Introduction
Laryngeal carcinoma is one of the most common types
of head and neck cancer. Greater than 1.5 million individuals are
diagnosed with head and neck squamous cell carcinoma annually
worldwide, with ~25% represented by patients with laryngeal
squamous cell carcinoma (LSCC) (1). Although progress has been made in the
diagnosis and treatment of laryngeal carcinoma, significant
improvements in survival remain to be achieved (2,3).
The Trop2 gene (also termed TACSTD2) is located on
1p32. It encodes for a single-pass transmembrane protein of 35.7
kDa, which contains a conserved motif involved in Trop2-mediated
signaling (4,5). A previous study demonstrated that a
phosphatidylinositol 4,5-bis phosphate-binding sequence is present
in this motif (6). A conserved
serine residue within this sequence is phosphorylated by protein
kinase C (PKC) (6). Thus, PKC and
mitogen-activated protein kinases (MAPKs), including extracellular
signal-regulated kinase 1/2 (ERK1/2), may be associated with
Trop2-mediated tumor cell activity (7). Trop2 is involved in the regulation of
cell adhesion and its overexpression has been observed in a variety
of epithelial cells, whereas in healthy human somatic cells and
tissues, expression is either low or absent (8). It has been demonstrated that elevated
expression of Trop2 in pancreatic, stomach, oral and cervical
cancer is correlated with poor survival (9–12).
In a previous study, it was demonstrated that the expression of
Trop2 in laryngeal carcinoma is an independent prognostic factor
(13). However, the biological
significance of Trop2 in the development of LSCC remains to be
fully elucidated.
In the present study, the role of Trop2 in laryngeal
carcinoma was investigated. In order to establish this role, Trop2
expression was suppressed in the Hep2 human laryngeal carcinoma
cell line using small interfering RNA (siRNA), and the effects of
its knockdown on proliferation, migration and invasiveness were
examined. The interaction between Trop2 and the ERK/MAPK signaling
pathway were also investigated.
Materials and methods
Clinical samples
A total of four paired fresh laryngeal carcinoma
tissues and adjacent non-cancerous tissues were collected from The
Head and Neck Department of The Affiliated Hospital of Nantong
University (AHNU, Nantong, China). The paraffin-embedded laryngeal
carcinoma tissues were collected from the Department of Pathology
of the AHNU. The current study was approved by the Medical Ethics
Committee of the AHNU and samples were collected with informed
patient consent.
Cell culture
The Hep2 human laryngeal carcinoma cell line was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China) and maintained in RPMI-1640 (Gibco
Life Technologies, Grand Island, NY, USA) with 10% fetal bovine
serum (FBS; Hangzhou Sijiqing Biological Engineering Materials Co.,
Ltd., Hangzhou, China), 100 U/ml penicillin and 100 mg/ml
streptomycin (Gibco Life Technologies) at 37°C in a humidified
atmosphere containing 5% CO2.
siRNA transfection
Hep2 cells in the logarithmic growth phase were
harvested and sub-cultured into 6-well plates. At 70–80%
confluence, cells were transfected with Trop2 siRNAs (Table I; Guangzhou Ribobio Co., Ltd.,
Guangzhou, China) at 100 nmol using Lipofectamine 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA). A non-targeting siRNA was
used as a negative control (NC; Guangzhou Ribobio Co., Ltd.). After
24 h, fluorescence microscopy (BX51; Olympus Corporation, Tokyo,
Japan) was used to examine transfection efficiency. Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
used to examine Trop2 mRNA expression profiles of the transfected
cells, and the siRNA that induced the maximal suppression was
selected for subsequent analysis.
 | Table ICandidate siRNA sequences of
Trop2. |
Table I
Candidate siRNA sequences of
Trop2.
| Name | Sequences
(5′–3′) | Position in Trop2
mRNA (bp) |
|---|
| Trop2-S1 |
GUGUCCCACCAACAAGAUGTT | 443 |
| Trop2-S2 |
CCAAGUGUCUGCUGCUCAATT | 550 |
| Trop2-S3 |
GCACGCUCAUCUAUUACCUTT | 1100 |
| Trop2-NC |
UUCUCCGAACGUGUCACGUTT | |
RT-qPCR
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen Life Technologies). First-strand complementary
DNA synthesis was then performed using the Reverse Transcription
System kit (Thermo Fisher Scientific, Pittsburgh, PA, USA)
according to the manufacturer’s instructions. RT-qPCR was performed
using the SYBR Green kit (Thermo Fisher Scientific) to examine
Trop2 mRNA levels, in addition to those of GAPDH, which served as
the endogenous control for normalization. The relative expression
levels of Trop2 mRNA were calculated using the comparative
2−ΔΔCt method. The qPCR was conducted on an Applied
Biosystems 7500 Real-Time PCR system (Applied Biosystems, Foster
City, CA, USA). The following RT-qPCR conditions were used: 2 min
at 94°C, followed by 40 cycles of 15 sec at 94°C, 25 sec at 58°C
and 30 sec at 72°C. Each experiment was performed three times in
duplicate. Primer sequences are presented in Table II.
