Introduction
Patients with type 2 diabetes have a higher risk of
developing atherosclerosis due to dyslipidemia (1), which results in complications,
including cardiovascular disease and stroke. High-density
lipoprotein (HDL) is a critical vascular-protective factor in lipid
metabolism. In addition to reverse cholesterol transportation, HDL
also has other protective functions (2), including promoting nitric oxide (NO)
production and flow-induced vasodilation, stimulating endothelial
cell (EC) proliferation and migration, and preventing EC apoptosis.
These processes are important in neovascularization and vascular
repair. Furthermore, HDL stimulates capillary tube formation in
vitro by increasing p42/44 mitogen-activated protein kinase
(MAPK) activity (3–5). Apoptosis of ECs is triggered by
exposure to inflammatory factors, resulting in disruption of the
endothelial monolayer integrity. HDL inhibits tumor necrosis
factor-α (TNF-α)-induced EC apoptosis (6). However, the effects of HDL may be
altered or reversed, particularly in certain pathophysiological
circumstances (7). Diabetic
(D)-HDL exhibits reduced anti-oxidative ability and an impaired
ability to stimulate NO production in ECs (7–9). In
addition, a previous study demonstrated that HDL isolated from
diabetic patients is dysfunctional in stimulating EC migration and
proliferation due to downregulation of scavenger receptor-B1
(SR-BI) expression (10).
There is an urgent requirement to develop
therapeutic agents that modulate the function of HDL particles, in
addition to increasing HDL-cholesterol (HDL-c) levels. Current
laboratory testing in clinics only provides a direct quantity of
HDL-c protein, however, the antiatherosclerotic functions of HDL
particles change despite no alteration in the level of HDL-c during
certain disease states, including diabetes and coronary heart
disease (11,12). A previous study of cholesteryl
ester transfer protein inhibitor, torcetrapib demonstrated a marked
increase in the HDL-c level, however, increased morbidity and
mortality rates were off-target effects (13). In addition to elucidating the
changed functions of HDL in diabetic states, changes in HDL action
in response to therapeutic strategies targeting the lipoprotein
require investigation.
Herbal medicines are widely administered to treat
diabetes in China. Naoxintong (NXT) is a compound of 16 herbal
medicines (Radix Astragali, Radix Angelicae Sinensis, Radix
Paeoniae Rubra, Radix Salviae Miltiorrhizae, Rhizoma Chuanxiong,
Semen Persicae, Flos Carthami, Resina Olibani, Myrrha, Caulis
Spatholobi, Radix Achyranthis Bidentatae, Ramulus Cinnamomi,
Ramulus Mori, Pheretima, Scorpio and Hirudo), which has been
recognized as a treatment for coronary heart disease, qi
deficiency, blood stasis syndrome (in traditional Chinese medicine)
and cerebrovascular diseases in clinical trials (14). It is an approved therapeutic agent
for stroke by China Food and Drug Administration (15). Furthermore, NXT combined with
aspirin may enhance the antiplatelet effect in patients with
cardio-cerebrovascular diseases (16). In the present study, the HDL level
and its functions in EC proliferation, migration, anti-apoptosis
and angiogenesis during NXT intervention were investigated.
Materials and methods
Study design
Between August 1, 2014 and November 1, 2014, 30
healthy control subjects (35–70 years) and 69 patients who met the
diagnostic criteria (17,18) of type 2 diabetes mellitus (T2DM;
50–80 years) were recruited following informed consent. Control
subjects were healthy individuals without large artery
atherosclerosis, diabetics or hypertension. Carotid ultrasonography
was performed on each patient. A high-resolution B-mode ultrasound
machine (E8, X300PE; GE Healthcare, Little Chalfont, UK) was used
to examine the common carotid arteries. The region from the
beginning of the bifurcation bulb to 15 mm distal was examined.
Atherosclerotic plaques were defined as focal structures
encroaching into the arterial lumen by 0.5 mm or 50% of the
surrounding intima-media thickness (IMT) value or an IMT value
>1.5 mm. Carotid atherosclerosis was defined as the presence of
atherosclerotic plaques in any of the aforementioned arterial
segments. A total of 69 patients were divided into the diabetes
with atherosclerosis group (n=42) and diabetes without
atherosclerosis group (n=27) according to the results of
sonography. The present study was approved by the Institutional
Review Board and the Ethics Committee of Peking University First
Hospital (Beijing, China). The patients were administered NXT
(Shandong Buchang Pharmaceutical Co., Ltd., Xian, China) orally at
a dose of 1.2 g per day for 3 months. Healthy subjects without
cardiovascular risk factors or disease were included in the study
as baseline control and were not subjected to any intervention.
NXT (batch no. Z20025001) is composed of 16 herbs.
The herbal materials were powdered and assembled into capsules.
Five major compounds of NXT were analyzed by ultra-performance
liquid chromatography for intestinal absorption, these were
hydroxysafflor yellow A, paeoniflorin, ferulic acid, salvianolic
acid B and ligustilide (19,20).
Lipoprotein preparation
Following a 12-h fast peripheral venous blood
samples (4 ml) were drawn from the healthy control subjects and the
patients into ethylenediamine tetraacetic acid (EDTA) tubes and
kept in a refrigerator at −80°C prior to the NXT intervention. In
addition, samples were drawn following 90 days of treatment
following a 12-h fast. Plasma (2 ml) was isolated by centrifugation
at 4°C and 1,000 x g for 15 min. HDL (1.063–1.210 g/ml) were
isolated by ultracentrifugation as previously described (21). Briefly, the plasma density was
adjusted to 1.3 g/ml with KBr and normal saline (1.006 g/ml) was
layered over the adjusted plasma to form a discontinuous NaCl/KBr
density gradient. The tubes loaded with the sample and gradient
were placed in a P40ST rotor of an ultracentrifuge (CP70MX;
Hitachi, Tokyo, Japan) and were centrifuged at 350,000 × g for 3.5
h at 4°C. The HDL layer was collected. The purity of HDL was
evaluated by 12% SDS-PAGE and western blot analysis using goat
anti-apoA-I polyclonal antibody (DiaSorin, Stillwater, OK, USA) and
quantified through the measurement of apoA-I content by
nephelometry (Dimension XPand; Dade Behring, Marburg, Germany). HDL
was dialyzed with 0.01 M phosphate-buffered saline (PBS; 0.1%
EDTA-Na2) for 72 h. The purity of HDL and the level of
apolipoprotein A-I (apoA-I) in HDL was determined.
