Introduction
Breast cancer, the fifth leading cause of
cancer-associated mortality worldwide, has high rates of recurrence
and metastasis, which makes the disease difficult to eradicate
(1). Although improved screening
techniques have assisted in detecting breast cancer at early stages
and advances in treatment have markedly improved patient survival
rates, tumor invasion and metastasis remain the major contributors
in breast cancer-associated mortality (2). Therefore the inhibition of metastasis
offers a potential strategy to minimize the spread of breast tumors
and improve patient survival rates.
Tumor metastasis is the primary cause of mortality
in patients with cancer, which occurs when malignant cells degrade
the cellular basement membrane and spread to distant organs,
resulting in the formation of secondary tumors (3). Degradation of the basement membrane
and extracellular matrix (ECM) is achieved by various proteases,
including matrix metalloproteinases (MMPs) (4), of which MMP-2 and MMP-9 are
frequently overexpressed in malignant tumors and directly affect
cancer cell invasion (5).
Traditional oriental herbs offer potential therapies
for various diseases, including diabetes mellitus, cancer and
hypertension (6–9). In addition, increasing evidence has
demonstrated that certain herbs improve the efficacy and reduce the
side effects of conventional cancer therapies in breast and
prostate cancer (10,11).
Ampelopsis japonica (AJ), a well-known
traditional oriental herb, has long been used as an
anti-inflammatory and antibacterial agent against skin inflammation
and ulcerous diseases or burns of the skin, respectively (12–14),
and as an antitumor agent (15).
However, few studies have been performed in this area and the
mechanisms underlying the antitumor activity of AJ remain to be
elucidated. The aim of the present study was to investigate the
effects of EAJ on the invasion and migration of MDA-MB-231 breast
cancer cells in vitro and to elucidate the associated
signaling pathways.
Materials and methods
Chemicals and materials
All plastic materials, including cell culture
vessels, flasks, Petri dishes, roller bottle and multi-well plates,
were purchased from BD Falcon Labware (Franklin Lakes, NJ, USA).
Fetal bovine serum (FBS), phosphate-buffered saline (PBS) and
penicillin G/streptomycin were obtained from Gibco-BRL (Grand
Island, NY, USA). RPMI-1640 medium was purchased from Welgene
(Daegu, Korea). The CCK-8 assay kit was obtained from Dojindo
Molecular Technologies, Inc. (Rockville, MD, USA). High-performance
liquid chromatography (HPLC)-grade reagents, acetonitrile and water
were obtained from J.T. Baker Chemical Co. (Phillipsburg, NJ, USA).
Catechin, resveratrol standards and other chemicals were purchased
from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of the extract
AJ was purchased from Omniherb Co. (Yeoungcheon,
Korea) as a dried herb and was authenticated based on microscopic
and macroscopic characteristics by the Classification and
Identification Committee of the Korea Institute of Oriental
Medicine (KIOM; Daejeon, Korea). The committee was composed of nine
experts in the fields of plant taxonomy, botany, pharmacognosy and
herbology. A voucher specimen (no. KIOM 108-29A) was deposited at
the herbarium of the Department of Herbal Resources Research in
KIOM. The dried bark of AJ (200 g) was extracted twice using 70%
ethanol (with 2 h reflux) and the extract was concentrated under
reduced pressure. The decoction was then filtered, lyophilized and
stored at 4°C until further use. The yield of dried extract from
the initial crude materials was ~15.56% (w/w). The lyophilized
powder was dissolved in 10% dimethyl sulfoxide and then filtered
through a 0.22 μm syringe filter to create a stock solution.
Cell culture
Human breast (MDA-MB-231) and liver (HepG2 and
Hep3b) cancer cell lines were obtained from the American Type
Culture Collection (ATCC; Rockville, MD, USA). The MDA-MB-231 cells
were cultured in RPMI-1640 and liver cancer cells (HepG2 and Hep3b)
were cultured in Eagle’s Minimum Essential Medium (EMEM; ATCC)
supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin in a humidified atmosphere of 5% CO2 at
37°C. The medium was replaced every three days.
Cell viability assay
WST-8, the active agent in the Cell Counting kit-8
(CCK-8) cell viability assessment, is reduced to yellow
water-soluble formazan by the dehydrogenase released from
mitochondria. A CCK-8 assay kit was used to determine the cell
viability of AJ ethanol extract (EAJ)-treated cells and the
absorbance was measured at 450 nm using a Benchmark Plus Microplate
Spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
HepG2, Hep3b and MDA-MB-21 cells were exposed to various
concentrations of EAJ (0, 25, 50, 100, 200 or 400 μg/ml) for 24 h.
MDA-MB-231 cells were exposed to the various concentrations of EAJ
(0, 10, 25, 50, 100, 150, 200, 300 or 400 μg/ml) for 9, 12 or 24 h.
Cell viability was then assessed using a CCK-8 assay. Cytotoxicity
was expressed as a percentage of the absorbance measured in the
untreated control cells.
