Introduction
Gastric cancer is one of the leading causes of
cancer-related mortality worldwide (1). The present therapeutic options of
chemotherapy and targeted drug treatment are unsatisfactory in the
treatment of patients with advanced gastric cancer and the
development of more efficacious and individualized therapies
remains a major challenge to be overcome.
The traditional Chinese medicine Chan Su, which is
isolated from secretions of the skin and parotid venom glands of
the Chinese and black-spectacled toads, contains the active
component bufalin (2,3). Previous studies have demonstrated
that bufalin exhibits significant antitumor activity, via the
induction of apoptosis and inhibition of proliferation, in a number
of tumor types, including lung cancer, breast cancer, hepatic
carcinoma and leukemia. In these cancer types, inhibition of the
phosphoinositide 3-kinase (PI3K)/Akt pathway activation was found
to be the predominant mechanism by which bufalin induced apoptosis
(4–10). In accordance with this, a previous
study from this laboratory found that downregulation of Bcl/Bax,
activation of Caspase-3 and inhibition of the PI3K/Akt signaling
pathway occurred during bufalin-induced apoptosis in gastric cancer
(11). However, it was shown that
not all gastric cancer cells were sensitive to bufalin, suggesting
that other factors may have an antagonistic effect on
bufalin-induced apoptosis.
Secreted protein acidic and rich in cysteine (SPARC)
is a protein that is associated with embryonic development,
remodeling, cell turnover and tissue repair (12). SPARC is normally secreted by
stromal cells but is also produced by cancer cells, including in
pancreatic, breast, prostate and gastric cancer (13–16).
Recent studies have reported a positive correlation between
overexpression of stromal-derived SPARC and the response to
nanoparticle albumin-bound (NAB) drugs in certain tumors. Phase II
and III studies have revealed that NAB-paclitaxel was significantly
more effective and well-tolerated than conventional docetaxel and
paclitaxel in patients with metastatic breast cancer and advanced
pancreatic cancer. This increased efficacy is likely to be a result
of stromal SPARC directly increasing accumulation of NAB-paclitaxel
in tumor tissues via binding to albumin (17–20).
However, recent studies have suggested that intracellular SPARC is
also important in the regulation of apoptosis and cell
proliferation (21–24). Silencing of SPARC expression
significantly suppresses tumor cell proliferation and induces
apoptosis via modulation of the expression of Bcl-2, Bax and
proliferating cell nuclear antigen in human ovarian cancer,
melanoma and leukemia (22,25,26).
As a number of the apoptosis-related proteins regulated by SPARC
are also involved in bufalin-induced apoptosis, the present study
investigated the possibility that SPARC may regulate
bufalin-induced apoptosis in gastric cancer cells.
Materials and methods
Reagents and antibodies
Rabbit anti-Caspase-3 (cat. no. sc-7148; 1:500),
anti-Bax (cat. no. sc-493; 1:1,000), anti-Src (cat. no. sc-8995;
1:1,000), anti-cyclin-dependent kinase (cdk)2 (cat. no. sc-748;
1:500), anti-Cyclin B1 (cat. no. sc-752; 1:1,000), anti-Cyclin A
(cat. no. sc-596; 1:500), anti-Cyclin E (cat. no. sc-481; 1:1,000)
and anti-actin (cat. no. sc-7210, 1:2,000) polyclonal antibodies
were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Rabbit anti-Akt (cat. no. 4691; 1:1,000), anti-phospho-(p)Akt
(cat. no. 4058; 1:1,000), anti-extracellular signal-regulated
kinase (ERK; cat. no. 4348; 1:1,000) anti-phospho-ERK (cat. no.
4370; 1:2,000), anti-phospho-Src (cat. no. 12432; 1:1,000),
anti-poly(ADP-ribose) polymerase (PARP; cat. no. 5625, 1:1,000) and
anti-Bcl-2 (cat. no. 2870; 1:1,000) monoclonal antibodies were
obtained from Cell Signaling Technology (Danvers, MA, USA). A mouse
anti-cytochrome c (cat. no. 556433, 1:500) monoclonal
antibody was obtained from BD Biosciences (Franklin Lakes, NJ,
USA). Bufalin was obtained from Sigma-Aldrich (St. Louis, MO,
USA).
Cell cultures
The SGC7901, MGC803, BGC823 and MKN45 human gastric
cancer cell lines were obtained from the Type Culture Collection of
the Chinese Academy of Sciences (Shanghai, China). Cells were
cultured in RPMI-1640 medium (Gibco Life Technologies, Grand
Island, NY, USA) supplemented with 10% heat-inactivated fetal
bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin
at 37.0°C in 5% CO2. Cells were subcultured for two to
three days in order to maintain a log-phase growth for
experiments.
