Introduction
Chronic myeloid leukemia (CML) is characterized by
the presence of the Philadelphia (Ph+) chromosome
[t(9;22) q34;q11)] (1). This
chromosome harbors the constitutively active, oncogenic tyrosine
kinase (TK) Breakpoint Cluster Region-Abelson murine leukemia viral
proto-oncogene 1 (BCR-ABL1), which is responsible for leukemic cell
transformation (2–4). Imatinib mesylate
(Glivec®/Gleevec®, Novartis, Basel,
Switzerland) is a potent and selective inhibitor of BCR-ABL1. It
was initially licensed in 2001 (5–10),
and has since rapidly become the standard front-line treatment for
CML, leading to high response rates (11). However, imatinib shows off-target
effects on TKs other than BCR-ABL1, such as platelet-derived growth
factor and colony-stimulating factor 1 receptor, which are involved
in the bone remodeling cycle (12). Previous studies have revealed that
prolonged imatinib treatment in adult CML patients may cause
hypophosphatemia and altered bone mineralization (13–15),
whereas pediatric CML patients develop growth retardation in ≤70%
of cases (16,17).
Growth delay due to long-term imatinib intake is
increasingly observed (11,12,16,18,19),
and is more prominent in patients who began treatment with imatinib
at prepubertal age (12). In
addition, pediatric patients exhibit reduced serum levels of
25-hydroxyvitamin D3 (25-OH-VD3; calcidiol)
and 1,25-dihydroxyvitamin D3
(1,25-(OH)2-VD3; calcitriol) (20) whilst on imatinib treatment. In
humans, vitamin D3 (VD3) is synthesized by
keratinocytes in the skin, by UVB-induced photolysis of
7-dehydrocholesterol (7-DHC), which results in the formation of
previtamin D3, followed by a thermal isomerization step
(21). Thereafter, VD3
is enzymatically hydroxylated to calcidiol by cytochrome P450
(CYP450) isoenzymes CYP2R1 and/or CYP27A1 (22) in the liver, and further metabolized
to hormonally active calcitriol by CYP27B1 (23–25)
in the kidney (Fig. 1). In order
to investigate the calcitriol pathway and its modulation, the HaCaT
human keratinocyte cell line was established by Lehmann (26) as a cellular model, thus
demonstrating for the first time that HaCaT cells were capable of
hydroxylating calcidiol to calcitriol.
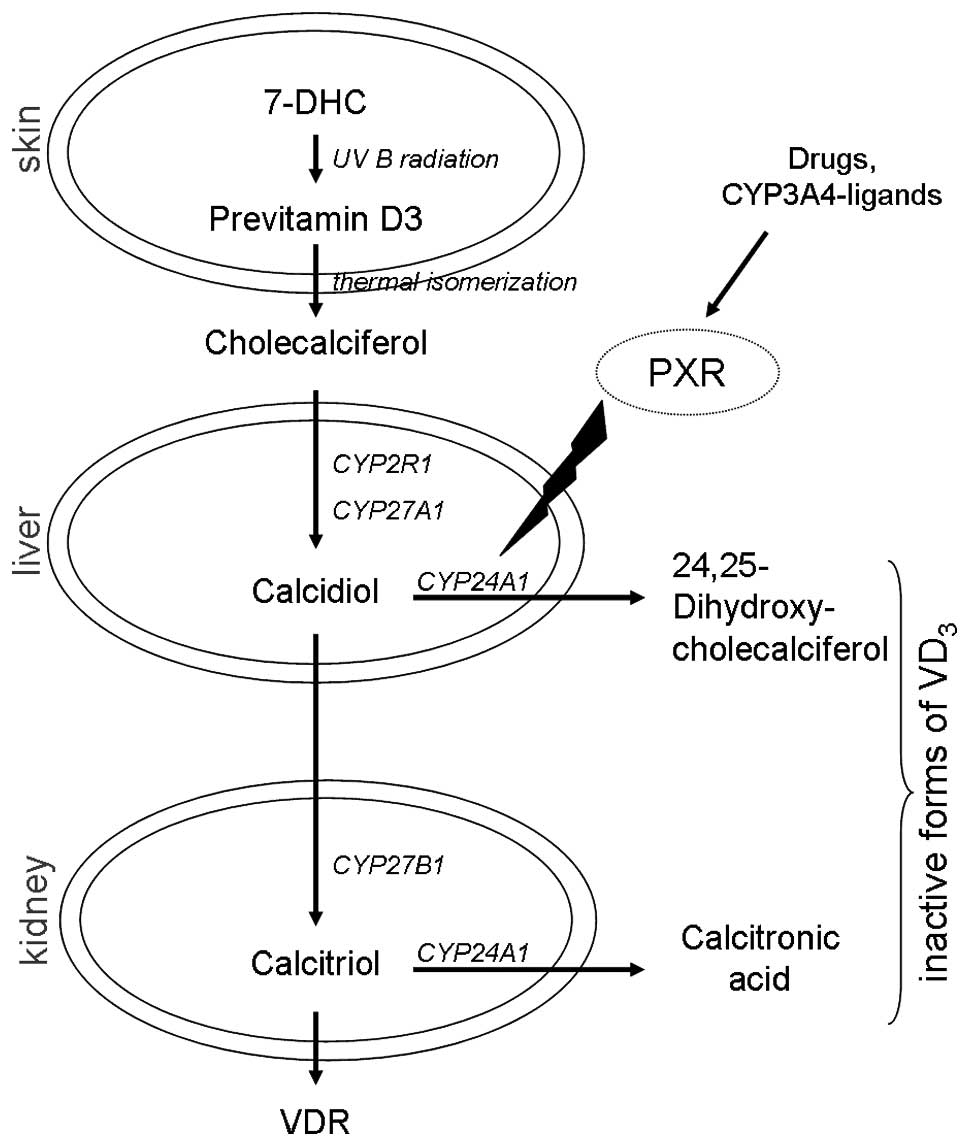 | Figure 1Vitamin D cascade and the enzymes
involved. Modulation of CYP24A1 by citamin D metabolites or other
compounds, such as PXR, may generate a high expression and vitamin
D deficiency, as detected in various tumor tissues [modified after
Schuster et al (32)]. VDR,
