Introduction
As the most powerful antigen-presenting cell (APC),
dendritic cells (DCs) have a critical role in the stimulation of
immune tolerance. Upon capturing of antigens, DCs migrate to the
lymph nodes and transfer antigenic epitopes to T cells, which leads
to activation of T cells (1–3). The
surface expression of major histocompatibility complex (MHC) and
co-stimulatory molecules, including CD40, CD80 and CD86, are
upregulated in DCs upon their functional transformation from
'tolerogenic' to 'immunogenic' DCs (4).
The gap junction is a specialized intercellular
connection between a multitude of cell-types of higher organisms.
Connexins (Cx) are members of a diverse family of proteins that are
differentially produced by a number of cell types (5,6). Two
connexin-based hemichannels are coupled to form a gap junction
between two adjacent cells (7,8). Gap
junctions formed by Cx (≤1 kDa in size) have an important role in
transmitting signals between cells of the immune system (9–14).
As one of the principal connexins in the immune system, the
expression of connexin43 (Cx43) is upregulated in human monocytes
and DCs at inflammatory sites to form gap junctions when immune
cells are exposed to inflammatory factors (15). Neijssen et al (16) found that monocytes or DCs
interacted with their environment to source metabolic or electronic
information from the neighboring cells upon detecting inflammation.
These results revealed a novel mechanism of cross-presentation
through gap junctions. Therefore, suppression of Cx43 may be an
important strategy for immune therapy.
RNA interference (RNAi), which sequence-specifically
targets mRNAs with short double-stranded (ds)RNAs, has been
successfully employed in various mammalian cell lines as well as in
primary cells, including T lymphocytes and antigen-presenting
cells. In previous studies, knockdown of various cytokines has
successfully generated desired immune responses (17,18).
DNA vectors, adenoviruses as well as lentiviruses have been widely
applied for small interfering (si) RNA delivery into a variety of
cell systems (19,20). Among the methods for the delivery
of siRNA into DCs, chemical transfection (e.g. using lipid-based
reagents) and viral vectors are routinely used (21,22).
However, chemical transfection reagents are prone to causing
allergic reactions and the application of lentiviral vectors may
result in unspecific immune responses (23). In order to circumvent these
undesired effects, a transfection method exclusively relying on
physical reactions is urgently required to downregulate specific
protein expression. Jantsch et al (24) previously established an
electroporation protocol to deliver siRNA molecules into bone
marrow-derived dendritic cells (BM-DCs) without affecting the
immunoresponse of DCs.
The present study reported on the application an
efficient method of electroporation to knock down Cx43 expression
in BM-DCs by using siRNA. Three prospective dsRNAs targeting Cx43
were applied and reverse-transcription quantitative polymerase
chain reaction (RT-qPCR) was used to identify the dsRNA with the
most potent Cx43 knockdown ability following transfection under
electroporation conditions. After knockdown of Cx43 expression, the
surface antigens of DCs were assessed by flourescence-assisted cell
sorting (FACS) and their capacity to activate T cells was evaluated
using a mixed lymphocyte reaction (MLR). The results of the present
study provided evidence that Cx43 has an important role in the
immunomodulation of DCs.
Materials and methods
Generation of BM-DCs
A total of 20 adult male C57BL/6 and BALB/c mice (2
months old; weight, 25 g), obtained from the Animal Laboratory of
Zhejiang University (Hangzhou, China) were raised under a 12-h
light/dark cycle at 22±1°C, 60±5% humidity, and access to food and
water ad libitum. The present study was approved by the
ethics committee of the College of Medicine, Zhejiang University
(Hangzhou, China).
The animals were sacrificed by decapitation under
anesthesia with 1% sodium pentobarbital (Merck, Darmstadt,
Germany). BM-DCs were generated from bone marrow progenitor cells
according to the protocol of a previous study (25). Briefly, bone marrow cells were
flushed from the femurs and tibias of C57BL/6 mice, followed by
washing and culture in six-well plates (2×106 cells/ml)
in 2 ml RPMI 1640 medium containing 2 mM l-glutamine and 10% fetal calf
serum (FCS) (all from Gibco-BRL, Invitrogen Life Technologies,
Inc., Carlsbad, CA, USA), supplemented with recombinant
Granulocyte-macrophage colony-stimulating factor (GM-CSF; 20 ng/ml;
PeproTech, Rocky Hill, NJ, USA) and recombinant mouse interleukin
(IL)-4 (10 ng/ml; PeproTech). All cultures were incubated at 37°C
in an incubator containing a humidified atmosphere with 5%
CO2. After 48 h of culture, non-adherent granulocytes
were removed by washing and fresh medium was added. DCs were
cultured for six days as immature DCs. Activation with
lipopolysaccharide (LPS; 100 ng/ml; Sigma-Aldrich, St. Louis, MO,
USA) was performed after eight days of culture, and following 48 h,
DCs differentiated into mature DCs (mDC).
siRNA synthesis
siRNA sequences were selected in accordance with a
previously described method (26,27).