Wound healing motility assay
The MDA-MB-231 cells were plated in 12-well plates
at a density of 5×105 cells/well and cultured in medium
containing 10% FBS until the cells were reached ~70–80% confluence
as a monolayer. The monolayer was carefully wounded using a yellow
pipette tip and the cellular debris was removed by washing with
RPMI-1640. The wounded monolayer was incubated with or without EAJ
(25, 50, 100 or 150 μg/ml) for 12 and 24 h in RPMI-1640 medium
containing 10% FBS, as a chemoattractant or 1% FBS, as a control.
Images were captured of the cell migration into the wounded area
using phase contrast microscopy (Olympus IX53; Olympus Corp.,
Tokyo, Japan). The migratory cells were quantified and the
percentage of inhibition was expressed based on the untreated
control wells. The degree of wound closure or invasion was
determined using inverted microscopy (Olympus IX53) and ImageJ
analysis (version, IJ 1.46r; National Institutes of Health,
Bethesda, MA, USA) to measure percent closure of the wounded area
within the captured images. Briefly, from the wound area, the
average wound width was obtained by dividing the area by the length
of the analyzed region. The obtained wound widths were plotted
against time in Microsoft Excel software (2010; Microsoft Corp.,
Redmond, WA, USA), and a linear fit was generated for each dataset.
The slope of the linear fit was used as a measure of cell
migration.
Cell invasion assay
The MDA-MB-231 cells were evaluated in Transwell
inserts with 8 μm pores (Corning Life Science, Inc, Tewksbury, MA,
USA). The upper surface of the Transwell membrane was coated with
Matrigel (5 μg), while the lower compartment of the chamber was
filled with 500 μl RPMI-1640 medium containing 1% FBS (control).
The cells (5×105) were seeded onto the upper part of
each Transwell in serum-free RPMI-1640 medium containing different
concentrations of EAJ (50, 100 or 150 μg/ml) for 24 h. The cells
were allowed to invade through the Matrigel for 24 h at 37°C in 5%
CO2/95% air and were then stained with Giemsa for 15
min. The upper surface of the membrane was scraped with a cotton
swab and, when the invasive cells reached the bottom surface of the
porous filter, images were captured and the cells were counted. The
percentage inhibition was expressed as a percentage of the
untreated control wells.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The cells were lysed, and total RNA was isolated
with an easy-BLUE total extraction kit (Intron, Seoul, Korea)
according to the manufacturer’s instructions. For cDNA synthesis, 1
μg total RNA was mixed with Maxime RT premix (Intron) containing
oligo dT primers and DEPC-treated water to a final volume of 20 μl,
and incubated at 45°C for 60 min. The reaction was stopped by heat
inactivation at 95°C for 5 min. RT-qPCR was performed using a
Rotor-Gene 3000 system (Corbett Research, Sydney, Australia) with
SYBR Green Master mix (Qiagen, Tokyo, Japan). All reactions were
carried out according to the following protocol: 94°C for 2 min,
followed by 35 cycles of 94°C for 20 sec, 60°C for 20 sec and 72°C
for 30 sec. The percentage of target gene expression relative to
the control was normalized to the expression of glyceraldehyde
3-phosphate dehydrogenase (GAPDH; internal control) using
2−ΔΔCt analysis. Primers for the target genes were
designed using Primer 3 software (Table I) (16).
 | Table ISequences of the primers used in
reverse transcription quantitative polymerase chain reaction
analysis. |
Table I
Sequences of the primers used in
reverse transcription quantitative polymerase chain reaction
analysis.
| Gene | Forward | Reverse | Accession number | Length (bp) |
|---|
| MMP-2 |
CAGAATACCATCGAGACCAT |
ATGTGATCTGGTTCTTGTCC | NM_004530 | 106 |
| MMP-9 |
CCACTACTGTGCCTTTGAGT |
TCCCATCCTTGAACAAATAC | NM_004994 | 102 |
| TIMP1 |
TGGACTCTTGCACATCACTA |
GATGGATAAACAGGGAAACA | NM_003254 | 133 |
| TIMP2 |
GCTCTGTTGATTTTGTTTCC |
CTGCTTGTCAACTTTCAACA | NM_003255 | 125 |
| GAPDH |
TCAAGCTCATTTCCTGGTAT |
GTGAGGGTCTCTCTCTTCCT | NM_002046 | 141 |
High-performance liquid chromatography
analysis
The samples were analyzed using reverse phase
high-performance liquid chromatography with the Waters Alliance
2695 system (Waters Co., Milford, MA, USA) coupled with a 2996
Photodiode Array Detector (Waters Co.). A Phenomenex Luna C18
column (250×4.6 mm; particle size, 5 μm; Phenomenex, Torrance, CA,
USA) was used as the stationary phase and the mobile phase was
composed of 0.1% (v/v) trifluoroacetic aqueous solution (A)
and acetonitrile (B). The elution conditions were as follows: At 0
min, the mobile phase consisted of 90% A/10% B and was held for 10
min. Between 10 and 40 min, a gradient was applied to 60% A/40% B.
