Introduction
Colorectal cancer (CRC) ranks third in worldwide
cancer incidence and second in mortality, with ~1.2 million new
patients diagnosed per year (1).
In China, CRC has become the third leading cause of mortality due
to tumor disease. Genetic and environmental risk factors, including
lifestyle and nutrition modulate colon cancer development (2). Chemotherapeutic compounds used for
the treatment of CRC include oxaliplatin (L-OHP), 5-flurouracil and
irinotecan (3). While chemotherapy
can improve the survival rate of CRC patients, resistance emerges
in certain patients, with a typical survival of <6 months once
resistance is identified. The detection of genes associated with
CRC may facilitate the development of targeted therapies to improve
CRC prognosis, reduce resistance and adverse events and establish
individualized treatment programs for CRC.
The nitrogen permease regulator like-2 (NPRL2) gene
is a candidate tumor suppressor gene identified in the 3p21.3
region. Genomic abnormalities have been identified in this region
in various types of human cancer (4,5).
Certain studies suggest that the NPRL2 gene may be a tumor
suppressor and that its inactivation may promote tumorigenesis
(4–6). The NPRL2 gene is composed of 11 exons
and encodes a 380 amino acid protein. Multiple spliced isoforms of
NPRL2 are expressed in different tissue types. However, the
mechanism by which NPRL2 mediates tumor suppression remains to be
elucidated. Previous studies have suggested that NPRL2 is involved
in DNA mismatch repair, cell cycle checkpoint signaling and the
regulation of apoptosis (4,6). In
certain tumor cell lines, overexpression of NPRL2 induces apoptosis
and inhibits proliferation (7).
NPRL2 has been demonstrated to increase
susceptibility to anticancer drugs and apoptosis (7,8).
Previous studies have reported that NPRL2 is a potential biomarker
for predicting response to cisplatin, the prognosis of patients
with lung cancer and other types of cancer and as a molecular
therapeutic agent for enhancing and resensitizing the response of
nonresponders to cisplatin treatment (7,8).
However, how NPRL2 suppresses tumor proliferation and whether NPRL2
can affect the sensitivity of cells to chemotherapy remains to be
elucidated. In the present study, the colon cancer cell line HCT116
was used to observe the effects of the NPRL2 signaling pathway on
apoptosis induced by the chemotherapeutic drug L-OHP to further
elucidate the role of the NPRL2 signaling pathway in increased
L-OHP sensitivity in these cells as part of the search for an
effective treatment for CRC.
Materials and methods
Cell culture
The colon cancer cell line HCT116 was purchased from
the Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (HyClone Laboratories, Inc., Logan, UT, USA) and 1%
penicillin/streptomycin (Beyotime Institute of Biotechnology,
Haimen, China) in a humidified atmosphere of 5% CO2 at
37 °C. Cells were passaged every 2–3 days through digestion with
0.25% trypsin. Logarithmically growing cells were prepared.
Transductions and assay
The full length human NPRL2 gene (GenBank serial
number: NM_006545) was purchased from Shanghai Genechem Co., Ltd.
(Shanghai, China) as a fusion with enhanced green fluorescence
protein (eGFP) in the GV208 vector. The lentiviral vector system
consisted of GV208 and the pHelper 1.0 and pHelper 2.0 packaging
vectors. The three vectors were cotransfected into 293T cells in
serum-free medium using Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA). The medium was changed to
complete medium after 8 h of incubation. High-titer recombinant
lentiviruses encoding NPRL2 were harvested 48 h after transfection.
HCT116 cells in the log phase were seeded at 5×05
cells/well in 96-well plates and transduced with NPRL2-GFP or GFP
lentiviruses in serum-free medium. Polybrene was added to improve
the transduction efficiency. After 8 h, the medium was changed to
complete medium. At 72 h after transduction, GFP expression was
examined by fluorescence microscopy (Nikon TE2000; Nikon
Corporation, Tokyo, Japan) and a luciferase assay was performed in
HCT116 cells. The protein expression levels were analyzed 72 h
after transduction. All experiments were performed in triplicate
and representative results are reported.
Cell viability assay
Non-transduced and transduced cells were dispersed
and seeded at 5×103 cells/well in 96-well microplates.
After 24 h, freshly prepared L-OHP (Sanofi-Synthelabo, Paris,
France) was used to determine the optimal concentration and time
course of the HCT116 cell response to L-OHP. Cell viability was
assessed using a cell counting kit-8 assay (Dojindo Laboratories,
Kumamoto, Japan) following various concentrations of drug treatment
(2.5, 5, 10, 20 and 40 μg/ml L-OHP) or duration in culture
(24, 48 and 72 h). The absorbance value at 450 nm was read using a
microplate reader (Multiskan MK3; Thermo Fisher Scientific Inc.,
Rockford, IL, USA). At least three independent experiments were
performed in quadruplicate.