Isolation of human umbilical vein ECs
(HUVECs) and cell culture
HUVECs were isolated from umbilical veins of fresh
umbilical cords as previously described (22). Umbilical cords were donated by
pregnant volunteers (n=3; 26–28 years) following written informed
consent on June 20, 2014 from the Department of Gynecology (Peking
University First Hospital). The umbilical vein was washed with PBS
three times and perfused with 100 U/ml collagenase type IA
(Sigma-Aldrich, St. Louis, MO, USA). Following incubation at 37°C
for 15 min, HUVECs were collected and added to complete EC medium
(ECM; Sciencell Research Laboratories, Carslbad, CA, USA). HUVECs
were resuspended in ECM following centrifugation for 5 min at 25°C
and 100 × g. HUVECs were detached with 0.05% trypsin (Sciencell
Research Laboratories), at a ratio of 1:3. Cells were cultured in a
humidified atmosphere of 5% CO2 at 37°C for 3–5 days
until confluence.
Cell proliferation assay
The bromodeoxyuridine (BrdU; (Roche, Basel,
Switzerland) assay was performed as described previously (15). HUVECs incubated with different
types of HDL at 100 µg/ml apoA-I concentration for 12, 24,
36 or 48 h. The cells were labeled with BrdU labeling solution
(Roche) and then fixed with paraformaldehyde (Sigma-Aldrich).
Following incubation with peroxidase-conjugated anti-BrdU working
solution (Roche) for 90 min at 37°C, the cells were washed with
washing buffer three times and substrate solution,
3,3′,5,5′-tetramethylbenzidine (Roche) was added. The absorbance of
each well was measured at a wavelength of 450 nm with an ELISA
plate reader (Model 550; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Wound healing assay
Wound healing and Transwell assays were used to
analyze HUVEC migration. The procedure was conducted as described
previously (10). The HUVEC
monolayer was scraped using a pipette tip and then incubated with
EC medium containing 1% bovine serum albumin (BSA) alone or with
different types of HDL at an apoA-I concentration of 100
µg/ml for 18 h. Images of the migrated cells were captured
and quantified in 10 random fields (CK40; Olympus Corp., Tokyo,
Japan), and results were repeated for 15 individuals from each
group.
Transwell experiments
Quantitative migration assays of HUVECs were
performed using a modified Boyden chamber (Minicell; EMD Millipore,
Billerica, MA, USA) with an 8.0-µm pore polycarbonate filter
inserted. Cells were treated as described in the wound healing
assay. Following migration for 5 h, migrated cells were fixed and
stained with crystal violet. Migrated cells were visualized in 10
high-powerfields (magnification, ×100), and images were captured
for each chamber and quantified in 5 random fields. Results were
repeated for 10 individuals from each group.
Anti-TNF-α-induced apoptosis assay
HUVECs were seeded at a density of 5×105
cells/well in 6-well plates and cultured to 70% confluence. HUVECs
were pretreated with serum-free ECM with different types of HDL at
100 µg/ml apoA-I concentration for 18 h, and then incubated
in the presence of TNF-α (200 U/ml; BD Biosciences, Franklin Lakes,
NJ, USA) for 24 h. Cells were washed with PBS three times and fixed
with 4% paraformaldehyde for 10 min at room temperature.
Subsequently, cells were washed twice with PBS and stained with
Hoechst 33258 (Beijing TransGen Biotech Co., Ltd., Beijing, China)
for 20 min at room temperature. Following three washes with PBS,
cells were observed using a microscope (LSM 510; Zeiss GmbH, Jena,
Germany).
Caspase-3 activity assay
Caspase-3 activity in the cells was detected using a
Caspase-3 Colorimetric assay kit (Beyotime Institute of
Biotechnology, Haimen, China). Cells were treated as described
above. Cell lysates were centrifuged at 10,000 × g for 15 min at
4°C, the supernatants were collected and the protein concentration
determined by BCA Protein assay kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Cellular extracts (30 µg) were then
incubated in a 96-well microtitre plate with 20 ng Ac-DEVD-pNA
(Beyotime Institute of Biotechnology) for 2 h at 37°C. Caspase
activity was measured by cleavage of the Ac-LEVD-pNA substrate to
pNA, the absorbance of which was measured at a wavelength of 405
nm. Relative caspase activity was calculated as a ratio of emission
of fluorescence of treated cells to untreated cells.
Tube formation assays
The effects of different HDL treatments on in
vitro angiogenesis were assessed by Matrigel tube formation
assay as described previously (23). Each well of pre-chilled 96-well
plates was coated with 50 µl Matrigel (BD Biosciences) and
incubated at 37°C for 30 min for solidification. Following removal
of any fluid, ~4×104 HUVEC cells were seeded and
cultured in free ECM containing different types of HDL at a
concentration of 100 µg/ml apo-AI. Images were digitally
captured using an Olympus microscope (Olympus Corp.) 4 h after
seeding. Tube formation was assessed by counting the length of
tubes from 10 random fields (magnification ×100). Results were
repeated for 10 individuals from each group.
Western blotting analysis
HUVECs were cultured in 12-well plates and treated
with ECM or the various types of HDL at an apoA-I concentration of
100 µg/ml for 15 min for the indicated times. Following
administration of HDL for 15 min, the cells were lysed with
radioimmunoprecipation assay buffer [(Beijing TransGen Biotech Co.,
Ltd.) 50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium
deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 0.1% EDTA and 1%
Triton X-100]. Cell protein samples were separated by
centrifugation (4°C, 10,000 × g for 20 min), and the concentration
was detected by Coomassie brilliant blue (Sigma-Aldrich). The
protein samples (40 µg) were subjected to 10%
SDS-polyacrylamide gel (Beijing TransGen Biotech Co., Ltd.)
electrophoresis (100 V for 120 min) and transferred onto
nitrocellulose membranes (EMD Millipore) according to standard
protocols. The membranes were blocked for 2 h with 1% BSA
containing 0.05% Tween 20 in Tris-buffered saline (Beijing TransGen
Biotech Co., Ltd.). Membranes were incubated with primary
antibodies, including monoclonal rabbit anti-human phosphorylated
(p)-Akt (phospho S47; cat. no. ab81283; Abcam, Cambridge, MA, USA;
1:1,000), monoclonal rabbit p-extracellular signal-regulated kinase
(ERK)1/2 (Cell Signaling Technology, Inc.; 1:1,000; T202/Y204; cat.
no. 4377), monoclonal rabbit anti-ERK1/2 (cat. no. 9102; Cell
Signaling Technology, Inc.; 1:1,000) and rabbit monoclonal
anti-AKT1/2/3 antibody (cat. no. ab179463; Abcam) overnight at 4°C
followed by horseradish peroxidase-conjugated goat-anti-rabbit IgG
secondary antibody (cat. no. SC-2004; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA; 1:1,000). Protein bands were detected by
electrochemiluminescence using the Super Signal West Pico kit
(Pierce Biotechnology, Inc.).