This was followed by washing with 100% B for 5 min and a 15 min
equilibration period in 90% A/10% B. The separation temperature was
maintained at 40°C throughout the analysis, with a flow rate of 1.0
ml/min and an injection volume of 20 μl. Identification was based
on the retention time and ultraviolet (UV) spectra compared with
that of commercial standards, catechin and resveratrol. The
components were quantified based on peak areas at the maximal
wavelength. Calibration curves of the standards, ranging between
6.25 and 200 μg/ml (six levels), revealed good linearity with
R2 values >0.99 (peak areas, vs. concentration).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA).
One-way and two-way analysis of variance were followed by Dunnett’s
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of EAJ on the proliferation of
MDA-MB-231 cells
The cytotoxic effect of EAJ on three cancer cell
lines (HepG2, Hep3b and MDA-MB-231) was evaluated using a CCK-8
assay. The results demonstrated that EAJ reduced the viability of
each of these cell lines in a concentration-dependent manner
(Fig. 1A). In addition, EAJ was
shown to have a weaker effect on the viability of MDA-MB-231 cells
compared with the HepG2 and Hep3b cells. EAJ reduced the viability
of the MDA-MB-231 cells to 70% of the control in a concentration-
and time-dependent manner, with significant reduction in viability
following exposure to 200–400 μg/ml EAJ for 12 or 24 h (Fig. 1B). Based on these results,
non-cytotoxic concentrations of EAJ (25–150 μg/ml) were used for
further experiments. The lack of cytotoxicity was verified by
assessing cell morphology (Fig.
1C).
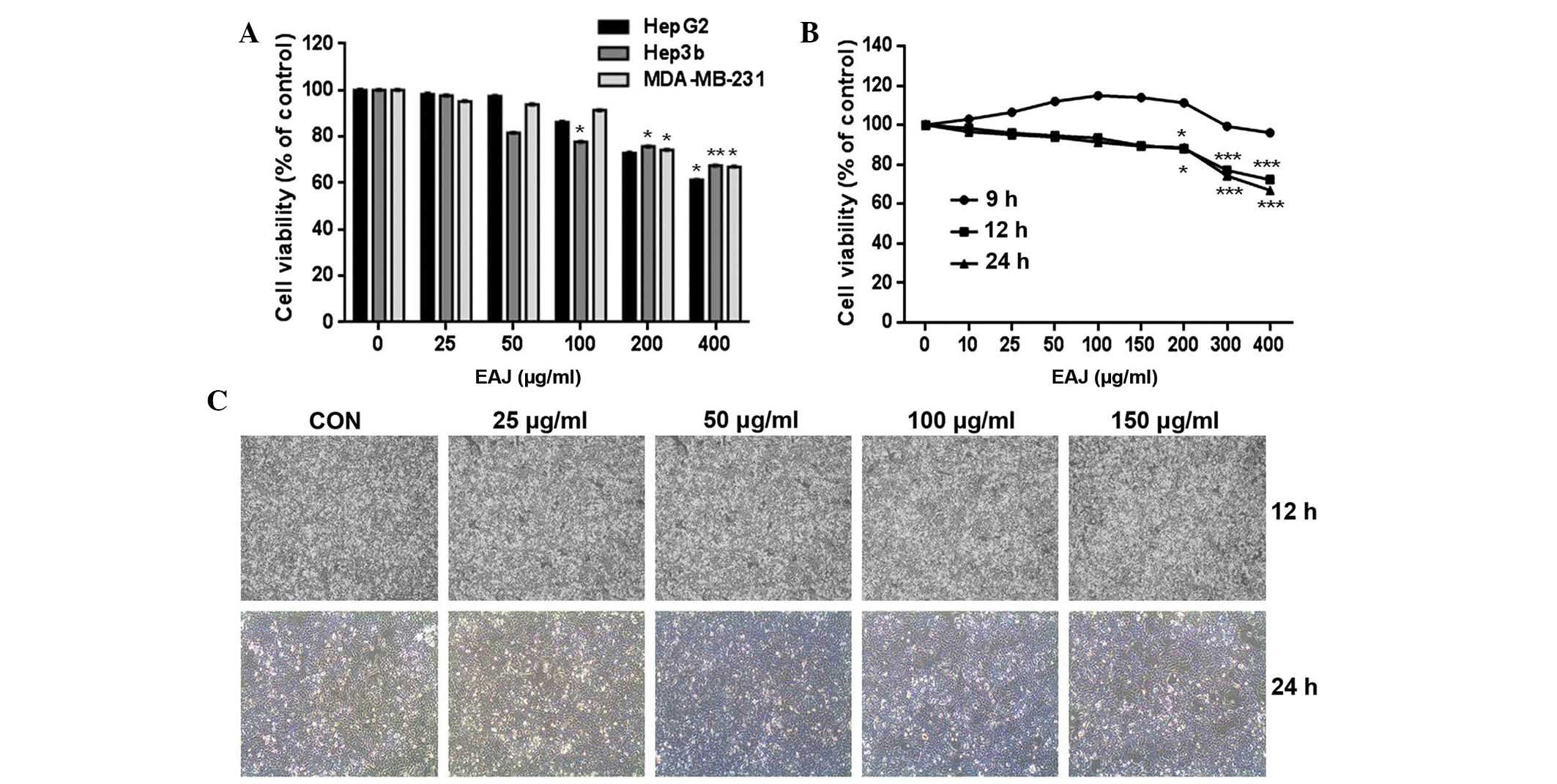 | Figure 1EAJ reduces the viability of
MDA-MB-231 breast cancer cells. (A) HepG2, Hep3b and MDA-MB-21
cells were exposed to various concentrations of EAJ (0, 25, 50,
100, 200 or 400 μg/ml) for 24 h. (B) MDA-MB-231 cells were exposed
to the indicated concentrations of EAJ for 9, 12 or 24 h. Cell
viability was then assessed using a Cell Counting kit-8 assay.