Small interfering (si)R NA
interference
SPARC and scrambled control siRNA were obtained from
Shanghai GeneChem Co., Ltd. (Shanghai, China). The siRNA sequences
used were as follows: Forward: 5′-GCCACUUCUUUGCCACAAAT)-3′ and
reverse: 5′-TTTGTGGCAAAGAAGTGGC-3′ for SPARC-specific siRNA; and
forward: 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse:
5′-ACGUGACACGUUCGGAGAATT-3′ for scrambled control siRNA. SGC7901
cells were seeded at 2.5×105/well into 6-well plates and
transfected with 5 μl/well Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s
instructions.
Cytotoxicity assays
Cell viability was measured using the
3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT) assay. SGC7901 cells were transfected with SPARC-specific or
scrambled control siRNA following 30 h culture in a 6-well plate,
seeded into a 96-well plate at a cell density of 5,000 per well and
incubated overnight. Cell cultures, including the initial SGC7901
and MGC803 cells and the transfected SGC7901 cells were treated
with varying concentrations of bufalin (20, 40, 80, 160, 320
nmol/l) for 20 h and 20 μl of 5 mg/ml MTT solution
(Sigma-Aldrich) was added to each well, prior to incubation for a
further 4 h at 37.0°C. Following removal of the culture medium,
cells were lysed in 200 μl dimethylsulfoxide, and the
optical density (OD) was measured at 570 nm using a microplate
reader (Bio-Rad, Hercules, CA, USA). The following formula was used
to calculate cell viability: Cell viability (%) = (OD of the
experimental sample/OD of the control group) × 100.
Cell cycle phase and mitochondrial
membrane potential analysis
Phase distributions of the cell cycle and cell
apoptosis were determined by flow cytometry. Cells were seeded at
2.5×105/well in 6-well plates and transfected with SPARC
siRNA or scrambled control siRNA at 30 h. They were then exposed to
bufalin (100 and 200 nmol/l doses) and incubated for 6 and 24 h in
separate plates. Cells were trypsinized, washed once with
phosphate-buffered saline (PBS) and then fixed with cold 70%
ethanol overnight. Fixed cells were washed twice with PBS,
incubated with 20 μg/ml ribonuclease A (RNase A) at 37.0°C
for 30 min and stained with 10 μg/ml propidium iodide for 30
min in darkness. In addition to this, the mitochondrial membrane
potential was determined by means of the cationic lipophilic
fluorochrome DIOC6. Cells were collected and incubated with 20 nm
DIOC6 (Molecular Probes Life Technologies, Carlsbad, CA, USA) for
15 min in darkness. The fluorescence intensity of the cells was
detected using a BD FACSCalibur cytometer (BD Biosciences, San
Jose, CA, USA) and the cell cycle distribution was analyzed using
WinMDI 2.9 software (Scripps Research Institute, La Jolla, CA,
USA).
Western blot analysis
Following administration of treatments at the time
points indicated, the cells were washed twice with ice-cold PBS,
lysed in 1% Triton lysis buffer on ice and quantified using the
Lowry method (27). Proteins (40
μg) were separated using a 10% SDS-polyacrylamide gel and
transferred electrophoretically onto polyvinylidene difluoride
membranes (Millipore, Bedford, MA, USA). The membranes were blocked
with 5% non-fat milk in Tris-buffered saline with Tween-20 for 1.5
h at room temperature, and subsequently incubated with primary
antibodies targeting SPARC, Caspase-3, PARP, Bax, Bcl-2, cytochrome
c, Cyclin B1, Cyclin A, Cyclin E, Cdk2, p-AKT, p-ERK, p-Src,
AKT, ERK and Src, at 4°C overnight, prior to incubation with
horseradish peroxidase-conjugated anti-rabbit (cat. no. sc-2491;
1:2,000) or mouse (cat. no. sc-2072; 1:2,000) secondary antibody
(Santa Cruz Biotechnology, Inc.) for 1 h at room temperature.
Protein bands were visualized with an enhanced chemiluminescence
reagent (Super Signal Western Pico Chemiluminescence substrate,
Pierce Biotechnology Inc., Rockford, IL, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
The significance of any differences between the groups was assessed
by Student’s t-test. Statistical analyses were performed using SPSS
version 16.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference. All
means were calculated from at least three independent
experiments.