Statistical analysis
All experiments were reproduced in triplicate. In
the majority of experiments, the control sample was defined as
100%, and for each sample, increase or decrease of the percentage
in other samples compared with the control was calculated. Data
were presented as the mean ± standard error of the mean.
Differences were compared with two-tailed Student's t-test using
GraphPad Prism software (version 5.0; GraphPad Software, Inc., La
Jolla, CA, USA) and P<0.05 was considered to indicate a
statistically significant difference.
Result
Participants
All the patients underwent ultrasonography of the
carotid and intracranial arteries. The diabetic patients were
divided into two groups, depending on these ultrasound findings: i)
individuals free of atherosclerotic stenosis, the diabetic group
and ii) individuals with atherosclerotic stenosis, the
diabetic-atherosclerosis group (24). HDLs obtained from diabetic groups
prior to NXT therapy were designated D-HDL baseline (BL) and
D-atherosclerosis (As)-HDL BL. HDL obtained from the two groups
following 3 months of NXT therapy were designated D-HDL M3 and
D-As-HDL M3. Baseline demographic and clinical characteristics of
the diabetic patients and healthy subjects are presented in
Table I. No significant difference
was observed in age, gender, body mass index, MAP and lipid profile
between the diabetic and diabetic-atherosclerosis groups. The NXT
treatment was well-tolerated by all subjects in the two groups and
there were no significant side-effects observed during therapy. All
the patients underwent the anti-diabetic therapy (insulin or oral
drugs, including metformin, sulfonylurea, α-glucosidase and
thiazolidinediones). Statins were prescribed to 5 patients of the
diabetic-atherosclerosis group and 4 of the diabetic group.
Anti-hypertensive therapy was prescribed to 15 patients of the
diabetic-atherosclerosis group and to 14 of the diabetic group.
Characteristics of the patients in the two groups prior to and
following treatment are presented in Table II. Compared with the baseline
characteristics, there were no significant changes observed in the
lipid profile except for the low density lipoprotein (LDL)-c level
in the diabetic-atherosclerosis group, which was decreased
(P=0.043). In addition, the hemoglobin A1c level in patients of the
two groups decreased significantly (P<0.01).
 | Table ICharacteristics of healthy subjects
and diabetic patients. |
Table I
Characteristics of healthy subjects
and diabetic patients.
| Characteristic | Healthy control
(n=30) | Diabetic patients
with atherosclerosis (n=42) | Diabetic patients
without atherosclerosis (n=27) |
|---|
| Age, years | 55.50±1.21 | 66.10±1.29 | 63.31±1.82 |
| Gender,
male/female | 16/14 | 17/13 | 20/10 |
| Body mass index,
kg/m2 | 23.49±0.40 | 24.34±0.31 | 23.96±0.37 |
| Mean arterial
pressure, mmHg | 92.92±0.80 | 99.92±0.91 | 101.70±1.44 |
| Fasting glucose,
mmol/l | 4.65±0.15 | 7.87±0.40a | 8.70±0.61a |
| Hemoglobin A1c,
% | 4.50±0.50 | 9.49±0.20a | 9.05±0.26a |
| LDL cholesterol,
mmol/l | 2.30±0.30 | 2.76±0.15 | 2.74±0.14 |
| HDL cholesterol,
mmol/l | 1.45±0.10 | 1.24±0.06a | 1.22±0.07a |
| Triglycerides,
mmol/l | 1.67±0.35 | 1.98±0.29 | 1.88±0.58 |
| Total cholesterol,
mmol/l | 3.80±0.19 | 4.45±0.19 | 4.54±0.18 |
| Anti-hypertensive
agents | 0/30 | 15/30 | 14/30 |
| Statin therapy | 0/30 | 5/30 | 4/30 |
| Anti-diabetes
therapy | 0/30 | 30/30 | 30/30 |
 | Table IICharacteristics of diabetic patients
prior to and following naoxintong treatment. |
Table II
Characteristics of diabetic patients
prior to and following naoxintong treatment.
| Characteristic | Diabetic patients
with atherosclerosis (n=42)
| Diabetic patients
without atherosclerosis (n=27)
|
|---|
| Prior to | Following | P-value | Prior to | Following | P-value |
|---|
| Fasting glucose,
mmol/l | 7.87±0.40 | 7.06±0.23 | 0.09 | 8.70±0.61 | 7.80±0.51 | 0.27 |
| Hemoglobin A1c,
% | 9.49±0.21 | 7.06±0.23 | <0.01 | 9.05±0.26 | 7.29±0.26 | <0.01 |
| LDL-c, mmol/l | 2.76±0.15 | 2.38±0.10 | 0.04 | 2.74±0.14 | 2.53±0.34 | 0.52 |
| HDL-c, mmol/l | 1.24±0.06 | 1.29±0.05 | 0.19 | 1.22±0.07 | 1.21±0.07 | 0.95 |
| Triglycerides,
mmol/l | 1.98±0.29 | 2.02±0.39 | 0.94 | 1.88±0.58 | 1.63±0.27 | 0.81 |
| Total cholesterol,
mmol/l | 4.45±0.19 | 4.58±0.19 | 0.65 | 4.54±0.18 | 4.05±0.45 | 0.24 |
NXT intervention improves proliferative
effects of HDL on ECs in diabetic patients
The HUVECs were stimulated by different types of HDL
in a time-dependent manner and similar patterns of action were
observed in cell proliferation. Normal (N)-HDL (100 µg/ml
apo-AI) demonstrated an increase in relative cell number for 36 h,
while D-HDL BL and D-As-HDL BL demonstrated diminished increases.
HUVEC proliferation was increased by 27% in the D-HDL M3 group when
compared with the D-HDL BL group at 48 h (Fig. 1). While the D-As-HDL M3 group
demonstrated an increased ability (by 10%) to stimulate cell
proliferation compared with D-As-HDL BL at 48 h (Fig. 1).
