Introduction
Lung cancer is the most common type of cancer and
the most prevalent cause of cancer-associated mortality worldwide,
as well as the leading cause of mortality in Chinese males
(1). At present, there are >150
million lung cancer patients worldwide, with >one million novel
cases diagnosed per year (2).
Psychological distress, including depression, has previously been
associated with increased lung cancer incidence and mortality
(3). In addition, diminishing the
effect of psychological stress through social support, including
the presence of a social network or psychological intervention, was
reported to increase the survival time and decrease the metastatic
rate of cancer patients (4).
Numerous epidemiological studies have provided
evidence that chronic psychological stress may have negative
influences on the onset, progression and mortality rate of various
types of cancer (4–6). A meta-analysis of 165 longitudinal
studies revealed that psychosocial factors and stressful life
experiences were associated with an increased incidence of cancer
as well as decreased survival rates (6). Furthermore, psychosocial
interventions have been shown to improve quality of life among
cancer patients (7). However,
there is only limited evidence to suggest that chronic stress
enhances tumorigenesis in vivo, the molecular mechanisms of
which remain to be elucidated (8).
Psychological factors have been thought to be
associated with the downregulation of cellular immune responses,
including the number and type of lymphocytes in circulation,
proliferative responses in vitro as well as antibody levels
post-immunization (9). These
proposed mechanisms were suggested to be involved in cancer
defense, as cellular immunity were reported to have an important
role in the defense against cancer cells (10). Following initiation of tumor
development, angiogenesis has a critical role in the growth and
metastasis of tumors through the constitutive expression of several
angiogenic genes, including vascular endothelial growth factor
(VEGF) and matrix metalloproteinases (MMPs), particularly MMP-2 and
MMP-9, which are involved in extracellular matrix degradation and
are crucial for the endothelial cell migration, organization and
hence, angiogenesis (11). In
vitro studies have shown that tumor tissue stimulated the
production of VEGF via β-adrenergic signaling; this process was
blocked by the β-blocker propranolol (12). In addition, it has been
demonstrated that stress hormones modulated the migration and
invasion of cancer cells through stimulating the production of MMPs
in tumor as well as stromal cells, and also served as
chemoattractants for processes such as cell migration (13,14).
For example, norepinephrine (NE) and epinephrine (E) were found to
significantly stimulate the production of MMP-2 and MMP-9 in
ovarian cancer cells (15). MMPs
were reported to facilitate tumor cell invasion and contribute to
lung cancer progression; MMP-2 and MMP-9 were found to be
differentially regulated in patient samples of lung tumor cells,
although the physiological factors which modulate lung cancer MMP
expression remain to be elucidated (16).
Stress is a complex process involving environmental
and psychosocial factors, which are known to initiate cascade
information processing in the peripheral as well as the central
nervous system (17). Animal
models which replicate the pathogenesis of human disease are
essential for understanding the effects of stress on cancer,
amongst other diseases, as well as for investigating potential
therapeutic interventions for the effective prevention of tumor
progression or further disease complications. In the present study,
an established mouse model of repeated social defeat stress (RSDS),
which was previously determined to mimic numerous symptoms of
depression in humans (18–20), was used to investigate the effect
of stress on the growth and metastasis of a Lewis lung carcinoma
(LLC)-bearing tumor model in C57BL/6J mice in vivo.
Materials and methods
Animals
A total of 32 male C57BL/6J mice, aged six weeks,
were purchased from Shanghai Laboratory Animal Center (Shanghai,
China) and housed in the animal experiment center of the Fudan
University (Shanghai, China). Male CD-1 mice, aged four months,
were obtained from Vital River (Beijing, China) and housed in
individual cages. The mice were housed at four per cage in an
aseptic room at a constant temperature (23°C) under a 12-h
light/dark cycle (light on from 7:00 AM to 7:00 PM) with ad
libitum access to sterilized food and water. All animal
experiments were approved by the Fudan University Animal Care and
Use Committee. Every effort was made to minimize the number of
animals used and their suffering.
RSDS paradigm
The social defeat stress model was performed as
previously described (19,20). In brief, male C57BL/6J mice were
used as experimental mice while resident mice were CD-1 retired
breeders, selected for their attack latencies which are reliably
<30 sec. Every day, each experimental mouse was introduced into
the home cage of a novel resident aggressor for 5 min and was
physically defeated. Then, the resident home cage was divided into
two halves using a perforated plexiglass partition to allow sensory
contact but preventing further physical contact. Residents and
intruders were maintained in sensory contact for 24 h. Experimental
mice were submitted to social defeat for 10 consecutive days.