Results
SPARC reduces the sensitivity of gastric
cancer cells to bufalin
SPARC expression was measured in four gastric cancer
cell lines. SGC7901 cells expressed the highest levels of SPARC,
whilst the MGC803, BGC823 and MKN45 cell lines had markedly lower
levels of expression (Fig. 1A).
SGC7901 cells (high SPARC expression) and MGC803 cells (low SPARC
expression) were selected for the following experiments. The
viability of MGC803 and SGC7901 cell lines treated with varying
concentrations of bufalin was assessed via an MTT assay. The
IC50 value for the viability of SGC7901 cells treated
with bufalin was >800 nmol/l following 24 h treatment. This is
approximately a 5-fold increase compared with the IC50
value of 160±0.87 nmol/l for the viability of MGC803 cells treated
under the same conditions (P<0.001; Fig. 1B). Flow cytometric analysis showed
that 200 nmol/l bufalin induced apoptosis in 21.63±1.76% of MGC803
cells at 24 h compared with 6.027±2.85% of SGC7901 cells
(P<0.001; Fig. 1C). To further
investigate whether SPARC influences the sensitivity of gastric
cancer cells to bufalin, SGC7901 cells were transfected with
SPARC-specific or scrambled control siRNA and treated with varying
concentrations of bufalin over 24 h. Compared with parental SGC7901
cells and scrambled siRNA control cells, knockdown of SPARC
significantly decreased the IC50 value of cell viability
following treatment with bufalin from 919.6±2.928 to 159.1±1.598
nmol/l (P<0.001; Fig. 1D).
Consistent with this, the degree of bufalin-induced apoptosis in
these cells also significantly increased, from 7.02±2.12 to
23.42±0.60% (P<0.001; Fig. 1E).
These results suggest that higher levels of SPARC reduced the
sensitivity of gastric cancer cells to bufalin treatment.
 | Figure 1Effect of SPARC expression on the
sensitivity of gastric cancer cells to bufalin. (A) SPARC
expression was measured in four gastric cancer cell lines. Lane 1,
MGC803; lane 2, SGC7901; lane 3, BGC823; lane 4, MKN45.
Immunoblotting was conducted using a rabbit polyclonal SPARC
antibody (1:200). (B) Viability of MGC803 and SGC7901 cell lines
treated with varying concentrations of bufalin (20, 40, 80, 160 and
320 nmol/l) for 24 h was assessed via an MTT assay. (C) Following
incubation with bufalin (100 and 200 nmol/l) for 24 h, cell
apoptosis as a sub-G1 fraction of SGC7901 and MGC803 cells was
analyzed by flow cytometry. Cells were stained with propidium
iodide. (D) Parental SGC7901 cells were transfected with
SPARC-specific or scrambled control siRNA for 30 h, and then
treated with bufalin (20–320 nmol/l) for 24 h. The cell viability
was examined using an MTT assay. (E) Apoptosis of the cells as
described in (D) was assessed by flow cytometry after treatment
with or without 200 nmol/l bufalin at the indicated time-points (24
h). Columns indicate the mean percentage of apoptotic cells and
bars indicate standard deviation. *P<0.05. SPARC,
secreted protein acidic and rich in cysteine; MTT, 3-(4,5-dimethyl
thiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay; siRNA, small
interfering RNA. |
SPARC suppresses bufalin-induced
activation of the intrinsic apoptosis pathway
To further investigate the effect of SPARC on
bufalin-induced apoptosis in gastric cancer cells, the activation
of apoptosis-related proteins was measured by western blot
analysis. SGC7901 cells with high SPARC expression and MGC803 cells
with low SPARC expression were treated with bufalin (100 and 200
nmol/l doses) for 24 h. In MGC803 cells bufalin treatment markedly
increased cleavage of Caspase-3 and PARP, the release of
cytoplasmic cytochrome c and the ratio of Bax/Bcl-2. Minimal
or no change in the levels of these proteins was observed in
SGC7901 cells (Fig. 2A). SGC7901
cells transfected with SPARC or scrambled control siRNA were then
treated with bufalin at the doses and times indicated in Fig. 2B. Activation of
mitochondrial-associated proteins, cytochrome c and Bax, was
significantly increased in SPARC-knockdown cells (Fig. 2B), as determined using ImageJ
software. Furthermore, flow cytometry was conducted to measure the
mitochondrial membrane potential (Δψ M) in these cells. Treatment
of MGC803 cells with bufalin (200 nmol/l) for 24 h reduced Δψ M to
a greater degree than with bufalin treatment of SGC7901 cells
(Fig. 2C). However, knockdown of
SPARC significantly reduced Δψ M in SGC7901 cells treated with
bufalin (Fig. 2D). These findings
suggest that SPARC antagonizes bufalin-induced apoptosis through
suppression of the intrinsic apoptotic pathway.