 | Table IIPrimers for Trop2 mRNA detection by
reverse transcription-quantitative polymerase chain reaction. |
Table II
Primers for Trop2 mRNA detection by
reverse transcription-quantitative polymerase chain reaction.
| Name | Primers |
|---|
| Trop2 | Fwd:
5′-TATTACCTGGACGAGATTCCCC-3′ |
| Rev:
5′-CCCCGACTTTCTCCGGTTG-3′ |
| GAPDH | Fwd:
5′-TGCACCACCAACTGCTTAGC-3′ |
| Rev:
5′-GGCATGGACTGTGGTCATGAG-3′ |
Western blot analysis
Whole-cell lysates were prepared using
radioimmunoprecipitation assay buffer with protease inhibitors
(Thermo Fisher Scientific, Beijing, China) and protein
concentrations were quantitated using the Bradford method (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Subsequently, proteins were
separated using 7% SDS-PAGE and blotted using standard procedures.
Visualization of the specific proteins on polyvinylidene fluoride
membranes (Bio-Rad Laboratories, Inc.) was accomplished with an
enhanced chemiluminescence reaction (Pierce Biotechnology, Inc.,
Rockford, IL, USA), followed by scanning using the Odyssey Infrared
Imaging system (Li-Cor Biosciences, Lincoln, NE, USA) and analysis
using Quantity One 1-D Analysis version 4.62 software (Bio-Rad
Laboratories, Inc.). Relative target protein expression was
determined using the formula: Gray scale value of target
protein/gray scale value of β-actin (Actin). The goat anti-human
polyclonal antibody against Trop2 (1:500; cat. no. AF650) and
horseradish peroxidase (HRP)-conjugated chicken anti-goat
immunoglobulin (Ig)G antibody (1:2,000; cat. no. HAF019) were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA). The
following anti-human antibodies: Rabbit polyclonal anti-ERK 1/2
(1:750; cat. no. sc-292838), rabbit polyclonal phosphorylated-ERK
1/2 (1:500; cat. no. sc-23759-R), rabbit polyclonal anti-cyclin D1
(1:1,000; cat. no. sc-717), mouse monoclonal anti-p27 (1:1,000;
cat. no. sc-1641), rabbit polyclonal anti-actin (1:1,000; cat. no.
sc-7210), goat anti-rabbit IgG-HRP (1:2,500; cat. no. sc-2004) and
goat anti-mouse IgG-HRP (1:2,500; cat. no. sc-2005) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All
primary antibodies were used at an incubation temperature of 4°C
overnight and all secondary antibodies were used for 2 h at an
ambient temperature.
Immunohistochemical staining
Briefly, the sections were deparaffinized,
rehydrated and subjected to antigen retrieval by boiling in 10 mM
citrate buffer, pH 6.0 for 15 min, prior to blocking in 10% normal
goat serum. The sections were then incubated with human TROP-2
Polyclonal Ab (cat. no. AF650; R&D Systems, Inc.; dilution
1:50) overnight at 4°C. Subsequently, the sections were stained
using the Anti-Goat HRP-DAB Cell and Tissue Staining kit (cat. no.
CTS008; R&D Systems, Inc.) and counterstained with hematoxylin.
The images were captured using an Olympus BX-51 light microscope
system (Olympus Corporation).
Cell viability
Hep2 laryngeal carcinoma cells were transferred into
96-well plates at a density of 1×103 cells/well in a
volume of 200 μl. Following cultivation for 12, 24, 36 and
48 h, 20 μl MTT (5 g/l; Sigma-Aldrich, St. Louis, MO, USA)
was added to each well. Following incubation for a further 4 h, the
supernatant was discarded, 150 μl dimethyl sulfoxide was
added to each well and the resulting mixture was agitated under
ambient conditions for 10 min, or until the newly formed crystals
were dissolved completely. The absorbance of each well was then
determined at 570 nm using a microplate spectrophotometer
(Multiskan GO; Thermo Fisher Scientific, Beijing, China).
Triplicates were prepared for each sample and each time point. The
average values were used to prepare a growth curve.
Cell invasion
Hep2 cells in the logarithmic growth phase were used
to prepare a single cell suspension of ~3×105 cells/ml.