Flow cytometric analysis of the cell
cycle and apoptosis
Cells transduced with NPRL2 were treated with 10
μg/ml L-OHP for 48 h and harvested. Following
trypsinization, the cells were washed with phosphate-buffered
saline (PBS; Beyotime Institute of Biotechnology) and subsequently
fixed in 85% ethanol. Following fixation, the cells were washed
with PBS/1% fetal calf serum (FCS; HyClone Laboratories, Inc.),
resuspended in PBS/1% FCS containing 10 μg/ml propidium
iodide (PI) and 250 μg/ml RNase A (Multisciences Biotech
Co., Ltd., Hangzhou, China) and incubated for 30 min at 37°C.
Apoptosis was evaluated using flow cytometry with Annexin
V-fluorescein isothiocyanate and PI (KeyGEN Biotech Co., Ltd.,
Nanjing, China) staining. Following gating on the CD24+
subpopulation, the cells were analyzed for positive Annexin V
staining. Positive rates of CD24+ apoptosis were
detected using a FACScan flow cytometer (Becton Dickinson, Franklin
Lakes, NJ, USA) following the addition of CD24 monoclonal
antibodies (Ebioscience, San Diego, CA, USA).
Western blot analysis
Cellular protein extracts were separated by
electrophoresis in a 12 or 8% SDS-polyacrylamide gel (Beyotime
Institute of Biotechnology) and electrophoretically transferred
onto a polyvinylidene fluoride membrane (Millipore, Bedford, MA,
USA). The membranes were blocked overnight with 5% non-fat dried
milk and incubated overnight at 4°C with the following antibodies:
Rabbit monoclonal anti-GAPDH (cat. no. 2118; 1:1,500), rabbit
monoclonal anti-pyruvate dehydrogenase kinase, isozyme 1 (PDK1)
(cat. no. 13037; 1:1,000), mouse monoclonal anti-p-Akt (cat. no.
4051; 1:1,000), rabbit monoclonal anti-mammalian target of
rapamycin (mTOR)(p) (cat. no. 5536; 1:1,000), rabbit polyclonal
anti-p70S6K(P) (cat. no. 9209; 1:1,000) and rabbit monoclonal
anti-4E-binding protein 1 (4E -BP1) (cat. no. 9456; 1:1,000)
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA)
or mouse monoclonal anti-NPRL2 (cat. no. sc-376986; 1:1,000),
rabbit polyclonal anti-phosphatidylinositol 3-kinase (PI3K)(p)
