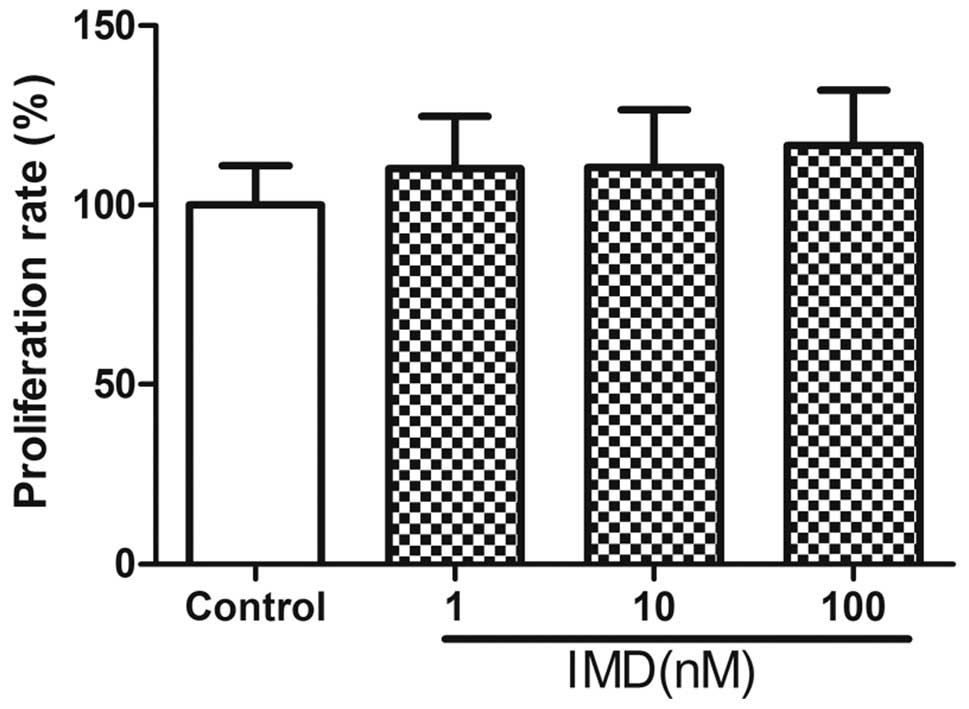
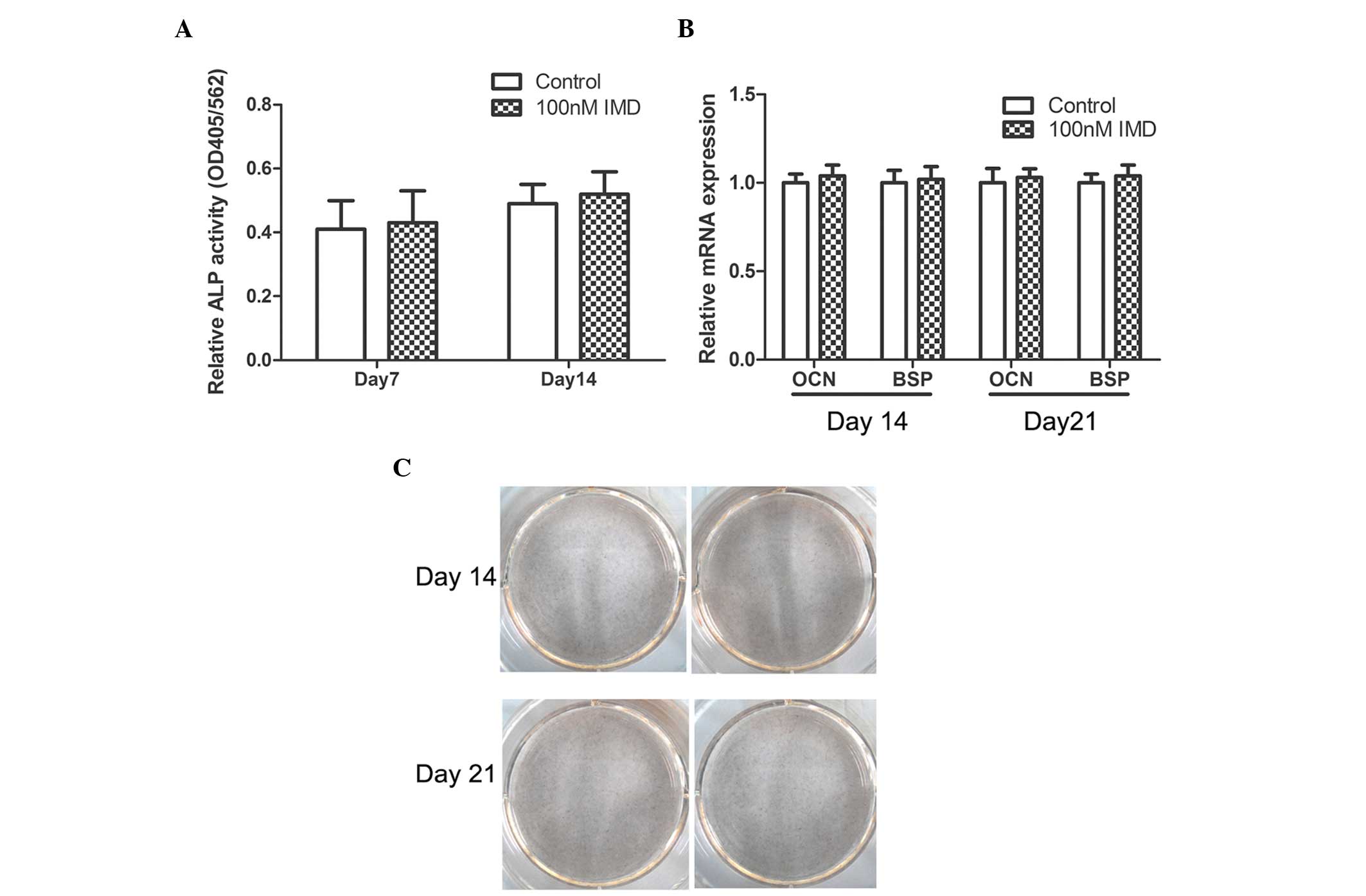
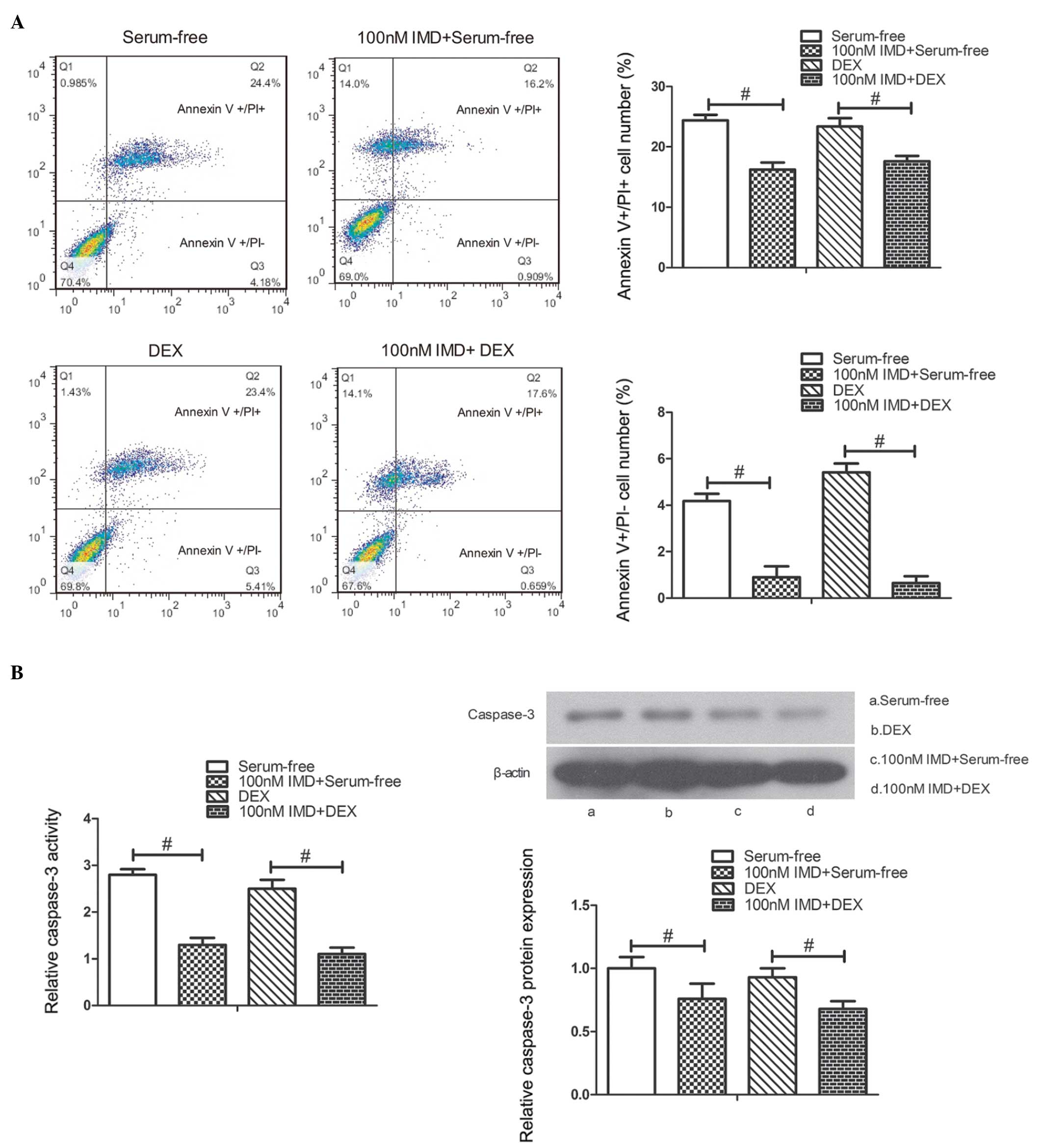
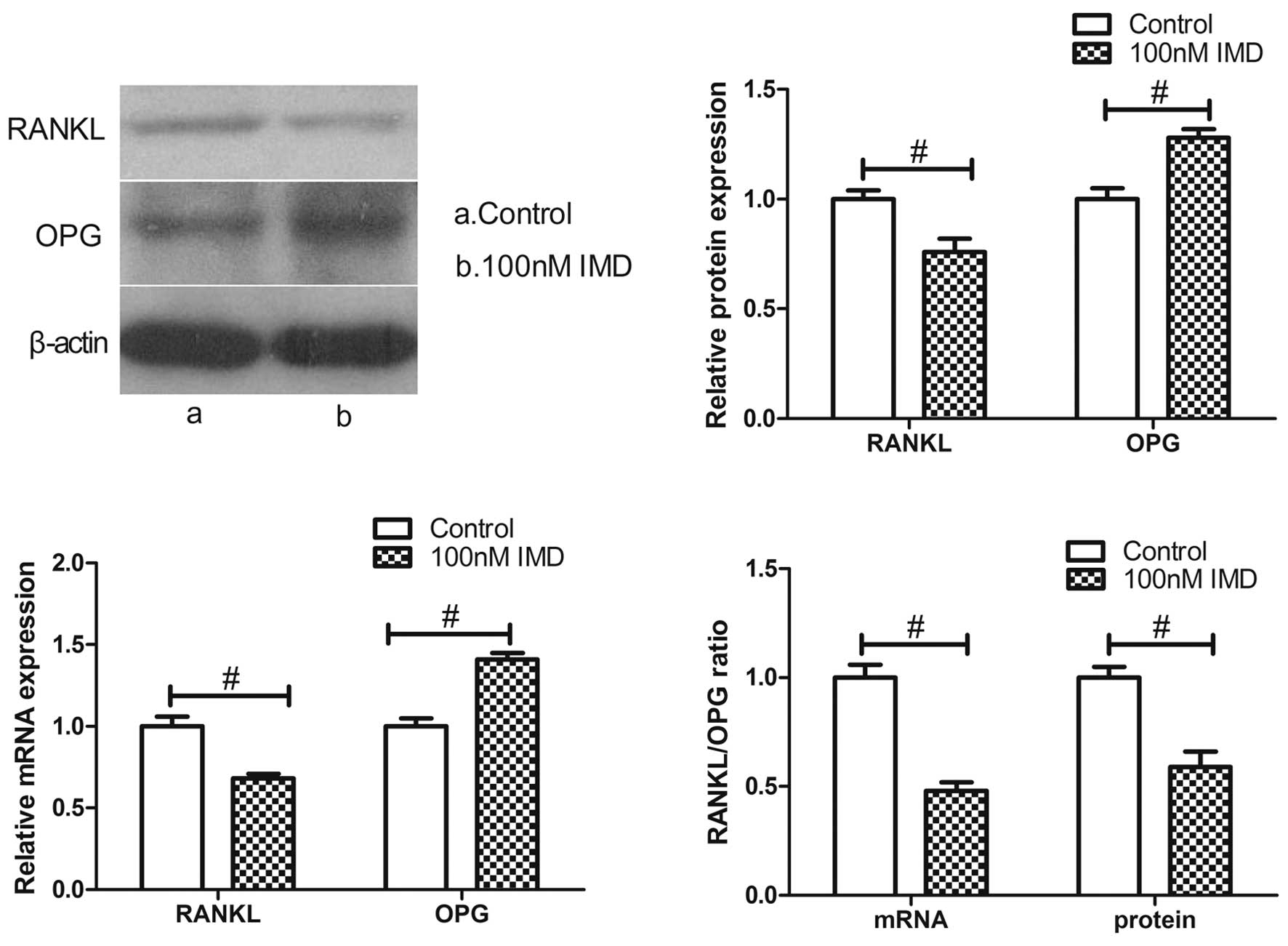
|
1
|
Roh J, Chang CL, Bhalla A, Klein C and Hsu
SY: Intermedin is a calcitonin/calcitonin gene-related peptide
family peptide acting through the calcitonin receptor-like
receptor/receptor activity-modifying protein receptor complexes. J
Biol Chem. 279:7264–7274. 2004. View Article : Google Scholar
|
|
2
|
Takei Y, Inoue K, Ogoshi M, Kawahara T,
Bannai H and Miyano S: Identification of novel adrenomedullin in
mammals: A potent cardiovascular and renal regulator. FEBS Lett.
556:53–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chauhan M, Balakrishnan M, Yallampalli U,
Endsley J, Hankins GD, Theiler R and Yallampalli C: Adrenomedullin
2/intermedin regulates HLA-G in human trophoblasts. Biol Reprod.