Transwell chambers (Costar Transwell; Corning Incorporated,
Tewksbury, MA, USA) were sterilized for 2 h in an ultra-clean
cabinet under ultraviolet radiation. Maintaining sterile
conditions, the upper side of the 24-well Transwell chambers were
coated with 50 μl of 1 mg/ml Matrigel gum (Matrigel™; BD
Biosciences, Franklin Lakes, NJ, USA). Following solidification at
4°C, the mixture was hydrated with serum-free RPMI 1640 medium for
30 min at 37°C to provoke its reor-ganization into a basement
membrane-like structure over the microporous membrane. The single
cell suspensions (100 μl) were then transferred onto the
upper chambers of the micro-porous membranes while 15% FBS-RPMI
1640 medium (500 μl) was added to the lower chamber. Five
replicates were analyzed for each group. The chamber was removed
following cultivation at 37°C for 12, 24 and 36 h. The residual
medium and cells of the upper chamber were removed carefully with a
swab. The chamber was then dried under ambient conditions for 30
min, followed by staining with 0.1% crystal violet at 37°C for 30
min. The number of cells on the underside of the chamber was
counted in six fields of view (magnification, ×50; Olympus
BX51).
Wound scratch assay
Hep2 cells in the logarithmic growth phase were
transferred into six-well culture plates at a density of
5×105 cells/well. Four parallel samples for each
transfection group were prepared. Once the cells had reached 60–70%
confluence, a straight-line scratch (2 mm in width) was created
along the longitudinal axis in the center of each well using a
20-μl pipette tip. Floating cells that were scraped off in
the process were removed by washing the plates with
phosphate-buffered saline three times. The remaining cells were
placed in 1% FBS-RPMI 1640 medium (2 ml/well) for 24 h, then the
medium was then replaced with 10% FBS-RPMI 1640 for continued
cultivation. The scratch width was examined under the microscope
(Olympus BX51) at 0, 24, 48 and 72 h later. Three independent
experiments were performed in which all experimental and control
groups were analyzed in triplicate.
Statistical analysis
Statistical analyses were conducted using SPSS
software, version 18.0 (SPSS, Inc., Chicago, IL, USA) and data were
expressed as the mean ± standard error. Pairwise comparisons were
analyzed using Student’s t-test. Multi-group differences were
measured using a one way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
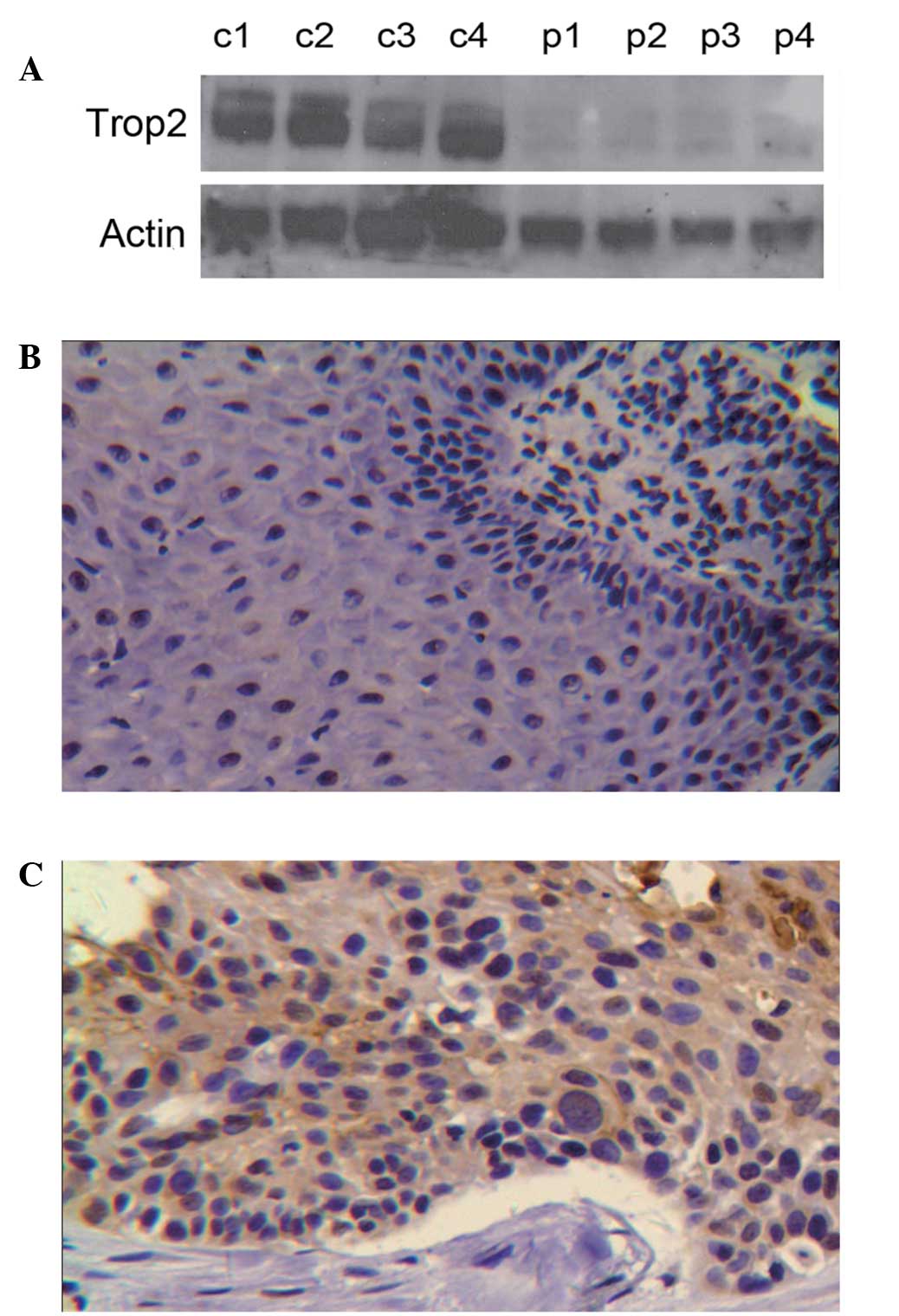
Trop2 expression in LSCC tissues
Trop2 protein expression levels in four fresh
laryngeal carcinoma tissue samples and paracancerous tissues
(control) were analyzed by western blotting (Fig. 1A). Trop2 was observed to be
elevated in the carcinoma tissue compared with the control tissue.