(cat. no. sc-134986; 1:1,000), rabbit polyclonal anti-caspase-3
(cat. no. sc-7148; 1:1,000) and mouse monoclonal anti-caspase-9
(cat. no. sc-56073; 1:2,000) obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Following washing with
Tris-buffered saline and Tween 20, the membranes were incubated
with a horseradish peroxidase-linked goat anti-rabbit (cat. no.
A0208; 1:1,000) or goat anti-mouse (cat. no. A0216; 1:1,000)
immunoglobulin G secondary antibodies (Beyotime Institute of
Biotechnology). The proteins were visualized by enhanced
chemiluminescence using an integrated automatic chemiluminescent
imaging and analysis system (Sage Creation Science Co., Ltd.,
Beijing, China).
Statistical analysis
All experimental data are presented as the mean ±
standard error of the mean. Differences between samples were
analyzed using the two-tailed Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
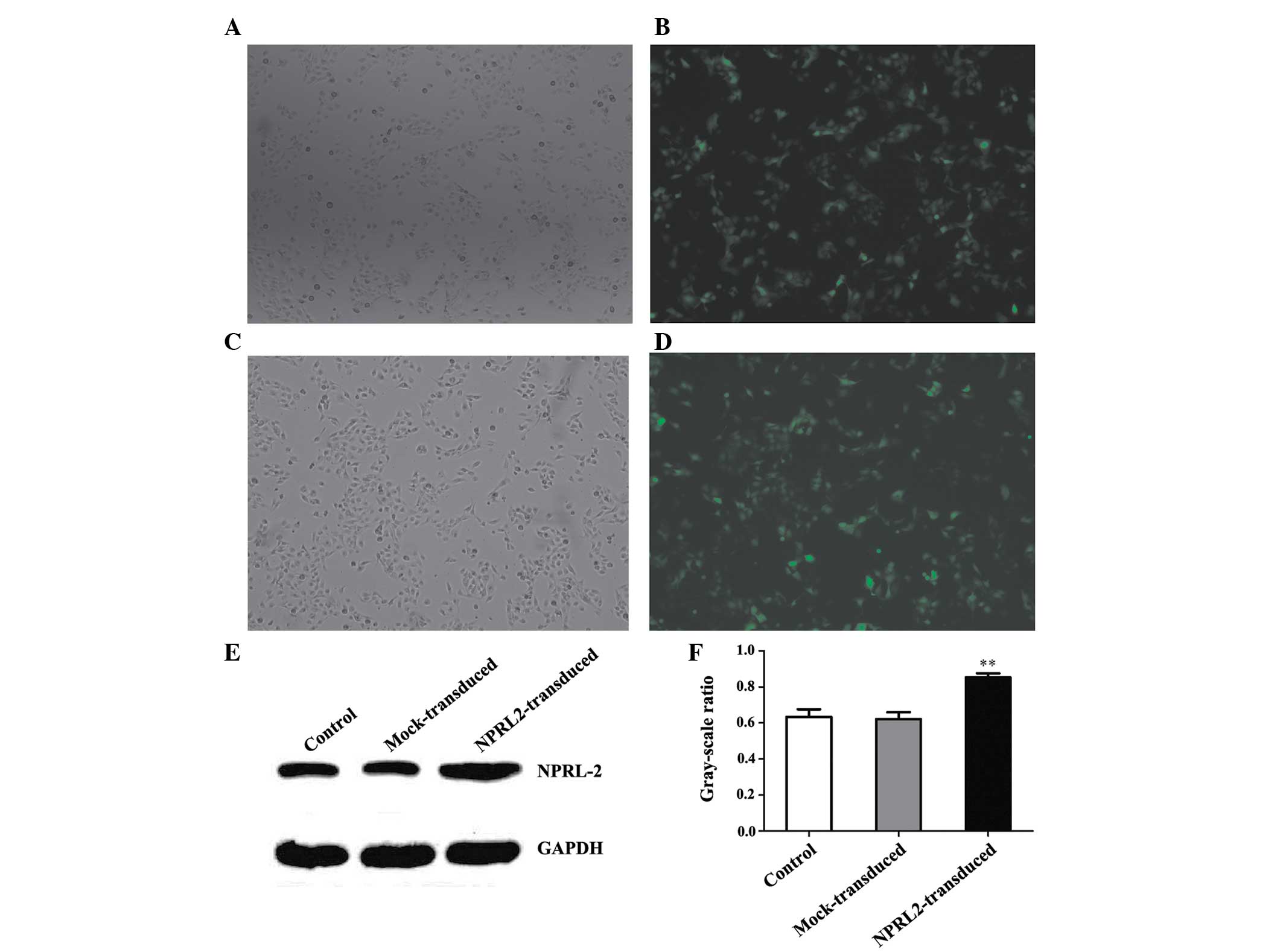
Lentiviral transduction of NPRL2
Transduction efficiency was evaluated 72 h after
NPRL2 transduction. eGFP was expressed in cells following
lentiviral transduction at different multiplicities of infection
(MOIs). The transduction efficiency (average proportion of
GFP-expressing cells compared with the total cell count) was
>70% at an MOI of 10 (P<0.05; Fig. 1A–D). Protein expression levels were
analyzed at 72 h post-transduction. NPRL2 expression was higher in
the transduced cells than in the negative control (NC) and mock
cells (P<0.01; Fig. 1E and
F).
NPRL2 overexpression increases the L-OHP
sensitivity of HCT116 cells
To investigate the role of NPRL2 in L-OHP-induced
cytotoxicity, NPRL2 was transduced into HCT116 cells. The IC50 of
L-OHP was lower in cells transduced with NPRL2 than in NC cells
(P<0.05), indicating that overexpression of NPRL2 markedly
increases the sensitivity of HCT116 cells to L-OHP (Fig. 2A). Cell survival was assayed
following NPRL2 transduction and treatment with 10 μg/ml
L-OHP for an additional 24, 48 and 72 h, which confirmed that the
effects of NPRL2 on L-OHP sensitivity were time dependent (Fig. 2B).
