Introduction
Atherosclerosis (AS) is a pathological process
associated with the majority of cardiocerebrovascular diseases, and
is a predominant cause of morbidity and mortality worldwide
(1). Oxidized low-density
lipoproteins (ox-LDLs) are known to be of importance in the
pathogenesis of AS. However, the precise mechanism underlying LDL
oxidation has yet to be fully elucidated (2).
All three major cell types of the human
atherosclerotic lesions, particularly macrophages are hypothesized
to be the principal mediators of LDL oxidation (3,4).
Macrophages have been shown to modulate LDL oxidation through the
production of reactive oxygen species (5), lipoxygenase (6), and myeloperoxidase (MPO) (7). MPO is a highly cationic protein that
is able to bind to endothelial cells, leukocytes and LDL (2). Sokolov et al (8) concluded that the likely site of
interaction with MPO is the amino acid stretch 445–456 of apoB-100
though mimicking 3 kinds of apoB-100 fragments. Numerous studies
have suggested that MPO adsorbs onto the surface of LDL, promoting
the oxidation of amino acid residues and the formation of oxidized
lipoproteins (9,10).
In vitro, various antioxidants are able to
inhibit LDL oxidation, such as vitamin E and probucol. In
vivo, serum or interstitial fluid is able to markedly inhibit
LDL oxidation by cells due to the presence of vitamin C in human
plasma, interstitial fluid and arterial walls (11). As early as 1990, Heiple et
al (12) hypothesized that
macrophages were able to create a closed compartment on the plasma
membrane and substrate that excludes proteins in the surrounding
medium, thereby protecting cells from external factors. This may
explain the ineffectiveness of antioxidants in clinical therapy as
compared with in vitro studies.
Lipid rafts are membrane microdomains characterized
by a high content of sphingolipids and cholesterol, and a low
content of protein (13). Lipid
rafts have been shown to participate in numerous important steps of
atherogenesis, such as inflammation (14), apo-A1-mediated cholesterol efflux
(15) and the secretion of
pro-inflammatory cytokines by immune cells (16). Lipid rafts in macrophages are
important for vesicular trafficking, transmembrane signal
transduction, protein translocation and cytoskeletal rearrangements
(17). In response to various
stimuli, numerous molecules move into or out of the lipid rafts.
These molecules include, but are not limited to, toll-like receptor
4 (18) and class A scavenger
receptor (19), which affect
macrophage functions. However, the mechanism underlying the effect
of LDL on the translocation and identity of their target molecules
in lipid rafts remains unknown.
The present study demonstrated that native-LDL
promotes lipid raft clustering in macrophages, and identified lipid
raft-associated proteins by label-free quantitative proteomic
analysis, in order to gain further insight into LDL oxidation.
Materials and methods
Materials
Methyl-β-cyclodextr in (MβCD) and anti-neutrophil
myeloperoxidase [MPO; mouse anti-goat polyclonal IgG (H+L)]
antibodies were obtained from Santa Cruz Biotechnology, Inc.
(1:100, cat. no. sc-16129, Dallas, TX, USA), along with lipid-raft
disruptor filipin. Alexa Fluor 488-cholera toxin subunit B (CTXB)
and Alexa Fluor 594-labeled chicken anti-Goat IgG (H+L) secondary
antibody were purchased from Invitrogen (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Anti-flotillin-1 antibody was purchased
from BD Biosciences (1:1000, cat no. 61802; Franklin Lakes, NJ,
USA). Polyclonal anti-ERp29 rabbit anti-mouse antibody was obtained
from Abcam (1:3000, ab11420; Cambridge, MA, USA). Optiprep was
obtained from Axis-Shield, Inc. (Norton, MA, USA). High glucose
Dulbecco's modified Eagle's medium (DMEM) was purchased from GE
Healthcare Life Sciences (Logan, UT, USA). An Enhanced
Chemiluminescence (ECL) kit was obtained from PerkinElmer Inc.
(Waltham, MA, USA). Human LDL was purchased from ProSpec-Tany
TechnoGene, Ltd. (Ness Ziona, Israel).
Cell culture and oxidation of native LDL
in Raw264.7 cells
Raw264.7 mouse macrophages were purchased from the
China Centre for Type Culture Collection (Wuhan, China). The cells
were cultured in high glucose DMEM supplemented with 10% fetal
bovine serum (Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 mg/ml streptomycin (both from Thermo Fisher Scientific,
Inc.) at 37°C and in 5% CO2. When cell density reached
70–80% confluence, the cells were washed three times with
phosphate-buffered saline and pre-incubated for 2 h in serum-free
DMEM for LDL oxidation. The Raw264.7 cell line was incubated with
native-LDL (100 µg/ml) at 37°C for 3, 6, 12 and 24 h.
Cholesterol depletion
To disrupt lipid raft membrane domains, membrane
cholesterol was depleted by treating the Raw264.7 cells with DMEM
supplemented with 5 mM MβCD for 30 min or 100 nM filipin for 15 min
at 37°C.
Thiobarbituric acid assay (TBA)
The TBA assay was used to assess the extent of
cell-mediated LDL oxidation as described previously (20). TBA reacts with malondialdehyde
(MDA) and MDA-like derivatives to form TBA reactive species, which
may be quantified by spectrophotometry at 535 nm using a UV-2000
spectophotometer [UNICO (Shanghai) Scientific Instrument Co.,
Ltd.]. Data are presented as MDA equivalents (nM MDA/mg
protein).
Confocal analysis of lipid rafts and
their colocalization with LDL and MPO in Raw264.7 cells
Detection of lipid rafts was performed as described
previously (21). Briefly, the
Raw264.7 cells were fixed with 4% paraformaldehyde (Sigma-Aldrich)
and double stained with Alexa Fluor 488-CTXB (which attaches to
ganglioside GM1 and thus does not require a second antibody), or
incubated with anti-MPO antibody followed by Alexa
Fluor-594-labeled secondary antibody, prior to being visualized
under a DM6000 confocal microscope (Leica Microsystems GmbH,
Wetzlar, Germany).