Three sequences specific for the Cx43 gene were selected:
Cx43-targeting dsRNA 1 (5′-CCCACCTTTGTGTCTTCCATA-3′), 2
(5′-GCAGATTGAAATCAAGAAGTT-3′) and 3 (5′-CCTGCTGAGAACCTACATCAT-3′).
Non-silencing control siRNA (5′-TTCTCCGAACGTGTCACGT-3′) is an
irrelevant siRNA with random nucleotides. All siRNAs were
synthesized and annealed by Shanghai GeneChem Limited Co.
(Shanghai, China).
Electroporation of DCs
DCs were harvested and washed with RPMI 1640 and
phosphate-buffered saline at room temperature. The cells were
suspended in optimized minimum essential medium (Opti-MEM;
Invitrogen Life Technologies, Inc.) at a concentration of
4×107 cells/ml. 7.5 µg siRNA duplexes were
transferred to a 4-mm cuvette (Tiancheng Company, Hangzhou, China)
and filled up to a final volume of 100 µl with Opti-MEM. 100
µl cell suspension (containing 4×106 cells) was
added and immediately pulsed using a Gene Pulser II apparatus
(Bio-Rad Laboratories, Inc., Hercules, CA, US). Pulse conditions
were 300 V, 150 µF and 100 Ω. Immediately after ~2 sec
electroporation, the cells were transferred into medium
supplemented with GM-CSF and IL-4.
RT-qPCR
After gene silencing, mRNA was extracted from DCs
using TRIzol (Invitrogen Life Technologies, Inc.) according to the
manufacturer's instructions. The SuperScript Pre-amplification
system kit (Invitrogen Life Technologies, Inc.) was used for the
generation of the first-strand cDNA. In brief, 0.5 µg oligo
(dT) (12–18 bp) and 200 U SuperScript-2 reverse transcriptase were
incubated with 2 µg DNA-free total RNA for 50 min at 42°C in
the presence of 0.5 mM desoxynucleotide triphosphate, 10 mM
dithiothreitol and 1X first-strand buffer. The following primers
(Sangon Biotech Co., Ltd., Shanghai, China) were used: Cx43 (519
bp) sense, 5′-CCCCACTCTCACCTATGTCTCC-3′ and anti-sense,
5′-ACTTTTGCCGCCTAGCTATCCC-3′; and GAPDH (172 bp) sense,
5′-ATTCAACGGCACAGTCAAGG-3′ and anti-sense,
5′-GCAGAAGGGGCCGGAGATGA-3′. qPCR was performed on an ABI 7900 PCR
Instrument (PerkinElmer, Inc., Waltham, MA, USA) in a 20 µl
volume, using 5x Universal SYBR Green PCR Master Mix (Takara Bio
Inc., Otsu, Japan). GAPDH was used as an internal control. PCR was
performed based on following protocol: Initial denaturation at 95°C
for 5 min, followed by 30 cycles of denaturation at 95°C for 0.5
min, re-naturation at 60°C for 0.5 min and extension at 72°C for
0.5 min, followed by a final extension at 72°C for 10 min. Results
were obtained from at least three independent experiments performed
in triplicate. The expression levels were quantified using the ΔΔCT
method.
Cell viability assay
The cell viability after electroporation was
determined using Trypan blue staining. Dendritic cells were
collected 48 h after electroporation, stained with Trypan blue
(Beyotime Institute of Biotechnology, Inc., Haimen, China) and
counted using a hematocytometer (Hangzhou Kanna Tech Co. Ltd,
Hangzhou, China). Four different populations of electroporated
cells were generated: i) DC; ii) DC electroporated without siRNA;
iii) DC electroporated with non-silencing control siRNA; iv) DC
electroporated with Cx43-targeting siRNA 2.
Western blot analysis
DCs (4×106) were lysed in 150 µl
lysis buffer containing 10% glycerol, 2 mM EDTA (pH 8.0), 0.5%
Nonidet P-40, 137 mM NaCl and 50 mM Tris-HCl (pH 8.0) (Beyotime
Institute of Biotechnology, Inc.). Protein concentration was
determined using a bicinchoninic acid assay (Beyotime Institute of
Biotechnology, Inc.) and 20 µg protein was separated using
12% SDS-PAGE following transfer onto a nitrocellulose membrane. The
following primary antibodies were used for western blot analysis,
and incubated at 4°C overnight: Mouse anti-Cx43 monoclonal antibody
(mAb; Sigma-Aldrich; 1:8,000) and mouse anti-GAPDH mAb (clone 6c5,
Kang Chen, China; 1:1,000). After incubation with goat anti-mouse
horseradish peroxidase-labeled immunoglobulin G secondary
antibodies (1:10,000, 2 h at room temperature), the blots were
washed three times with phosphate-buffered saline for 10 min,
signal detection was performed using an enhanced chemiluminescence
western blotting substrate (Pierce Biotechnology, Inc., Rockford,
IL, USA), and the blots were scanned using Bio-Rad ChemiDoc XRS
(Bio-Rad Laboratories, Inc.).