vitamin D receptor; VD3, vitamin D3; 7-DHC,
7-dehydrocholesterole; PXR, pregnane x receptor; CYP2R1, cytochrome
P450 family 2, subfamily R, polypeptide 1 (vitamin D
25-hydroxylase); CYP27A1, cytochrome P450, family 27, subfamily A,
polypeptide 1 (vitamin D 25-hydroxylase); CYP27B1, cytochrome P450,
family 27, subfamily B, polypeptide 1 (1α-Hydroxylase); CYP24A1,
cytochrome P450, family 22, subfamily a, polypeptide 1
(1,25-dihydroxyvitamin D3 24-hydroxylase). |
Calcitriol is essential in regulating blood levels
of calcium and phosphorus (27)
and has a key role during bone mineralization (28–30).
Numerous studies have identified an association of vitamin
D3 deficiency (as indicated by low calcidiol/calcitriol
blood levels) with impaired growth, particularly during puberty and
prepuberty (28,31). However, the detailed mechanisms
causing growth delay during imatinib therapy are currently
speculative. The aim of the present study was to investigate the
effects of the TK inhibitor (TKI) imatinib on vitamin D3
metabolism in the HaCaT human keratinocyte cell line.
Materials and methods
Cell culture protocol
The HaCaT human keratinocyte cell line was purchased
from Leibniz Institute DSMZ-German Collection of Microorganisms and
Cell Cultures (Braunschweig, Germany). The cells were seeded at a
density of 1×105 cells/cm2 and grown in
Dulbecco’s modified Eagle’s medium (DMEM; Gibco Life Technologies
GmbH, Darmstadt, Germany), supplemented with 10% fetal bovine serum
(FBS; Gibco Life Technologies GmbH) in a 95% humidified atmosphere
containing 5% CO2, at 37°C for 48 h. The media was
subsequently replaced by serum-free DMEM for 18 h, in order to
induce synchronization of the cell cycle. The cells were then grown
in FBS-supplemented DMEM for 8 h, until they had reached 80–90%
confluency. To investigate the metabolism of vitamin D3,
the cells were seeded at a density of 5×104
cells/cm2 in culture dishes (Ø, 30 mm).
Vitamin D3 assay
To investigate vitamin D3 metabolism the HaCaT cells
(5×104 cells/cm2) were incubated with 25 μM
7-DHC (dissolved in 100% ethanol; Sigma-Aldrich, Steinheim,
Germany) as a substrate, and exposed to UVB (300 nm; application
rate, 75 mJ/cm2). Irradiation of the cells was performed
using a tuneable high intensity monochromator (FWHM, 5 nm; Müller
Optik-Elektronik, Moosinning, Germany) over 15 min. At the start of
irradiation the cells were incubated with imatinib (supplied by
Novartis, Basel, Switzerland), at a concentration of 1 μM
[dissolved in 100% dimethylsufloxide (DMSO)] for 24, 48, or 72 h.