Isolation of detergent-free lipid
rafts
Detergent-free rafts were prepared using the
OptiPrep gradient method previously described by Macdonald and Pike
(22). Following centrifugation at
52,000 × g for 90 min, cloudiness was observed throughout the
gradient. A diffuse band was observed about one-third of the way
down the gradient, and a distinct band was apparent at the
interface of the 20% end of the gradient and the 25% OptiPrep
bottom layer. The gradients were fractionated into 0.67 ml
fractions (each in a new tube), and the distribution of various
proteins was assessed by western blotting as described below.
Western blotting
Total protein from each fraction was determined
using a bicinchoninic acid assay kit (Thermo Fisher Scientific,
Inc.). The proteins were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis prior to being
electrophoretically transferred onto nitrocellulose membranes (GE
Healthcare Life Sciences). The membranes were blocked using 5%
non-fat milk in Tris-buffered saline with Tween 20 (TBST) for 1 h,
incubated with primary antibodies against anti-flotillin-1, ERp29
and transferrin (1:2,000; cat. no. ab84036, Abcam) in TBST
overnight at 4°C, followed by incubation with horseradish
peroxidase-conjugated goat anti-rabbit/anti-mouse secondary
antibodies [1:1,000; cat. nos. A0208, A0216; IgG (H+L); Beyotime
Institute of Biotechnology, Haimen, China]. Prior to being washed
three times with TBST. Protein bands were visualized on X-ray film
(Kodak, Rochester, NY, USA) and quantified using Image J software
(version 1.48U; National Institutes of Health, Bethesda, MA,
USA).
Mass spectrometry analysis
In-gel digestion with trypsin was performed as
previously described (23–25). Peptide samples were resuspended in
loading buffer (containing 0.1% formic acid, 0.03% trifluoroacetic
acid and 1% acetonitrile) and loaded onto a 15 cm nano-HPLC column
(internal diameter 100 µm) packed with Reprosil-Pur 120
C18-AQ 1.9 µm beads (Dr Maisch HPLC GmbH, Ammerbuch,
Germany), and eluted for 120 min with 4–80% buffer B reverse phase
gradient (buffer A, 0.1% formic acid and 1% acetonitrile in water;
buffer B, 0.1% formic acid in acetonitrile) generated by a
NanoAcquity UPLC system (Waters Corporation, Milford, MA, USA). The
peptides were ionized with 2.0 kV electrospray ionization voltage
from a nano-ESI source (Thermo Fisher Scientific, Inc.) on a hybrid
LTQ XL Orbitrap mass spectrometer (Thermo Fisher Scientific,
Inc.).
Data-dependent acquisition of centroid MS spectra at
30,000 resolution and MS/MS spectra were obtained in the LTQ
following collision-induced dissociation (collision energy, 35%;
activation Q, 0.25; and activation time, 30 msec) for the top 10
precursor ions, with charge determined by the Sage Sorcerer SEQUEST
(version 3.5; Sage-N Research, Inc., Milpitas, CA, USA) acquisition
software to be z≥2. Dynamic exclusion of peaks already sequenced at
30 sec with early expiration for two count events with
signal-to-noise >2. Automatic gating control was set to 150 msec
maximum injection time. The Sage Sorcerer SEQUEST (version 3.5;
Sage-N Research, Inc.) was used to search and match MS/MS spectra
to a complete semi-tryptic mouse proteome database (NCBI reference
sequence revision 54, with 34,421 target entries; http://www.ncbi.nlm.nih.gov/refseq/) and a
pseudo-reversed decoys sequences (26,27)
with a 20 ppm mass accuracy threshold. Static modifications for
cysteine carbamidomethyl (+57.021465) and dynamic modifications for
oxidized methionine (+15.99492) were included. Only b and y ions
were considered for scoring (Xcorr), and Xcorr along with ΔCn were
dynamically increased for groups of peptides organized by a
combination of trypticity (fully or partial) and precursor ion
charge state in order to remove false positive hits along with
decoys until a false discovery rate (FDR) of <1% was
achieved.
The FDR was estimated by the number of decoy matches
(nd) and total number of assigned matches (nt): FDR=2nd/nt,
assuming mismatches in the original database were the same as in
the decoy database (28).
Quantification of proteins was based on the comparison of extracted
ion current intensities for identified peptides as previously
described (29).
Protein-protein interaction analysis
The upregulated proteins were analyzed using the
Panther classification system (http://www.pantherdb.org/) and were assigned to
various functional groups.
Statistical analysis
All values are presented as the mean ± standard
deviation. Statistical analyses were performed using GraphPad Prism
software (version 5; GraphPad Software, Inc., La Jolla, CA, USA).