Flow cytometric analysis
Phenotypic analysis of isolated or cultured DCs was
performed on a FACScan flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA). All antibodies (fluorescein
isothiocyanate-conjugated anti-mouse CD40 and CD80 antibodies, and
PE-conjugated anti-mouse MHC-II and CD86 antibodies) were purchased
from eBioscience, Inc. (San Diego, CA, USA). DC subsets were
analyzed by means of two- or three-color staining with various
combinations of mouse antibodies. All FACS analyses were performed
using appropriate isotype controls (eBioscience, Inc).
MLR analysis
DCs from the different experimental groups were
treated with 30 µg/ml mitomycin C (Sigma-Aldrich) at 37°C
for 1 h, washed twice with RPMI 1640 and seeded into flat-bottom
96-well culture plates (104/well) for use as stimulator
cells. Recipient T cells (0.2−1×106/well) from BALB/c
mice were added to the DCs in a total volume of 200 µl RPMI
1640 containing 10% FCS, followed by co-culture in a humidified
atmosphere with 5% CO2 at 37°C for 72 h. 3 h prior to
the end of the culture, Cell Counting kit-8 solution (Beyotime
Institute of Biotechnology, Inc.) was added to each well of the
plate (10 µl/well). At the end of the culture, the
absorbance at 450 nm was measured using a microplate reader 3550
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). MLR and RT-qPCR data were
analyzed using one-way analysis of variance followed by the
Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Silencing of Cx43 in DCs
In order to identify an siRNA duplex that is able to
reduce the expression of Cx43, three candidate dsRNAs were
synthesized. Following dsRNA-mediated knockdown with
electroporation, transcripts of Cx43 were detected by RT-qPCR.
After transfection for 24 h, a marked downregulation of Cx43 mRNA
levels in Cx43 dsRNA-transfected cells, but not in scrambled
siRNA-transfected cells, was observed compared to that in the
untreated DCs (Fig. 1).
Cx43-targeting dsRNA 2 was the most effective siRNA to block Cx43
expression at the mRNA level. The protein levels of Cx43 were also
detected in the transfection groups. Consistent with the effects on
Cx43 mRNA expression, Cx43 protein was also significantly reduced
after transfection with Cx43-targeting dsRNA 2 (Fig. 2).
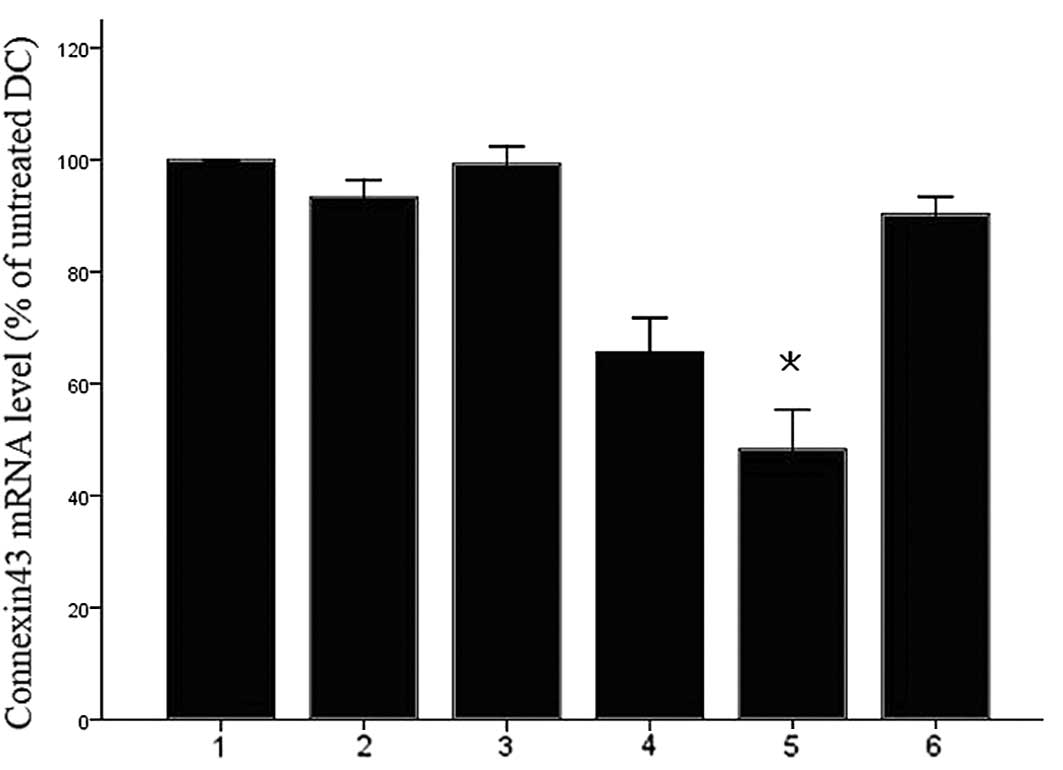 | Figure 1Identification of an efficient and
specific siRNA duplex for connexin43 knockdown. DCs were
electroporated with 7.5 µg of various connexin43-siRNA
duplexes and maturation was induced 4 h after electroporation.