Further analysis by immunohistochemistry (IHC) demonstrated that
Trop2 protein was predominantly expressed in the membrane of
laryngeal carcinoma cells with a small quantity of cytoplasmic
expression (Fig. 1B and C).
Knockdown of Trop2 expression in Hep2
cells by siRNA
To investigate the role of Trop2 in LSCC, it was
first silenced in Hep2 cells using siRNA transfection. Following
this, cDNA generated from the Hep2 nontransfected cells,
NC-transfected cells, or those transfected with three different
Trop2 siRNA sequences (Trop2-S1, Trop2-S2 and Trop2-S3) was used as
a template for RT-qPCR. GAPDH was used as an internal reference.
The relative levels of Trop2 mRNA in the Trop2-S1, Trop2-S2 and
Trop2-S3 groups were 0.50±0.18, 0.80±0.14 and 0.85±0.12,
respectively, compared with the NC group (Fig. 2A). Statistical analysis revealed
that, in comparison with the NC group, Trop2 expression was
significantly reduced (P<0.05) following transfection with the
three siRNAs, while the greatest inhibitory effect was observed
with Trop2-S1 transfection. The difference between the NC
(1.00±0.02) group and nontransfected group was not statistically
significant (P>0.05). The Trop2-S1 group was thus selected for
further assays. Similarly, as demonstrated by western blotting, the