The paired, two-tailed Student's t-test was used to analyze the
significance of the difference between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Native-LDL promotes lipid raft clustering
in macrophages
Previous studies have demonstrated that ox-LDL
induced aggregation of gp91phox (30) and Fas (31) in lipid rafts. In order to examine
whether native-LDL induces lipid raft clustering, GM1 ganglioside
(the predominant lipid raft component) was stained using
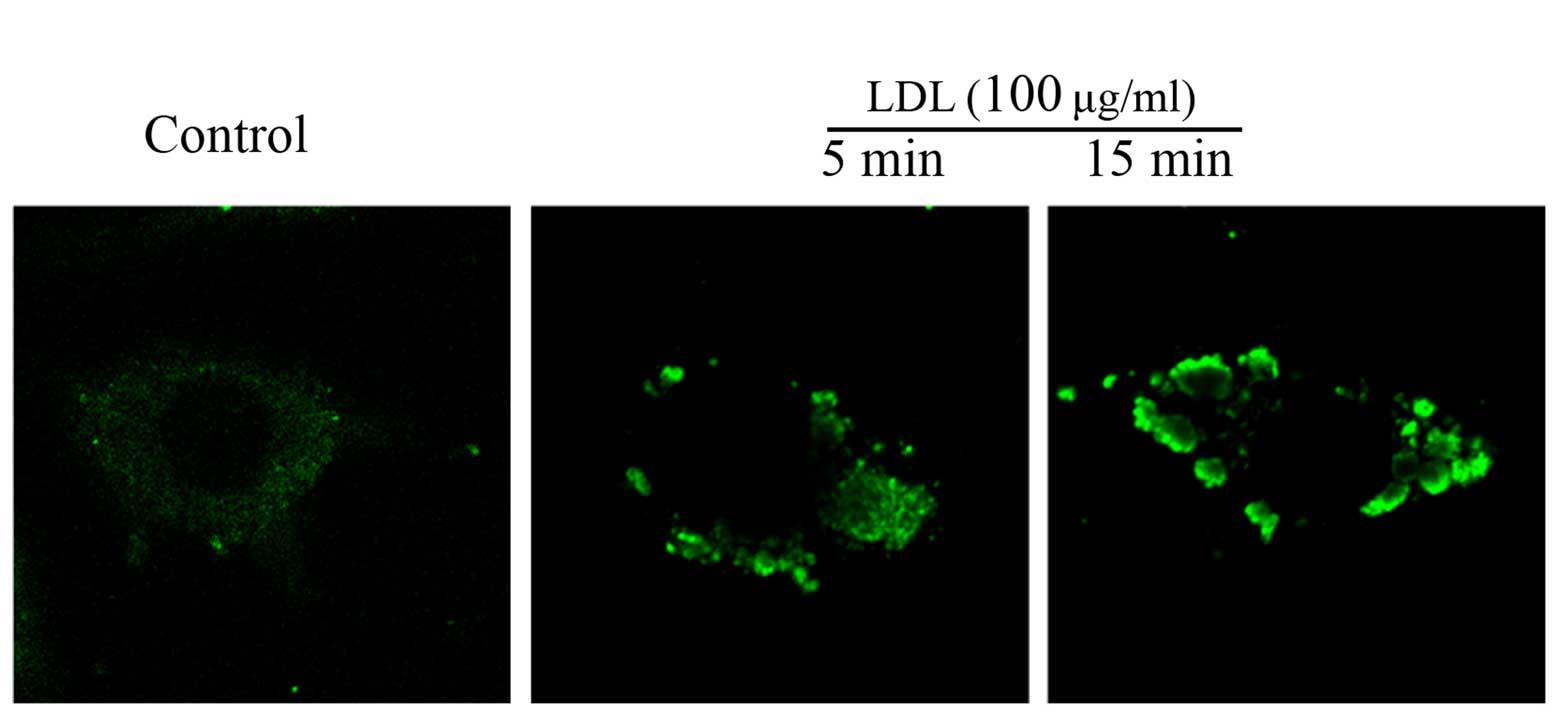
Alexa-488-labeled CTXB. As shown in Fig. 1, native-LDL stimulation caused an
aggregation of GM1, exhibited as green dots or patches. These
results suggest that treatment with native-LDL led to the formation
of lipid rafts in Raw264.7 cells.
Native-LDL-induces aggregation of MPO in
lipid raft clusters
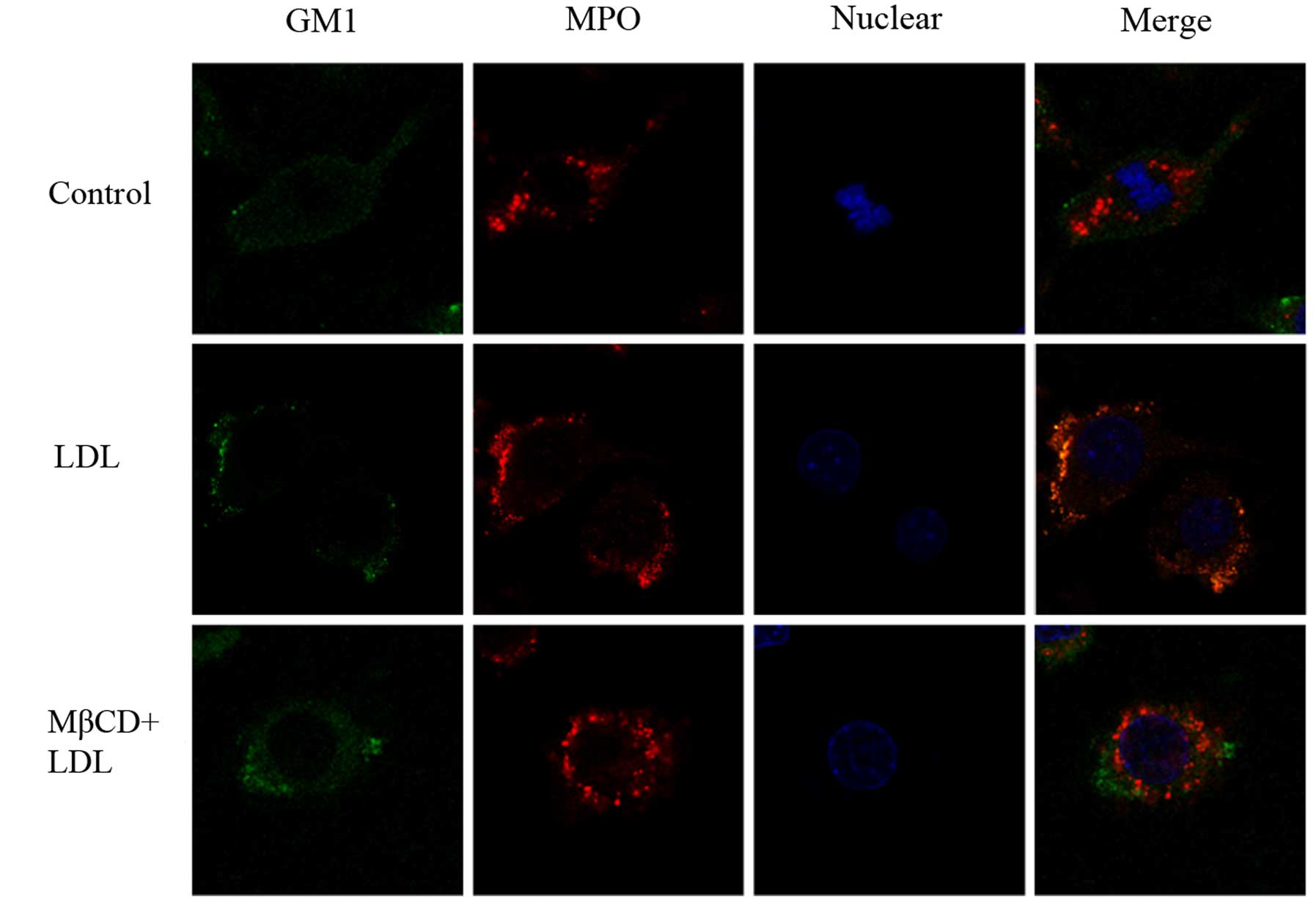
To examine whether MPO was able to aggregate in
lipid raft clusters following native-LDL stimulation, Raw264.7
cells were stained with Alexa-488 CTXB and Alexa-594-conjugated
anti-MPO, and the distribution of MPO in lipid raft clusters was
visualized by confocal microscopy. As shown in Fig. 2, MPO, an important enzyme involved
in the oxidation of LDL, was distributed in both the membrane and
cytosol of cells. After LDL treatment, MPO was redistributed in the
plasma membrane and colocalized with GM1, so LDL increased MPO
translocation into lipid rafts. This colocalization was blocked in
MβCD-treated cells.