Control animals were housed in identically partitioned cages with a
paired mouse from the same genotype and switched to opposite sides
of the partition daily.
Social avoidance evaluation
Social avoidance evaluation was performed 11 days
following the last social defeat using a video tracking system
(EthovisionXT with Social Interaction Module; Noldus Information
Technology, Wageningen, Netherlands) in a two-trial social
interaction task. Testing was performed under red-light conditions
in a room isolated from external sound sources. The social
interaction test consisted of two separate trials: Trial 1
(no-target) and Trial 2 (target present). In Trial 1 (2.5 min),
experimental mice explored an open-field arena containing an empty
wire mesh cage on one edge of the arena. In Trial 2 (2.5 min),
experimental mice were re-introduced to the arena, now with an
unfamiliar CD-1 aggressor positioned in the mesh cage. Noldus video
tracking system software was used to measure the time spent in the
interaction zone surrounding the target box. Interaction ratios
were calculated as the time spent in the interaction zone in the
‘target present’ condition as a percentage of the time spent in the
zone in the ‘no target’ condition.
Spontaneous metastasis of lung cancer
model
The LLC cell line was obtained from Experimental
Center of Shanghai Chest Hospital (Shanghai, China) and maintained
in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) containing
10% fetal bovine serum (FBS; Gibco Life Technologies, Grand Island,
NY, USA), 100 KU/l penicillin and 100 mg/l streptomycin (Beyotime
Institute of Biotechnology, Jiangsu, China) at 37°C in humidified
atmosphere of 5% CO2/95% air with medium replacement
every two days. Cells in the logarithmic growth phase were used for
experiments in the present study. Following seven-day
acclimatization, mice were randomly divided into four groups (eight
mice per group) as follows: Control group (C), stress group (S),
tumor group (T) and tumor-stress group (T-S). Mice in the S and T-S
groups were exposed to RSDS for 10 days, as described above, while
the mice in the C and T groups were exposed to control conditions.
Subsequently, 1×106 LLC cells (0.l ml single-cell
suspension) were inoculated subcutaneously into the right axilla of
mice in the T and T-S groups and the same volume of PBS (0.1 ml) as
a control was injected into the C and S groups. Following seven
days, the subcutaneous tumors were measured using calipers thrice
weekly and the tumor volume was calculated using the following
formula: V=1/2(ab2), where a represents the largest
tumor diameter and b the smallest diameter. On day 29 the mice were
sacrificed by cervical dislocation and tumors were harvested and
weighed. Following blood collection, the lungs and tumors were
rapidly excised and stored at −80°C until assays were performed.
The schematic diagram of the experimental procedures is shown in
Fig. 1A.
Assessment of serum VEGF levels
Serum levels of VEGF were measured by ELISA. Blood
was collected by intracardiac puncture and separated in a
refrigerated centrifuge at 2,810 × g for 10 min at 4°C. Serum was
separated from the clotted blood by centrifugation and analyzed for
VEGF using the Mouse VEGF-A Platinum ELISA kit (eBiosciences, Inc.,
San Diego, CA, USA) according to the manufacturer’s
instructions.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the mouse tumors using
TRIzol reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. Samples containing 0.5 μg total
RNA were reverse-transcribed using oligo d(T) and a PrimeScript RT
reagent kit (Takara Bio, Inc., Shiga, Japan). The resulting
complementary DNA was then subjected to qPCR (IQ-5 System; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer’s instructions. PCR amplification was conducted with
the following cycling conditions: Enzyme activation at 95°C for 15
min, followed by 40 cycles consisting of denaturation for 15 sec at
95°C, annealing for 30 sec at 58–64°C depending on the primers, and
elongation for 30 sec at 72°C. The primer sequences used were as
follows: VEGF receptor 2 (VEGFR2) sense,
5′-GCTGTGAACGCTTGCCTTATGATG-3′ and anti-sense,
5′-GCTCGCTGTGTGTTGCTCCTTC-3′; L1 cell adhesion molecule (L1CAM)
sense, 5′-CTCCTCATCCTGCTCATCCTCTG-3′ and anti-sense,
5′-GCCTTCTCTTCATTGTCACTCTCC-3′; and GAPDH sense,
5′-AACTTGGCATTGTGGAAGG-3′ and anti-sense,
5′-GGATGCAGGGATGATGTTCT-3′. The primers were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China) and the data were analyzed
using the 2(−∆∆Ct) method.