Following the incubation, the media and detached keratinocytes were
collected and calcitriol was extracted using methanol : chloroform
(1:1) (Merck, Darmstadt, Germany). The levels of calcitriol were
determined quantitatively from the organic phase using a
commercially available enzyme assay (1,25-Dihydroxy Vitamin D EIA;
IDS, Frankfurt, Germany). All experiments were performed four times
and the results were normalized to 1×106 cells. Control
experiments with ethanol and DMSO were conducted in order to
identify any interactions with solvents or other components.
To determine whether the VD3 processing
CYP450 isoenzymes CYP2R1, CYP27A1 and CYP27B1 were inhibited by
imatinib, specific inhibitors of the CYP450 isoenzyme family
(VID400 and ketoconazole) were applied concomitantly. These
experiments were conducted without irradiation. The HaCaT cells
were incubated for 0, 2 or 4 h with either 5 μM cholecalciferol or
5 μM calcidiol (both dissolved in 100% ethanol) as a substrate.
Prior to substrate incubation, the cells were incubated for 1 h
with 200 nM VID400 or 10 μM ketoconazole (both dissolved in 100%
ethanol), with or without 1 μM imatinib.
Statistical analysis
Statistical analysis at defined time points of
incubation was performed using one-way analysis of variance with
Bonferoni adjustment to evaluate the effects of IMA-treated samples
compared with untreated controls, using the GraphPad Prism 6.0
software (GraphPad Software, Inc., San Diego, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Inhibitory effects of imatinib on
calcitriol synthesis
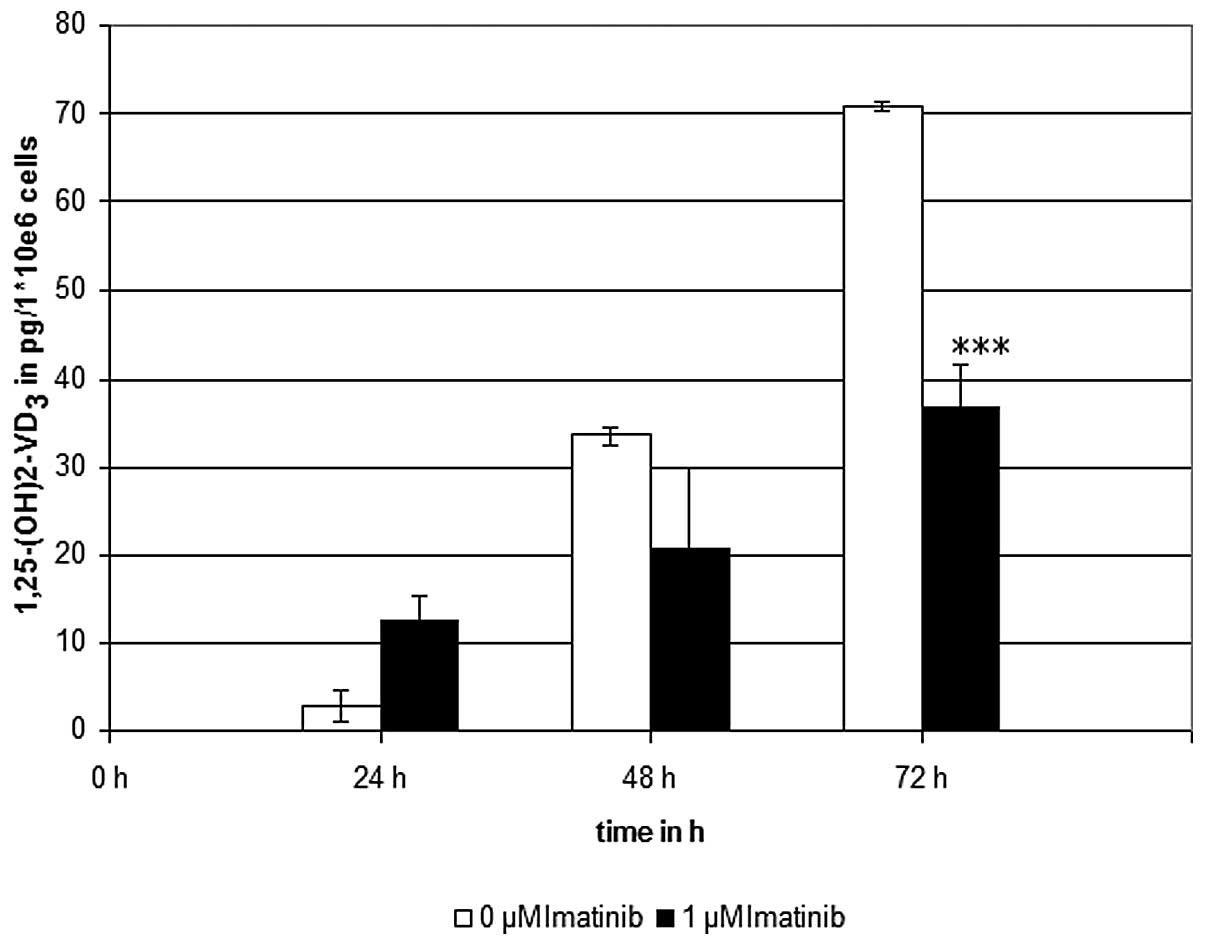
Imatinib incubation at the clinically effective
concentration of 1 μM, significantly reduced the calcitriol levels
to ~50%, as compared with the controls, which were not treated with
the TKI (Fig. 2). To verify these
results, control experiments were conducted in the presence of
7-DHC without irradiation, and in the absence of 7-DHC with
irradiation. Furthermore, to screen out any interactions of the
solvents used, control experiments with ethanol and DMSO were
conducted. As expected, no generation of calcitriol was detectable
in the control experiments.
