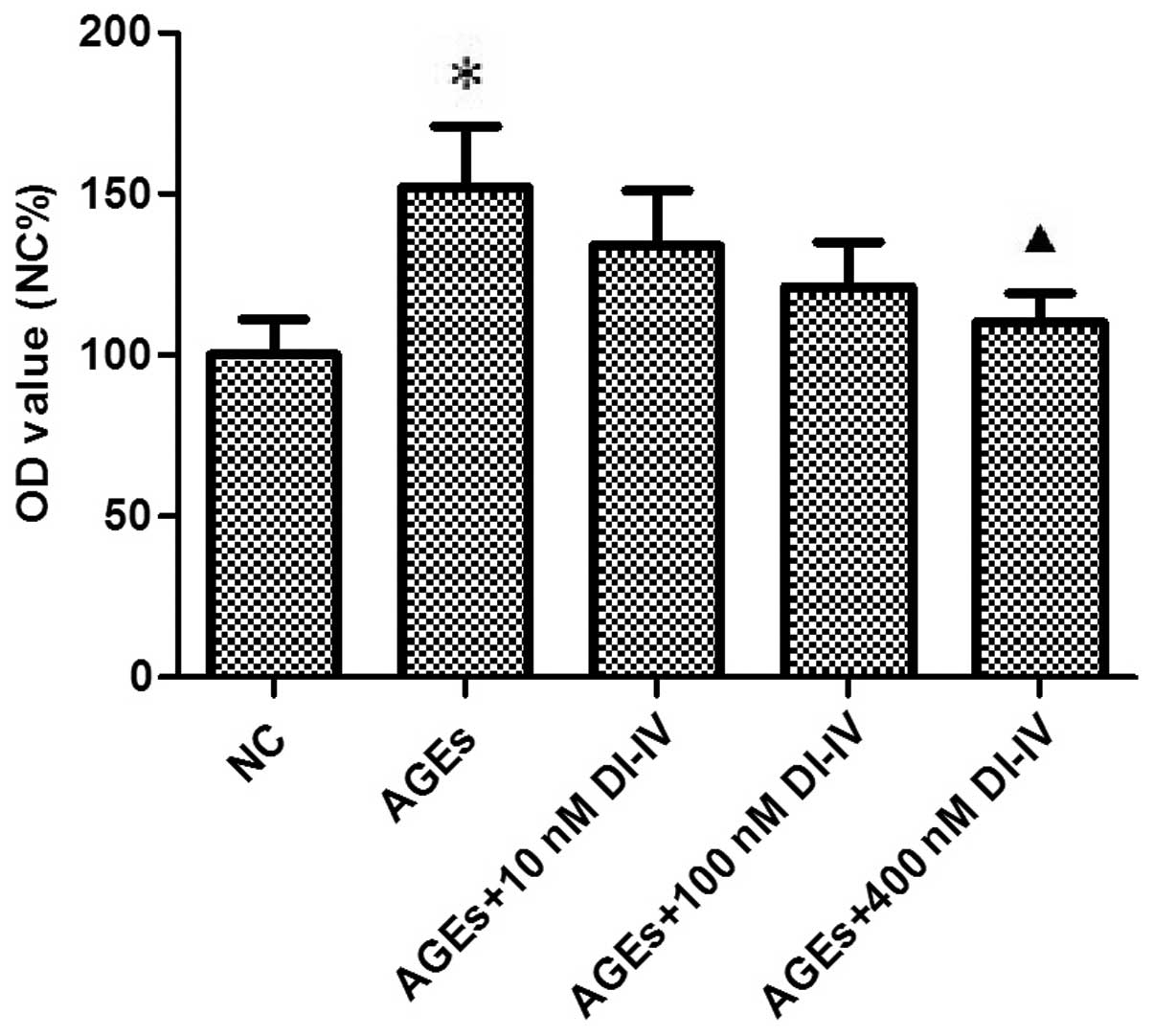
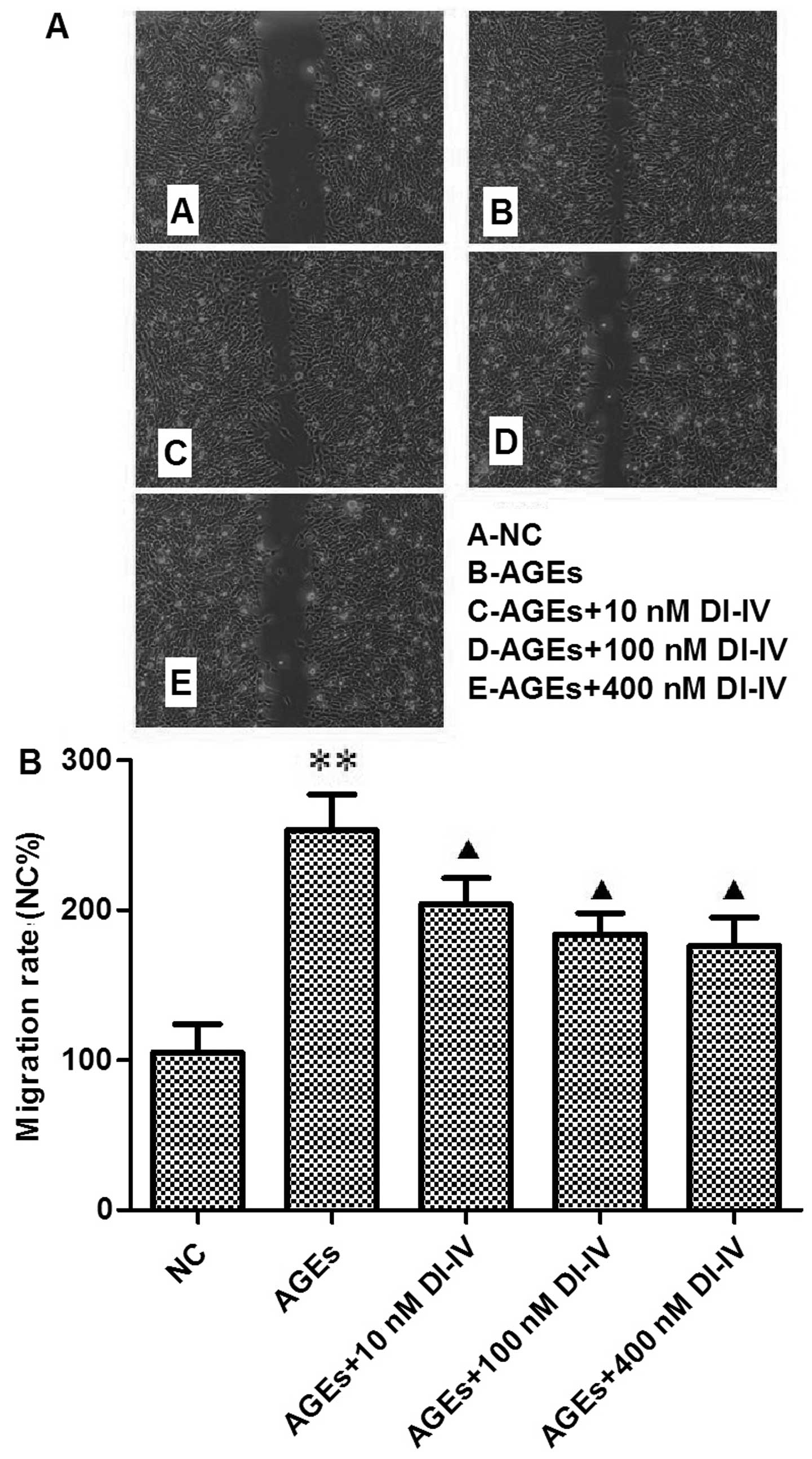
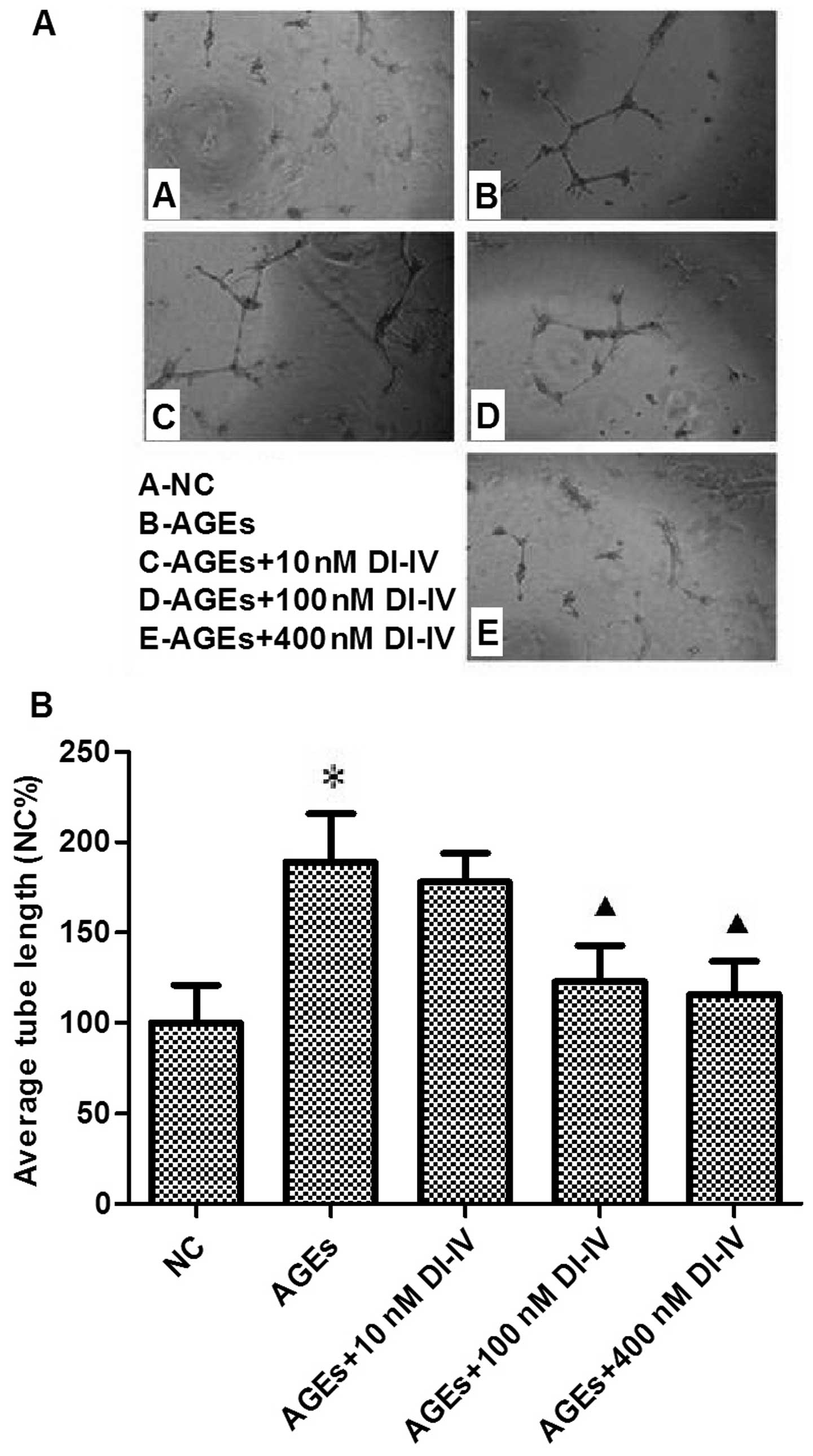
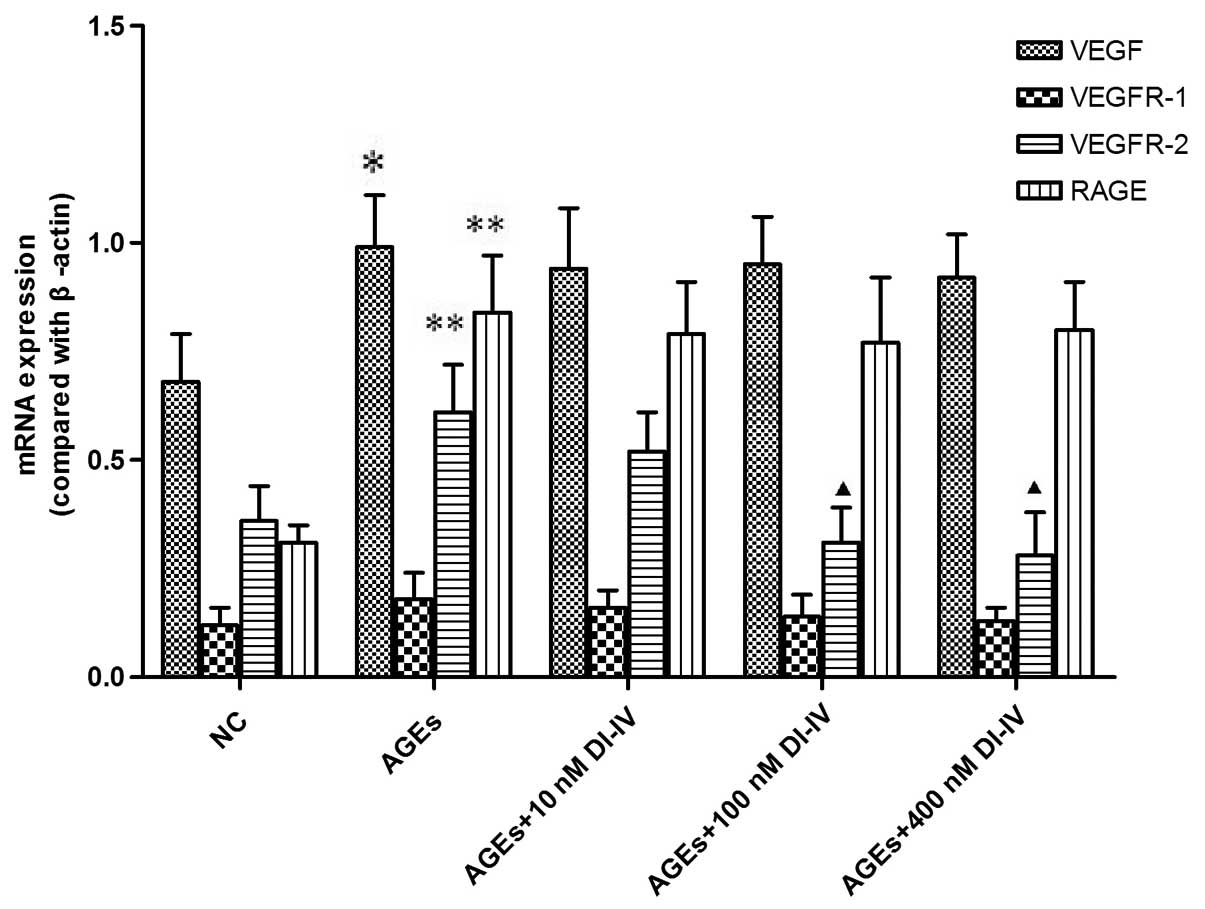
|
1
|
Danaei G, Finucane MM, Lu Y, Singh GM,
Cowan MJ, Paciorek CJ, et al; Global Burden of Metabolic Risk
Factors of Chronic Diseases Collaborating Group (Blood Glucose).
National, regional, and global trends in fasting plasma glucose and
diabetes prevalence since 1980: systematic analysis of health
examination surveys and epidemiological studies with 370