Western blot analysis
Mouse tumors were dissected, homogenized and
resuspended in radioimmunoprecipitation assay lysis buffer. Lysates
were kept on ice for 30 min, vortexed, disrupted by 4 × 30-sec
bursts of supersonication with a 550 Sonic Dismembrator (Thermo
Fisher Scientific, Hampton, NH, USA) and then centrifuged at 10,800
× g for 20 min at 4°C. Supernatants were collected and quantified
for protein levels with a Bicinchoninic Acid Protein Assay kit
(Beyotime Institute of Biotechnology) and a Synergy 2 Multi-Mode
microplate reader (562nm; BioTek Instruments, Inc., Winooski, VT,
USA). In brief, supernatants were boiled in 2X SDS loading buffer
(Beyotime Institute of Biotechnology), separated using 10% SDS-PAGE
and transferred to nitrocellulose membranes (Bio-Rad Laboratories,
Inc.). The membrane was blocked with 5% non-fat milk (Beyotime
Institute of Biotechnology) for 1 h at room temperature and then
incubated overnight at 4°C with the following primary antibodies:
Mouse monoclonal anti-phosphorylated extracellular signal-regulated
kinase (pERK; 1,000; sc-7383; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), rabbit polyclonal anti-MMP-2 (1:2,000; ab124294;
Abcam, Shanghai, China), rabbit monoclonal anti-MMP-9 (1:3,000;
EP1255Y; Abcam) and anti-β-actin (1:1,000; AA132; Beyotime
Institute of Biotechnology) antibodies. The blots were then
incubated for 2 h at room temperature with goat anti-rabbit (A0208)
and goat anti-mouse (A0216) horseradish peroxidase-conjugated goat
secondary antibodies (1:3,000; Beyotime Institute of Biotechnology)
in blocking buffer (Beyotime Institute of Biotechnology). Target
proteins were detected using an enhanced chemiluminescence Plus
Western Blotting Detection System (Amersham Pharmacia Biotech,
Uppsala, Sweden) and images of the blots were captured using a
molecular imager (GelDoc XR system; Bio-Rad Laboratories, Inc.).
The images were analyzed using Quantity One image analysis
software, version 4.2.1 (Bio-Rad Laboratories, Inc.). The optical
densities of the target protein were normalized to that of β-actin.
Western blot experiments were replicated three times.
Statistical analysis
Values are presented as the mean ± standard error of
the mean. Data analysis was performed using one-way analysis of
variance or, for comparison of two groups, Student’s t-test,
conducted using SPSS, version 20.0 (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
RSDS causes behavioral sensitization to
stress
Mice were subjected to the ten-day social defeat
procedure and then tested for social approach or avoidance behavior
in a social interaction test. This was measured by comparing the
time a mouse spent in an interaction zone with a social target to
the time in that zone in the absence of a social target, as
previously described (21). As
shown in Fig. 1B and C, in the
absence of an aggressor, the control and RSDS mice spent a
comparable length of time in the interaction zone (P>0.05). By
contrast, when an aggressor was introduced into the cage, control
mice showed a dramatic increase in their interaction time, while
chronically defeated mice showed a significant reduction in their
interaction time (P<0.01) compared with that in the absence of
an aggressor, which implied the successful establishment of the
RSDS model.
RSDS promotes lung cancer
progression
The effects of RSRS on lung cancer progression were
investigated. Mice in the T and T-S groups were injected with LLC
cells and the same volume of PBS was injected into the C and S
groups as a control. No tumor formation was observed in mice in the
C and S groups. As shown in Fig.
2A, tumors were first observed on day 7 and the volume of
tumors increased until day 29, on which the mice were sacrificed.