Trop2 expression in the Trop2-S1 group was significantly reduced
compared with the NC group after 48 h (Fig. 2B).
Downregulation of Trop2 inhibits
viability of Hep2 cells
Hep2 cell viability was examined using the MTT assay
at 12, 24, 36 and 48 h following Trop2 suppression. Optical density
(at 570 nm) of Hep2 cells reduced following knockdown of Trop2 and
the difference was statistically significant
(*P<0.05) at 24, 36 and 48 h (Fig. 3).
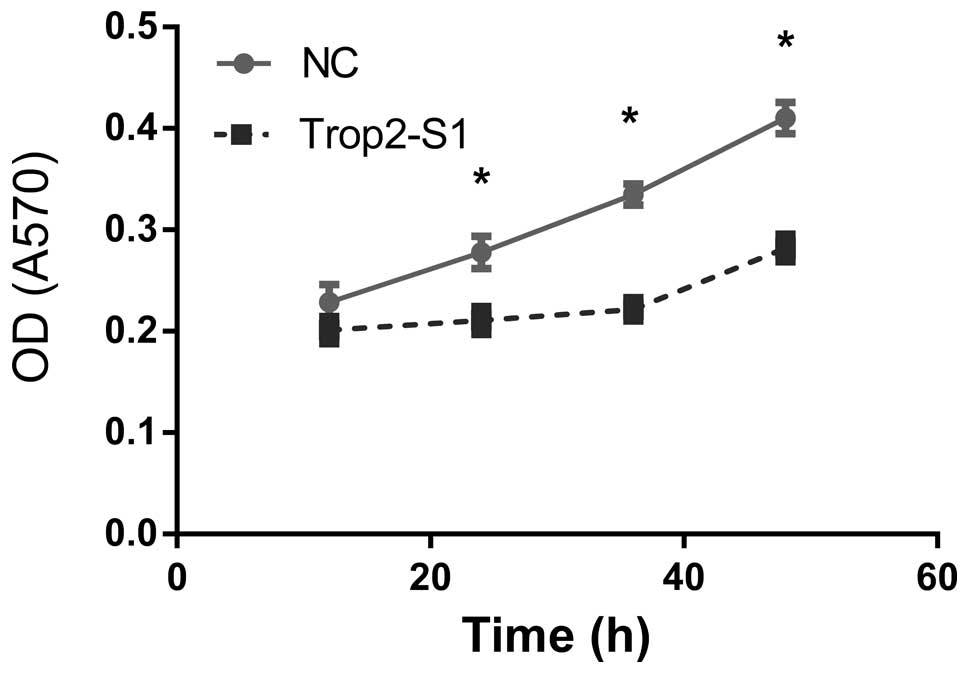 | Figure 3Downregulation of Trop2 in Hep2 cells
inhibits viability. OD was measured at 12, 24, 36 and 48 h
following transfection of Hep2 cells with control (NC) or Trop2
(Trop2-S1) using the MTT assay. At 24 h, OD was 0.21±0.02 and
0.23±0.03 in Trop2 suppressed cells and NC, respectively; OD
reduced by 8.6%. At 36 h, OD was 0.24±0.03 and 0.33±0.02 in Trop2
suppressed cells and NC, respectively; OD reduced by 27.2%. At 48
h, OD was 0.29±0.04 and 0.41±0.03 in Trop2 suppressed cells and NC,
respectively; OD reduced by 29.2%. *P<0.05. OD,
optical density; NC, negative control. |
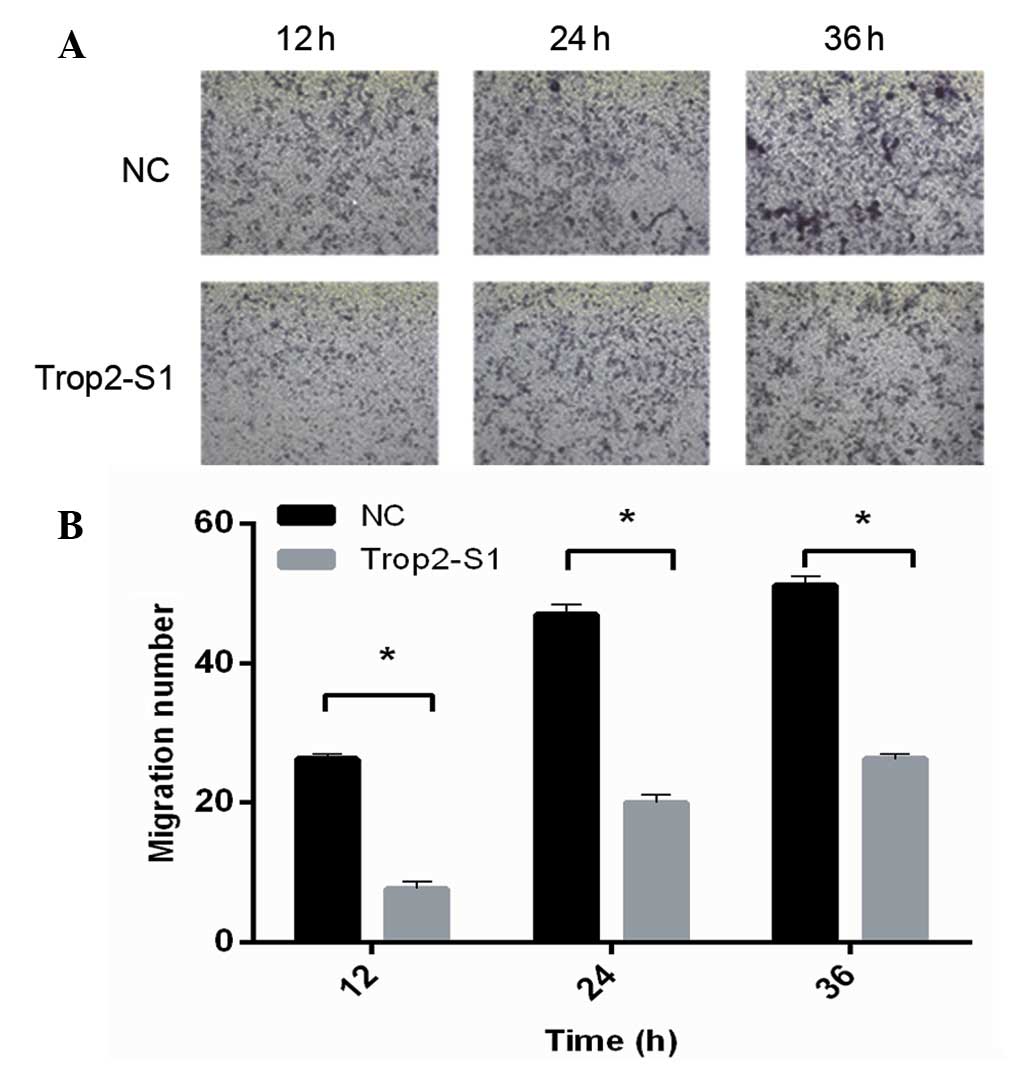
Downregulation of Trop2 inhibits cell
invasion
The effect of siRNA Trop2-S1 on the invasive
capability of Hep2 cells was analyzed using the Transwell method.