After 24 h of maturation, connexin43 mRNA was quantified by
reverse-transcription quantitative polymerase chain reaction with
GAPDH as an internal control. The normalized ratio of connexin43 to
GAPDH in untreated cells was set as 100%. DCs electroporated
without any siRNA demonstrated no significant downregulation of
connexin43 mRNA. A significant knockdown of connexin43 mRNA by up
to 45% was detected for DC plus connexin43 target 2 dsRNA. Values
are expressed as the mean ± standard deviation of three independent
experiments. *P<0.05 vs. untreated cells. Lanes 1,
DC; 2, DC electroporated without siRNA; 3, DC plus non-silencing
control siRNA; 4, DC connexin43-targeting dsRNA 1; 5, DC plus
connexin43-targeting dsRNA 2; 6, DC plus connexin43-targeting dsRNA
3. siRNA, small interfering RNA; DC, dendritic cells; dsRNA,
double-stranded RNA. |
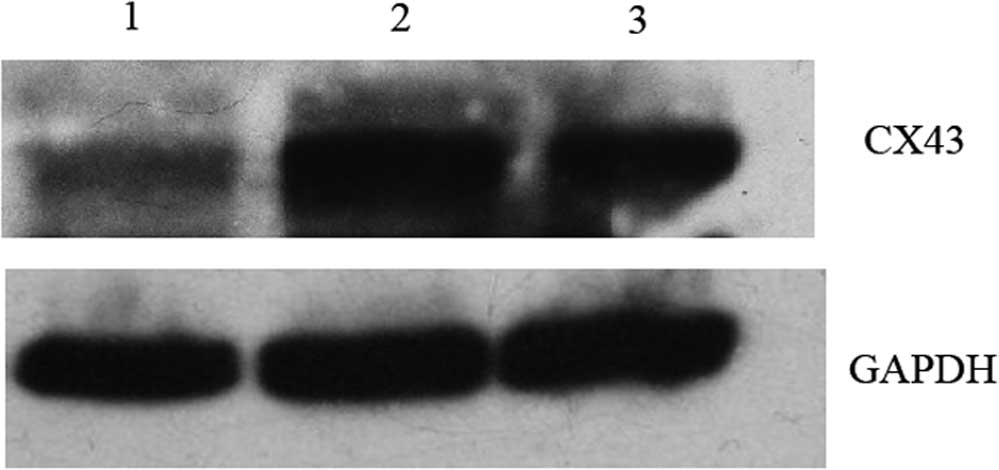 | Figure 2Western blot analysis of Cx43. Effects
of siRNA on Cx43 levels in DC. Lanes: 1, DC plus Cx43-targeting
dsRNA 2; 2, DC; 3, DC electroporated without siRNA. DCs
electroporated with Cx43-targeting dsRNA 2 showed markedly reduced
Cx43 levels when compared with those of untreated and
electroporated cells. After 48 h, the total cellular protein
extract of electro-porated DCs was separated by 12% SDS-PAGE and
Cx43 was detected using an anti-Cx43 Ab. Subsequently, the
membranes were stripped and re-probed with an anti-GAPDH Ab. While