Tumor growth was significantly faster, with a markedly higher final
tumor volume in the T-S group compared with that of the T group;
the final tumor volume was 69.2% (P<0.001) larger in the T-S
group as compared with that of the T group. As shown in Fig. 2B, the mean weight of the tumors in
the T-S mice was increased by 258% compared with that of the T
mice. In addition, tumor nodules were also detected in the lungs of
the T and T-S groups when the mice were sacrificed 19 days
following LLC cell injection. As shown in Fig. 2C, RSDS increased the number of
tumor nodules on the lung by 128% (P<0.01), compared with that
of the mice in the T group.
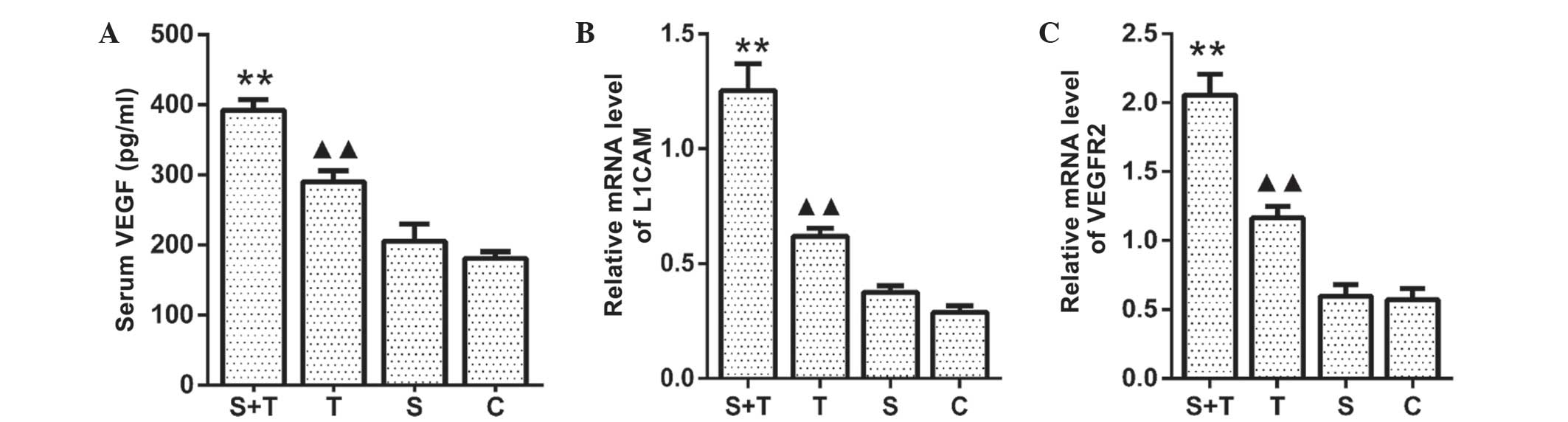
RSDS promotes the expression of VEGF,
VEGFR2 and L1CAM
As previously described (10), the growth and metastasis of tumors
is closely associated with angiogenesis. In the present study, it
was investigated whether RSDS influenced the mRNA expression of
VEGFR2 and L1CAM in the lung tissues and the serum levels of VEGF.
As shown in Fig. 3A, serum VEGF
levels were significantly higher in the T-S group compared with
those of the unstressed T mice (P<0.05). Consistent with this,
the mRNA expression of L1CAM and VEGFR2 in the lung tissue from the
T-S mice was significantly increased compared with that in the T
group (P<0.05) (Fig. 3B and C).
By contrast, no significant differences were observed in mRNA
expression of VEGFR2 and L1CAM in the lung tissues or the serum
levels of VEGF between the S and C groups (P<0.05). These
results suggested that angiogenic processes mediated the effects of
RSDS on tumor growth in LLC cell-bearing mice.
RSDS upregulates pERK, MMP-2 and MMP-9
protein expression
In order to verify whether upregulation of VEGF and
VEGFR2 resulted in the activation of the gene transcription of
downstream targets, western blot analysis was used to determine the
expression levels of angiogenesis-associated proteins, including
pERK, MMP-2 and MMP-9 in normal lung tissue from the C and S groups
as well as tumor tissue from the T and T-S groups. The results
showed that RSDS induced a significant increase in pERK, MMP-2 and
MMP-9 protein expression levels compared with levels in the
unstressed T group (P<0.01). No significant differences were
noted in protein levels of pERK, MMP-2 and MMP-9 in normal lung
tissues between the S and C groups (P>0.05) (Fig. 4).