85:1232–1239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi K, Kikuchi K, Maruyama Y, Urabe
T, Nakajima K, Sasano H, Imai Y, Murakami O and Totsune K:
Immunocytochemical localization of adrenomedullin 2/intermedin-like
immunore-activity in human hypothalamus, heart and kidney.
Peptides. 27:1383–1389. 2006. View Article : Google Scholar
|
|
5
|
Takahashi K, Morimoto R, Hirose T, Satoh F
and Totsune K: Adrenomedullin 2/intermedin in the
hypothalamo-pituitary-adrenal axis. J Mol Neurosci. 43:182–192.
2011. View Article : Google Scholar
|
|
6
|
Chauhan M, Yallampalli U, Dong YL, Hankins
GD and Yallampalli C: Expression of adrenomedullin 2
(ADM2)/intermedin (IMD) in human placenta: Role in trophoblast
invasion and migration. Biol Reprod. 81:777–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McLatchie LM, Fraser NJ, Main MJ, Wise A,
Brown J, Thompson N, Solari R, Lee MG and Foord SM: RAMPs regulate
the transport and ligand specificity of the
calcitonin-receptor-like receptor. Nature. 393:333–339. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan CS, Yang JH, Cai DY, Zhao J, Gerns H,
Yang J, Chang JK, Tang CS and Qi YF: Cardiovascular effects of
newly discovered peptide intermedin/adrenomedullin 2. Peptides.
26:1640–1646. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujisawa Y, Nagai Y, Miyatake A, Takei Y,
Miura K, Shoukouji T, Nishiyama A, Kimura S and Abe Y: Renal
effects of a new member of adrenomedullin family, adrenomedullin2,
in rats. Eur J Pharmacol. 497:75–80. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holmes D, Campbell M, Harbinson M and Bell
D: Protective effects of intermedin on cardiovascular, pulmonary
and renal diseases: Comparison with adrenomedullin and CGRP. Curr
Protein Pept Sci. 14:294–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Granholm S, Lundberg P and Lerner UH:
Expression of the calcitonin receptor, calcitonin receptor-like
receptor, and receptor activity modifying proteins during
osteoclast differentiation. J Cell Biochem. 104:920–933. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naot D and Cornish J: The role of peptides
and receptors of the calcitonin family in the regulation of bone
metabolism. Bone. 43:813–818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Granholm S, Henning P and Lerner UH:
Comparisons between the effects of calcitonin receptor-stimulating
peptide and intermedin and other peptides in the calcitonin family
on bone resorption and osteoclastogenesis. J Cell Biochem.