Lipid raft disruptors attenuate LDL
oxidation in Raw264.7 cells
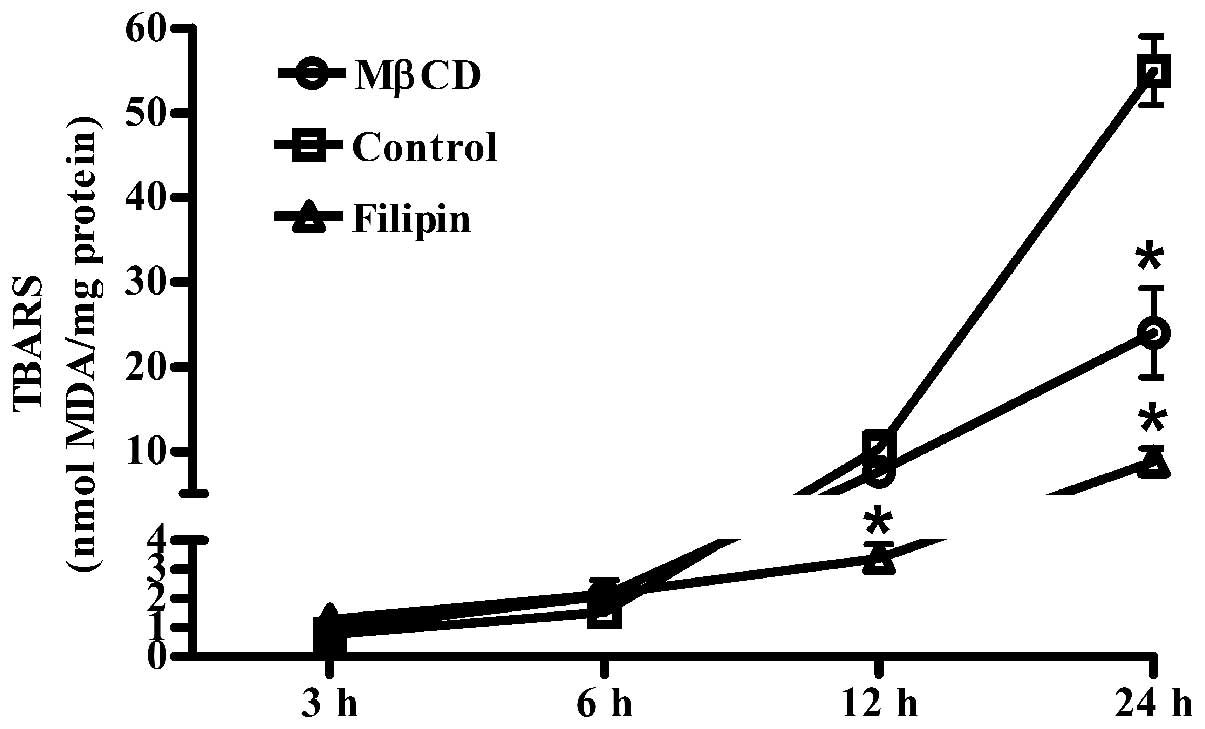
The role of lipid rafts in macrophage-mediated LDL
oxidation was investigated. Fig. 3
shows that Raw264.7 cells oxidized human LDL over a 24 h time
period, which was significantly decreased following treatment with
MβCD and filipin compared with the controls.
Total protein concentration in lipid
rafts was increased following treatment with LDL in Raw264.7
cells
Lipid rafts were isolated and purified from
non-treated and LDL-stimulated Raw264.7 cells using OptiPrep
gradient centrifugation. As shown in Fig. 4, low protein concentration levels
were present in the lipid raft fractions (fraction 6–8). The
highest protein concentration levels were located at the bottom of
the gradient. However, an increase in total protein concentration
levels was observed in fraction 7, and the total protein levels in
the lipid rafts fractions significantly increased following
treatment with LDL (P<0.05).