Discussion
Previous studies have revealed that psychological
stress may be a potential modulator of cancer progression and this
has become an important novel field of cancer research (22). Clinical and experimental animal
studies indicated that stress, chronic depression and other
psychological factors may influence the pathogenesis and
progression of cancer. In the present study, an established RSDS
mouse model, based on previous social defeat studies (18,19),
was used to explore the influence of stress on tumor growth and
metastasis. The mechanisms by which chronic stress promoted
tumorigenesis have previously been investigated (23). The results of the present study
demonstrated that RSDS significantly increased the weight and
volume of primary tumors as well as the number of the lung
metastatic nodules. In addition, serum VEGF levels were
significantly higher in the T-S group compared with those of the
unstressed T mice. Furthermore, tumors in stressed animals showed
markedly enhanced mRNA expression of VEGFR2 and L1CAM as well as
protein levels of pERK, MMP-2 and MMP-9. These data suggested that
RSDS contributed to lung cancer progression, angiogenesis and
metastasis, which was partially associated with increased VEGF
secretion and therefore activated the ERK signaling pathway,
resulting in the induction of MMP-2 and MMP-9 expression.
Cancer metastasis, which is resistant to
conventional therapies, is the primary cause of cancer-associated
mortality (11). In addition, the
result of cancer metastasis is dependent upon the tumor cells
themselves as well as on the organ micro-environment (24). Therefore, treatments should be
aimed at targeting the cancer cells as well as the host factors
which contribute to the progressive growth and survival of
metastatic cancer cells. Previous clinical and epidemiological
studies have reported that psychosocial factors, including stress,
chronic depression and lack of social support, may be risk factors
for cancer progression (6,15,25).
However, there have been limited studies into the role of
psychosocial factors in cancer initiation, although certain studies
have reported that these factors may have a negative role in the
progression of the cancer (26,27).
Studies have suggested that the chronicity of a negative effect
sustains the activation of the pathways it mediates, which may be
associated with cancer progression (28). Numerous studies have associated
high levels of social support with improved clinical outcomes in
cancer patients; in breast cancer patients, social support was
associated with prolonged survival rates in multiple large-scale
studies (29,30), although negative findings were
noted in certain studies (31). In
view of the fact that there is still controversy over the role of
psychosocial factors on carcinoma metastasis, further studies are
required in order to verify this hypothesis and thereby provide
more effective means for the potential treatment of cancer.
Based on previous findings, it was hypothesized that
psychological factors reduce the body’s immune system and have a
positive role in the initiation and progression of cancer (19). Therefore, in the present study, an
established RSDS mouse model, which was previously shown to mimic
numerous symptoms of depression in humans (18,19),
was used to investigate the effect of stress on tumor growth and
metastasis in a LLC-bearing tumor model.
Animal models which replicate the pathogenesis of
human disease are essential for understanding the effects of stress
on cancer, amongst other diseases (17). The consideration of chronic
psychological stress due to socio-economic factors and
disease-associated anxiety is an important, but overlooked aspect
of cancer intervention that requires inclusion in preventative and
therapeutic strategies for the successful treatment of lung cancer.
The majority of previous studies have used various forms of chronic
stress in order to replicate the behavioral adaptations associated
with depression in animal models (32). These models include chronic
unpredictable stress, restraint stress or foot-shock stress, which
may be followed by behavioral measures of anhedonia (e.g., sucrose
preference) or behavioral despair (e.g., forced swim test and tail
suspension test) (33). However,
all of these models are inconsistent with the etiology of the
depression and cannot address the various validities which are
required to produce an effective animal model of depression. The
RSDS model used in the present study is performed on the basis of
the etiology of the depression; it is comparable with the process
of psychological factors acting on the human and better simulates
the pathogenesis of human depression. In addition, another feature
of this model is that social avoidance induced by 10 days of social
defeat can be reserved through chronic, but not acute,
anti-depressant treatments (18,33).
A previous study, which examined psychological distress and its
association with the site of cancer reported that primary lung
cancer was markedly associated with psychological distress in
cancer patients (34). In
addition, numerous studies have revealed that psychological
distress was more prevalent in patients with lung cancer compared
with other types of cancer (35).
Therefore, in the present study, the RSDS model was combined with
the spontaneous metastasis of lung carcinoma through inoculation of
LLC cells in mice. This enabled the observation of the effect of
psychological factors on the growth and metastasis of lung
carcinoma.