Effects of selective inhibitors in
combination with imatinib on the vitamin D3 cascade
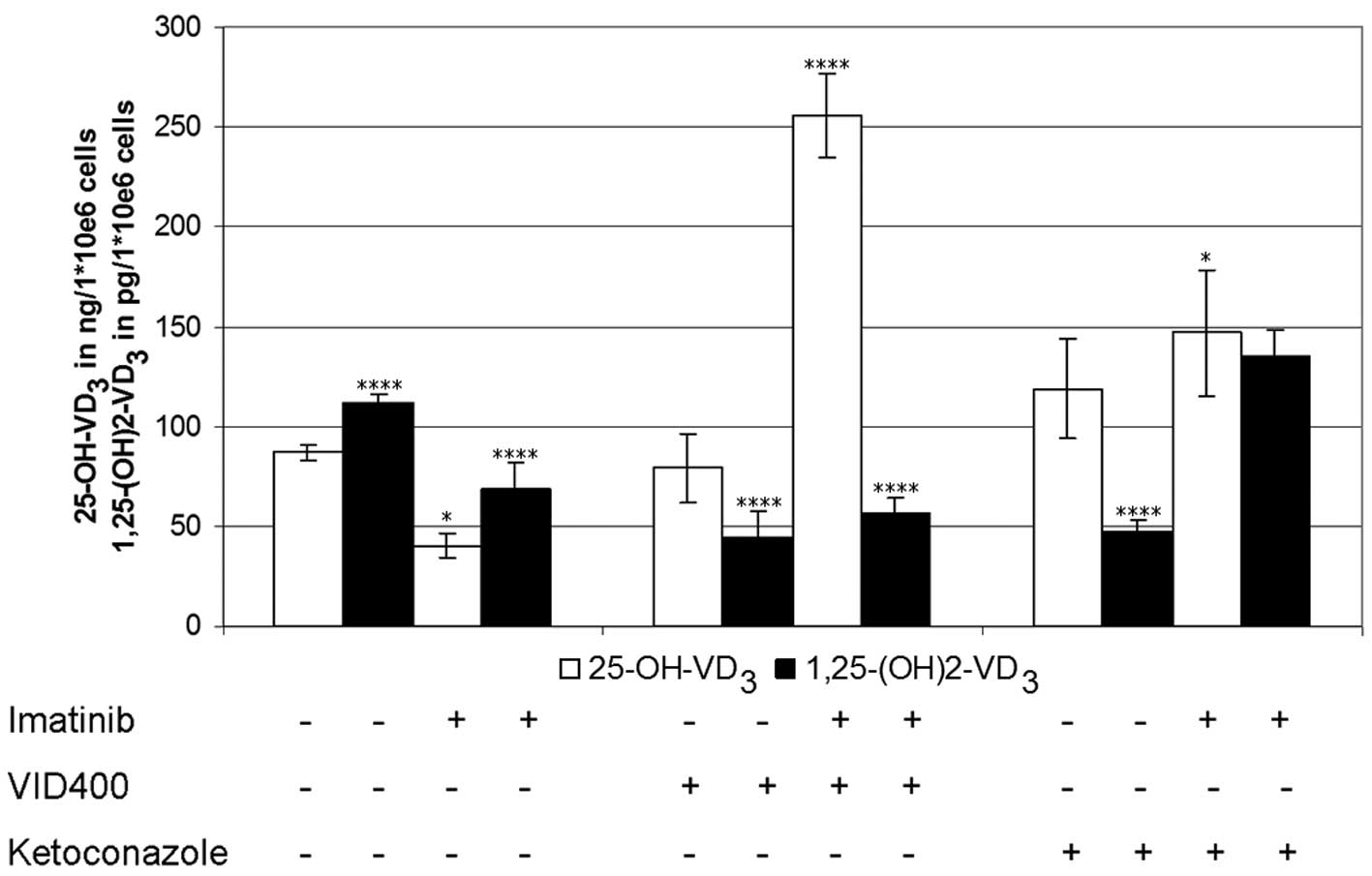
Using cholecalciferol as the vitamin D3
synthesis-starting substrate, the levels of calcidiol and
calcitriol in the cells exposed to imatinib over 4 h were lowered
to 50% that of the controls (Fig.