SPARC expression overcomes
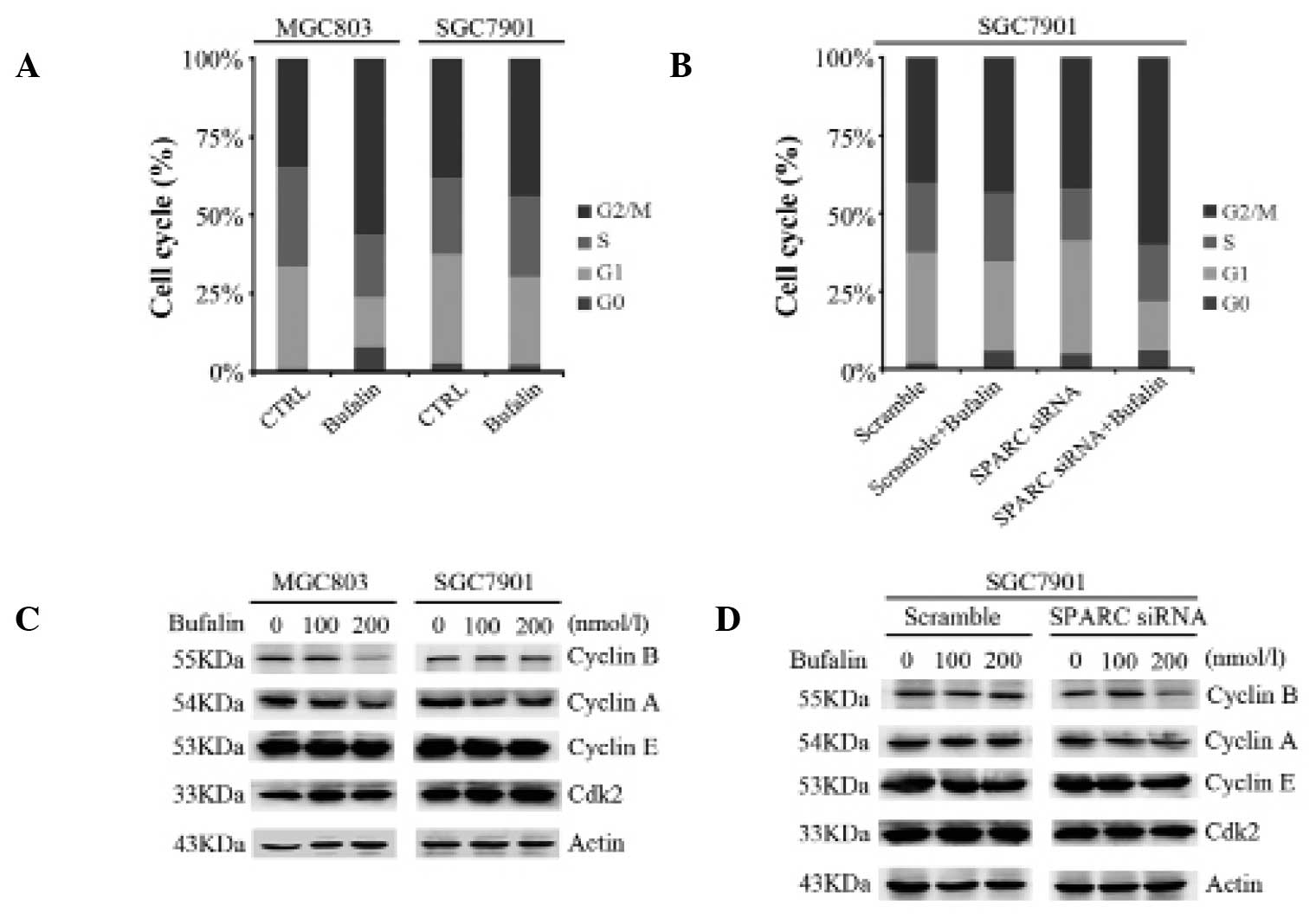
bufalin-induced cell cycle arrest at G2/M phase
In order to examine whether SPARC affects
bufalin-induced cell cycle arrest in gastric cancer cell lines,
MGC803 and SGC7901 cells were incubated with 100 or 200 nmol/l
bufalin for 6 h. Flow cytometry was conducted to assess the cell
cycle state of these cells. The percentage of cells in G2/M phase
increased from 34.59 to 56.16% in MGC803 cells treated with 200
nmol/l bufalin, while a smaller increase from 38.19 to 44.05% was
observed in SGC7901 cells (Fig.