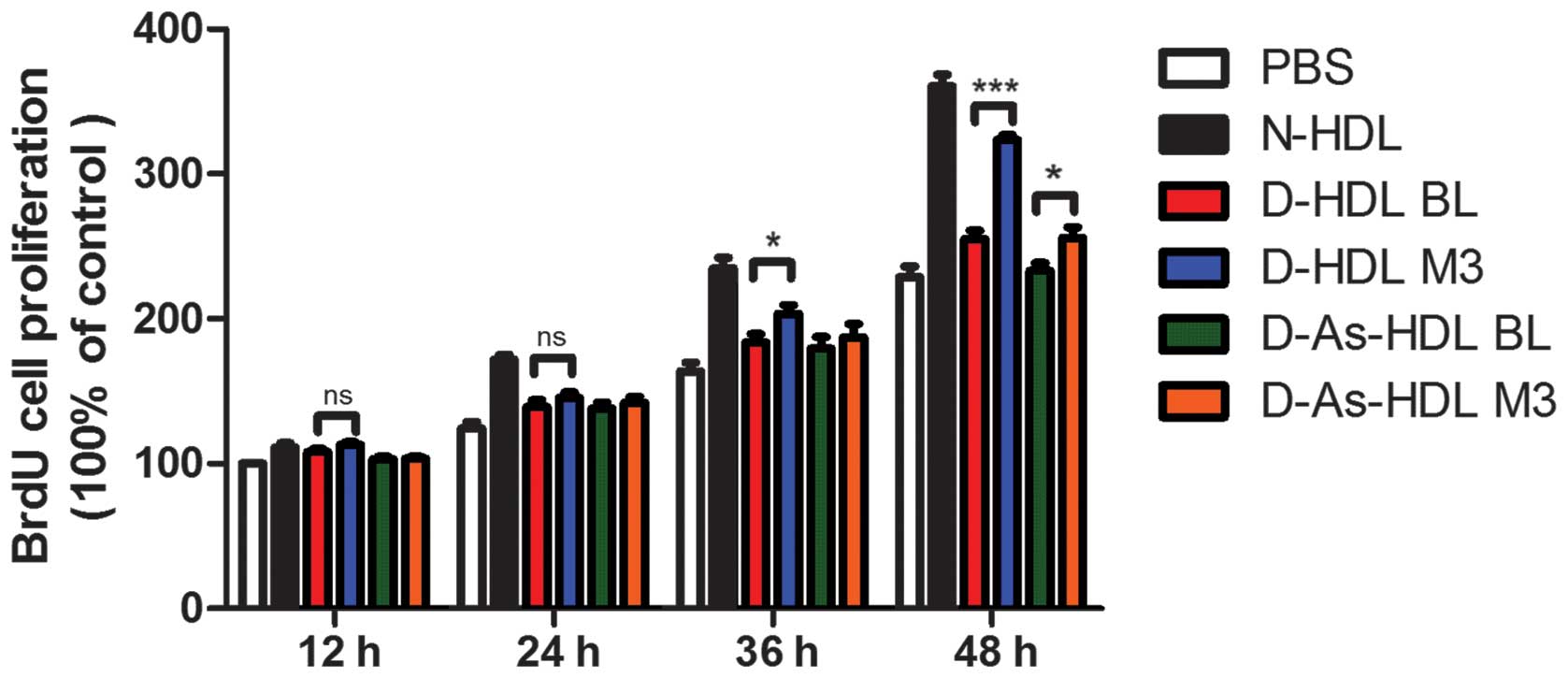 | Figure 1NXT therapy improves proliferative
effects of HDL on endothelial cells in diabetic patients. Human
umbilical vein endothelial cells were treated with N-HDL, D-HDL BL,
D-HDL M3, D-As-HDL BL, D-As-HDL M3 (n=10 each) in 100 mg/ml
apolipoprotein A-I for 12, 24, 36 or 48 h and cell proliferation
was analyzed using a BrdU proliferation assay. Data are presented
as mean ± standard error of the mean; *P<0.05,
***P<0.001 (Student's t-test). NXT, naoxintong; HDL,
high-density lipoprotein; N, normal; D, diabetic; BL, baseline; M3,
3 months of NXT; BrdU, bromodeoxyuridine; PBS, phosphate-buffered
saline; As, atherosclerosis. |
NXT intervention improves
D-HDL-associated HUVEC migration
Wound healing and Transwell assays were performed to
analyze HUVEC migration (Fig. 2).
Compared with N-HDL, D-HDL BL and D-As-HDL BL were less effective
(38 and 50%, respectively) at stimulating HUVEC migration in the
wound healing assay (Fig. 2A and
C). D-HDL M3 stimulated 22% greater cell migration compared
with D-HDL BL, and the D-As-HDL M3 group demonstrated 15% more
migration when compared with D-As-HDL BL group (Fig. 2C).
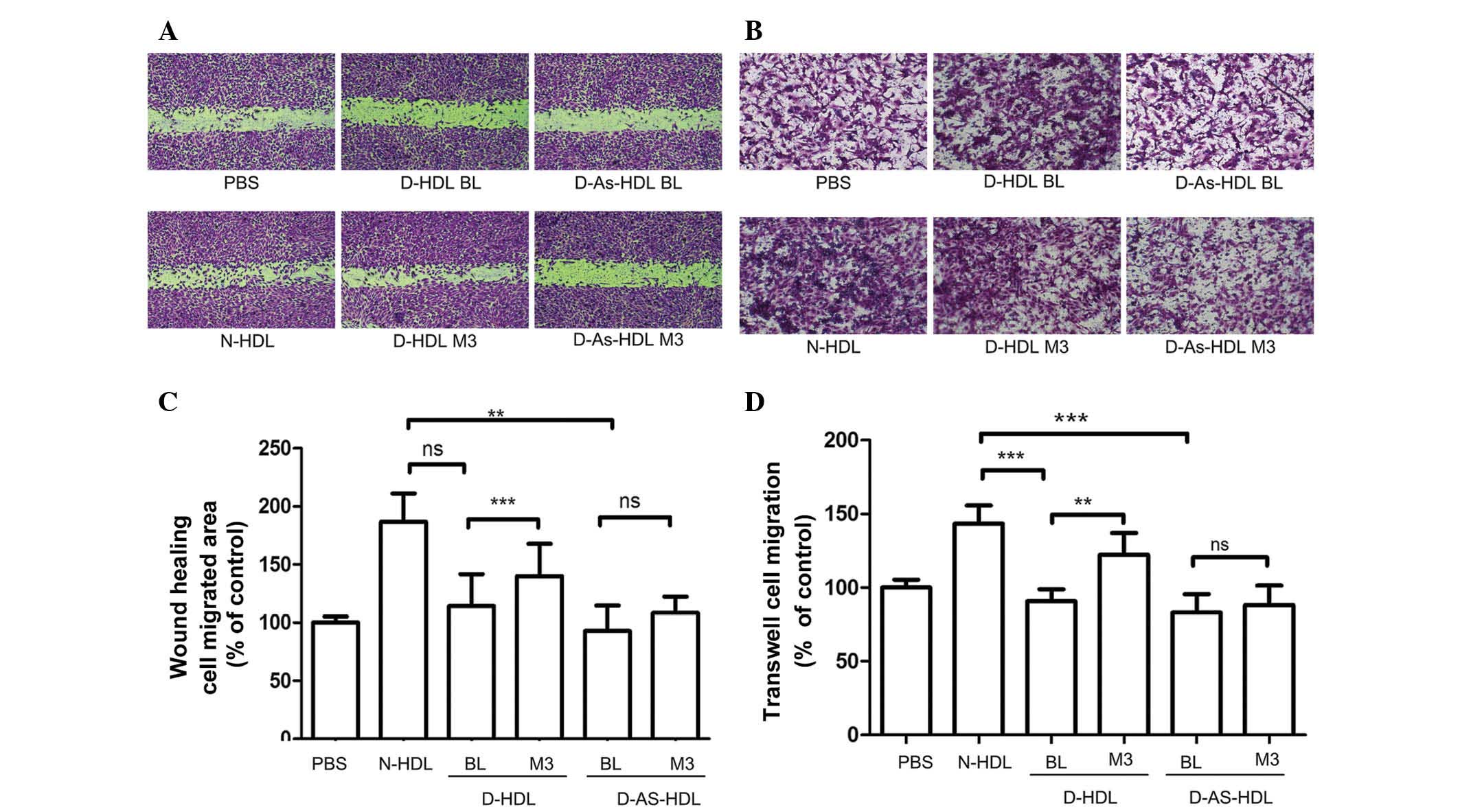 | Figure 2NXT therapy improves the effects of
HDL on endothelial migration in diabetic patients. HUVECs were
treated with N-HDL, D-HDL BL, D-HDL M3, D-As-HDL BL, D-As-HDL M3 in
100 mg/ml apolipoprotein A-I. (A) Representative images of HUVEC
migration under different HDL treatments in the wound healing
assay. (B) Representative images of HUVEC migration under different