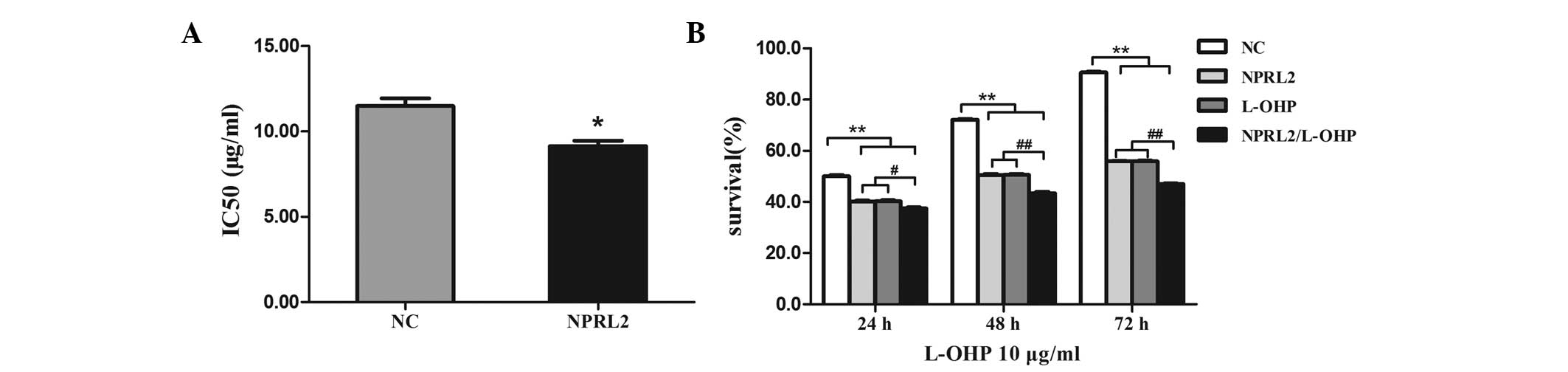 | Figure 2NPRL2 increases the sensitivity of
HCT116 cells to L-OHP. (A) The IC50 of L-OHP was lower in cells
transduced with NPRL2 than in NC cells. *P<0.05, as
compared with the NC cells. (B) Cell survival was assayed following
NPRL2 transduction and treatment with 10 μg/ml L-OHP for an
additional 24, 48 and 72 h. #P<0.05, compared with
NPRL2-transduced cells, cells treated with L-OHP for 24 h;
##P<0.01, compared with NPRL2-transduced cells, cells
treated with L-OHP for 48 and 72 h; **P<0.01,
compared with NPRL2-transduced cells, cells treated with L-OHP,
NPRL2-transduced cells treated with L-OHP for 24, 48 and 72 h. NC,
negative control; NPRL2, nitrogen permease regulator-like 2; L-OHP,
oxaliplatin. |
NPRL2 overexpression increases L-OHP
sensitivity by inhibiting cell growth
Cell survival assays revealed that the cell cycle
was arrested in the G1 phase and that there was a partial decrease
in the S phase population following NPRL2 transduction in HCT116
cells (P<0.05). In addition, L-OHP significantly inhibited the
growth of NPRL2-transduced cells compared with NC cells (P<0.01;
Fig. 3).
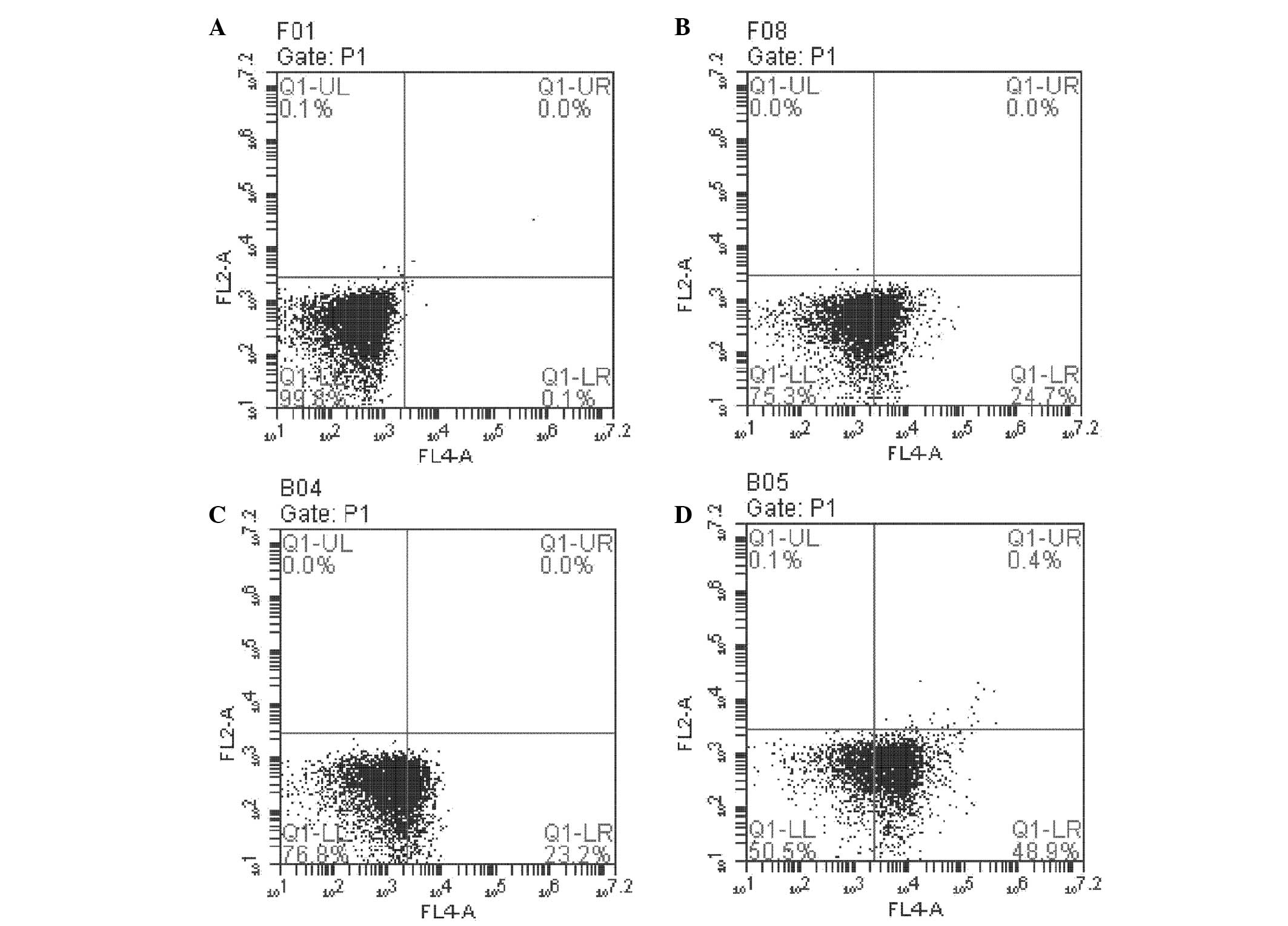
NPRL2 overexpression increases L-OHP
sensitivity by promoting apoptosis
Flow cytometry was performed to investigate the role
of NPRL2 in L-OHP-induced apoptosis. The number of apoptotic cells
was greater in NPRL2-transduced and L-OHP cells than in NC cells
(P<0.05). In addition, the combination of NPRL2 overexpression
and L-OHP treatment promoted apoptosis more significantly than
either perturbation alone (P<0.01; Fig. 4). NPRL2 and L-OHP also decreased
the rate of CD24-positive apoptotic HCT116 cells (P<0.05). In
addition, L-OHP significantly decreased the proportion of
CD24-positive apoptotic HCT116 cells among cells overexpressing
NPRL2 compared with NC cells (P<0.01; Fig. 5).
NPRL2 promotes HCT116 cell sensitivity to
L-OHP by inhibiting the PI3K/Akt/mTOR signaling pathway
The protein expression in the following four
different groups of cells was evaluated by western blot analysis:
Negative control cells, NPRL2-transduced cells, HCT116 cells
treated with 10 μg/ml L-OHP and grown for 48 h and
NPRL2-transduced HCT116 cells treated with 10 μg/ml L-OHP
and grown for 48 h. Overexpression of NPRL2 and L-OHP treatment