3). Treatment with the CYP450 inhibitors VID400 and/or
ketoconazole, in the absence of imatinib, had nearly no effect on
calcidiol levels (range, 90–110 ng/1×106 cells), whereas
calcitriol levels decreased to 60% of the control values. Treatment
with imatinib in the presence of VID400, resulted in increased
calcidiol levels by 600% but had no effects on calcitriol
synthesis. Treatment with ketoconazole and imatinib resulted in
increased levels of calcitriol, by 200% (Fig. 3).
Furthermore, the experiments were repeated using
calcidiol as the substrate and analyzed in the same way as
previously described, resulting in calcitriol levels concordant
with those described in Fig. 3,
with the exception of ketoconazole. The cells incubated with
imatinib in the absence of VID400 or ketoconazole had lower
calcitriol levels, as compared with those incubated without
imatinib. Identical levels were detected in the presence of VID400
without imatinib, whereas the combination of VID400 and imatinib
increased the levels. In the presence of ketoconazole the
calcitriol levels, with and without imatinib exposure, were
decreased.
Discussion
During imatinib treatment longitudinal growth
retardation has been identified as a frequent side effect in
children (13–15,32–43).
Jaeger et al (20)
investigated biochemical skeletal markers in 17 pediatric patients
with CML (age, 4–17 years) undergoing imatinib treatment and
reported low serum levels of vitamin D3, as well as
impaired bone metabolism. However, children undergoing treatment
for various types of cancer frequently exhibit vitamin
D3 deficiencies (44,45).
The reason for this may be a lack of sun exposure or poor
nutrition, but may also be due to drug interactions, or a
combination of these factors (44).
In humans, vitamin D3 has a primary role
in maintaining extracellular ionized calcium levels and bone
mineralization (46). In children,
vitamin D3 is required for growth and also for the
prevention of rickets (47). In
addition, vitamin D3 is an important immunomodulator,
that has been shown to have antiproliferative effects, potentiate
apoptosis and inhibit angiogenesis (45). Pediatric oncology patients have a
higher prevalence of vitamin D3 hypovitaminosis
(20,45). The present study aimed to
investigate the reasons for vitamin D3 deficiency and
recognize the potentially causative mechanisms for low vitamin D
serum levels and growth retardation in prepubertal patients with
CML. The results of the present study demonstrate an inhibitory
effect of imatinib on the synthesis of calcidiol and calcitriol
during vitamin D3 synthesis in human keratinocytes,
leading to decreased levels by 50%. This finding is in concordance
with the published clinical data of Jaeger et al (20).
To identify the potential target of imatinib within
the vitamin D3 cascade, the synthesis of calcidiol and
calcitriol was examined in confluent HaCaT cells treated with two
well-known specific CYP450 inhibitors: VID400 and ketoconazole.
While ketoconazole is known to be a general inhibitor of P450
enzymes, VID400 specifically blocks CYP24A1, thus allowing the
identification of the potential target of imatinib within the
vitamin D3 cascade with enzymes involved, such as
CYP24A1, CYP27A1 and CYP27B1 (Fig.
1). Experiments were conducted in combination with and without
imatinib using cholecalciferol as a substrate, therefore no
irradiation of the cells was required. As previously described,
VID400 at a concentration of 200 nM, may dose-dependently inhibit
CYP24A1 activity, and partially inhibit CYP27B1 by 30% (48). Ketoconazole, at a concentration of
10 μM, is a general inhibitor of the CYP450 isoenzymes (49), including vitamin D hydroxylating
enzymes, such as CYP24A1, CYP27A1 and CYP27B1 (50). The present study demonstrated that
exposure to VID400 alone stabilized the levels of endogenously
produced calcitriol.
It has been shown that VID400 results in increased
expression of Cyp24 (451,52). CYP24 catalyzes the metabolism of
calcidiol and calcitriol. The activity is regulated by a negative
feedback loop dependent on calcitriol concentration, resulting in
decreased calcitriol levels. It has previously been suggested, that
in cancer cells, particularly in prostate cancer, a rapid breakdown
of calcitriol levels is caused by an overactive CYP24 (53). The combination of the CYP24
inhibitors tested with imatinib, resulted in increased levels of
calcidiol levels. These results suggest that besides the inhibition
of CYP24 by VID400, the activity of CYP27B1 is impaired by
imatinib, resulting in an intracellular accumulation of calcidiol.