HDL treatments in wound healing assay. (C) Quantification of wound
healing assay (n=15 each). (D) Cell migration quantification based
upon an 8-h incubation in the Transwell migration assay (n=10
each). **P<0.01, ***P<0.001 (Student's
t-test). NXT, naoxintong; HDL, high-density lipoprotein; N, normal;
D, diabetic; BL, baseline; M3, 3 months of NXT; PBS,
phosphate-buffered saline; As, atherosclerosis; HUVEC, human
umbilical vein endothelial cell; ns, no significance. |
Results of the Transwell assay were similar. D-HDL
M3 had a greater ability (by 35%) to stimulate cell migration
compared with D-HDL BL (Fig. 2B and
D). The D-As-HDL BL group stimulated less cell proliferation
than the D-As-HDL M3 group (Fig.
2D).
NXT therapy improves the
endothelial-associated angiogenesis effects of HDL
Compared with N-HDL, D-HDL BL and D-As-HDL BL were
51 and 61%, respectively, less effective in tube formation
(Fig. 3A and B). Following 3
months of NXT therapy, the D-HDL M3 and D-As-HDL M3 groups
demonstrated increased ability to stimulate cell tube formation
(increase of 54 and 30%, respectively) when compared with the D-HDL
BL and D-As-HDL BL groups (Fig.
3B).
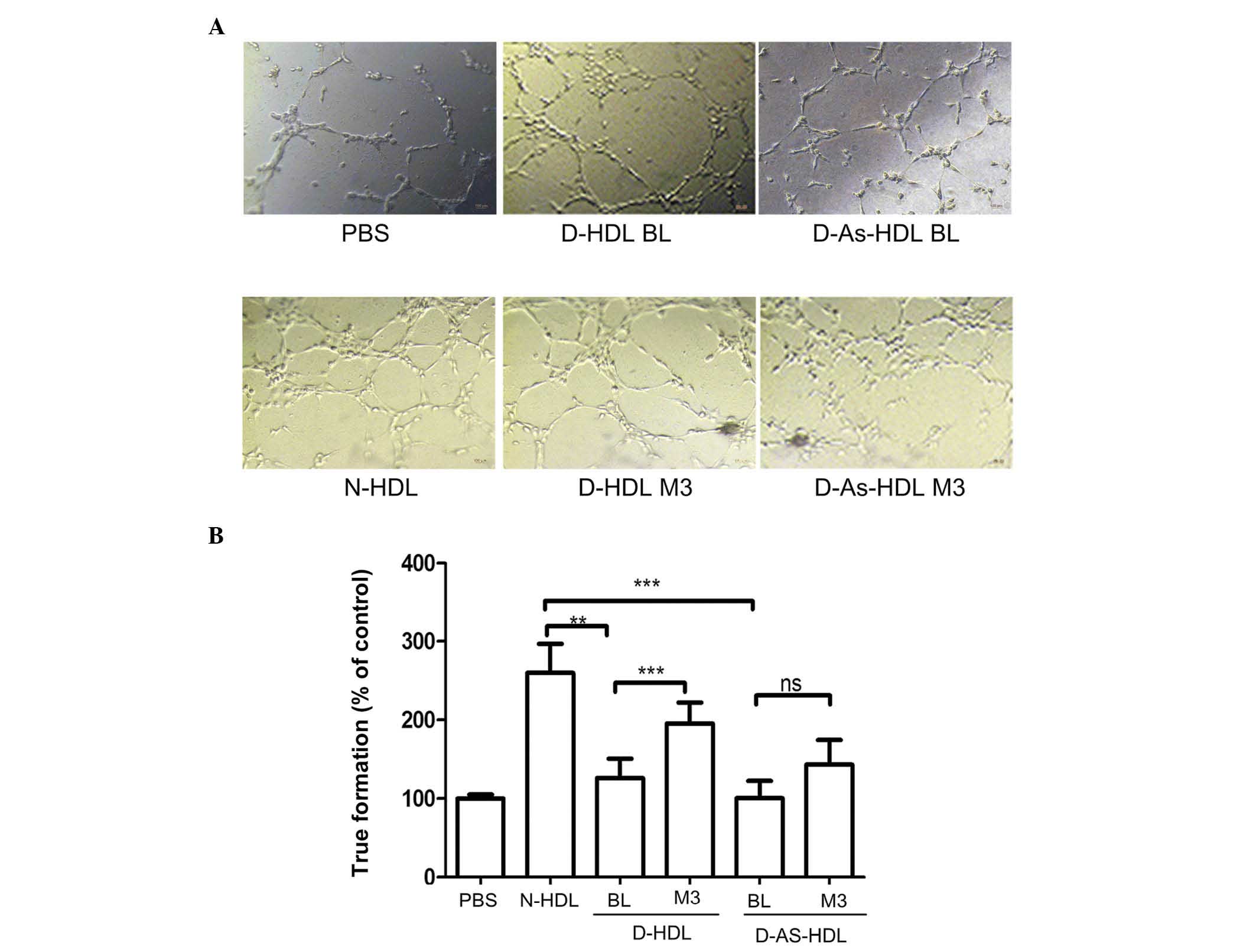 | Figure 3NXT therapy improves the
endothelial-associated angiogenesis effect of HDL in diabetic
patients. (A) N-HDL, D-HDL BL, D-HDL M3, D-As-HDL BL, D-As-HDL M3
was added to each well in 100 mg/ml apolipoprotein A-I and images
of tube formation were captured after 3 h. (B) The tube formation
was evaluated by counting 10 random fields (magnification, ×100).
