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sadowska AM: N-Acetylcysteine mucolysis in
the management of chronic obstructive pulmonary disease. Ther Adv
Respir Dis. 6:127–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dodd-o JM, Welsh LE, Salazar JD, et al:
Effect of NADPH oxidase inhibition on cardiopulmonary
bypass-induced lung injury. Am J Physiol Heart Circ Physiol.
287:H927–H936. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Franco-Gou R, Roselló-Catafau J and
Peralta C: Protection against lung damage in reduced-size liver
transplantation. Crit Care Med. 34:1506–1513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan J, Frey RS and Malik AB: TLR4
signaling induces TLR2 expression in endothelial cells via
neutrophil NADPH oxidase. J Clin Invest. 112:1234–1243. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poinas A, Gaillard J, Vignais P and
Doussiere J: Exploration of the diaphorase activity of neutrophil
NADPH oxidase. Eur J Biochem. 269:1243–1252. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Auten RL, Richardson RM, White JR, Mason
SN, Vozzelli MA and Whorton MH: Nonpeptide CXCR2 antagonist
prevents neutrophil accumulation in hyperoxia-exposed newborn rats.
J Pharmacol Exp Ther. 299:90–95. 2001.PubMed/NCBI
|
|
20
|
Auten RL, Whorton MH and Nicholas Mason S:
Blocking neutrophil influx reduces DNA damage in hyperoxia-exposed
newborn rat lung. Am J Respir Cell Mol Biol. 26:391–397. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hõrak P, Sild E, Soomets U, Sepp T and
Kilk K: Oxidative stress and information content of black and
yellow plumage coloration: an experiment with greenfinches. J Exp
Biol. 213:2225–2233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manni ML, Epperly MW, Han W, et al:
Leukocyte-derived extracellular superoxide dismutase does not
contribute to airspace EC-SOD after interstitial pulmonary injury.
Am J Physiol Lung Cell Mol Physiol. 302:L160–L166. 2012. View Article : Google Scholar
|
|
23
|
Keegan MT and Pickering BW: Critical care
issues following orthotopic liver transplantation. Minerva
Gastroenterol Dietol. 56:305–330. 2010.PubMed/NCBI
|
|
24
|
Yamaoka S, Kim HS, Ogihara T, et al:
Severe Vitamin E deficiency exacerbates acute hyperoxic lung injury
associated with increased oxidative stress and inflammation. Free
Radic Res. 42:602–612. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weng TI, Wu HY, Kuo CW and Liu SH:
Honokiol rescues sepsis-associated acute lung injury and lethality
via the inhibition of oxidative stress and inflammation. Intensive
Care Med. 37:533–541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Compton CN, Franko AP, Murray MT, Diebel
LN and Dulchavsky SA: Signaling of apoptotic lung injury by lipid
hydroperoxides. J Trauma. 44:783–788. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kupatt C, Habazettl H, Goedecke A, et al:
Tumor necrosis factor-alpha contributes to ischemia- and
reperfusion-induced endothelial activation in isolated hearts. Circ
Res. 84:392–400. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kennedy TP, Rao NV, Hopkins C, Pennington
L, Tolley E and Hoidal JR: Role of reactive oxygen species in
reperfusion injury of the rabbit lung. J Clin Invest. 83:1326–1335.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
den Hengst WA, Gielis JF, Lin JY, Van
Schil PE, De Windt LJ and Moens AL: Lung ischemia-reperfusion
injury: a molecular and clinical view on a complex
pathophysiological process. Am J Physiol Heart Circ Physiol.
299:H1283–H1299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adkins WK and Taylor AE: Role of xanthine
oxidase and neutrophils in ischemia-reperfusion injury in rabbit
lung. J Appl Physiol (1985). 69:2012–2018. 1990.
|
|
31
|
Al-Mehdi AB, Zhao G, Dodia C, et al:
Endothelial NADPH oxidase as the source of oxidants in lungs
exposed to ischemia or high K+. Circ Res. 83:730–737.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zulueta JJ, Yu FS, Hertig IA, Thannickal
VJ and Hassoun PM: Release of hydrogen peroxide in response to
hypoxia-reoxygenation: role of an NAD(P)H oxidase-like enzyme in
endothelial cell plasma membrane. Am J Respir Cell Mol Biol.
12:41–49. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bernard GR, Wheeler AP, Arons MM, et al: A
trial of antioxidants N-acetylcysteine and procysteine in ARDS. The
Antioxidant in ARDS Study Group. Chest. 112:164–172. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takao Y, Mikawa K, Nishina K and Obara H:
Attenuation of acute lung injury with propofol in endotoxemia.
Anesth Analg. 100:810–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shao H, Li J, Zhou Y, et al:
Dose-dependent protective effect of propofol against mitochondrial
dysfunction in ischaemic/reperfused rat heart: role of cardiolipin.
Br J Pharmacol. 153:1641–1649. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang B, Shravah J, Luo H, Raedschelders K,
Chen DD and Ansley DM: Propofol protects against hydrogen
peroxide-induced injury in cardiac H9c2 cells via Akt activation
and Bcl-2 up-regulation. Biochem Biophys Res Commun. 389:105–111.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ware LB: Pathophysiology of acute lung
injury and the acute respiratory distress syndrome. Semin Respir
Crit Care Med. 27:337–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park HR, Kamau PW and Loch-Caruso R:
Involvement of reactive oxygen species in brominated diphenyl
ether-47-induced inflammatory cytokine release from human
extravillous trophoblasts in vitro. Toxicol Appl Pharmacol.
274:283–292. 2014. View Article : Google Scholar
|
|
39
|
Luo C, Yuan D, Li X, et al: Propofol
Attenuated Acute Kidney Injury after Orthotopic Liver
Transplantation via Inhibiting Gap Junction Composed of Connexin
32. Anesthesiology. Sept 24–2014.(Epub ahead of print). PubMed/NCBI
|
|
40
|
Ma L, Wu X, Chen W and Fujino Y: Propofol
has anti-inflammatory effects on alveolar type II epithelial cells.
Acta anaesthesiologica Scandinavica. 54:362–369. 2010. View Article : Google Scholar
|

