untreated DCs and electroporated DCs showed almost equal expression
levels of Cx43, DCs electroporated with Cx43-targeting 2 dsRNA
showed markedly reduced levels of Cx43. The blots are
representative of two independent experiments with cells from
different mice. DC, dendritic cells; dsRNA, double-stranded RNA;
Cx, connexin; Ab, antibody. |
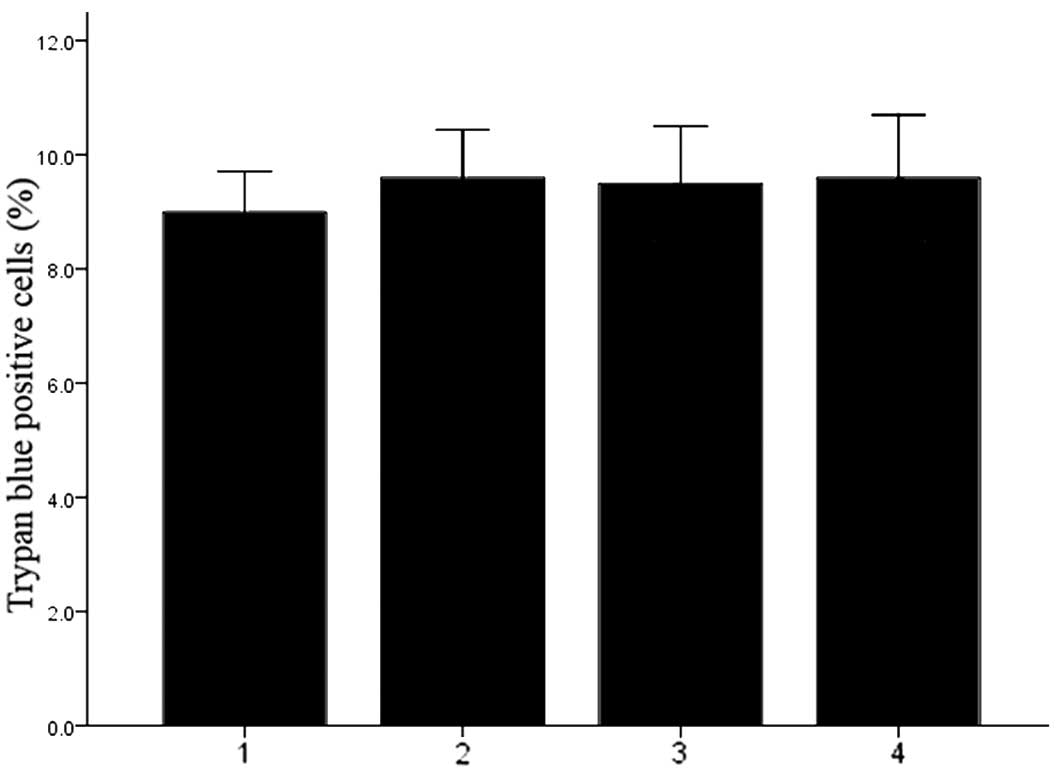
Transfection of DCs with Cx43-targeting
dsRNA under electroporation does not affect cell viability
The viability of DCs was determined by Trypan blue
staining. As shown in Fig. 3, the
viability was similar among all experimental groups, indicating
that electroporation-mediated Cx43 knockdown did not affect the
viability of DCs (P>0.05).
Transfection of DCs with Cx43-targeting
dsRNA under electroporation decrease the expression of surface
antigens
After six days of culture, transfected DCs were
stimulated with LPS for 48 h and subsequently collected. The
expression of surface co-stimulatory molecules was assessed by FACS
analysis. The surface expression of CD40, CD80, MHC-II and CD86 on
untreated DCs, DCs that were electroporated without siRNA and DCs
that were electroporated in the presence of Cx43-targeting dsRNA
was assessed following 48 h. As shown in Fig. 3, the fraction of cells expressing
CD40, MHC-II, CD80 and CD86 was decreased in DCs with Cx43
knockdown compared with that in the other groups.