3A). In accordance with prior results in this study, knockdown
of SPARC in SGC7901 cells followed by exposure to 200 nmol/l
bufalin for 6 h resulted in a greater number of cells arresting in
G2/M phase compared with cells transfected with scrambled control
siRNA (60.07% and 43.19%, respectively; Fig. 3B). The expression levels of the
cell cycle-related proteins Cyclin B1, Cyclin A, Cyclin E and cdk2
were also measured. Levels of Cyclin B1 and Cyclin A were reduced
in MGC803 cells and SGC7901 cells with knockdown of SPARC, compared
with parental SGC7901 cells (Fig. 3C
and D). These results indicate that SPARC expression overcomes
bufalin-induced cell cycle arrest at the G2/M phase.
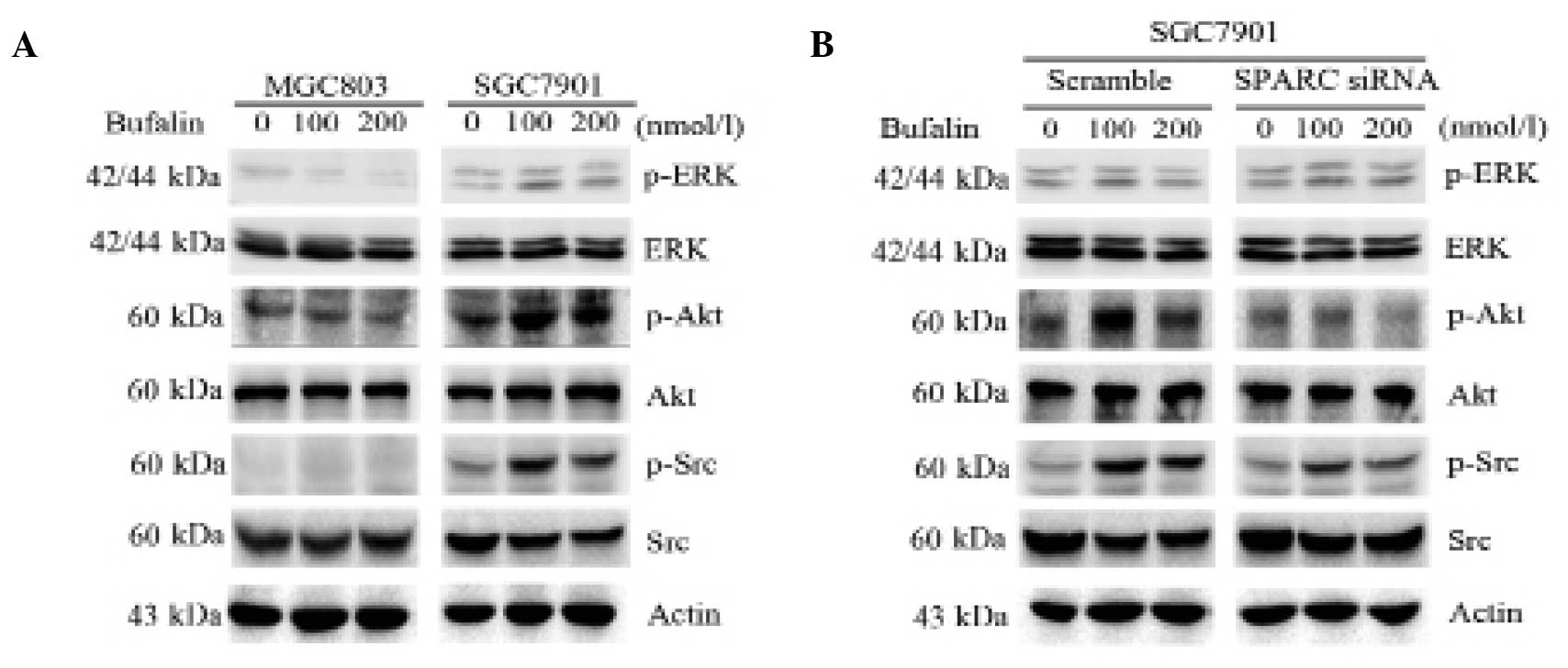
SPARC enhances the activation of survival
signal pathways in bufalin-resistant gastric cancer cells
To investigate whether SPARC influenced survival
signaling pathways during treatment of MGC803 and SGC7901 cells
with bufalin, the phosphorylation of Src, Akt and ERK was assessed.