**P<0.01, ***P<0.001 (Student's
t-test). NXT, naoxintong; HDL, high-density lipoprotein; N, normal;
D, diabetic; BL, baseline; M3, 3 months of NXT; PBS,
phosphate-buffered saline; As, atherosclerosis; ns, no
significance. |
NXT therapy improves the anti-apoptotic
effects of D-HDL
HUVECs exhibited marked apoptosis following TNF-α
treatment. N-HDL treatment reduced rates of apoptosis by 45%
(Fig. 4A and B). D-HDL BL and
D-As-HDL BL demonstrated reduced ability to protect HUVECs from
apoptosis; D-HDL BL and D-As-HDL BL were 48 and 35%, respectively,
less effective when compared with N-HDL (Fig. 4B). Following 3 months of NXT
therapy, the D-HDL M3 group exhibited an increased ability (by 34%)
to inhibit cell apoptosis when compared with D-HDL BL (Fig. 4B). While, the increasing ability in
inhibiting cell apoptosis of D-As-HDL M3 was 23% (Fig. 4B).
 | Figure 4NXT therapy improves the
anti-apoptotic effects of D-HDL for HUVECs following TNF-α
treatment. HUVECs were pre-incubated with N-HDL, D-HDL BL, D-HDL
M3, D-As-HDL BL, D-As-HDL M3 in 100 mg/ml apolipoprotein A-I for 18
h. TNF-α was added to each well and incubated for 24 h. (A)
Representative images of apoptotic cells stained by Hoechst 33258
(objective lens magnification, ×100). (B) The apoptotic cells were
evaluated by counting 10 random fields. (C) Caspase-3 activity in
cell lysates was determined. (n=6). *P<0.05,
**P<0.01 (Student's t-test). NXT, naoxintong; HDL,
high-density lipoprotein; HUVECs, human umbilical vein endothelial
cells; TNF-α, tumor necrosis factor-α; N, normal; D, diabetic; BL,
baseline; M3, 3 months of NXT; PBS, phosphate-buffered saline; As,
atherosclerosis; ns, no significance. |
HUVECs treated with N-HDL + TNF-α exhibited 48% less
caspase-3 activity in cell lysates than cells treated with TNF-α
alone (Fig. 4C). The D-HDL BL and
D-As-HDL BL pretreatment groups exhibited 72 and 64%, respectively
more caspase-3 activity when compared with N-HDL (Fig. 4C). Following the 3-month NXT
therapy, the D-HDL M3 group exhibited 53% less caspase-3 activity
when compared with D-HDL BL, while no change in the caspase-3
activity level was exhibited in the D-As-HDL M3 group (Fig. 4C).
Diminished capacity of D-HDL to activate
Akt and ERK1/2 phosphorylation was abrogated by NXT therapy
Akt and ERK1/2 phosphorylation had been demonstrated
to be important in signal transduction for EC proliferation,
migration and angiogenesis (25,26).
The present study analyzed whether NXT therapy increased D-HDL
function by increasing Akt and ERK1/2 phosphorylation (Fig. 5). Compared with N-HDL, D-HDL BL and
D-As-HDL BL demonstrated reduced phosphorylation of Akt (D-HDL BL,
28%; D-As-HDL BL, 51%). HUVECs stimulated by D-HDL M3 exhibited 36%
more Akt phosphorylation than those stimulated by D-HDL BL
(Fig. 5A and C). HUVECs stimulated
by D-HDL M3 exhibited 26% more Akt phosphorylation than those
stimulated by D-HDL BL. However, no significant difference in Akt
phosphorylation was observed between HUVECs stimulated by D-As-HDL
M3 and those stimulated by D-As-HDL BL (Fig. 5A and C).