Values are presented as the mean ± standard deviation (n=3).
*P<0.05, **P<0.01 and
***P<0.001, vs. control. (C) Cell morphology was
examined under an inversion microscope following treatment of
MDA-MB-231 cells with the indicated concentrations of EAJ
magnification, ×200). EAJ, Ampelopsis japonica ethanol
extract; CON, control (0 μg/ml EAJ). |
Effect of EAJ on the migration of
MDA-MB-231 cells
Cell migration is an important process in tumor
metastasis. As shown by the wound healing assay in Fig. 2A, 25–150 μg/ml EAJ significantly
inhibited MDA-MB-231 cell migration following incubation for 24 h,
whereas only those in 100 and 150 μg/ml EAJ were significantly
inhibited after 12 h of incubation. As indicated by the
densitometric analyses (Fig. 2B),
150 μg/ml EAJ decreased the migration of the MDA-MB-231 cells by
~60 and 80% at 12 and 24 h, respectively. These results suggested
that EAJ may effectively inhibit the migration of the highly
invasive MDA-MB-231 cells.
Effect of EAJ on the invasion of
MDA-MB-231 cells
Interactions between components of the cell matrix
are important in cancer invasion. In the Transwell cell invasion
assay, the motility and invasive ability of the MDA-MB-231 cells