It was identified that Trop2 downregulation by Trop2-S1 was
associated with the significantly reduced invasive capability of
Hep2 cells (P<0.05) at all of the time-points measured. As
demonstrated in Fig. 4,
quantification indicated that for the Trop2-S1 and NC groups,
respectively, the number of invasive cells at 12 h
post-transfection was 7.02±1.26 and 23.94±0.98; at 24 h was
16.79±0.92 and 41.86±1.05; and at 36 h was 24.97±1.62 and
50.06±0.90. Thus, these data suggest that Trop2 is required for the
invasive capacity of Hep2 laryngeal cells.
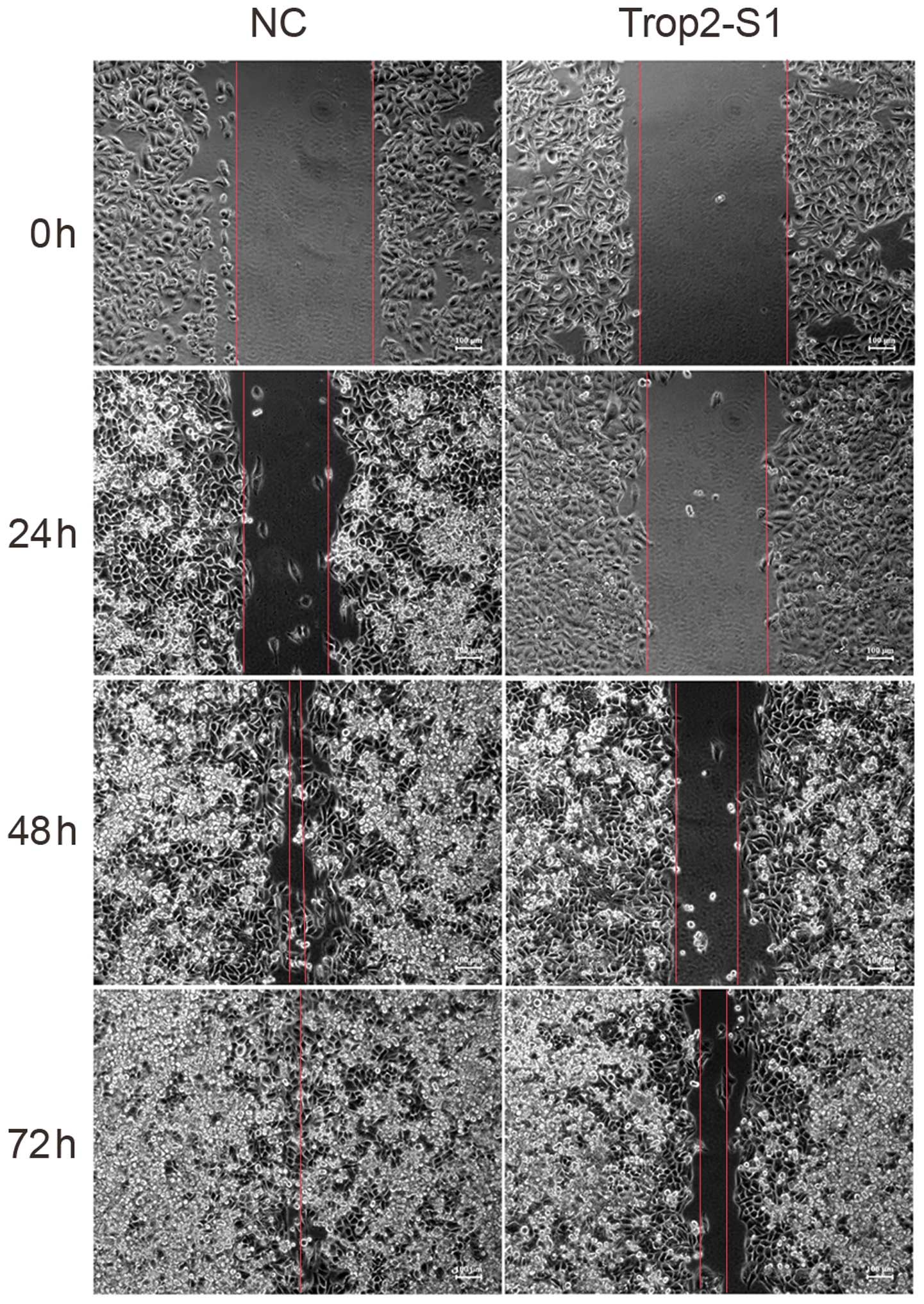
Downregulation of Trop2 reduces cell
migration
Next, the role of Trop2 in Hep2 cell migration was
investigated. As demonstrated in the wound scratch assay,
downregulation of Trop2 was associated with reduced Hep2 migration.
The migratory capacity of cells transfected with Trop2-S1 was lower
than that of the NC group at 24, 48 and 72 h (Fig. 5).