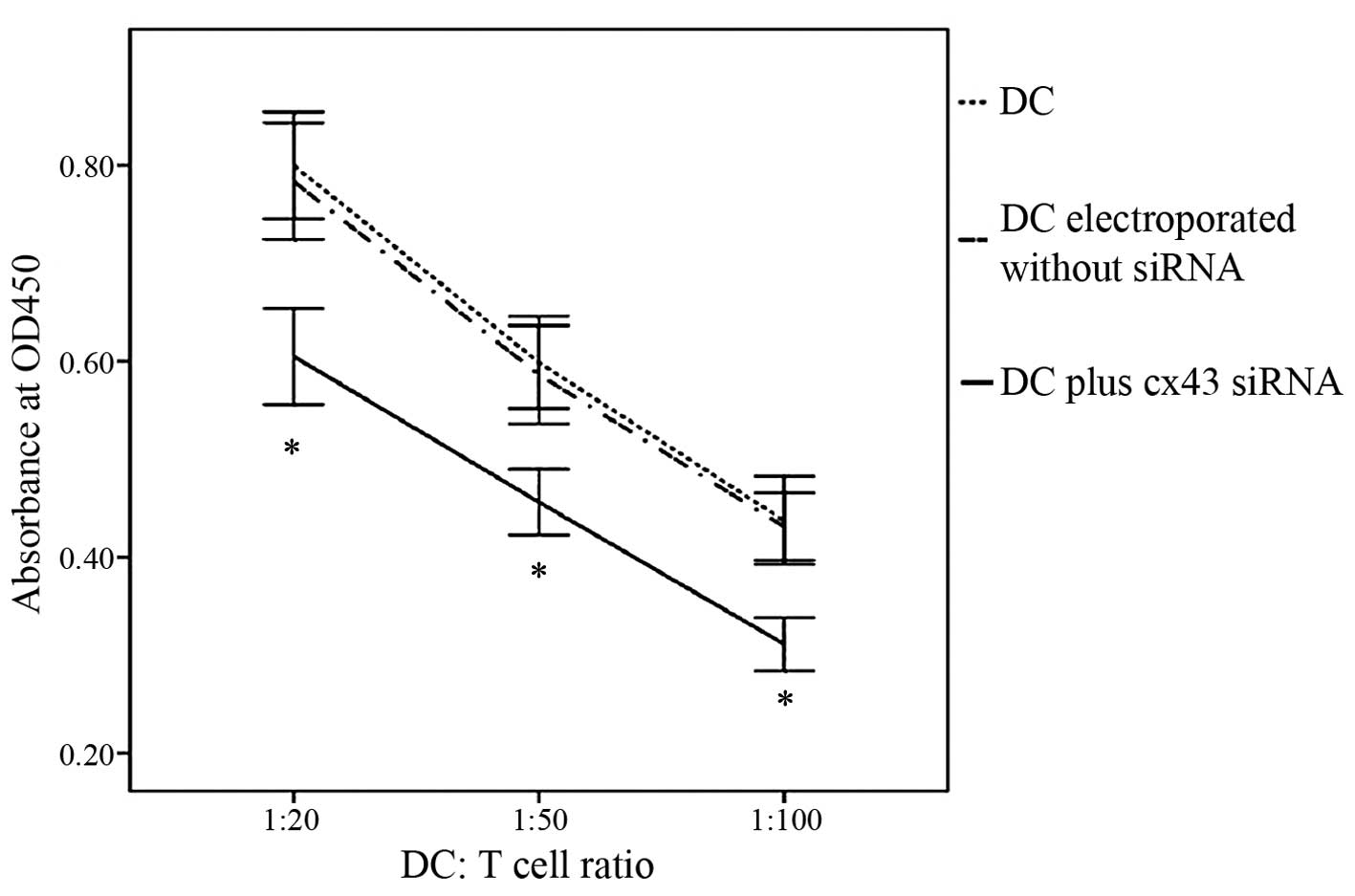
Cx43-knockdown DCs show a reduced
capacity to stimulate T cells
In order to assess whether Cx43 knockdown affected
the stimulatory effects of DCs on T-cell proliferation, an
allogeneic MLR was performed. DCs that were electroporated in the
presence of Cx43-targeting dsRNA 2 exhibited a markedly reduced
capacity to stimulate T-cell proliferation compared with that of
untreated DCs or those subjected to electroporation only (Fig. 5). These results demonstrated that
Cx43 had a specific role in DC-mediated T-cell stimulation.
Discussion
In response to inflammatory reactions, DCs or
monocytes/macrophages upregulate Cx43 expression and form gap
junctions at the inflammation site in order to link and therefore
aggregate cells of identical as well as of different types
(15,16). A recent study by our group further
supported that Cx43 has critical roles in mediating the maturation
of DCs as well as T-cell stimulation.
Mendoza-Naranjo et al (28) reported that gap junction formation
was inhibited by a specific Cx-mimetic peptide, which binds to Cx43
extracellular loop 1 at the plasma membrane surface, which hampered
human DCs from acquiring melanoma antigens from adjacent cells and
inhibited melanoma-specific T-cell activation. Consistently with
these results, gap junction inhibitors also affected the antigen
transfer between human DCs (29,30).
These findings suggested that membrane expression of Cx43 in DCs is
closely associated with antigen transfer. A further study revealed
that Cx43 is required for correcting T-cell maturation as
demonstrated in Cx43-deficient mice (31).
For treatment regimens of various conditions,
including autoimmune diseases, organ transplantation and cancer,
immunomodulation is critical. Since the immunogenic potential of
DCs is among the most important factors in generating potent
cytotoxic T lymphocytes (32), the
development of strategies to suppress DCs is expected to improve
current immunotherapies. siRNA-based knockdown of endogenous
immunostimulatory factors is a promising strategy for modulation of
the immunogenic functions of DCs for use in therapeutic strategies
such as immunosuppression.