Quantitative proteomics of lipid
raft-associated proteins induced by LDL in Raw264.7 cells
To identify differentially regulated lipid
raft-associated proteins following LDL stimulation, label-free
quantitative proteomics analysis was performed on lipid raft
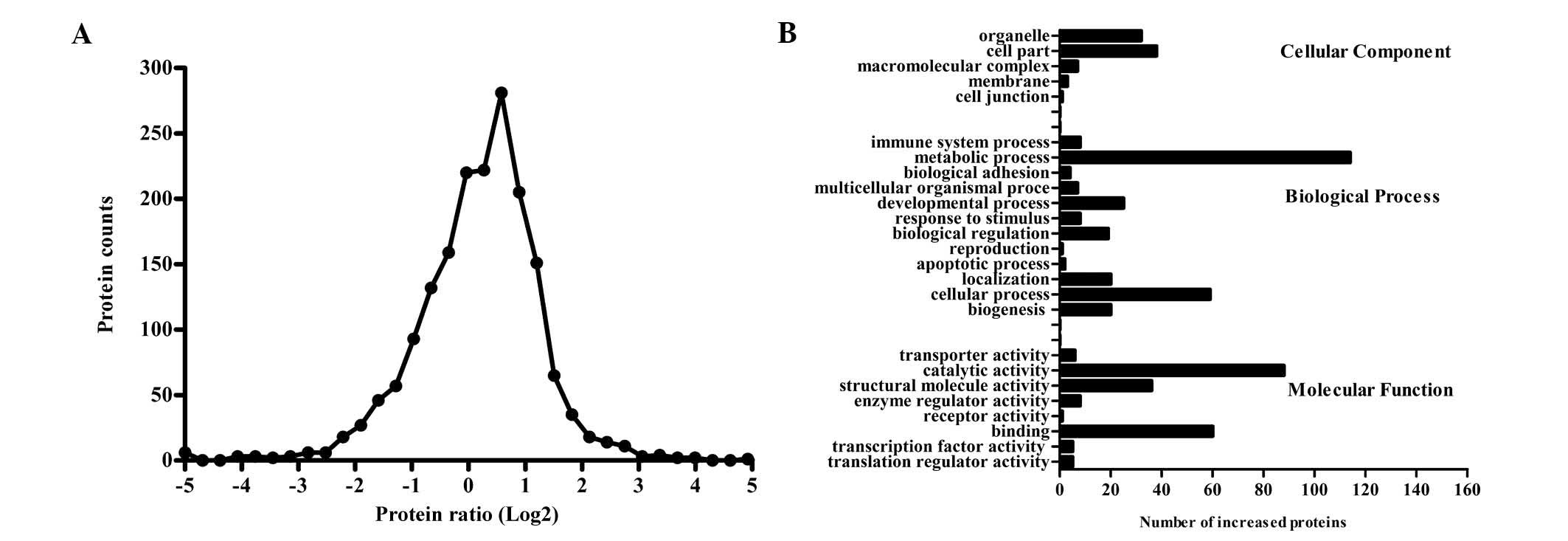
fractions of macrophages. The distribution of the ratios of protein
abundance between the LDL-stimulated macrophages and the resting
state is shown in Fig. 5A. The
present study identified 1,449 proteins with ≥1 unique peptides
with quantifiable abundance measurements, of which many were lipid
raft marker proteins, including flotillins and
glycosylphosphatidylinositol-anchored proteins. From the identified
proteins, a fold change of ≥2 was used to define differential
regulation. Out of the 1,449 proteins, 204 and 203 proteins were
shown to be downregulated and upregulated following LDL
stimulation, respectively. Protein groups were then sorted
according to biological processes, cellular components and
molecular function GO categories (Fig.
5B). Upregulated proteins comprised GO terms associated with
metabolic processes and response to stimuli. Notably, the results
also demonstrated enrichment for biological adhesion, localization,
and enzyme regulator activity. Furthermore, apoB100 was identified
in lipid rafts when the protein spectra were matched to a human
proteome database, which indicated the human origin of LDL.
The 203 upregulated proteins were analyzed using
STRING. Numerous interaction groups were apparent, such as Hmox-1 -
Bax, Pfn - Cap-1. Notably, ERp29 was associated with
calreticulin.
Validation of the label-free
quantification of lipid raft proteins by immunoblotting
To further validate the proteomic identification
results, the expression levels of one of the upregulated proteins,
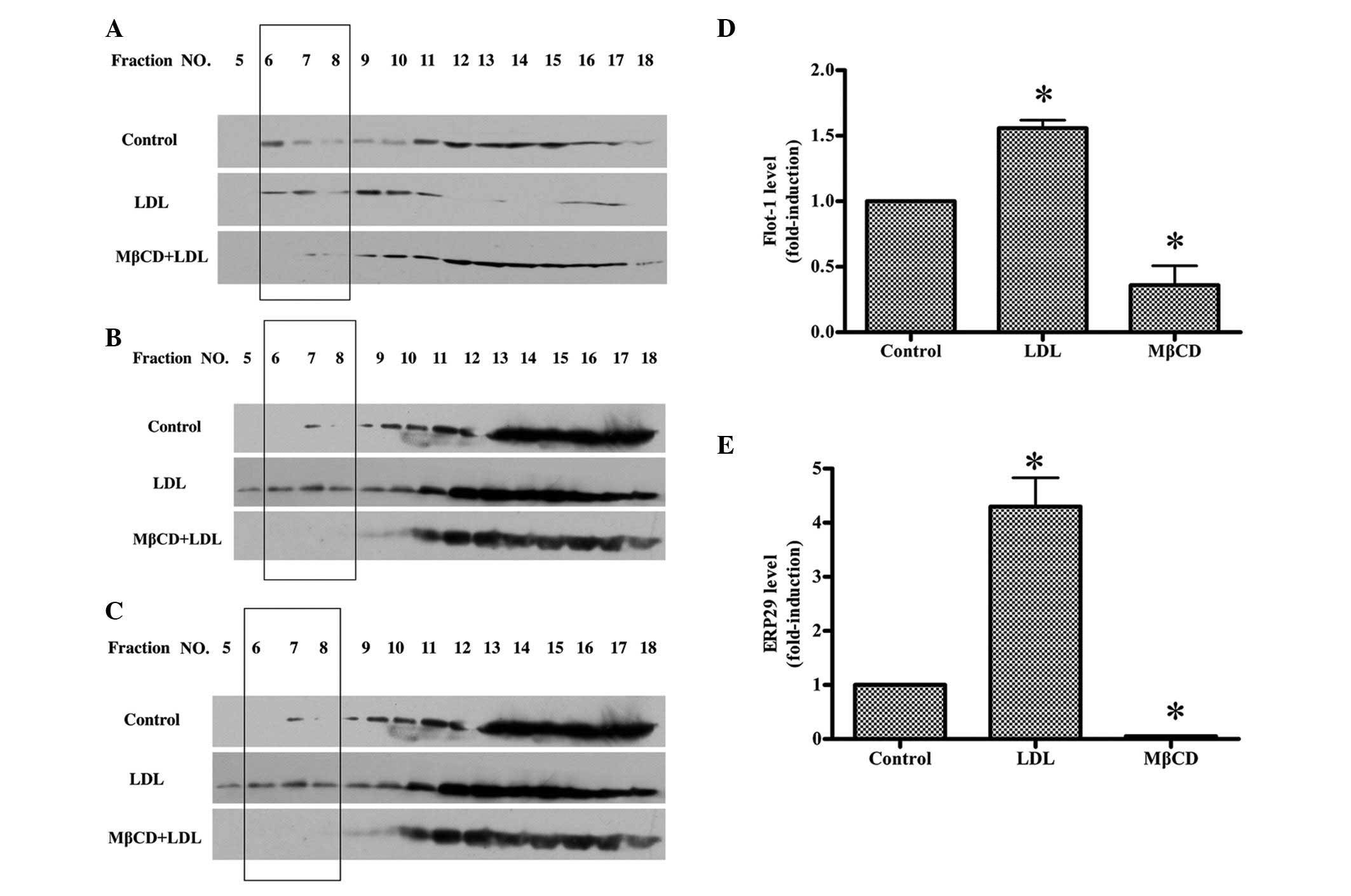
ERp29, were analyzed by western blotting. The floated low density
fractions 6, 7 and 8 (lipid rafts observed one-third of the way
down the gradient) represented the lipid raft fractions following
gradient ultra-centrifugation of the cell lysates. As shown in
Fig. 6A, the proteins from various
fractions were immunoblotted for flotillin-1, a well-documented
marker protein of lipid rafts using an anti-flotillin-1 antibody.