were reduced in a concentration-dependent manner (Fig. 3A). In addition, quantitative
analysis of these results demonstrated that exposure of the cells
to 50, 100, 150 μg/ml EAJ for 24 h significantly reduced cell
attachments to the Matrigel compared with the untreated control
cells (Fig. 3B).
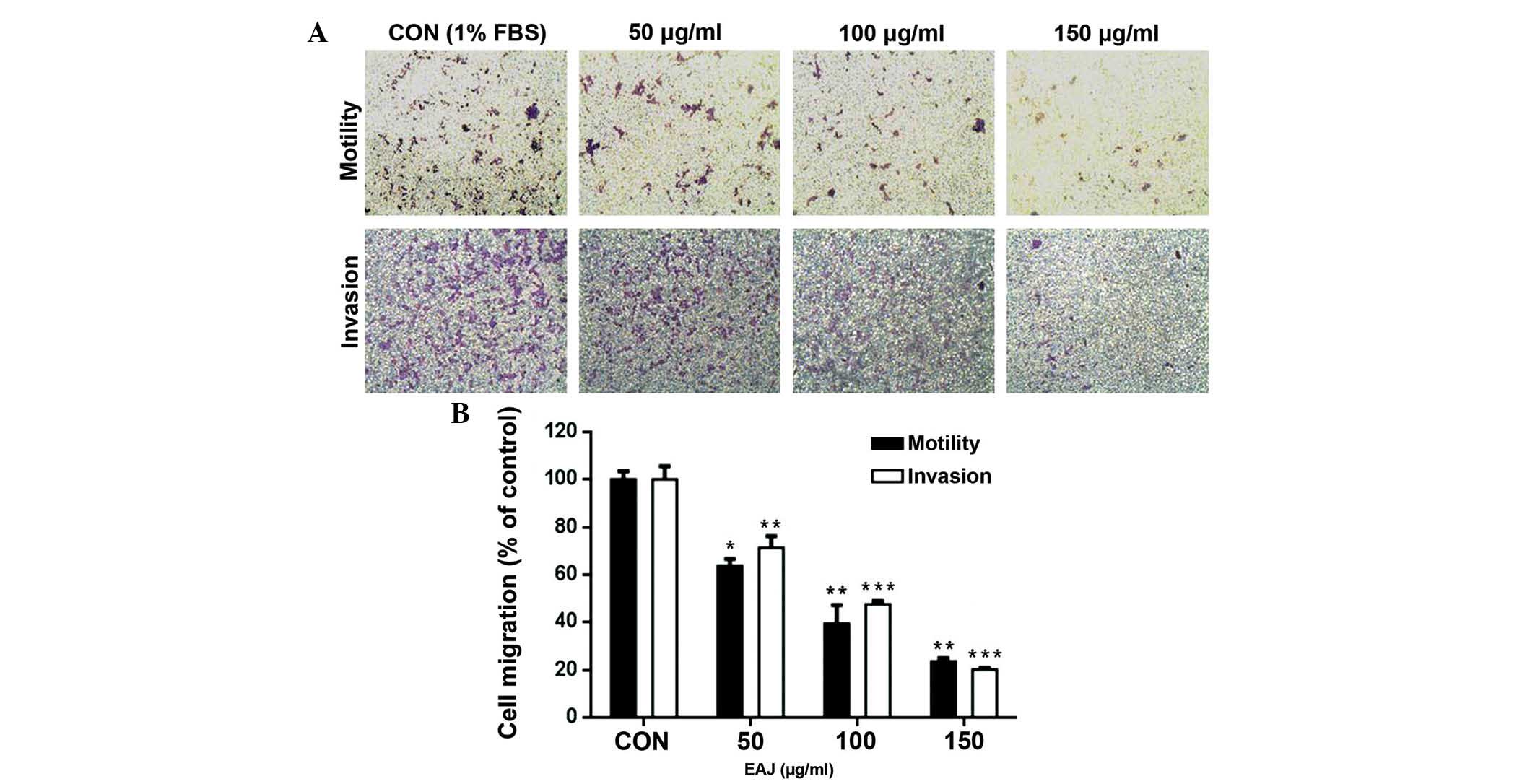 | Figure 3EAJ inhibits the invasion of
MDA-MB-231 breast cancer cells. Transwell inserts, containing
MDA-MB-231 cells and 1% serum, were placed in separate chambers
with 50, 100 or 200 μg/ml EAJ with 10% FBS and incubated for 24 h
at 37°C. The cells which crossed the Matrigel were fixed and
stained with Giemsa and the number of cells were counted in six
randomly selected fields. (A) Representative images of MDA-MB-231
cell invasion in the Transwell culture system (magnification,
×200). (B) Quantification of the cells, which crossed the
Matrigel-coated membrane. Values are presented as the mean ±
standard deviation (n=3). *P<0.05,
**P<0.01 and ***P<0.001, vs. control.
EAJ, Ampelopsis japonica ethanol extract; FBS, fetal bovine
serum; CON, control (10% FBS) |
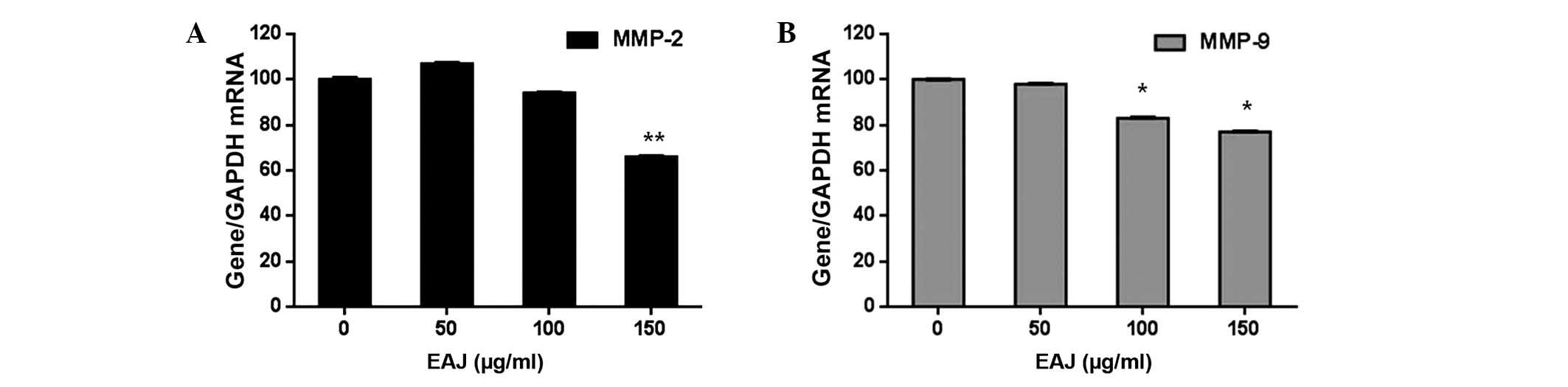
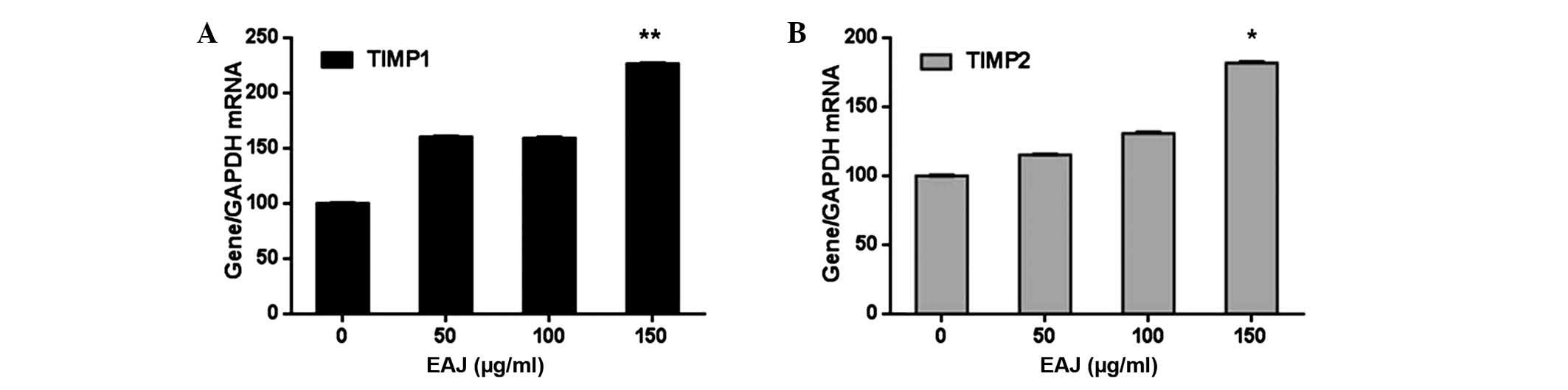