The present study described an efficient method to
regulate Cx43 expression via electroporation-mediated siRNA
transfection into immature DCs. A specific siRNA duplex was
identified with the ability to knockdown Cx43 at the mRNA and
protein level. Due to the fact that RNAi is a potent method that
requires only a small number of dsRNA molecules per cell to silence
repression, RNAi technology is superior to anti-sense
oligonucleotide, genetic engineering or antibody-blocking
approaches (32). Of note, under
certain circumstances, siRNAs may directly stimulate immunity by
initiating DC maturation (33,34);
however, this is not a general effect of the siRNA but is dependent
on the sequence of the duplex and is therefore considered to be an
additional biological activity of siRNA. For this reason, these
siRNAs are referred to as immunostimulatory siRNAs (isRNAs)
(33). In the present study, a
marginal increase in Cx43 mRNA expression levels in DCs transfected
with non-silencing siRNA was demonstrated.
APCs acquire information in the form of antigens
from infected cells in their periphery and subsequently migrate to
lymph nodes to specifically stimulate cytotoxic T lymphocytes by
antigen presentation and expression of specific co-stimulatory
molecules, leading to the activation and expansion of the cytotoxic
T-lymphocytes (35,36). Activated monocytes have been
demonstrated to acquire antigenic information in the form of
peptides from influenza-infected cells through the gap junction
(16). The present study showed
that knockdown of Cx43 in DCs significantly reduced their capacity
to stimulate T-cell proliferation, thereby contributing to the
elucidation of the function of Cx43 in DCs during the activation of
T-cell proliferation.
The present study was the first to demonstrate that
RNAi can be successfully applied to block Cx43 expression in DCs.
siRNA-mediated silencing of Cx43 equipped DCs with 'tolerogenic'
properties. This approach may be a promising strategy for
immunotherapy.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 30670866).
References
|
1
|
Hart DN: Dendritic cells: Unique leukocyte
populations which control the primary immune response. Blood.
90:3245–3287. 1997.PubMed/NCBI
|
|
2
|
Shortman K and Liu YJ: Mouse and human
dendritic cell subtypes. Nat Rev Immunol. 2:151–161. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mellman I and Steinman RM: Dendritic
cells: Specialized and regulated antigen processing machines. Cell.
106:255–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steinman RM, Hawiger D and Nussenzweig MC:
Tolerogenic dendritic cells. Annu Rev Immunol. 21:685–711. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bennett MV, Contreras JE, Bukauskas FF and
Sáez JC: New roles for astrocytes: Gap junction hemichannels have
something to communicate. Trends Neurosci. 26:610–617. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saez JC, Berthoud VM, Branes MC, Martinez
AD and Beyer EC: Plasma membrane channels formed by connexins:
Their regulation and functions. Physiol Rev. 83:1359–1400. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harris AL: Emerging issues of connexin
channels: Biophysics fills the gap. Q Rev Biophys. 34:325–472.
2001. View Article : Google Scholar
|
|
8
|
Evans WH, De Vuyst E and Leybaert L: The
gap junction cellular internet: Connexin hemichannels enter the
signalling limelight. Biochem J. 397:1–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krenács T and Rosendaal M:
Immunohistological detection of gap junctions in human lymphoid
tissue: Connexin43 in follicular dendritic and lymphoendothelial
cells. J Histochem Cytochem. 43:1125–1137. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krenacs T and Rosendaal M: Gap-junction
communication pathways in germinal center reactions. Dev Immunol.
6:111–118. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nihei OK, Campos de Carvalho AC, Spray DC,
Savino W and Alves LA: A novel form of cellular communication among
thymic epithelial cells: Intercellular calcium wave propagation. Am
J Physiol Cell Physiol. 285:C1304–C1313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alves LA, Nihei OK, Fonseca PC, Carvalho
AC and Savino W: Gap junction modulation by extracellular signaling
molecules: The thymus model. Braz J Med Biol Res. 33:457–465. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong CW, Christen T and Kwak BR: Connexins
in leukocytes: Shuttling messages? Cardiovasc Res. 62:357–367.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oviedo-Orta E, Errington RJ and Evans WH:
Gap junction intercellular communication during lymphocyte
transendothelial migration. Cell Biol Int. 26:253–263. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eugenín EA, Brañes MC, Berman JW and Sáez
JC: TNF-alpha plus IFN-gamma induce connexin43 expression and
formation of gap junctions between human monocytes/macrophages that
enhance physiological responses. J Immunol. 170:1320–1328. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neijssen J, Herberts C, Drijfhout JW,
Reits E, Janssen L and Neefjes J: Cross-presentation by
intercellular peptide transfer through gap junctions. Nature.