downregulated PDK1, 4E-BP1, phosphorylated PI3K, Akt, mTOR and
p70S6K in HCT116 cells. In addition, the combination of NPRL2
overexpression and L-OHP treatment resulted in significantly
greater downregulation of these genes compared with either
perturbation alone (P<0.01). Furthermore, L-OHP upregulated
caspase-3 and caspase-9 to promote apoptosis in
NPRL2-overexpressing cells compared with cells subjected to either
perturbation alone or NC cells (P<0.01; Fig. 6).
 | Figure 6Western blot analysis of the protein
levels of PI3K(p), PDK1, p-Akt, mTOR(p), p70S6K(p), 4E-BP1,
caspase-3, caspase-9 and GAPDH. (A) Protein expression levels in
the four different groups of cells (NC cells; NPRL2-transduced
cells; HCT116 cells treated with 10 μg/ml L-OHP and grown
for 48 h; NPRL2-transduced HCT116 cells treated with 10
μg/ml L-OHP and grown for 48 h). (B) Gray-scale ratio
comparison between the four different groups of cells (NC cells;
NPRL2-transduced cells; HCT116 cells treated with 10 μg/ml
L-OHP and grown for 48 h; NPRL2-transduced HCT116 cells treated
with 10 μg/ml L-OHP and grown for 48 h). The downregulation
of the expression of PDK1, 4E-BP1, phosphorylated PI3K, Akt, mTOR
and p70S6K was observed in cells overexpressing NPRL2 and treated
with L-OHP compared with NC cells, NPRL2-transduced cells and cells
treated with 10 μg/ml L-OHP. NPRL2 overexpression resulted
in increased levels of caspase-3 and caspase-9 compared with NC
cells. In addition, combined NPRL2 overexpression and L-OHP
treatment significantly upegulated apoptosis compared with either
perturbation alone. Western blot analysis was performed using GAPDH
as a loading control. **P<0.01, compared with NC
cells, NPRL2-transduced cells, HCT116 cells treated with 10
μg/ml L-OHP and grown for 48 h. NPRL2, nitrogen permease
regulator-like 2; L-OHP, oxaliplatin; NC, negative control; PI3K,
phosphatidylinositol 3-kinase; PDK1, pyruvate dehydrogenase kinase,
isozyme 1; mTOR, mammalian target of rapamycin; 4E-BP1, 4E-binding
protein 1. |
Discussion
CRC is the third leading cause of cancer-associated
mortality and the second overall cause in males and females
combined in the USA (9). A deep
understanding of the dietary, lifestyle and medical risk factors
for this malignancy has been achieved (10,11).