The degree of phosphorylation of Src, Akt and ERK was markedly
increased in SGC7901 cells treated with bufalin alone (100 and 200
nmol/l). By contrast, in MGC803 cells Akt phosphorylation did not
visibly change (Fig. 4A). Notably,
knockdown of SPARC in SGC7901 cells greatly suppressed activation
of Src and Akt, but not ERK, when compared with cells transfected
with scrambled control siRNA (Fig.
4B). These results suggest that SPARC enhances the activation
of survival signal pathways in bufalin-resistant gastric cancer
cells.
Discussion
Extracellular SPARC has received marked attention in
cancer research due to its high affinity for albumin, which
facilitates the targeting of nanoparticle albumin-bound drugs to
tumor cells (17–20). Recently there has been an increased
focus on the influence of intracellular levels of SPARC on the
sensitivity of tumor cells to drugs (28–30).
To the best of our knowledge, the present study demonstrates for
the first time that SPARC suppresses bufalin-induced intrinsic
apoptosis signals and G2/M cell cycle arrest, whilst concurrently
promoting the activation of survival signaling pathways.
Previous reports concerning apoptosis regulation by
SPARC are contradictory. One study found that SPARC promoted
activation of the intrinsic apoptosis pathway via Bid, and
decreased the ratio of Bcl-2 and Bax in colorectal cancer cells
(30). However, SPARC has also
been shown to protect against tyrosine kinase inhibitor-mediated
apoptosis of chronic myeloid leukemia cells and to suppress the
mitochondrial pathway in two human melanoma cell lines (26,28)
To date, to the best of our knowledge, there is no data regarding
the influence of SPARC on bufalin-induced apoptosis. In this study,
MGC803 and SGC7901 gastric cancer cells exhibited different
sensitivities to bufalin, and the level of expression of SPARC was
negatively correlated with this sensitivity. It has been reported
that the Fas/Fas ligand pathway and the mitochondrial pathway are
involved in bufalin-triggered apoptosis (8). The current study demonstrated that
bufalin-resistant SGC7901 cells with high SPARC expression had
reduced mitochondrial integrity, reduced release of cytoplasmic
cytochrome c and an increased Bcl-2:Bax ratio. Additionally,
knockdown of SPARC restored bufalin-induced apoptosis. These
findings suggest that SPARC may be crucial in resisting
bufalin-induced apoptosis in gastric cancer.
Bufalin treatment led to the arrest of
hepatocellular carcinoma cells in G2/M phase as a result of
modulation of Cyclin B1 (6). Tumor
cell-derived SPARC may bypass the G2/M checkpoint, thereby
facilitating loss of control of the cell cycle, by reducing
expression of Cyclin B1 in melanoma (31). In the present study, it was
observed that low SPARC expression was associated with a greater
percentage of cells in G2/M phase following bufalin treatment.
Consistent with this, knockdown of SPARC led to an increase in G2/M
arrest in SGC7901 cells treated with bufalin. In addition,
expression of Cyclin B1 and Cyclin A, but not cdk2 and Cyclin E,
decreased following silencing of SPARC. These results indicate that
SPARC overcomes bufalin-induced G2/M arrest via regulation of the
expression of Cyclin proteins.
Several reports have indicated that inhibition of
certain tumor cell survival pathways, including the PI3K and
mitogen-activated protein kinase pathways, enhances bufalin-induced
apoptosis (32,33). Furthermore, activity of Akt was
shown to be increased in bufalin-insensitive hepatocellular
carcinoma cells (34). Bufalin has
also been shown to act synergistically with Akt inhibitors to
enhance apoptosis in lung cancer cells (33). SPARC was demonstrated to
significantly suppress activation of Akt in hepatic and ovarian
carcinoma (35,36). However, SPARC has also been
reported to reduce tumor cell apoptosis and promote tumor cell
survival by upregulating p-Akt in malignant glioma and melanoma
(23,37). In this study, it was observed that
Akt pathways, but not ERK pathways, were markedly activated in
gastric cancer cells with high levels of expression of SPARC. By
contrast, the degree of Akt phosphorylation was lower in gastric
cancer cells with low levels of SPARC expression. Notably,
increased Src phosphorylation was also observed in SGC7901 gastric
cancer cells with high levels of SPARC. It was also observed that
knockdown of SPARC in SGC7901 cells inhibited bufalin-induced Src
phosphorylation. An earlier study showed that SPARC inhibits
cellular migration and invasion via the activation of Src in
medulloblastoma cells (38).
However, there have been no reports on the effect of the
association between SPARC and Src on drug sensitivity in tumor
cells. The results presented in the current study indicate that
SPARC is involved in gastric cancer cell resistance to bufalin via
activation of the Akt and Src pathways.