Stress commonly occurs due to an imbalance between
environmental requirements and an individual’s abilities, and is
often defined as the experience of a negative life event (36). Recently, studies have documented
that stress may promote tumor incidence and progression (37). Metastasis is a complex process that
requires the development of several hallmarks in order to occur,
including angiogenesis, proliferation, invasion, embolization and
evasion of immune system surveillance (38). Increasing evidence has suggested
that the stress response may have roles is numerous aspects of this
cascade, as cellular and molecular studies have associated stress
with several processes known to be involved in cancer angiogenesis
and metastasis (39). Angiogenesis
involves the release of pro-angiogenic factors. VEGF is an
angiogenic molecule which has important roles in embryogenesis,
physiologic angiogenesis and the neovascularization of
malignancies. Previous clinical studies have reported associations
between increased levels of social support and lower VEGF
expression in sera and tumor tissues (40). Furthermore, angiogenesis was found
to be significantly upregulated in tumors in stressed mice compared
with those in control mice; in addition, VEGF mRNA and protein
levels were significantly elevated in tumor samples from mice
exposed to daily stress (23).
Consistent with these results, these present study demonstrated
that serum VEGF levels were significantly increased in the T-S
group compared with those in the unstressed T mice; in addition,
the expression of VEGFR2 and L1CAM mRNA in lung tissue from the T-S
group was significantly increased compared with that in the T
group.
MMPs are a family of endoproteinases, which are
essential for the degradation of the extracellular matrix and
contribute to cancer initiation, growth, invasion and metastasis
(41). The gelatinases A (MMP-2)
and B (MMP-9) have been reported to be associated with an increased
malignancy of tumor cells, as they are able to degrade type-IV
collagen in the basement membrane (42), which was reported to be important
for the invasive and metastatic potential in lung carcinoma
(43). However, to date, the
physiological factors involved in the modulation of MMP expression
in lung cancer have remained to be elucidated. The results of the
present study identified chronic stress as a novel regulator of MMP
expression, which selectively upregulated MMP-9 and MMP-2 in the
tumors of stressed animals. The ERK1/2 signaling pathways are
important molecular pathways downstream of VEGF, which participate
in regulating the expression of numerous genes (44). However, it has not yet been
elucidated whether VEGF regulates the expression of MMP-2/9 through
the ERK pathway. It was reported that estrogen increased the
expression of VEGF, and thus activated ERK1/2 signaling to induce
MMP-2/9 expression (45). In order
to verify these findings in the present study, western blot
analysis was used to detect the expression of pERK; the results
showed that RSDS upregulated the expression of pERK in the tumor
tissue of stressed mice. These data suggested that RSDS may
contribute to lung cancer progression, angiogenesis and metastasis,
which was found to be, in part, associated with increased VEGF
secretion and therefore activated the ERK pathway, resulting in the
induction of MMP-2 and MMP-9 protein expression.
Future studies are required in order to further
explore the specific molecular mechanism by which stress hormones
modulate the interplay between tumor and stromal cells in the tumor
microenvironment, resulting in the regulation of various signaling
pathways associated with cancer progression. The elucidation of
these pathways is therefore essential for the development of novel
approaches to prevent and treat the deleterious effects of stress
biology on cancer growth and metastasis.
In conclusion, the results of the present study
expanded on the current understanding of the general pathways by
which stress regulates cancer pathogenesis. These results have
demonstrated that RSDS significantly promoted tumor growth,
metastasis and angiogenesis, which was partially associated with
increased VEGF secretion and the subsequent activation of the ERK
signaling pathway, resulting in the induction of MMP-2 and MMP-9
protein expression. However, further studies are required in order
to elucidate the psychoneuroimmunological details of the influence
of chronic stress on lung cancer progression.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81173390 and
81102562), the National Basic Science Program of China (no.
2009CB523000) and the Chinese Ministry of Education Fund for Doctor
Discipline Scientific Research (no. 20110071120072).
References
|
1
|
Zou XN, Lin DM, Wan X, et al: Histological
subtypes of lung cancer in chinese males from 2000 to 2012. Biomed
Environ Sci. 27:3–9. 2014.PubMed/NCBI
|
|
2
|
de Mello RA, Costa BM, Reis RM and
Hespanhol V: Insights into angiogenesis in non-small cell lung
cancer: molecular mechanisms, polymorphic genes and targeted
therapies. Recent Pat Anticancer Drug Discov. 7:118–131. 2012.