In China, with the improvement of living standards and alterations
in diet, the incidence of CRC has gradually increased. However,
half of CRC treatment remains unsuccessful.
For patients with advanced colonic cancer, surgery
and systemic chemotherapy are the most common treatment methods.
L-OHP is an alkylating agent that is cell cycle non-specific and
most active during the resting phase of the cell cycle. This drug
forms a coordination metal salt complex and inhibits DNA synthesis
in cancer cells. Although L-OHP is widely used for the treatment of
advanced malignancies, long-term treatment outcomes are
unsatisfactory. Cancer recurrence is frequently observed in
patients who have undergone chemotherapy and recurrent cancers are
frequently highly malignant and drug resistant. Furthermore, tumor
chemotherapy is often associated with side effects, complicating
the limitation of the long-term effects of chemotherapy (12–17).
In addition, tumor chemoresistance can develop as a result of
decreased drug uptake, increased drug efflux, activation of
detoxifying systems or DNA repair mechanisms and/or evasion of
drug-induced apoptosis. Improving the sensitivity of chemotherapy
and overcoming L-OHP resistance are critical requirements in cancer
therapy.
The NPRL2 gene has potent tumor suppressive activity
in vitro and in vivo and has been suggested to be
involved in DNA mismatch repair, cell cycle checkpoint signaling
and regulation of the apoptotic pathway (5,18).
Overexpression of NPRL2 inhibits proliferation and induces
apoptosis in a variety of tumor cell lines (19). In the present study, it was
demonstrated that NPRL2 overexpression increases L-OHP sensitivity
in HCT116 cells. Initially, it was observed that the IC50 of L-OHP
was lower in cells transduced with NPRL2 than in NC cells
(P<0.05). The present study determined that the effects of NPRL2
on L-OHP sensitivity were time dependent and that the
overexpression of NPRL2 promotes apoptosis in a time-dependent
manner, thereby inhibiting cell proliferation. Following NPRL2
transduction in HCT116 cells, the cell cycle was arrested in the G1
phase and there was a partial decrease in cells in the S phase
(P<0.05). In addition, L-OHP significantly inhibited cell growth
in NPRL2-transduced cells compared with NC cells (P<0.01). These
data further confirm that NPRL2 overexpression increases L-OHP
sensitivity by inhibiting cell growth. Flow cytometric analysis
revealed an increase in apoptotic cells due to NPRL2 transduction
and L-OHP treatment compared with NC cells (P<0.05). In
addition, combined NPRL2 overexpression and L-OHP treatment
promoted apoptosis more significantly than either perturbation
alone. These results indicate that an essential function of
exogenous NPRL2 involves activation of the DNA damage checkpoint
pathway, which regulates not only cell-cycle checkpoints but also
DNA repair, genome maintenance, senescence and apoptosis (20).
NPRL2 may enhance L-OHP sensitivity via additional
mechanisms, which require examination in future studies. In the
present study, it was demonstrated that NPRL2 is a new potential
therapeutic molecule. Compared with traditional drugs, NPRL2 has a
more modulatory role. Combined NPRL2 overexpression and L-OHP
treatment effectively downregulated the phosphorylation of PI3K,
Akt and mTOR and the mTOR downstream target proteins phospho-p70S6K
(Thr389) and 4E-BP1 (Thr37/46). The PI3K/Akt/mTOR signaling axis is
critical in proliferation, apoptotic resistance, angiogenesis and
metastasis and is central to the development and maintenance of CRC
(21). Previous studies have
reported the potential for the PI3K/Akt/mTOR network to be
therapeutically targeted at multiple molecular levels (22,23).
PI3K is activated upon the binding of growth factors to their
cognate receptors. Activated PI3K leads to Akt activation via
phosphorylation at Ser473 and Thr308 (24). Akt activates several downstream
targets, including mTOR. Deregulation of mTOR signaling occurs in
several types of human tumor, including colon cancer (21). mTOR associates with Raptor (mTORC1
complex) to phosphorylate p70S6K, which in turn phosphorylates
4E-BP1, leading to increased cell proliferation (25). 4E-BP1 is considered to be a
funneling factor through which transforming signals converge,
channeling oncogenic proliferative signals regardless of the
specific upstream oncogenic alteration (26). Phospho-p70S6K is cytoplasmic and
its nuclear immunopositivity is a common feature of various types
of tumor. Phospho-p70S6K stimulates ribosome rearrangement into
active polysomes and increases the capacity of the translational
events essential for the G1/S transition of the cell cycle
(27). These findings suggest that
NPRL2 overexpression enhances L-OHP sensitivity by downregulating
the functions of the PI3K/Akt/mTOR network, leading to inhibition
of cell proliferation and G1 cell cycle arrest. Furthermore, L-OHP
upregulates caspase-3 and caspase-9 to promote apoptosis in
NPRL2-overexpressing cells compared with either perturbation alone
and NC cells (P<0.01). Furthermore, the present study
demonstrated that the NPRL2-mediated increase in L-OHP sensitivity
that induces apoptosis in HCT116 cells is associated with
significant activation of caspase-3 and caspase-9.