434:83–88. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar P, Ban HS, Kim SS, Wu H, Pearson T,
Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, et al: T
cell-specific siRNA delivery suppresses HIV-1 infection in
humanized mice. Cell. 134:577–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chhabra A, Chakraborty NG and Mukherji B:
Silencing of endogenous IL-10 in human dendritic cells leads to the
generation of an improved CTL response against human melanoma
associated antigenic epitope, MART-1 27–35. Clin Immunol.
126:251–259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Armouche A, Singh J, Naito H,
Wittköpper K, Didié M, Laatsch A, Zimmermann WH and Eschenhagen T:
Adenovirus-delivered short hairpin RNA targeting PKCalpha improves
contractile function in reconstituted heart tissue. J Mol Cell
Cardiol. 43:371–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baba K, Goto-Koshino Y, Mizukoshi F,
Setoguchi-Mukai A, Fujino Y, Ohno K and Tsujimoto H: Inhibition of
the replication of feline immunodeficiency virus by lentiviral
vector-mediated RNA interference in feline cell lines. J Vet Med
Sci. 70:777–783. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu G, Ng H, Akasaki Y, Yuan X, Ehtesham
M, Yin D, Black KL and Yu JS: Small interference RNA modulation of
IL-10 in human monocyte-derived dendritic cells enhances the Th1
response. Eur J Immunol. 34:1680–1687. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, He J and Chang LJ: Alteration of T
cell immunity by lentiviral transduction of human monocyte-derived
dendritic cells. Retrovirology. 1:–37. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prechtel AT, Turza NM, Theodoridis AA,
Kummer M and Steinkasserer A: Small interfering RNA (siRNA)
delivery into monocyte-derived dendritic cells by electroporation.
J Immunol Methods. 311:139–152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jantsch J, Turza N, Volke M, Eckardt KU,
Hensel M, Steinkasserer A, Willam C and Prechtel AT: Small
interfering RNA (siRNA) delivery into murine bone marrow-derived
dendritic cells by electroporation. J Immunol Methods. 337:71–77.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sumimoto H, Tsuji T, Miyoshi H, Hagihara
M, Takada-Yamazaki R, Okamoto S, Ikeda Y, Takahashi T and Kawakami
Y: Rapid and efficient generation of lentivirally gene-modified
dendritic cells from DC progenitors with bone marrow stromal cells.
J Immunol Methods. 271:153–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsue H, Yao J, Matsue K, Nagasaka A,
Sugiyama H, Aoki R, Kitamura M and Shimada S: Gap junction-mediated
intercellular communication between dendritic cells (DCs) is
required for effective activation of DCs. J Immunol. 176:181–190.
2006. View Article : Google Scholar
|
|
28
|
Mendoza-Naranjo A, Saéz PJ, Johansson CC,
Ramírez M, Mandakovic D, Pereda C, López MN, Kiessling R, Sáez JC
and Salazar-Onfray F: Functional gap junctions facilitate melanoma
antigen transfer and cross-presentation between human dendritic
cells. J Immunol. 178:6949–6957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guan X, Cravatt BF, Ehring GR, Hall JE,
Boger DL, Lerner RA and Gilula NB: The sleep-inducing lipid
oleamide deconvolutes gap junction communication and calcium wave
transmission in glial cells. J Cell Biol. 139:1785–1792. 1997.
View Article : Google Scholar
|
|
30
|
Guan X, Wilson S, Schlender KK and Ruch
RJ: Gap-junction disassembly and connexin 43 dephosphorylation
induced by 18 beta-glycyrrhetinic acid. Mol Carcinog. 16:157–164.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Montecino-Rodriguez E, Leathers H and
Dorshkind K: Expression of connexin 43 (Cx43) is critical for
normal hemato-poiesis. Blood. 96:917–924. 2000.PubMed/NCBI
|
|
32
|
Ichim TE, Zhong R and Min WP: Prevention
of allograft rejection by in vitro generated tolerogenic dendritic
cells. Transpl Immunol. 11:295–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hornung V, Guenthner-Biller M, Bourquin C,
Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S,
de Fougerolles A, et al: Sequence-specific potent induction of
IFN-alpha by short interfering RNA in plasmacytoid dendritic cells
through TLR7. Nat Med. 11:263–270. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prechtel AT, Turza NM, Theodoridis AA and
Steinkasserer A: CD83 knockdown in monocyte-derived dendritic cells
by small interfering RNA leads to a diminished T cell stimulation.
J Immunol. 178:5454–5464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Randolph GJ, Sanchez-Schmitz G and Angeli
V: Factors and signals that govern the migration of dendritic cells
via lymphatics: Recent advances. Springer Semin Immunopathol.
26:273–287. 2005. View Article : Google Scholar
|
|
36
|
Sumen C, Mempel TR, Mazo IB and von
Andrian UH: Intravital microscopy: Visualizing immunity in context.
Immunity. 21:315–329. 2004.PubMed/NCBI
|