country-years and 2.7 million participants. Lancet. 378:31–40.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fong DS, Aiello LP, Ferris FL and Klein R:
Diabetic Retinopathy. Diabetes Care. 27:2540–2553. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller JW, Adamis AP and Aiello LP:
Vascular endothelial growth factor in ocular neovascularization and
proliferative diabetic retinopathy. Diabetes Metab Rev. 13:37–50.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldin A, Beckman JA, Schmidt AM and
Creager MA: Advanced glycation end products: sparking the
development of diabetic vascular injury. Circulation. 114:597–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato T, Wu X, Shimogaito N, et al: Effects
of high-AGE beverage on RAGE and VEGF expressions in the liver and
kidneys. Eur J Nutr. 48:6–11. 2009. View Article : Google Scholar
|
|
6
|
Yamagishi S, Matsui T, Nakamura K, et al:
Olmesartan blocks advanced glycation end products (AGE)-induced
angiogenesis in vitro by suppressing receptor for AGE (RAGE)
expression. Microvasc Res. 75:130–134. 2008. View Article : Google Scholar
|
|
7
|
De Groot PG and Meijers JC:
β(2)-Glycoprotein I: evolution, structure and function. J Thromb
Haemost. 9:1275–1284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Passam FH, Qi JC, Tanaka K, Matthaei KI
and Krilis SA: In vivo modulation of angiogenesis by beta 2
glycoprotein I. J Autoimmun. 35:232–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu P, Passam FH, Yu DM, Denyer G and
Krilis SA: Beta2-glycoprotein I inhibits vascular endothelial
growth factor and basic fibroblast growth factor induced
angiogenesis through its amino terminal domain. J Thromb Haemost.