Notably, a previous study also demonstrated that
combined NPRL2 transduction and L-OHP treatment led to a
significant decrease in the proportion of CD24+
apoptotic HCT116 cells, indicating that NPRL2 overexpression causes
downregulation of the proportion of CD24+ cells. CD24 is
a sialoglycoprotein that is anchored to the cell surface by a
glycosyl phosphatidylinositol linkage (28). This protein is a ligand for
P-selectin, an adhesion receptor found on activated endothelial
cells and platelets; thus, it may contribute to the metastasizing
capacity of CD24-expressing tumor cells (29,30).
In gastric cancer, an association between high CD24 expression and
lymph node metastasis, venous invasion and lymphatic invasion has
been observed (31). CD24
expression tended to be higher in cell lines derived from
differentiated gastric carcinomas, including those derived from
lymph node metastases (32).
Downregulation of CD24 by NPRL2 overexpression may significantly
reduce tumor invasiveness and the metastatic capacity of HCT116
cells.
In conclusion, transfection of colon cancer cells
with NPRL2 resulted in a significant inhibition of tumor cell
growth. The present study also demonstrated that NPRL2 affects the
PI3K/Akt/mTOR pathway. It was confirmed that NPRL2 enhances L-OHP
sensitivity by inhibiting proliferation and promoting apoptosis and
may potentially serve as a therapeutic target for overcoming L-OHP
resistance in colon cancer. These mechanisms are likely active in
other types of cancer and may be exploited for the development of
novel cancer therapies.
References
|
1
|
Perazzo F, Piaggio F, Krupitzki H, et al:
Clinical-pathological features and gene profile in colorectal
cancer. Medicina (B Aires). 73:417–422. 2013.In Spanish.
|
|
2
|
Ahmed FE: Gene-gene, gene-environment
& multiple interactions in colorectal cancer. J Environ Sci
Health C Environ Carcinog Ecotoxicol Rev. 24:1–101. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel BB and Majumdar AP: Synergistic role
of curcumin with current therapeutics in colorectal cancer:
minireview. Nutr Cancer. 61:842–846. 2009. View Article : Google Scholar
|
|
4
|
Lerman MI and Minna JD: The 630-kb lung
cancer homozygous deletion region on human chromosome 3p21.3:
identification and evaluation of the resident candidate tumor
suppressor genes. Cancer Res. 60:6116–6133. 2000.PubMed/NCBI
|
|
5
|
Wistuba II, Behrens C, Virmani AK, Mele G,
et al: High resolution chromosome 3p allelotyping of human lung
cancer and preneoplastic/preinvasive bronchial epithelium reveals
multiple, discontinuous sites of 3p allele loss and three regions
of frequent breakpoints. Cancer Res. 60:1949–1960. 2000.PubMed/NCBI
|
|
6
|
Li J, Wang F, Haraldson K, Protopopov A,
et al: Functional characterization of the candidate tumor
suppressor gene NPRL2/G21 located in 3p21.3C. Cancer Res.
64:6438–6443. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schenk PW, Brok M, Boersma AW, et al:
Anticancer drug resistance induced by disruption of the
Saccharomyces cerevisiae NPR2 gene: a novel component involved in
cisplatin- and doxorubicin-provoked cell kill. Mol Pharmacol.
64:259–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueda K, Kawashima H, Ohtani S, et al: The
3p21.3 tumor suppressor NPRL2 plays an important role in
cisplatin-induced resistance in human non-small-cell lung cancer
cells. Cancer Res. 66:9682–9690. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan AT and Giovannucci EL: Primary
prevention of colorectal cancer. Gastroenterology. 138:2029–2043.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poynter JN, Haile RW, Siegmund KD, et al:
Colon Cancer Family Registry: Associations between smoking, alcohol
consumption and colorectal cancer, overall and by tumor
microsatellite instability status. Cancer Epidemiol Biomarkers
Prev. 18:2745–2750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bostick RM, Potter JD, Kushi LH, Sellers
TA, Steinmetz KA, McKenzie DR, Gapstur SM and Folsom AR: Sugar,
meat and fat intake and non-dietary risk factors for colon cancer
incidence in Iowa women (United States). Cancer Causes Control.