MAPK/ERK signaling pathway is involved in
Trop2-mediated invasion of Hep2 cells
To further evaluate the mechanism underlying Trop2
function in LSCC, the activity of the ERK/MAPK signaling pathway
and associated proteins was examined in Hep2 cells with and without
silencing of Trop2 by western blotting. Compared with the NC group,
ERK, p-ERK and cyclin D1 expression were downregulated, while
upregulation of p27 protein expression was observed in Hep2 cells
following the downregulation of Trop2 (Fig. 6).
Discussion
In a previous study, a total of 109
paraffin-embedded laryngeal carcinoma tissue specimens were
analyzed using a tissue microarray for Trop2 protein expression,
which was observed to be significantly increased compared with that
of paracancerous tissue (13). In
addition, increased Trop2 expression was observed to negatively
correlate with the overall survival of patients with LSCC and was
an independent prognostic factor for LSCC (13). In the current study, the Trop2
protein expression levels in fresh laryngeal carcinoma and
paracancerous tissues were examined using western blotting, and
Trop2 expression in carcinoma tissues was observed to be
significantly increased compared with that of paracancerous
tissues. The expression profile of laryngeal carcinoma tissue was
also examined by IHC, which identified that Trop2 protein is
predominantly expressed in the membranes of laryngeal carcinoma
tissue with a small quantity of cytoplasmic expression. It has been
previously demonstrated that the TP63/TP53L, ERG, GRHL1/Get-,
HNF1A/TCF-1, SPI1/PU.1, WT1 and GLIS2, FOXM1 and FOXP3
transcription factor networks, which are mediated by HNF4A, can
regulate the expression of Trop2 in tumor tissues (14). In addition, Trop2 has been reported
to be regulated by the epigenetic regulatory factor miRNA-125b in
head and neck tumors (15). A
previous study demonstrated that overexpression of Trop2 is
sufficient to drive cancer growth in various species (16). Upregulation of Trop-2 has been
observed to quantitatively stimulate tumor growth, suggesting that
it serves an oncogenic role in tumor development (16).
In the current study, Trop2 expression levels were
suppressed in Hep2 laryngeal cancer cells and the resulting effects
on proliferation, migration and invasiveness were examined. A total
of three siRNAs (Trop2-S1, -S2 and -S3) directed against Trop2 mRNA
by transfection into the Hep2 cells were screened. The results
demonstrated that Trop2-S1 reduced Trop2 mRNA expression to 51% of
that of the control (NC group). In addition, as validated by the
western blotting assay, Trop2-S1 also reduced the expression of
Trop2 protein. These results suggest that out of the three siRNAs
analyzed, Trop2-S1 achieved the optimal silencing effect. Following
silencing of Trop2 for 48 h, the MTT assay was conducted in order
to examine Hep2 cell viability. It was observed that viability
reduced 29.2% following Trop2 suppression. These results suggest
that Trop2 regulates the proliferation and growth of Hep2
cells.
Previous studies have suggested that elevated Trop2
expression levels correlate with metastasis in a variety of tumor
types (6,12). Thus, the migration and invasiveness
of Hep2 cells were analyzed using the Transwell assay following the
silencing of Trop2. The results demonstrated that the migratory and
invasive abilities of Hep2 cells were reduced significantly
compared with that measured in the control groups. The wound
scratch assay also demonstrated that the migration of Hep2 cells
with Trop2 suppression was significantly reduced. Thus, these data
suggest that Trop2 regulates the invasive and migratory abilities
of laryngeal carcinoma cells.
Trop2 has also been observed to regulate the
activation of a number of important tumor-promoting growth factors,
such as nuclear factor-κB, cyclic adenosine monophosphate response
element-binding protein, Jun, retinoblastoma protein, signal
transducer and activator of transcription 1 (STAT1) and STAT3,
through the ERK/MAPK signaling pathway (14). In addition, Trop2 is involved in
the regulation of cyclin D1 and PKC activated cell growth. Cyclin
D1 is a cell cycle-dependent regulatory protein whose
overexpression can result in cell growth independent of growth
factors and malignant transformation of normal cells. It has been
demonstrated that cyclin D1 forms a complex with cyclin-dependent
kinase (CDK), which stimulates the expression of a number of
downstream genes. CDK kinase may mediate this phosphorylation event
and thereby the transition of cells from the G1 phase to
S phase, in addition to the subsequent initiation of the
self-division process (17).
The p27 protein is an inhibitor of cell
cycle-dependent kinases and suppresses the activities of the
majority of CDK-cyclin D1 complexes, subsequently inhibiting cell
transition from the G1 phase to S phase (18). Thus, while the p27 protein
maintains the quiescent state (G0 phase) of cells
through binding to the cyclin D1-CDK complex and regulating the
transition to the G1 phase, the ERK/MAPK signaling
pathway is critical to induce cells to leave the quiescent state
and initiate G1/S phase conversion (19).