6:1215–1223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu P, Passam FH, Yu DM, Denyer G and
Krilis SA: Beta2-glycoprotein I inhibits vascular endothelial
growth factor and basic fibroblast growth factor induced
angiogenesis through its amino terminal domain. J Thromb Haemost.
6:1215–1223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beecken WD, Ringel EM, Babica J, et al:
Plasmin-clipped beta (2)-glycoprotein-I inhibits endothelial cell
growth by down-regulating cyclin A, B and D1 and up-regulating p21
and p27. Cancer Lett. 296:160–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iverson GM, Victoria EJ and Marquis DM:
Anti-beta2-glycoprotein I (beta2-GPI) autoantibodies recognize an
epitope on the first domain of beta2-GPI. Proc Natl Acad Sci USA.
95:15542–15546. 1998. View Article : Google Scholar
|
|
13
|
Heo JW, Kim JH, Cho CS, et al: Inhibitory
activity of bevacizumab to differentiation of retinoblastoma cells.
PLoS One. 7:e334562012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nichol D and Stuhlmann H: EGFL7: a unique
angiogenic signaling factor in vascular development and disease.
Blood. 119:1345–1352. 2012. View Article : Google Scholar :
|
|
15
|
Shibuya M: Tyrosine kinase receptor
Flt/VEGFR family: its characterization related to angiogenesis and
cancer. Genes Cancer. 1:1119–1123. 2010. View Article : Google Scholar
|
|
16
|
Huang Q and Sheibani N: High glucose
promotes retinal endothelial cell migration through activation of
Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol.
295:C1647–C1657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elayappan B, Ravinarayannan H, Pasha SP,
Lee KJ and Gurunathan S: PEDF inhibits VEGF- and EPO-induced
angiogenesis in retinal endothelial cells through interruption of
PI3K/Akt phosphorylation. Angiogenesis. 12:313–324. 2009.
View Article : Google Scholar
|
|
18
|
Lou DA and Hu FN: Specific antigen and
organelle expression of a long-term rhesus endothelial cell line.
In Vitro Cell Dev Biol. 23:75–85. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du W, Yu W, Huang L, Zhao M and Li X:
Ephrin-a4 is involved in retinal neovascularization by regulating
the VEGF signaling pathway. Invest Ophthalmol Vis Sci.
53:1990–1998. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amrite AC, Ayalasomayajula SP, Cheruvu NP
and Kompella UB: Single periocular injection of celecoxib-PLGA
microparticles inhibits diabetes-induced elevations in retinal
PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci.
47:1149–1160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun T, Cao H, Xu L, et al: Insulin-like
growth factor binding protein-related protein 1 mediates
VEGF-induced proliferation, migration and tube formation of retinal
endothelial cells. Curr Eye Res. 36:341–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grigsby JG, Parvathaneni K, Almanza MA, et
al: Effects of tamoxifen versus raloxifene on retinal capillary
endothelial cell proliferation. J Ocul Pharmacol Ther. 27:225–233.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen P, Zhao J and Gregersen H:
Up-regulated expression of advanced glycation end-products and
their receptor in the small intestine and colon of diabetic rats.
Dig Dis Sci. 57:48–57. 2012. View Article : Google Scholar
|
|
24
|
Lee JJ, Hsiao CC, Yang IH, et al:
High-mobility group box 1 protein is implicated in advanced
glycation end products-induced vascular endothelial growth factor A
production in the rat retinal ganglion cell line RGC-5. Mol Vis.
18:838–850. 2012.PubMed/NCBI
|
|
25
|
Shibuya M: Vascular endothelial growth
factor (VEGF) and its receptor (VEGFR) dignaling in angiogenesis: A
crucial target for anti- and pro-angiogenic therapies. Genes
Cancer. 2:1097–1105. 2011. View Article : Google Scholar
|
|
26
|
Nakagawa H, Yasuda S, Matsuura E, et al:
Nicked {beta}2-glycoprotein I binds angiostatin 4.5 (plasminogen
kringle 1–5) and attenuates its antiangiogenic property. Blood.
114:2553–2559. 2009. View Article : Google Scholar : PubMed/NCBI
|