Effect of EAJ on the expression of MMP-2,
MMP-9, tissue inhibitor of MMP (TIMP)1 and TIMP2
Since the overexpression and activation of MMPs is
important in breast cancer cell invasion (17), the present study aimed to
investigate whether the anti-invasive activity of EAJ correlated
with the expression levels of MMP-2 and MMP-9. As shown in Fig. 4, EAJ inhibited the activities of
MMP-2 and MMP-9 in a concentration-dependent manner, with
significant reductions observed at 150 μg/ml EAJ for MMP-2 and 100
and 150 μg/ml EAJ for MMP-9. In addition, as the activity and
expression of MMPs are tightly regulated by endogenous TIMPs
(18), the transcription levels of
TIMP1 and TIMP2 were determined by RT-qPCR. As shown in Fig. 5, EAJ increased the expression
levels of TIMP1 and TIMP2 in a concentration-dependent manner, with
significant increases observed at 150 μg/ml EAJ, while the mRNA
expression levels of MMP-2 and MMP-9 decreased.
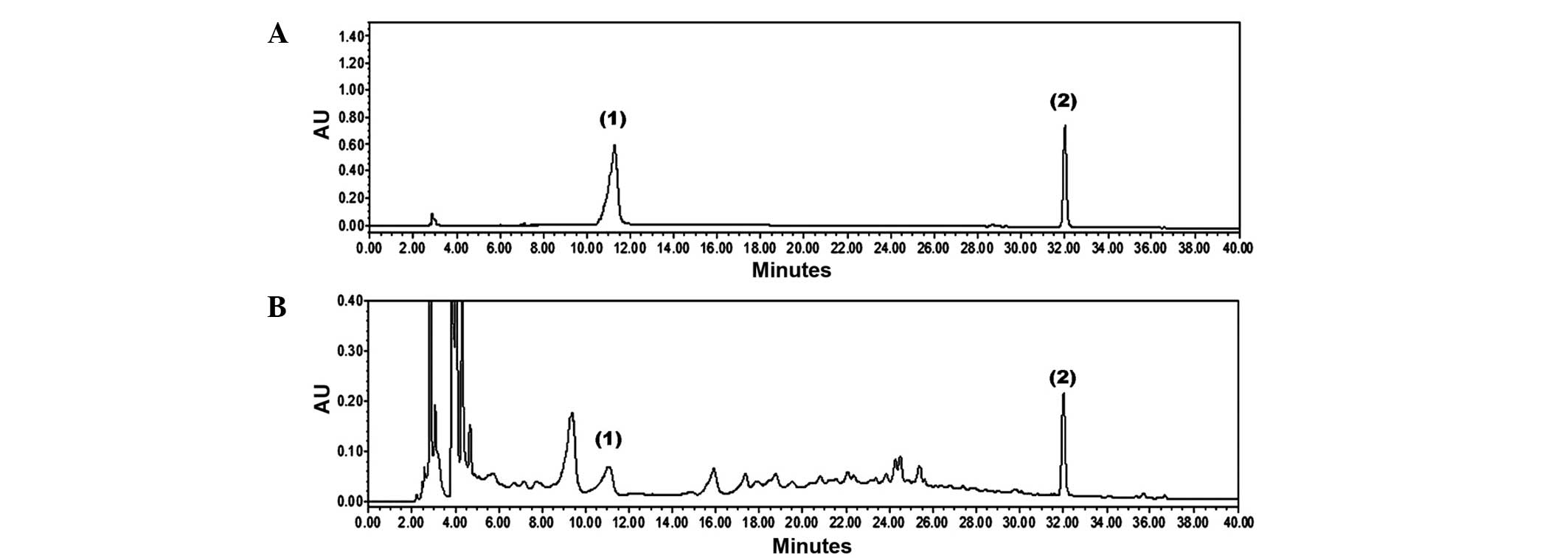
Identification and quantification of EAJ
components
The present study identified two components of EAJ,
catechin and resveratrol, using HPLC/PDA chromatograms and
comparing retention time and UV spectra with commercial standards.