This study demonstrates that SPARC protects against
bufalin-induced apoptosis in gastric cancer cells. This is achieved
by inhibition of the mitochondrial apoptosis pathway, including
downregulation of the release of cytoplasmic cytochrome c,
upregulation of the Bcl-2:Bax ratio, inhibition of cell cycle
arrest and activation of Src and Akt. Targeting SPARC expression
may prove useful in the development of novel indi-vidualized
therapeutic strategies to enable the effective use of bufalin in
gastric cancer.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81172369, 81372485
and 81372547) and the National Science and Technology Major Project
of the Ministry of Science and Technology of China (grant no.
2013ZX09303002).
References
|
1
|
Kanat O and O’Neil BH: Metastatic gastric
cancer treatment: a little slow but worthy progress. Med Oncol.
30:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hong Z, Chan K and Yeung HW: Simultaneous
determination of bufadienolides in the traditional Chinese medicine
preparation, liu-shen-wan, by liquid chromatography. J Pharm
Pharmacol. 44:1023–1026. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Panesar NS: Bufalin radioimmunoassays: in
search of the endogenous digitalis-like substance. J Immunoassay.
15:371–391. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang XH, Xu ZY, Gong YB, et al: Bufalin
reverses HGF-induced resistance to EGFR-TKIs in EGFR mutant lung
cancer cells via blockage of Met/PI3k/Akt pathway and induction of
apoptosis. Evid Based Complement Alternat Med. 2013:2438592013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan S, Qu X, Xu C, et al: Down-regulation
of Cbl-b by bufalin results in up-regulation of DR4/DR5 and
sensitization of TRAIL-induced apoptosis in breast cancer cells. J
Cancer Res Clin Oncol. 138:1279–1289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang DM, Liu JS, Tang MK, et al:
Bufotalin from Venenum Bufonis inhibits growth of multidrug
resistant HepG2 cells through G2/M cell cycle arrest and apoptosis.
Eur J Pharmacol. 692:19–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watabe M, Ito K, Masuda Y, Nakajo S and
Nakaya K: Activation of AP-1 is required for bufalin-induced
apoptosis in human leukemia U937 cells. Oncogene. 16:779–787. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi F, Inagaki Y, Gao B, et al: Bufalin and
cinobufagin induce apoptosis of human hepatocellular carcinoma
cells via Fas- and mitochondria-mediated pathways. Cancer Sci.
102:951–958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsiao YP, Yu CS, Yu CC, et al: Triggering
apoptotic death of human malignant melanoma a375.s2 cells by
bufalin: involvement of caspase cascade-dependent and independent
mitochondrial signaling pathways. Evid Based Complement Alternat
Med. 2012:5912412012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang WW, Yang JS, Pai SJ, et al: Bufalin
induces G(0)/G(1) phase arrest through inhibiting the levels of
cyclin D, cyclin E, CDK2 and CDK4, and triggers apoptosis via
mitochondrial signaling pathway in T24 human bladder cancer cells.
Mutat Res. 732:26–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D, Qu X, Hou K, et al: PI3K/Akt is
involved in bufalin-induced apoptosis in gastric cancer cells.
Anticancer Drugs. 20:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan Q and Sage EH: SPARC, a matricellular
glycoprotein with important biological functions. J Histochem
Cytochem. 47:1495–1506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seux M, Peuget S, Montero MP, et al:
TP53INP1 decreases pancreatic cancer cell migration by regulating
SPARC expression. Oncogene. 30:3049–3061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Azim HA Jr, Singhal S, Ignatiadis M, et
al: Association between SPARC mRNA expression, prognosis and
response to neoadjuvant chemotherapy in early breast cancer: a
pooled in-silico analysis. PLoS One. 8:e624512013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shin M, Mizokami A, Kim J, et al:
Exogenous SPARC suppresses proliferation and migration of prostate
cancer by interacting with integrin β1. Prostate. 73:1159–1170.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin J, Chen G, Liu Y, et al:
Downregulation of SPARC expression decreases gastric cancer
cellular invasion and survival. J Exp Clin Cancer Res. 29:592010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Desai N, Trieu V, Damascelli B and
Soon-Shiong P: SPARC expression correlates with tumor response to
albumin-bound paclitaxel in head and neck cancer patients. Transl
Oncol. 2:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Von Hoff DD, Ramanathan RK, Borad MJ, et
al: Gemcitabine plus nab-paclitaxel is an active regimen in
patients with advanced pancreatic cancer: a phase I/II trial. J
Clin Oncol. 29:4548–4554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Demeure MJ, Stephan E, Sinari S, et al:
Preclinical investigation of nanoparticle albumin-bound paclitaxel
as a potential treatment for adrenocortical cancer. Ann Surg.