View Article : Google Scholar
|
|
3
|
Al-Wadei HA, Plummer HR, Ullah MF, Unger
B, Brody JR and Schuller HM: Social stress promotes and
gamma-aminobutyric acid inhibits tumor growth in mouse models of
non-small cell lung cancer. Cancer Prev Res (Phila). 5:189–196.
2012. View Article : Google Scholar
|
|
4
|
Reiche EM, Nunes SO and Morimoto HK:
Stress, depression, the immune system and cancer. Lancet Oncol.
5:617–625. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antoni MH, Lutgendorf SK, Cole SW, et al:
The influence of bio-behavioural factors on tumour biology:
pathways and mechanisms. Nat Rev Cancer. 6:240–248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chida Y, Hamer M, Wardle J and Steptoe A:
Do stress-related psychosocial factors contribute to cancer
incidence and survival? Nat Clin Pract Oncol. 5:466–475. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Traeger L, Cannon S, Keating NL, et al:
Race by sex differences in depression symptoms and psychosocial
service use among non-Hispanic black and white patients with lung
cancer. J Clin Oncol. 32:107–113. 2014. View Article : Google Scholar :
|
|
8
|
Feng Z, Liu L, Zhang C, et al: Chronic
restraint stress attenuates p53 function and promotes
tumorigenesis. Proc Natl Acad Sci USA. 109:7013–7018. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zorrilla EP, Luborsky L, McKay JR, et al:
The relationship of depression and stressors to immunological
assays: a meta-analytic review. Brain Behav Immun. 15:199–226.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sahai E: Illuminating the metastatic
process. Nat Rev Cancer. 7:737–749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fidler IJ, Kim SJ and Langley RR: The role
of the organ micro-environment in the biology and therapy of cancer
metastasis. J Cell Biochem. 101:927–936. 2007. View Article : Google Scholar
|
|
12
|
Liao X, Che X, Zhao W, Zhang D, Bi T and
Wang G: The β-adrenoceptor antagonist, propranolol, induces human
gastric cancer cell apoptosis and cell cycle arrest via inhibiting
nuclear factor κB signaling. Oncol Rep. 24:1669–1676.
2010.PubMed/NCBI
|
|
13
|
Li S, Sun Y and Gao D: Role of the nervous
system in cancer metastasis. Oncol Lett. 5:1101–1111.
2013.PubMed/NCBI
|
|
14
|
Zou LB, Shi S, Zhang RJ, et al:
Aquaporin-1 plays a crucial role in estrogen-induced tubulogenesis
of vascular endothelial cells. J Clin Endocrinol Metab.
98:E672–E682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spiegel D and Giese-Davis J: Depression
and cancer: mechanisms and disease progression. Biol Psychiatry.
54:269–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patil N, Ahmed Kabeer Rasheed S, Abba M,
Hendrik Leupold J, Schwarzbach M and Allgayer H: A mechanistic
study on the metastasis inducing function of FUS-CHOP fusion
protein in liposarcoma. Int J Cancer. 134:2808–2819. 2014.
View Article : Google Scholar
|
|
17
|
Moreno-Smith M, Lutgendorf SK and Sood AK:
Impact of stress on cancer metastasis. Future Oncol. 6:1863–1881.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berton O, McClung CA, Dileone RJ, et al:
Essential role of BDNF in the mesolimbic dopamine pathway in social
defeat stress. Science. 311:864–868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu X, Wu J, Xia S, Li B and Dong J:
Icaritin opposes the development of social aversion after defeat
stress via increases of GR mRNA and BDNF mRNA in mice. Behav Brain
Res. 256:602–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Golden SA, Covington HR, Berton O and
Russo SJ: A standardized protocol for repeated social defeat stress
in mice. Nat Protoc. 6:1183–1191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krishnan V, Han MH, Graham DL, et al:
Molecular adaptations underlying susceptibility and resistance to
social defeat in brain reward regions. Cell. 131:391–404. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Payne JK: State of the science: stress,
inflammation, and cancer. Oncol Nurs Forum. 41:533–540. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thaker PH, Han LY, Kamat AA, et al:
Chronic stress promotes tumor growth and angiogenesis in a mouse
model of ovarian carcinoma. Nat Med. 12:939–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fidler IJ: The organ microenvironment and
cancer metastasis. Differentiation. 70:498–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spiegel D: Health caring Psychosocial
support for patients with cancer. Cancer. 74:1453–1457. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duijts SF, Zeegers MP and Borne BV: The
association between stressful life events and breast cancer risk: a
meta-analysis. Int J Cancer. 107:1023–1029. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Michael YL, Carlson NE, Chlebowski RT, et
al: Influence of stressors on breast cancer incidence in the
Women’s Health Initiative. Health Psychol. 28:137–146. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watson M, Haviland JS, Greer S, Davidson J
and Bliss JM: Influence of psychological response on survival in
breast cancer: a population-based cohort study. Lancet.