In the current study, Trop2 expression suppression
was demonstrated to result in reduced cyclin D1, ERK and p-ERK
expression, together with upregulation of p27 expression and
significant suppression of cell proliferation. Therefore, Trop2 is
suggested to exert its suppressive effects on Hep2 cell
proliferation through the suppression of ERK expression and
phosphorylation, and subsequent downregulation of cyclin D1
expression and upregulation of p27 expression.
In conclusion, as demonstrated by the current study,
Trop2 is suggested to regulate the growth, invasion and migration
of laryngeal carcinoma cells through the ERK/MAPK signaling
pathway. Therefore, although further studies are required,
including validation in animal studies, Trop2 is suggested as a
novel target for molecular therapy against laryngeal carcinoma.
Acknowledgments
The present study was supported by grants from the
Affiliated Hospital of Nantong University Postdoctoral Foundation
(grant no. 128385) and the Postdoctoral Foundation of Jiangsu
Province (grant no. 1302083C).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA. Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Chu EA and Kim YJ: Laryngeal cancer:
diagnosis and preoperative work-up. Otolaryngol Clin North Am.
41:673–695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dirix P, Lambrecht M and Nuyts S:
Radiotherapy for laryngeal squamous cell carcinoma: current
standards. Expert Rev Anticancer Ther. 10:1461–1469. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsujikawa M, Kurahashi H, Tanaka T, et al:
Identification of the gene responsible for gelatinous drop-like
corneal dystrophy. Nat Genet. 21:420–423. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calabrese G, Crescenzi C, Morizio E, Palka
G, Guerra E and Alberti S: Assignment of TACSTD1 (alias TROP1,
M4S1) to human chromosome 2p21 and refinement of mapping of TACSTD2
(alias TROP2, M1S1) to human chromosome 1p32 by in situ
hybridization. Cytogenet Cell Genet. 92:164–165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakanishi H, Taccioli C, Palatini J, et
al: Loss of miR-125b-1 contributes to head and neck cancer
development by dysregu-lating TACSTD2 and MAPK pathway. Oncogene.
33:702–712. 2014. View Article : Google Scholar
|
|
7
|
Cubas R, Zhang S, Li M, Chen C and Yao Q:
Trop2 expression contributes to tumor pathogenesis by activating
the ERK MAPK pathway. Mol Cancer. 9:2532010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kapoor S: TROP2 expression and its
evolving role in tumor pathogenesis in systemic tumors. Tumour
Biol. 34:1967–1968. 2013. View Article : Google Scholar
|
|
9
|
Fong D, Moser P, Krammel C, et al: High
expression of TROP2 correlates with poor prognosis in pancreatic
cancer. Br J Cancer. 99:1290–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fong D, Spizzo G, Gostner JM, et al:
TROP2: a novel prognostic marker in squamous cell carcinoma of the
oral cavity. Mod Pathol. 21:186–191. 2008.
|
|
11
|
Fang YJ, Lu ZH, Wang GQ, et al: Elevated
expressions of MMP7, TROP2, and survivin are associated with
survival, disease recurrence, and liver metastasis of colon cancer.
Int J Colorectal Dis. 24:875–884. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu T, Liu Y, Bao X, Tian J and Yang X:
Overexpression of TROP2 predicts poor prognosis of patients with
cervical cancer and promotes the proliferation and invasion of
cervical cancer cells by regulating ERK signaling pathway. PLoS
One. 8:e758642013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu H, Xu H, Zhang S, et al: Potential
therapeutic target and independent prognostic marker of TROP2 in
laryngeal squamous cell carcinoma. Head Neck. 35:1373–1378.
2013.
|
|
14
|
Guerra E, Trerotola M, Aloisi AL, et al:
The Trop-2 signalling network in cancer growth. Oncogene.
32:1594–1600. 2013. View Article : Google Scholar
|
|
15
|
Cubas R, Li M, Chen C and Yao Q: Trop2: a
possible therapeutic target for late stage epithelial carcinomas.
Biochim Biophys Acta. 1796:309–314. 2009.PubMed/NCBI
|
|
16
|
Trerotola M, Cantanelli P, Guerra E, et
al: Upregulation of Trop-2 quantitatively stimulates human cancer
growth. Oncogene. 32:222–233. 2013. View Article : Google Scholar
|
|
17
|
Nishi K, Inoue H, Schnier JB and Rice RH:
Cyclin D1 downregulation is important for permanent cell cycle exit
and initiation of differentiation induced by anchorage-deprivation
in human keratinocytes. J Cell Biochem. 106:63–72. 2009. View Article : Google Scholar
|
|
18
|
Gardner LB, Li Q, Park MS, Flanagan WM,
Semenza GL and Dang CV: Hypoxia inhibits G1/S transition through
regulation of p27 expression. J Biol Chem. 276:7919–7926. 2001.
View Article : Google Scholar
|
|
19
|
Kolch W: Meaningful relationships: the
regulation of the Ras/Raf/MEK/ERK pathway by protein interactions.
Biochem J. 351:289–305. 2000. View Article : Google Scholar : PubMed/NCBI
|