255:140–146. 2012. View Article : Google Scholar
|
|
20
|
Guarneri V, Dieci MV and Conte P:
Enhancing intracellular taxane delivery: current role and
perspectives of nanoparticle albumin-bound paclitaxel in the
treatment of advanced breast cancer. Expert Opin Pharmacother.
13:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Shi D, Liu X, Fang S, Zhang J and
Zhao Y: Targeting SPARC by lentivirus-mediated RNA interference
inhibits cervical cancer cell growth and metastasis. BMC Cancer.
12:4642012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Wang M, Xi B, et al: SPARC is a
key regulator of proliferation, apoptosis and invasion in human
ovarian cancer. PLoS One. 7:e424132012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fenouille N, Puissant A, Tichet M, et al:
SPARC functions as an anti-stress factor by inactivating p53
through Akt-mediated MDM2 phosphorylation to promote melanoma cell
survival. Oncogene. 30:4887–4900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gorantla B, Bhoopathi P, Chetty C, et al:
Notch signaling regulates tumor-induced angiogenesis in
SPARC-overexpressed neuroblastoma. Angiogenesis. 16:85–100. 2013.
View Article : Google Scholar
|
|
25
|
Seno T, Harada H, Kohno S, Teraoka M,
Inoue A and Ohnishi T: Downregulation of SPARC expression inhibits
cell migration and invasion in malignant gliomas. Int J Oncol.
34:707–715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Horie K, Tsuchihara M and Nakatsura T:
Silencing of secreted protein acidic and rich in cysteine inhibits
the growth of human melanoma cells with G arrest induction. Cancer
Sci. 101:913–919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sengupta S and Chattopadhyay MK: Lowry’s
method of protein estimation: Some more insights. J Pharm
Pharmacol. 45:801993. View Article : Google Scholar
|
|
28
|
Giallongo C, La Cava P, Tibullo D, et al:
SPARC expression in CML is associated to imatinib treatment and to
inhibition of leukemia cell proliferation. BMC Cancer. 13:602013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schultz CR, Golembieski WA, King DA, Brown
SL, Brodie C and Rempel SA: Inhibition of HSP27 alone or in
combination with pAKT inhibition as therapeutic approaches to
target SPARC-induced glioma cell survival. Mol Cancer. 11:202012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rahman M, Chan AP and Tai IT: A peptide of
SPARC interferes with the interaction between caspase8 and Bcl2 to
resensitize chemoresistant tumors and enhance their regression in
vivo. PLoS One. 6:e263902011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fenouille N, Robert G, Tichet M, et al:
The p53/p21Cip1/Waf1 pathway mediates the effects of SPARC on
melanoma cell cycle progression. Pigment Cell Melanoma Res.
24:219–232. 2011. View Article : Google Scholar
|
|
32
|
Jiang Y, Zhang Y, Luan J, et al: Effects
of bufalin on the proliferation of human lung cancer cells and its
molecular mechanisms of action. Cytotechnology. 62:573–583. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu Z, Sun H, Ma G, et al: Bufalin induces
lung cancer cell apoptosis via the inhibition of PI3K/Akt pathway.
Int J Mol Sci. 13:2025–2035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li H, Wang P, Gao Y, et al:
Na+/K+-ATPase α3 mediates sensitivity of
hepatocellular carcinoma cells to bufalin. Oncol Rep. 25:825–830.
2011.
|
|
35
|
Li Y, Chen L, Chan TH, et al: SPOCK1 is
regulated by CHD1L and blocks apoptosis and promotes HCC cell
invasiveness and metastasis in mice. Gastroenterology. 144:179–191.
2013. View Article : Google Scholar
|
|
36
|
Said N, Najwer I and Motamed K: Secreted
protein acidic and rich in cysteine (SPARC) inhibits
integrin-mediated adhesion and growth factor-dependent survival
signaling in ovarian cancer. Am J Pathol. 170:1054–1063. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu H, Xu Y, Chen Y, et al: RNA
interference against SPARC promotes the growth of U-87MG human
malignant glioma cells. Oncol Lett. 2:985–990. 2011.
|
|
38
|
Bhoopathi P, Gondi CS, Gujrati M, Dinh DH
and Lakka SS: SPARC mediates Src-induced disruption of actin
cytoskeleton via inactivation of small GTPases Rho-Rac-Cdc42. Cell
Signal. 23:1978–1987. 2011. View Article : Google Scholar : PubMed/NCBI
|