354:1331–1336. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Funch DP and Marshall J: The role of
stress, social support and age in survival from breast cancer. J
Psychosom Res. 27:77–83. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giraldi T, Rodani MG, Cartei G and Grassi
L: Psychosocial factors and breast cancer: a 6-year Italian
follow-up study. Psychother Psychosom. 66:229–236. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kroenke CH, Kubzansky LD, Schernhammer ES,
Holmes MD and Kawachi I: Social networks, social support and
survival after breast cancer diagnosis. J Clin Oncol. 24:1105–1111.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mandelblatt JS, Hurria A, McDonald BC, et
al: Cognitive effects of cancer and its treatments at the
intersection of aging: what do we know; what do we need to know?
Semin Oncol. 40:709–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsankova NM, Berton O, Renthal W, Kumar A,
Neve RL and Nestler EJ: Sustained hippocampal chromatin regulation
in a mouse model of depression and antidepressant action. Nat
Neurosci. 9:519–525. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dugan W, McDonald MV, Passik SD, Rosenfeld
BD, Theobald D and Edgerton S: Use of the Zung Self-Rating
Depression Scale in cancer patients: feasibility as a screening
tool. Psychooncology. 7:483–493. 1998. View Article : Google Scholar
|
|
35
|
Zabora J, BrintzenhofeSzoc K, Curbow B,
Hooker C and Piantadosi S: The prevalence of psychological distress
by cancer site. Psychooncology. 10:19–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Strom TQ and Kosciulek J: Stress,
appraisal and coping following mild traumatic brain injury. Brain
Inj. 21:1137–1145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saul AN, Oberyszyn TM, Daugherty C, et al:
Chronic stress and susceptibility to skin cancer. J Natl Cancer
Inst. 97:1760–1767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Szlosarek P, Charles KA and Balkwill FR:
Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer.
42:745–750. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fang BA, Kovačević Ž, Park KC, et al:
Molecular functions of the iron-regulated metastasis suppressor,
NDRG1, and its potential as a molecular target for cancer therapy.
Biochim Biophys Acta. 1845:1–19. 2014.
|
|
40
|
Schuller HM, Al-Wadei HA, Ullah MF and
Plummer HR: Regulation of pancreatic cancer by neuropsychological
stress responses: a novel target for intervention. Carcinogenesis.
33:191–196. 2012. View Article : Google Scholar :
|
|
41
|
Duffy MJ, Maguire TM, Hill A, McDermott E
and O’Higgins N: Metalloproteinases: role in breast carcinogenesis,
invasion and metastasis. Breast Cancer Res. 2:252–257. 2000.
View Article : Google Scholar
|
|
42
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shiraga M, Yano S, Yamamoto A, et al:
Organ heterogeneity of host-derived matrix metalloproteinase
expression and its involvement in multiple-organ metastasis by lung
cancer cell lines. Cancer Res. 62:5967–5973. 2002.PubMed/NCBI
|
|
44
|
Watanabe T, Takahashi A, Suzuki K, et al:
Epithelial-mesenchymal transition in human gastric cancer cell
lines induced by TNF-α-inducing protein of Helicobacter pylori. Int
J Cancer. 134:2373–2382. 2014. View Article : Google Scholar
|
|
45
|
Shan B, Li W, Yang SY and Li ZR: Estrogen
up-regulates MMP2/9 expression in endometrial epithelial cell via
VEGF-ERK1/2 pathway. Asian Pac J Trop Med. 6:826–830. 2013.
View Article : Google Scholar : PubMed/NCBI
|