5:38–52. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, et al: Chronic oxaliplatin resistance induces
epithelial-to-mesenchymal transition in colorectal cancer cell
lines. Clin Cancer Res. 12:4147–4153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU, et al: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Larco JE, Wuertz BR, Manivel JC and
Furcht LT: Progression and enhancement of metastatic potential
after exposure of tumor cells to chemotherapeutic agents. Cancer
Res. 61:2857–2861. 2001.PubMed/NCBI
|
|
15
|
Kajiyama H, Shibata K, Terauchi M,
Yamashita M, Ino K, et al: Chemoresistance to paclitaxel induces
epithelial-mesenchymal transition and enhances metastatic potential
for epithelial ovarian carcinoma cells. Int J Oncol. 31:277–283.
2007.PubMed/NCBI
|
|
16
|
Xiong W, Ren ZG, Qiu SJ, Sun HC, Wang L,
et al: Residual hepatocellular carcinoma after oxaliplatin
treatment has increased metastatic potential in a nude mouse model
and is attenuated by Songyou Yin. BMC Cancer. 10:2192010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamauchi K, Yang M, Hayashi K, Jiang P,
Yamamoto N, et al: Induction of cancer metastasis by
cyclophosphamide pretreatment of host mice: an opposite effect of
chemotherapy. Cancer Res. 68:516–520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zabarovsky ER, Lerman MI and Minna JD:
Tumor suppressor genes on chromosome 3p involved in the
pathogenesis of lung and other cancers. Oncogene. 21:6915–6935.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji L, Nishizaki M, Gao B, et al:
Expression of several genes in the human chromosome 3p21.3
homozygous deletion region by an adenovirus vector results in tumor
suppressor activities in vitro and in vivo. Cancer Res.
62:2715–2720. 2002.PubMed/NCBI
|
|
20
|
Zhou BB and Elledge SJ: The DNA damage
response: putting checkpoints in perspective. Nature. 408:433–439.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnson SM, Gulhati P, Rampy BA, Han Y,
Rychahou PG, Doan HQ, et al: Novel expression patterns of
PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J
Am Coll Surg. 210:767–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maira SM, Voliva C and Garcia-Echeverria
C: Class IA phosphatidylinositol 3-kinase: from their biologic
implication in human cancers to drug discovery. Expert Opin Ther
Targets. 12:223–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ekstrand AI, Jonsson M, Lindblom A, Borg A
and Nilbert M: Frequent alterations of the PI3K/AKT/mTOR pathways
in hereditary nonpolyposis colorectal cancer. Fam Cancer.
9:125–129. 2010. View Article : Google Scholar
|
|
24
|
Awasthi N, Yen PL, Schwarz MA and Schwarz
RE: The efficacy of a novel, dual PI3K/mTOR inhibitor NVP-BEZ235 to
enhance chemotherapy and antiangiogenic response in pancreatic
cancer. J Cell Biochem. 113:784–791. 2012. View Article : Google Scholar
|
|
25
|
Glienke W, Maute L, Wicht J and Bergmann
L: The dual PI3K/mTOR inhibitor NVP-BGT226 induces cell cycle
arrest and regulates Survivin gene expression in human pancreatic
cancer cell lines. Tumour Biol. 33:757–765. 2012. View Article : Google Scholar
|
|
26
|
Armegnol G, Rojo F, Castellví J, et al:
4E-binding protein 1: a key molecular ‘funnel factor’ in human
cancer with clinical implications. Cancer Res. 67:7551–7555. 2007.
View Article : Google Scholar
|
|
27
|
Xu G, Zhang W, Bertram P, et al:
Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR pathway in
common human tumors. Int J Oncol. 24:893–900. 2004.PubMed/NCBI
|
|
28
|
Lim SC and Oh SH: The role of CD24 in
various human epithelial neoplasias. Pathol Res Pract. 201:479–486.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sammar M, Aigner S, Hubbe M, Schirrmacher
V, Schachner M, Vestweber D and Altevogt P: Heat-stable antigen
(CD24) as ligand for mouse P-selectin. Int Immunol. 6:1027–1036.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aigner S, Ramos CL, Hafezi-Moghadam A,
Lawrence MB, Friederichs J, Altevogt P and Ley K: CD24 mediates
rolling of breast carcinoma cells on P-selectin. FASEB J.
12:1241–1251. 1998.PubMed/NCBI
|
|
31
|
Yong CS, Ou Yang CM, Chou YH, Liao CS, Lee
CW and Lee CC: CD44/CD24 expression in recurrent gastric cancer: A
retrospective analysis. BMC Gastroenterol. 12:952012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi M, Nakajima M, Ogata H, Domeki
Y, Ohtsuka K, Ihara K, Kurayama E, Yamaguchi S, Sasaki K, Miyachi K
and Kato H: CD24 expression is associated with progression of
gastric cancer. Hepatogastroenterology. 60:653–658. 2013.
|