112:3300–3312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uzan B, de Vernejoul MC and Cressent M:
RAMPs and CRLR expressions in osteoblastic cells after
dexamethasone treatment. Biochem Biophys Res Commun. 321:802–808.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Hsu WL, Chen CY, Tsauo JY and Yang RS:
Balance control in elderly people with osteoporosis. J Formos Med
Assoc. 113:334–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manolagas SC and Jilka RL: Bone marrow,
cytokines and bone remodeling. Emerging insights into the
pathophysiology of osteoporosis. N Engl J Med. 332:305–311. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brunner M, Jurdic P, Tuckerman JP, Block
MR and Bouvard D: New insights into adhesion signaling in bone
formation. Int Rev Cell Mol Biol. 305:1–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roodman GD: Advances in bone biology: The
osteoclast. Endocr Rev. 17:308–332. 1996.PubMed/NCBI
|
|
20
|
Roux S and Orcel P: Bone loss. Factors
that regulate osteoclast differentiation: An update. Arthritis Res.
2:451–456. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reid IR: Anti-resorptive therapies for
osteoporosis. Semin Cell Dev Biol. 19:473–478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Long F: Building strong bones: Molecular
regulation of the osteoblast lineage. Nat Rev Mol Cell Biol.
13:27–38. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsumura S, Hiranuma H, Deguchi A, Maeda
T, Jikko A and Fuchihata H: Changes in phenotypic expression of
osteoblasts after X irradiation. Radiat Res. 149:463–471. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuguchi T, Chiba N, Bandow K, Kakimoto
K, Masuda A and Ohnishi T: JNK activity is essential for Atf4
expression and late-stage osteoblast differentiation. J Bone Miner
Res. 24:398–410. 2009. View Article : Google Scholar
|
|
25
|
Kim HK, Cho SG, Kim JH, Doan TK, Hu QS,
Ulhaq R, Song EK and Yoon TR: Mevinolin enhances osteogenic genes
(ALP, type I collagen and osteocalcin), CD44, CD47 and CD51
expression during osteogenic differentiation. Life Sci. 84:290–295.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Q, Shao J, Chen W and Li YP:
Osteoclast differentiation and gene regulation. Front Biosci.
12:2519–2529. 2007. View
Article : Google Scholar
|
|
28
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin T, Gooi JH and Sims NA: Molecular
mechanisms in coupling of bone formation to resorption. Crit Rev
Eukaryot Gene Expr. 19:73–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khosla S: Minireview: The OPG/RANKL/RANK
system. Endocrinology. 142:5050–5055. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaji H, Kanatani M, Sugimoto T and Chihara
K: Statins modulate the levels of osteoprotegerin/receptor
activator of NFkappaB ligand mRNA in mouse bone-cell cultures. Horm
Metab Res. 37:589–592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giuliani N, Colla S, Sala R, Moroni M,
Lazzaretti M, La Monica S, Bonomini S, Hojden M, Sammarelli G,
Barillè S, et al: Human myeloma cells stimulate the receptor
activator of nuclear factor-kappa B ligand (RANKL) in T
lymphocytes: A potential role in multiple myeloma bone disease.
Blood. 100:4615–4621. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Udagawa N, Takahashi N, Akatsu T, Tanaka
H, Sasaki T, Nishihara T, Koga T, Martin TJ and Suda T: Origin of
osteoclasts: Mature monocytes and macrophages are capable of
differentiating into osteoclasts under a suitable microenvironment
prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci
USA. 87:7260–7264. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jimi E, Akiyama S, Tsurukai T, Okahashi N,
Kobayashi K, Udagawa N, Nishihara T, Takahashi N and Suda T:
Osteoclast differentiation factor acts as a multifunctional
regulator in murine osteoclast differentiation and function. J
Immunol. 163:434–442. 1999.PubMed/NCBI
|
|
35
|
Abdallah BM, Stilgren LS, Nissen N, Kassem
M, Jorgensen HR and Abrahamsen B: Increased RANKL/OPG mRNA ratio in
iliac bone biopsies from women with hip fractures. Calcif Tissue
Int. 76:90–97. 2005. View Article : Google Scholar
|