It was demonstrated that flotillin-1 localized not only in lipid
raft fractions, but also in non-raft fractions (high density
fractions). It should be noted that this was not due to
unsuccessful isolation of the lipid raft, but a phenomenon observed
in previous studies (22,32). LDL induced a significant increase
of the flotillin-1 into lipid raft, however, pre-treatment with
MβCD almost entirely disrupted the lipid raft fractions, as
determined by the lack of flotillin-1 in raft fractions. As shown
in Fig. 6B, the expression levels
of ERp29 in lipid rafts were significantly increased following
treatment with LDL. This increase in expression levels was
suppressed by MβCD pre-treatment. Notably, the transferrin
receptor, a marker for the non-raft plasma membrane, was
distributed at the bottom of the gradient (Fig. 6C), indicating that non-raft plasma
membrane did not significantly contaminate the major lipid raft
fractions. Bar graphs show the band density ratio of Flot-1
(Fig. 6D) and ERp29 (Fig. 6E), respectively.
Discussion
It has been demonstrated that ox-LDL-induces lipid
raft-redox signaling in the coronary arterial endothelium (30). The present study demonstrated that
LDL increases the formation of lipid rafts. Numerous changes
occurred in lipid rafts following treatment with LDL in
macrophages.
Oxidation of LDL has an important role in the
pathogenesis of atherosclerosis and vascular diseases. However, the
precise mechanisms underlying the role of LDL remain to be
elucidated, and the enzymes responsible for these mechanisms also
have yet to be identified (2).
Certain studies have suggested that LDL oxidation does not take
place in the blood circulation, and must occur in the arterial wall
due to the fact that blood contains numerous antioxidant molecules
(33,34). However, a previous study recently
reported results that supported the possibility of LDL oxidation in
the circulation (35). There may
be two major ways in which cell-mediated LDL oxidation may occur in
cells: i) Cell oxidative stress activated by microbial infection
causes normal levels of LDL oxidative damage, termed passive
oxidation; and ii) cell oxidative stress activated by high-level
LDL, termed active LDL oxidation (36). The results of the present study
demonstrated that LDL induced lipid raft clustering in macrophages.
These data suggested that LDL signaling may be associated with
lipid rafts.
MPO is an important enzyme in innate immunity and
defense against pathogens (37).
The first study to suggest that MPO is implicated in atherogenesis
was conducted in 1994 (38).
Numerous reactive oxygen species generated by MPO oxidize LDL, and
the interaction between MPO and LDL may enhance LDL oxidation
(39). The present study
demonstrated that MPO was aggregated in lipid rafts following LDL
cultivation with macrophages for 9 h. However, following
pretreatment with MβCD, a lipid raft disruptor, the colocalization
signals on the cell membrane were inhibited. These results
indicated that lipid rafts may be involved in LDL oxidation. To
further demonstrate this hypothesis, two lipid raft disruptors were
used to examine the association between lipid rafts and LDL
oxidation. It was demonstrated that lipid raft disruption
significantly inhibited LDL oxidation by macrophages.
Detergent-free and low density lipid rafts were
isolated from LDL-treated or untreated Raw264.7 cells following
density gradient centrifugation. The data demonstrated that the
detergent-free lipid rafts contained higher protein concentration
levels in the LDL-treated group, compared with the control group.
This suggested that certain proteins may translocate into lipid
rafts under LDL stimulation. To examine the possibility that LDL
induces the recruitment of non-lipid raft proteins in lipid rafts,
label-free quantitative proteomic analysis was undertaken to
profile the lipid raft proteome in control and LDL-treated Raw264.7
macrophages. A total of 203 significantly upregulated lipid
raft-associated proteins were identified. Functional classification
of the identified proteins in the lipid rafts revealed an increase
in proteins involved in biological adhesion, localization, and
enzyme regulator activity. These processes are closely associated
with LDL metabolism, including LDL oxidation.
Endoplasmic reticulum protein 29 (ERp29) is
ubiquitously expressed and has been characterized as a luminal ER
protein (40). The C-terminal
domain of ERp29 contains a novel helical fold which is able to
directly bind certain membrane proteins or hydrophobic secretory
proteins (41). Furthermore, ERp29
is a 4PBA-regulated ER chaperone that regulates wild-type cystic
fibrosis transmembrane conductance regulator (CFTR) biogenesis and
is able to promote F508-CFTR trafficking to the plasma membrane in
CF epithelial cells (42). The
results of the present study demonstrated that the levels of ERp29
increased in macrophage lipid rafts following LDL treatment.