This is in concordance with a previous in vivo study, where
it was shown that imatinib is metabolized by various liver CYP450
isoenzymes, mainly CYP3A4 and CYP3A5 (54). CYP3A4 is also known to be a human
microsomal vitamin D 25-hydroxylase (55), similar to CYP27B1.
Isolated ketoconazole exposure resulted in increased
calcidiol and decreased calcitriol levels, whereas the combination
with imatinib increased the levels of calcidiol and calcitriol.
Ketoconazole is also known to be a strong inhibitor of CYP3A4
(56), resulting in poor
metabolism of imatinib. Based on a drug interaction study,
co-administration of imatinib and inhibitors as well as inducers of
CYP3A4 activity (57), requires
careful monitoring of the patients to rule out toxic side effects,
or decreased TKI effects on the underlying CML.
To catalyze the 25-hydroxylation step in the liver,
at least six CYPs are involved in vivo, the most prominent
ones being CYP27A1 and CYP2R1 (58). CYP27B1 is responsible for the renal
1α-hydroxylation of vitamin D to hormonally active calcitriol. The
vitamin D synthesis cascade is a complex system with numerous
enzymes involved at diverse steps; therefore, various enzymes may
be affected by imatinib. Imatinib, the inhibitors (VID400 and/or
ketoconazole) and the substrates (cholecalciferol and/or calcidiol)
may all compete for binding to one or more CYPs in keratinocytes,
resulting in interference with vitamin D3 metabolism.
The results of the present study clearly indicate a competitive
inhibition of CYP27B1 by imatinib, as concomitant blocking of
CYP27B1 with VID400 resulted in elevated levels of calcidiol, but
decreased levels of calcitriol. However, the mechanism remains
poorly understood, and additional studies are required.
Acknowledgements
The authors of the present study would like to thank
Mr. Nick Zimmermann for assistance in technical work, as well as
Mr. Peter Knuschke for the introduction to solar radiation and for
scientific discussion. They are also grateful to Novartis Pharma AG
(Basel, Switzerland) for the supply of imatinib.
References
|
1
|
Tipping AJ, Mahon FX, Zafirides G, et al:
Drug responses of imatinib mesylate-resistant cells: synergism of
imatinib with other chemotherapeutic drugs. Leukemia. 16:2349–2357.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Capdeville R, Silberman S and Dimitrijevic
S: Imatinib: the first 3 years. Eur J Cancer. 38:S77–S82. 2002.
View Article : Google Scholar
|
|
3
|
Daley GQ, Van Etten RA and Baltimore D:
Induction of chronic myelogenous leukemia in mice by the
P210bcr/abl gene of the Philadelphia chromosome. Science.
247:824–830. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen MH, Williams G, Johnson JR, et al:
Approval summary for imatinib mesylate capsules in the treatment of
chronic myelogenous leukemia. Clin Cancer Res. 8:935–942.
2002.PubMed/NCBI
|
|
5
|
Champagne MA, Capdeville R, Krailo M, et
al: Imatinib mesylate (STI571) for treatment of children with
Philadelphia chromosome-positive leukemia: results from a
Children’s Oncology Group phase 1 study. Blood. 104:2655–2660.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Druker BJ, Tamura S, Buchdunger E, et al:
Effects of a selective inhibitor of the Abl tyrosine kinase on the
growth of Bcr-Abl positive cells. Nat Med. 2:561–566. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Druker BJ, Talpaz M, Resta DJ, et al:
Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine
kinase in chronic myeloid leukemia. N Engl J Med. 344:1031–1037.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grigg A and Hughes T: Role of allogeneic
stem cell transplantation for adult chronic myeloid leukemia in the
imatinib era. Biol Blood Marrow Transplant. 12:795–807. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Millot F, Guilhot J, Nelken B, et al:
Imatinib mesylate is effective in children with chronic myelogenous
leukemia in late chronic and advanced phase and in relapse after
stem cell transplantation. Leukemia. 20:187–192. 2006. View Article : Google Scholar
|
|
10
|
Roy L, Guilhot J, Krahnke T, et al:
Survival advantage from imatinib compared with the combination
interferon-alpha plus cytarabine in chronic-phase chronic
myelogenous leukemia: historical comparison between two phase 3
trials. Blood. 108:1478–1484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hobernicht SL, Schweiger B, Zeitler P,
Wang M and Hunger SP: Acquired growth hormone deficiency in a girl
with chronic myelogenous leukemia treated with tyrosine kinase
inhibitor therapy. Pediatr Blood Cancer. 56:671–673. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shima H, Tokuyama M, Tanizawa A, et al:
Distinct impact of imatinib on growth at prepubertal and pubertal
ages of children with chronic myeloid leukemia. J Pediatr.