As shown in Fig. 6, the HPLC
analysis of EAJ identified two standards, catechin and resveratrol,
which appeared at retention times of ~11.05 and 32.01 min,
respectively. The EAJ contained 3.15±0.07 mg/g catechin and
0.42±0.01 mg/g resveratrol.
Discussion
The invasion and metastasis of tumor cells occur
through several complex processes, including cell adhesion,
proteolytic extracellular matrix (ECM) degradation, cell migration
into the circulatory system through basement membranes and tumor
growth at the metastatic site (19). The dysregulation or inhibition of
one of several of these processes offers one approach in
antimetastatic therapy. The present study was the first, to the
best of our knowledge, to demonstrate the significant inhibition of
MDA-MB-231 cell invasion through the basement membrane following
treatment with non-cytotoxic concentrations of EAJ. In addition,
EAJ reduced cell motility, which is required for the migration of
cells from primary to secondary tumor sites.
MMPs, a family of zinc-dependent endopeptidases,
which degrade the collagen components of the ECM. MMPs also
regulate migration, invasion, proliferation and apoptosis in
various types of cell (20). MMP-2
and MMP-9, degrade the ECM and promote the release of key factors,
which are important in tumor angiogenesis and the growth of several
types of cancer (21–23). The results of the present study,
demonstrated that EAJ inhibited the mRNA expression levels of MMP-2
and MMP-9 at the transcriptional level. In addition, MMPs have been
implicated in the invasion of breast cancer cells, therefore, the
downregulation of MMP-2 and MMP-9 may mediate the EAJ-induced
inhibition of MDA-MB-231 cell invasion that was observed in the
present study.
According to the HPLC analysis performed in the
present study, EAJ contained catechin and resveratrol. Resveratrol,
as a member of the stilbene family, is primary ingredient of wine
(24,25), which has been previously used in
traditional Japanese and Chinese medicine for the treatment of
fungal diseases, various types of skin inflammation and
cardiovascular and liver diseases (26,27).
In addition, resveratrol has been found to have various therapeutic
benefits, including antioxidant, antiproliferation and antitumor
activities (28–30). Cathechin, green tea polyphenols,
also exhibits antioxidant and antitumor activities (31,32).
It has been shown to the molecular mechanisms of their anticancer
effects, including the suppression of cancer cell proliferation,
induction of apoptosis, and inhibition of tumor metastasis and
angiogenesis (33). The results of
the present study confirmed the presence of resveratrol and
catechin in EAJ at concentrations of 0.42 and 3.15 mg/g,
respectively, which, in part, explained the antitumor effect of
EAJ. However, no other active compound was detected in EAJ. Further
studies are required in order to clarify the pharmacological
mechanisms of EAJ and to identify other potential compounds
mediating its antimetastatic activity.
In conclusion, the present study indicated that EAJ
may potentially arrest the progression of tumors by inhibiting
invasion by suppressing the mRNA expression of MMP-2/−9 and
upregulation of the mRNA expression of TIMP1/2 These results
provided a theoretical foundation for the suitability of EAJ as a
potential therapeutic agent for the treatment of breast cancer
metastasis.
Acknowledgements
The present study was supported by grants from the
Construction of the Basis for Practical Application of Herbal
Resources (no. K12020), the ICT Fusional Construction of
Alternative Herbal Medicine Resources (no. K14410) and the Korea
Institute of Oriental Medicine to the Ministry of Science, ICT and
Future Planning (Daejeon, Korea).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Coughlin SS and Ekwueme DU: Breast cancer
as a global health concern. Cancer Epidemiol. 33:315–318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weber GF: Molecular mechanisms of
metastasis. Cancer Lett. 270:181–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johansson N, Ahonen M and Kähäri VM:
Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci.
57:5–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Köhrmann A, Kammerer U, Kapp M, Dietl J
and Anacker J: Expression of matrix metalloproteinases (MMPs) in
primary human breast cancer and breast cancer cell lines. BMC
Cancer. 9:1882009. View Article : Google Scholar
|
|
6
|
Wiwanitkit V: Thai ethnopharmacological
herbs for diabetes treatment: data collection and informatics
tracing for therapeutic property. Diabetes Metab Syndr. 5:103–104.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JH, Kim MH, Yang G, Huh Y, Kim SH and
Yang WM: Effects of topical application of Astragalus membranaceus
on allergic dermatitis. Immunopharmacol Immunotoxicol. 35:151–156.
2013. View Article : Google Scholar
|
|
8
|
Han MH, Lee WS, Lu JN, et al: Citrus
aurantium L. exhibits apoptotic effects on U937 human leukemia
cells partly through inhibition of Akt. Int J Oncol. 40:2090–2096.
2012.PubMed/NCBI
|
|
9
|
Mahassni SH and Al-Reemi RM: Apoptosis and
necrosis of human breast cancer cells by an aqueous extract of
garden cress (Lepidium sativum) seeds. Saudi J Biol Sci.