Through its protein binding and protein translocation functions,
ERp29 may participate in LDL oxidation by mediating associated
enzyme secretion or translocation into lipid rafts. A further
possibility is that ERp29 may provide binding sites for LDL or LDL
oxidation-associated proteins in lipid rafts. A previous study
determined by protein-protein interaction analyses that ERp29 was
associated with calreticulin, and the biosynthetic precursor,
apoproMPO, had transient interactions with the molecular chaperone
calreticulin (43). ERp29 may
participate in LDL oxidation through calreticulin and MPO. However,
the precise mechanism underlying ERp29 participation in LDL
oxidation through lipid rafts requires further investigation.
In conclusion, the present study demonstrated that
LDL induced lipid raft clustering in macrophages, and MPO
aggregation into lipid rafts. The inhibitory effect of LDL
oxidation is associated with the disruption of lipid rafts, thus
lipid raft disruptions attenuate LDL oxidation by macrophages.
Label-free quantitative proteomics analysis used in this study
showed that LDL induced the translocation of numerous proteins in
to and out of macrophage lipid rafts. These findings may provide
novel insights into the mechanism underlying LDL oxidation.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
81170273).
Abbreviations:
|
ox-LDL
|
oxidized low-density lipoprotein
|
|
MβCD
|
methyl-β-cyclodextrin
|
|
MPO
|
myeloperoxidase
|
|
ERp29
|
endoplasmic reticulum protein 29
|
References
|
1
|
Zoungas S, McGrath BP, Branley P, Kerr PG,
Muske C, Wolfe R, Atkins RC, Nicholls K, Fraenkel M, Hutchison BG,
et al: Cardiovascular morbidity and mortality in the
Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in
chronic renal failure: A multicenter, randomized, controlled trial.
J Am Coll Cardiol. 47:1108–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshida H and Kisugi R: Mechanisms of LDL
oxidation. Clin Chim Acta. 411:1875–1882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chisolm GM III, Hazen SL, Fox PL and
Cathcart MK: The oxidation of lipoproteins by
monocytes-macrophages. Biochemical and biological mechanisms. J
Biol Chem. 274:25959–25962. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Müller K, Carpenter KL and Mitchinson MJ:
Cell-mediated oxidation of LDL: Comparison of different cell types
of the atherosclerotic lesion. Free Radic Res. 29:207–220. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen K, Thomas SR and Keaney JF Jr: Beyond
LDL oxidation: ROS in vascular signal transduction. Free Radic Biol
Med. 35:117–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sparrow CP, Parthasarathy S and Steinberg
D: Enzymatic modification of low density lipoprotein by purified
lipoxygenase plus phospholipase A2 mimics cell-mediated oxidative
modification. J Lipid Res. 29:745–753. 1988.PubMed/NCBI
|
|
7
|
Malle E, Waeg G, Schreiber R, Gröne EF,
Sattler W and Grone HJ: Immunohistochemical evidence for the
myeloperoxidase/H2O2/halide system in human
atherosclerotic lesions: Colocalization of myeloperoxidase and
hypochlorite-modified proteins. Eur J Biochem. 267:4495–4503. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sokolov AV, Chekanov AV, Kostevich VA,
Aksenov DV, Vasilyev VB and Panasenko OM: Revealing binding sites
for myeloperoxidase on the surface of human low density
lipoproteins. Chem Phys Lipids. 164:49–53. 2011. View Article : Google Scholar
|
|
9
|
Carr AC, Myzak MC, Stocker R, McCall MR
and Frei B: Myeloperoxidase binds to low-density lipoprotein:
Potential implications for atherosclerosis. FEBS Lett. 487:176–180.
2000. View Article : Google Scholar
|
|
10
|
Sokolov AV, Ageeva KV, Cherkalina OS,
Pulina MO, Zakharova ET, Prozorovskii VN, Aksenov DV, Vasilyev VB
and Panasenko OM: Identification and properties of complexes formed
by myeloperoxidase with lipoproteins and ceruloplasmin. Chem Phys
Lipids. 163:347–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leake DS and Rankin SM: The oxidative
modification of low-density lipoproteins by macrophages. Biochem J.
270:741–748. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heiple JM, Wright SD, Allen NS and
Silverstein SC: Macrophages form circular zones of very close
apposition to IgG-coated surfaces. Cell Motil Cytoskeleton.
15:260–270. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simons K and Ikonen E: Functional rafts in
cell membranes. Nature. 387:569–572. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rentero C, Zech T, Quinn CM, Engelhardt K,
Williamson D, Grewal T, Jessup W, Harder T and Gaus K: Functional
implications of plasma membrane condensation for T cell activation.
PLoS One. 3:e22622008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaus K, Kritharides L, Schmitz G,
Boettcher A, Drobnik W, Langmann T, Quinn CM, Death A, Dean RT and
Jessup W: Apolipoprotein A-1 interaction with plasma membrane lipid
rafts controls cholesterol export from macrophages. FASED J.
18:574–576. 2004.
|
|
16
|
Lemaire-Ewing S, Prunet C, Montange T,
Vejux A, Berthier A, Bessède G, Corcos L, Gambert P, Néel D and
Lizard G: Comparison of the cytotoxic, pro-oxidant and
pro-inflammatory characteristics of different oxysterols. Cell Biol
Toxicol. 21:97–114. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brown DA and London E: Structure and
function of sphingolipid- and cholesterol-rich membrane rafts. J
Biol Chem. 275:17221–17224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Islam AS, Beidelschies MA, Huml A and
Greenfield EM: Titanium particles activate toll-like receptor 4
independently of lipid rafts in RAW264.7 murine macrophages. J
Orthop Res. 29:211–217. 2011. View Article : Google Scholar
|
|
19
|
Kiyanagi T, Iwabuchi K, Shimada K, Hirose
K, Miyazaki T, Sumiyoshi K, Iwahara C, Nakayama H, Masuda H, Mokuno
H, et al: Involvement of cholesterol-enriched microdomains in class
A scavenger receptor-mediated responses in human macrophages.