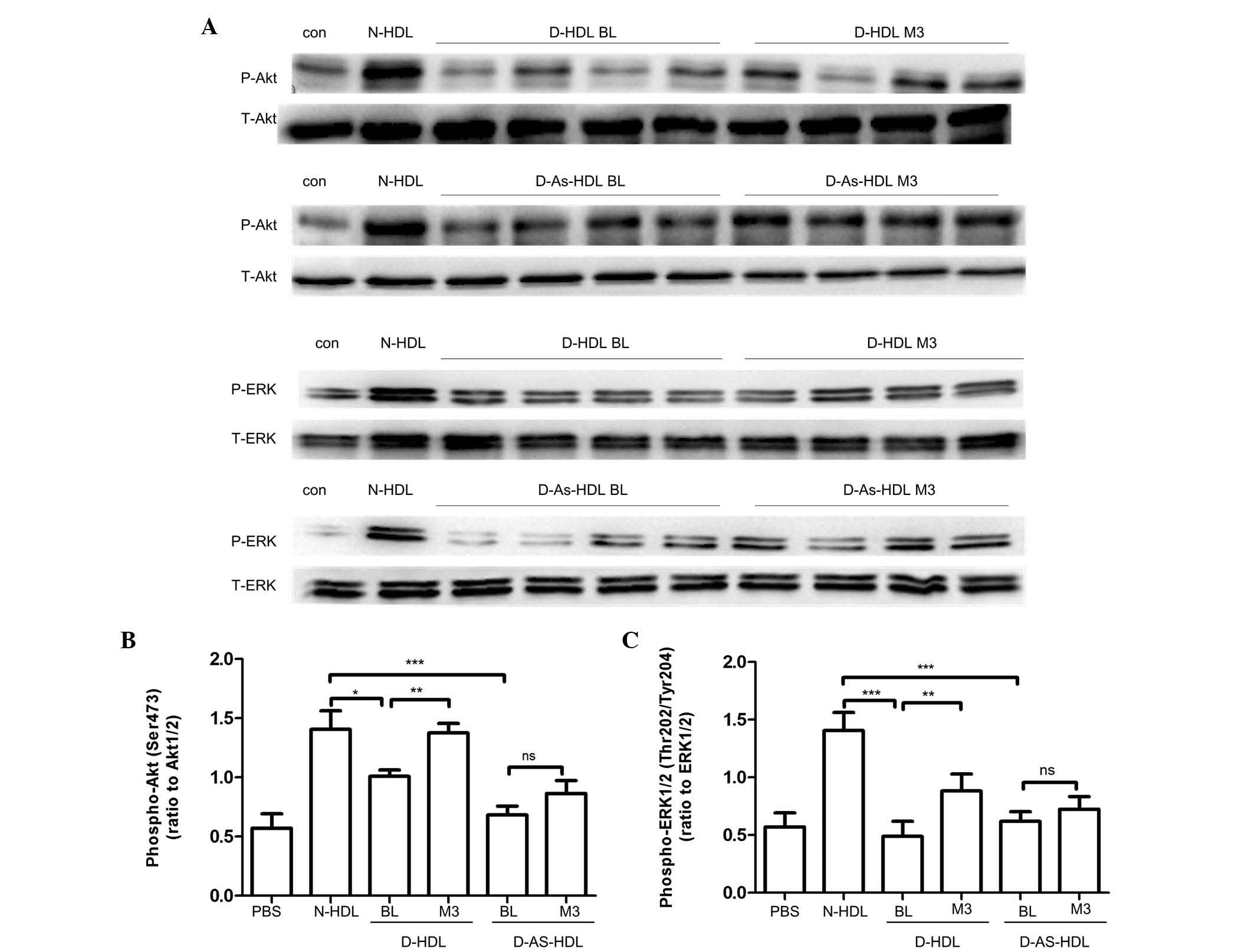 | Figure 5Diminished capacity to activate
phosphatidylinositol-4,5-bisphosphate 3-kinase/Akt and MAPK/ERK
signaling pathways of D-HDL was reversed by NXT therapy. (A) HUVECs
were treated with N-HDL, D-HDL BL, D-HDL M3, D-As-HDL BL, D-As-HDL
M3 in 100 mg/ml apolipoprotein A-I for 10 min. Cell lysates were
analyzed by western blotting using anti-p-Akt (Ser473) antibody,
anti-p-ERK1/2 (Thr202/Tyr204) antibody, anti-total-Akt antibody,
and anti-total-ERK1/2 antibody. Density of the (B) p-Akt and (C)
p-ERK1/2 bands was normalized to the total-Akt and total-ERK1/2
bands, respectively. *P<0.05, **P<0.01,
***P<0.001 (Student's t-test). HDL, high-density
lipoprotein; NXT, naoxintong; HUVECs, human umbilical vein
endothelial cells; N, normal; D, diabetic; BL, baseline; M3, 3
months of NXT; PBS, phosphate-buffered saline; As, atherosclerosis;
ns, no significance; p, phosphorylated; ERK, extracellular
signal-regulated kinase. |
ERK1/2 phosphorylation was also observed. Similarly,
compared with N-HDL, D-HDL BL and D-As-HDL BL reduced
phosphorylation of ERK1/2 by 34 and 26%, respectively (Fig. 5B and D). HUVECs stimulated by D-HDL
M3 demonstrated 80% greater ERK1/2 phosphorylation than those
stimulated by D-HDL BL (Fig. 5B and
D). HUVECs stimulated with D-As-HDL M3 exhibited 16% more
ERK1/2 phosphorylation when compared with those stimulated by
D-As-HDL BL (Fig. 5B and D).
Discussion
The present study indicated that HDL from patients
with T2DM exhibited a reduced capacity to stimulate EC
proliferation, migration, and anti-apoptotic and angiogenesis
functions, which are markedly different from the effects observed
with HDL from healthy subjects. Furthermore, HDL from patients with
T2DM at early and late stages of the disease present a progressive
loss of ability. Notably, NXT therapy improved the protective
properties of HDL isolated from diabetic patients on endothelial
function as, following NXT therapy, HDL from diabetic patients
exhibited an improved capacity to stimulate EC proliferation and
migration. In addition, HUVECs treated with D-HDL M3 and D-As-HDL
M3 demonstrated increased Akt and ERK phosphorylation, which may
explain the increase in migration. Therefore, NXT therapy may be a
promising strategy for restoring the direct protective functions of
HDL on ECs, rather than increasing HDL plasma levels.
HDL protects against atherosclerosis by reverse
cholesterol transportation, anti-inflammatory and anti-oxidative
effects, and endothelial protection. However, it has previously
been observed that the effects of HDL are heterogeneous (27). Modifying HDL with myeloperoxidase
improves the proliferative and migratory functions of ECs (28). Furthermore, HDL from coronary heart
disease patients exert a pro-inflammatory effect rather than an
anti-inflammatory effect (27).
HDL particles undergo modifications during the development of
diabetes and the function of D-HDL is controversial (11). D-HDL loses the ability to inhibit
oxidized LDL-induced vascular damage (29). The abnormalities of D-HDL are
triglyceride enrichment (30,31)
and glycation of apoA-I (32).
However, regarding the benefits of the phospholipid components of
HDL, HDL obtained from early-stage diabetes patients exerts
enhanced effect in activating the cyclooxygenase-2/prostacyclin
signaling pathway compared with healthy controls (33). In the present study, HDL from T2DM
patients lost the capacity to stimulate EC proliferation and
migration, which was consistent with a previous study (10). Furthermore, the anti-apoptotic and
angiogenesis ability of D-HDL was investigated, which, to the best
of our knowledge, has not yet been analyzed. In the Matrigel
angiogenesis assay, D-HDL demonstrated 51 to 61% reduced tube
length when compared with N-HDL. The HDL receptor, SR-BI is
required for the action on EC. The downstream process involves
proto-oncogene tyrosine-protein kinase Src activation and results
in parallel activation of the Akt kinase and mitogen-activated
kinases. A previous study demonstrated that cell surface levels of
SR-BI were decreased following incubation with D-HDL when compared
with N-HDL (10). LY294002, a
phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) inhibitor,
and PD98059, a MEK inhibitor, inhibit HDL-induced EC migration
(28). The present study suggests
that NXT therapy restores D-HDL function. Notably, HDL from
different diabetes patients demonstrated different responses to NXT
therapy. The majority of patients in the diabetes group had the
disease for ≤5 years. However, in the diabetes group with
atherosclerosis, the majority of patients had the disease for ≥10
years. In the diabetes group with atherosclerosis, no significant
change was observed between D-As-HDL BL and D-As-HDL M3 in the
migration and tube formation assays. Although the present study did
not investigate whether these two types of HDL possessed any
chemical differences, long-time glycation and an oxidative and
pro-inflammatory condition in these atherosclerosis patients may
result in modifications to HDL that cannot be reversed by the
short-term therapy used in the present study. Future studies on a
larger sample size and with a longer intervention period are
required to demonstrate whether treatments at different stages of
diabetes have the potential to modulate HDL function.