20:131–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang EJ, Lee WJ, Cho SH and Choi SW:
Proliferative effects of flavan-3-ols and propelargonidins from
rhizomes of Drynaria fortunei on MCF-7 and osteoblastic cells. Arch
Pharm Res. 26:620–630. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu J, Kim SH, Jiang C, Lee H and Guo J:
Oriental herbs as a source of novel anti-androgen and prostate
cancer chemopreventive agents. Acta Pharmacol Sin. 28:1365–1372.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong CH, Hur SK, Oh OJ, Kim SS, Nam KA and
Lee SK: Evaluation of natural products on inhibition of inducible
cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured
mouse macrophage cells. J Ethnopharmacol. 83:153–159. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bae KH: The medicinal Plants of Korea.
Hwang JS: Korea: Kyo-Hak Publishing Co; 328. 2000
|
|
14
|
Suh BI and Jeong GY: Herbology. Lee JG:
Korea: Dae Gu Hanny University; 108. 1987
|
|
15
|
Kim JH, Ju EM, Lee DK and Hwang HJ:
Induction of apoptosis by momordin I in promyelocytic leukemia
(HL-60) cells. Anticancer Res. 22:1885–1889. 2002.PubMed/NCBI
|
|
16
|
Koressaar T and Remm M: Enhancements and
modifications of primer design program Primer3. Bioinformatics.
23:1289–1291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong S, Park KK, Magae J, et al:
Ascochlorin inhibits matrix metalloproteinase-9 expression by
suppressing activator protein-1-mediated gene expression through
the ERK1/2 signalling pathway: inhibitory effects of ascochlorin on
the invasion of renal carcinoma cells. J Biol Chem.
280:25202–25209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cairns RA, Khokha R and Hill RP: Molecular
mechanisms of tumor invasion and metastasis: an integrated view.
Curr Mol Med. 3:659–671. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Ann Rev Cell Dev Biol.
17:463–516. 2001. View Article : Google Scholar
|
|
21
|
Liotta LA and Stetler-Stevenson WG: Tumor
invasion and metastasis: an imbalance of positive and negative
regulation. Cancer Res. 51(18 Suppl): 5054s–5059s. 1991.PubMed/NCBI
|
|
22
|
Kato Y, Yamashita T and Ishikawa M:
Relationship between expression of matrix metalloproteinase-2 and
matrix metalloproteinase-9 and invasion ability of cervical cancer
cells. Oncol Rep. 9:565–569. 2002.PubMed/NCBI
|
|
23
|
Gilabert-Estellés J, Ramón LA, España F,
et al: Expression of angiogenic factors in endometriosis:
relationship to fibrinolytic and metalloproteinase systems. Hum
Reprod. 22:2120–2127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weng CJ, Yang YT, Ho CT and Yen GC:
Mechanisms of apoptotic effects induced by resveratrol,
dibenzoylmethane and their analogues on human lung carcinoma cells.
J Agric Food Chem. 57:5235–5243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roy S, Sannigrahi S, Majumdar S, Ghosh B
and Sarkar B: Resveratrol regulates antioxidant status, inhibits
cytokine expression and restricts apoptosis in carbon tetrachloride
induced rat hepatic injury. Oxid Med Cell Longev. 2011:7036762011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnson WD, Morrissey RL, Usborne AL, et
al: Subchronic oral toxicity and cardiovascular safety pharmacology
studies of resveratrol, a naturally occurring polyphenol with
cancer preventive activity. Food Chem Toxicol. 49:3319–3327. 2001.
View Article : Google Scholar
|
|
27
|
Szekeres T, Saiko P, Fritzer-Szekeres M,
Djavan B and Jäger W: Chemopreventive effects of resveratrol and
resveratrol derivatives. Ann NY Acad Sci. 1215:89–95. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fabre KM, Saito K, DeGraff W, et al: The
effects of resveratrol and selected metabolites on the radiation
and antioxidant response. Cancer Biol Ther. 12:915–923. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scarlatti F, Sala G, Somenzi G, Signorelli
P, Sacchi N and Ghidoni R: Resveratrol induces growth inhibition
and apoptosis in metastatic breast cancer cells via de novo
ceramide signaling. FASEB J. 17:2339–2341. 2003.PubMed/NCBI
|
|
30
|
Kundu JK and Surh YJ: Cancer
chemopreventive and therapeutic potential of resveratrol,
mechanistic perspectives. Cancer Lett. 269:243–261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Castillo-Pichardo L and Dharmawardhane SF:
Grape polyphenols inhibit Akt/mammalian target of rapamycin
signaling and potentiate the effects of gefitinib in breast cancer.
Nutr Cancer. 64:1058–1069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Islam S, Nasrin S, Khan MA, et al:
Evaluation of antioxidant and anticancer properties of the seed
extracts of Syzygium fruticosum Roxb. growing in Rajshahi,
Bangladesh. BMC Complement Altern Med. 13:1422013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu Y, Deng Y, Lu BM, Liu YX, Li J and Bao
JK: Green tea catechins: a fresh flavor to anticancer therapy.
Apoptosis. 19:1–18. 2014. View Article : Google Scholar
|