159:676–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berman E, Nicolaides M, Maki RG, et al:
Altered bone and mineral metabolism in patients receiving imatinib
mesylate. N Engl J Med. 354:2006–2013. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fierro F, Illmer T, Jing D, et al:
Inhibition of platelet-derived growth factor receptorbeta by
imatinib mesylate suppresses proliferation and alters
differentiation of human mesenchymal stem cells in vitro. Cell
Prolif. 40:355–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fitter S, Dewar AL, Kostakis P, et al:
Long-term imatinib therapy promotes bone formation in CML patients.
Blood. 111:2538–2547. 2008. View Article : Google Scholar
|
|
16
|
Schmid H, Jaeger BA, Lohse J and Suttorp
M: Longitudinal growth retardation in a prepuberal girl with
chronic myeloid leukemia on long-term treatment with imatinib.
Haematologica. 94:1177–1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suttorp M, Yaniv I and Schultz KR:
Controversies in the treatment of CML in children and adolescents:
TKIs versus BMT? Biol Blood Marrow Transplant. 17:S115–S122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kimoto T, Inoue M and Kawa K: Growth
deceleration in a girl treated with imatinib. Int J Hematol.
89:251–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mariani S, Giona F, Basciani S, Brama M
and Gnessi L: Low bone density and decreased inhibin-B/FSH ratio in
a boy treated with imatinib during puberty. Lancet. 372:111–112.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jaeger BA, Tauer JT, Ulmer A, et al:
Changes in bone metabolic parameters in children with chronic
myeloid leukemia on imatinib treatment. Med Sci Monit.
18:CR721–CR728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lehmann B, Sauter W, Knuschke P, Dressler
S and Meurer M: Demonstration of UVB-induced synthesis of 1 alpha,
25-dihydroxyvitamin D3 (calcitriol) in human skin by microdialysis.
Arch Dermatol Res. 295:24–28. 2003.PubMed/NCBI
|
|
22
|
Lehmann B and Meurer M: Vitamin D
metabolism. Dermatol Ther. 23:2–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holick MF: Resurrection of vitamin D
deficiency and rickets. J Clin Invest. 116:2062–2072. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DeLuca HF: Overview of general physiologic
features and functions of vitamin D. Am J Clin Nutr.
80:1689S–1696S. 2004.PubMed/NCBI
|
|
26
|
Lehmann B: HaCaT cell line as a model
system for vitamin D3 metabolism in human skin. J Invest Dermatol.
108:78–82. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bogh MK, Schmedes AV, Philipsen PA,
Thieden E and Wulf HC: Interdependence between body surface area
and ultraviolet B dose in vitamin D production: a randomized
controlled trial. Br J Dermatol. 164:163–169. 2011. View Article : Google Scholar
|
|
28
|
Kremer R, Campbell PP, Reinhardt T and
Gilsanz V: Vitamin D status and its relationship to body fat, final
height, and peak bone mass in young women. J Clin Endocrinol Metab.
94:67–73. 2009. View Article : Google Scholar :
|
|
29
|
Davis CD and Dwyer JT: The ‘sunshine
vitamin’: benefits beyond bone? J Natl Cancer Inst. 99:1563–1565.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mathieu C and Badenhoop K: Vitamin D and
type 1 diabetes mellitus: state of the art. Trends Endocrinol
Metab. 16:261–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pettifor JM: Rickets and vitamin D
deficiency in children and adolescents. Endocrinol Metab Clin North
Am. 34:537–553. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schuster I, Egger H, Herzig G, Reddy GS,
Schmidt JA, Schüssler M and Vorisek G: Selective inhibitors of
vitamin D metabolism - new concepts and perspectives. Anticancer
Res. 26:2653–2668. 2006.PubMed/NCBI
|
|
33
|
Tibullo D, Giallongo C, La Cava P, et al:
Effects of imatinib mesylate in osteoblastogenesis. Exp Hematol.
37:461–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O’Sullivan S, Naot D, Callon K, et al:
Imatinib promotes osteoblast differentiation by inhibiting PDGFR
signaling and inhibits osteoclastogenesis by both direct and
stromal cell-dependent mechanisms. J Bone Miner Res. 22:1679–1689.