Atherosclerosis. 215:60–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q and Cathcart MK: Selective inhibition
of cytosolic phospholipase A2 in activated human monocytes.
Regulation of superoxide anion production and low density
lipoprotein oxidation. J Biol Chem. 272:2404–2411. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin S, Zhang Y, Yi F and Li PL: Critical
role of lipid raft redox signaling platforms in endostatin-induced
coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol.
28:485–490. 2008. View Article : Google Scholar
|
|
22
|
Macdonald JL and Pike LJ: A simplified
method for the preparation of detergent-free lipid rafts. J Lipid
Res. 46:1061–1067. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dammer EB, Fallini C, Gozal YM, Duong DM,
Rossoll W, Xu P, Lah JJ, Levey AI, Peng J, Bassell GJ and Seyfried
NT: Coaggregation of RNA-binding proteins in a model of TDP-43
proteinopathy with selective RGG motif methylation and a role for
RRM1 ubiquitination. PLoS One. 7:e386582012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seyfried NT, Gozal YM, Donovan LE,
Herskowitz JH, Dammer EB, Xia Q, Ku L, Chang J, Duong DM, Rees HD,
et al: Quantitative analysis of the detergent-insoluble brain
proteome in frontotemporal lobar degeneration using SILAC internal
standards. J Proteome Res. 11:2721–2738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Herskowitz JH, Gozal YM, Duong DM, Dammer
EB, Gearing M, Ye K, Lah JJ, Peng J, Levey AI and Seyfried NT:
Asparaginyl endopeptidase cleaves TDP-43 in brain. Proteomics.
12:2455–2463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu P, Duong DM and Peng J: Systematical
optimization of reverse-phase chromatography for shotgun
proteomics. J Proteome Res. 8:3944–3950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elias JE and Gygi SP: Target-decoy search
strategy for increased confidence in large-scale protein
identifications by mass spectrometry. Nat Methods. 4:207–214. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seyfried NT, Gozal YM, Donovan LE,
Herskowitz JH, Dammer EB, Xia Q, Ku L, Chang J, Duong DM, Rees HD,
et al: Quantitative analysis of the detergent-insoluble brain
proteome in frontotemporal lobar degeneration using silac internal
standards. J Proteome Res. 11:2721–2738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gozal YM, Duong DM, Gearing M, Cheng D,
Hanfelt JJ, Funderburk C, Peng J, Lah JJ and Levey AI: Proteomics
analysis reveals novel components in the detergent-insoluble
subproteome in Alzheimer's disease. J Proteome Res. 8:5069–5079.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei YM, Li X, Xiong J, Abais JM, Xia M,
Boini KM, Zhang Y and Li PL: Attenuation by statins of membrane
raft-redox signaling in coronary arterial endothelium. J Pharmacol
Exp Ther. 345:170–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gajate C and Mollinedo F: The antitumor
ether lipid ET-18-OCH3 induces apoptosis through translocation and
capping of Fas/CD95 into membrane rafts in human leukemic cells.
Blood. 98:3860–3863. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smart EJ, Ying YS, Mineo C and Anderson
RG: A detergent-free method for purifying caveolae membrane from
tissue culture cells. Proc Natl Acad Sci USA. 92:10104–10108. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stocker R and Keaney JF Jr: Role of
oxidative modifications in atherosclerosis. Physiol Rev.
84:1381–1478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Itabe H: Oxidative modification of LDL:
Its pathological role in atherosclerosis. Clin Rev Allergy Immunol.
37:4–11. 2009. View Article : Google Scholar
|
|
35
|
Delporte C, Van Antwerpen P, Vanhamme L,
Roumeguère T and Zouaoui Boudjeltia K: Low-density lipoprotein
modified by myeloperoxidase in inflammatory pathways and clinical
studies. Mediators Inflamm. 2013:9715792013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du F, Ping LY, He CY, Yu H, Cao J and Wu
JZ: Involvement of HNP-1 in different oxidation mechanisms in human
endothelial cells. Eur J Lipid Sci Technol. 113:430–435. 2011.
View Article : Google Scholar
|
|
37
|
Klebanoff SJ, Kettle AJ, Rosen H,
Winterbourn CC and Nauseef WM: Myeloperoxidase: A front-line
defender against phagocytosed microorganisms. J Leukoc Biol.
93:185–198. 2013. View Article : Google Scholar :
|
|
38
|
Daugherty A, Dunn JL, Rateri DL and
Heinecke JW: Myeloperoxidase, a catalyst for lipoprotein oxidation,
is expressed in human atherosclerotic lesions. J Clin Invest.
94:437–444. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carr AC, Myzak MC, Stocker R, McCall MR
and Frei B: Myeloperoxidase binds to low-density lipoprotein:
Potential implications for atherosclerosis. FEBS Lett. 487:176–180.
2000. View Article : Google Scholar
|
|
40
|
Zhang D and Richardson DR: Endoplasmic
reticulum protein 29 (ERp29): An emerging role in cancer. Int J
Biochem Cell Biol. 43:33–36. 2011. View Article : Google Scholar
|
|
41
|
Barak NN, Neumann P, Sevvana M,
Schutkowski M, Naumann K, Malesević M, Reichardt H, Fischer G,
Stubbs MT and Ferrari DM: Crystal structure and functional analysis
of the protein disulfide isomerase-related protein ERp29. J Mol
Biol. 385:1630–1642. 2009. View Article : Google Scholar
|
|
42
|
Suaud L, Miller K, Alvey L, Yan W, Robay
A, Kebler C, Kreindler JL, Guttentag S, Hubbard MJ and Rubenstein
RC: ERp29 regulates DeltaF508 and wild-type cystic fibrosis
transmembrane conductance regulator (CFTR) trafficking to the
plasma membrane in cystic fibrosis (CF) and non-CF epithelial
cells. J Biol Chem. 286:21239–21253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hansson M, Olsson I and Nauseef WM:
Biosynthesis, processing, and sorting of human myeloperoxidase.
Arch Biochem Biophys. 445:214–224. 2006. View Article : Google Scholar
|