2007. View Article : Google Scholar
|
|
35
|
Dewar AL, Zannettino AC, Hughes TP and
Lyons AB: Inhibition of c-fms by imatinib: expanding the spectrum
of treatment. Cell Cycle. 4:851–853. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dewar AL, Cambareri AC, Zannettino AC, et
al: Macrophage colony-stimulating factor receptor c-fms is a novel
target of imatinib. Blood. 105:3127–3132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dewar AL, Domaschenz RM, Doherty KV,
Hughes TP and Lyons AB: Imatinib inhibits the in vitro development
of the monocyte/macrophage lineage from normal human bone marrow
progenitors. Leukemia. 17:1713–1721. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Owen S, Hatfield A and Letvak L: Imatinib
and altered bone and mineral metabolism. N Engl J Med. 355:627–629.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
O’Sullivan S, Horne A, Wattie D, et al:
Decreased bone turnover despite persistent secondary
hyperparathyroidism during prolonged treatment with imatinib. J
Clin Endocrinol Metab. 94:1131–1136. 2009. View Article : Google Scholar
|
|
40
|
El Hajj Dib I, Gallet M, Mentaverri R, et
al: Imatinib mesylate (Gleevec) enhances mature osteoclast
apoptosis and suppresses osteoclast bone resorbing activity. Eur J
Pharmacol. 551:27–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grey A, O’Sullivan S, Reid IR and Browett
P: Imatinib mesylate, increased bone formation, and secondary
hyperparathyroidism. N Engl J Med. 355:2494–2495. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jönsson S, Olsson B, Ohlsson C, et al:
Increased cortical bone mineralization in imatinib treated patients
with chronic myelogenous leukemia. Haematologica. 93:1101–1103.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vandyke K, Fitter S, Dewar AL, Hughes TP
and Zannettino AC: Dysregulation of bone remodeling by imatinib
mesylate. Blood. 115:766–774. 2010. View Article : Google Scholar
|
|
44
|
Genc DB, Ozkan MA and Buyukgebiz A:
Vitamin D in childhood cancer: a promising anticancer agent?
Pediatr Endocrinol Rev. 10:485–493. 2013.PubMed/NCBI
|
|
45
|
Helou M, Ning Y, Yang S, et al: Vitamin D
deficiency in children with cancer. J Pediatr Hematol Oncol.
36:212–217. 2014. View Article : Google Scholar
|
|
46
|
Mithal A, Wahl DA, Bonjour JP, et al:
Global vitamin D status and determinants of hypovitaminosis D.
Osteoporos Int. 20:1807–1820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lips P: Vitamin D status and nutrition in
Europe and Asia. J Steroid Biochem Mol Biol. 103:620–625. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xie Z, Munson SJ, Huang N, et al: The
mechanism of 1,25-dihydroxyvitamin D(3) autoregulation in
keratinocytes. J Biol Chem. 277:36987–36990. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nguyen M, Boutignon H, Mallet E, et al:
Infantile hypercalcemia and hypercalciuria: new insights into a
vitamin D-dependent mechanism and response to ketoconazole
treatment. J Pediatr. 157:296–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Segersten U, Björklund P, Hellman P,
Akerström G and Westin G: Potentiating effects of nonactive/active
vitamin D analogues and ketoconazole in parathyroid cells. Clin
Endocrinol (Oxf). 66:399–404. 2007. View Article : Google Scholar
|
|
51
|
Schuster I, Egger H, Reddy GS and Vorisek
G: Combination of vitamin D metabolites with selective inhibitors
of vitamin D metabolism. Recent Results Cancer Res. 164:169–188.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schuster I, Egger H, Nussbaumer P and
Kroemer RT: Inhibitors of vitamin D hydroxylases:
structure-activity relationships. J Cell Biochem. 88:372–380. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yee SW, Campbell MJ and Simons C:
Inhibition of Vitamin D3 metabolism enhances VDR signalling in
androgen-independent prostate cancer cells. J Steroid Biochem Mol
Biol. 98:228–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Peng B, Lloyd P and Schran H: Clinical
pharmacokinetics of imatinib. Clin Pharmacokinet. 44:879–894. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gupta RP, Hollis BW, Patel SB, Patrick KS
and Bell NH: CYP3A4 is a human microsomal vitamin D 25-hydroxylase.
J Bone Miner Res. 19:680–688. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Takeshita A, Taguchi M, Koibuchi N and
Ozawa Y: Putative role of the orphan nuclear receptor SXR (steroid
and xenobiotic receptor) in the mechanism of CYP3A4 inhibition by
xenobiotics. J Biol Chem. 277:32453–32458. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dagher R, Cohen M, Williams G, et al:
Approval summary: imatinib mesylate in the treatment of metastatic
and/or unresectable malignant gastrointestinal stromal tumors. Clin
Cancer Res. 8:3034–3038. 2002.PubMed/NCBI
|
|
58
|
Cheng JB, Levine MA, Bell NH, Mangelsdorf
DJ and Russell DW: Genetic evidence that the human CYP2R1 enzyme is
a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA.
101:7711–7715. 2004. View Article : Google Scholar : PubMed/NCBI
|