The main components of NXT include Radix Astragali,
Angelicae Sinensis, Radix Paeoniae Rubra and Ligusticum
wallichii. Radix Astragali exerts immune-regulating effects and
a previous study indicated an Astragalus polysaccharide
suppresses the expression of adhesion molecules via the regulation
of the p38 MAPK signaling pathway in ECs (34). Radix Astragali, the major component
of NXT, has also been reported to exhibit antioxidant activities to
prevent LDL oxidation (35).
Previous studies demonstrate that NXT therapy had lipid lowering
effects (36), anti-inflammatory
effects (37), and anti-platelet
potential (38,39) in atherosclerosis patients. In the
present study, a novel therapeutic target of NXT therapy was
investigated. Following the 3-month NXT therapy, D-HDL demonstrated
an increased ability to stimulate HUVEC proliferation and
migration. However, the mechanism by which NXT therapy exerts these
effects on the endothelial function of HDL remains to be
elucidated.
In conclusion, the present study demonstrates that
HDL from diabetic patients has a markedly impaired protective
effect on endothelial function in vitro and that the level
of impairment is dependent on the stage of diabetes. The impaired
function was partly abrogated by NXT therapy.
Acknowledgments
The present study was supported by a 'Major New Drug
Development' grant from the National S&T Major Project of China
(grant. no. 2008ZX09312-017), a '973' National S&T Major
Project grant (grant no. 2011CB503900), the National Natural
Science Foundation of China (grant nos. 81370235 and 81170101) and
by the Natural Science Foundation of Beijing (grant no.
7122106).
Dr Yining Huang and Dr Lemin Zheng are the
guarantors of the present study, and take full responsibility for
the integrity of the data and the accuracy of data analysis.
References
|
1
|
Woodman RJ, Chew GT and Watts GF:
Mechanisms, significance and treatment of vascular dysfunction in
type 2 diabetes mellitus: Focus on lipid-regulating therapy. Drugs.
65:31–74. 2005. View Article : Google Scholar
|
|
2
|
Terasaka N, Wang N, Yvan-Charvet L and
Tall AR: High-density lipoprotein protects macrophages from
oxidized low-density lipo-protein-induced apoptosis by promoting
efflux of 7-ketocholesterol via ABCG1. Proc Natl Acad Sci USA.
104:15093–15098. 2007. View Article : Google Scholar
|
|
3
|
Tauber JP, Cheng J and Gospodarowicz D:
Effect of high and low density lipoproteins on proliferation of
cultured bovine vascular endothelial cells. J Clin Invest.
66:696–708. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tauber JP, Cheng J, Massoglia S and
Gospodarowicz D: High density lipoproteins and the growth of
vascular endothelial cells in serum-free medium. In Vitro.
17:519–530. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tamagaki T, Sawada S, Imamura H, Tada Y,
Yamasaki S, Toratani A, Sato T, Komatsu S, Akamatsu N, Yamagami M,
et al: Effects of high-density lipoproteins on intracellular pH and
proliferation of human vascular endothelial cells. Atherosclerosis.
123:73–82. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugano M, Tsuchida K and Makino N:
High-density lipoproteins protect endothelial cells from tumor
necrosis factor-alpha-induced apoptosis. Biochem Biophys Res
Commun. 272:872–876. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng L, Settle M, Brubaker G, Schmitt D,
Hazen SL, Smith JD and Kinter M: Localization of nitration and
chlorination sites on apolipoprotein A-I catalyzed by
myeloperoxidase in human atheroma and associated oxidative
impairment in ABCA1-dependent cholesterol efflux from macrophages.
J Biol Chem. 280:38–47. 2005. View Article : Google Scholar
|
|
8
|
Nobecourt E, Jacqueminet S, Hansel B,
Chantepie S, Grimaldi A, Chapman MJ and Kontush A: Defective
antioxidative activity of small dense HDL3 particles in type 2
diabetes: Relationship to elevated oxidative stress and
hyperglycaemia. Diabetologia. 48:529–538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sorrentino SA, Besler C, Rohrer L, Meyer
M, Heinrich K, Bahlmann FH, Mueller M, Horváth T, Doerries C,
Heinemann M, et al: Endothelial-vasoprotective effects of
high-density lipoprotein are impaired in patients with type 2
diabetes mellitus but are improved after extended-release niacin
therapy. Circulation. 121:110–122. 2010. View Article : Google Scholar
|
|
10
|
Pan B, Ma Y, Ren H, He Y, Wang Y, Lv X,
Liu D, Ji L, Yu B, Wang Y, et al: Diabetic HDL is dysfunctional in
stimulating endothelial cell migration and proliferation due to
down regulation of SR-BI expression. PLoS One. 7:e485302012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lüscher TF, Landmesser U, von Eckardstein
A and Fogelman AM: High-density lipoprotein: Vascular protective
effects, dysfunction, and potential as therapeutic target. Circ
Res. 14:171–182. 2014. View Article : Google Scholar
|
|
12
|
Rader DJ: Molecular regulation of HDL
metabolism and function: Implications for novel therapies. J Clin
Invest. 116:3090–3100. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yvan-Charvet L, Matsuura F, Wang N,
Bamberger MJ, Nguyen T, Rinninger F, Jiang XC, Shear CL and Tall
AR: Inhibition of cholesteryl ester transfer protein by torcetrapib
modestly increases macrophage cholesterol efflux to HDL.
Arterioscler Thromb Vasc Biol. 27:1132–1138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen C, Venketasubramanian N, Gan RN,
Lambert C, Picard D, Chan BP, Chan E, Bousser MG and Xuemin S:
Danqi Piantang Jiaonang (DJ), a traditional Chinese medicine, in
poststroke recovery. Stroke. 40:859–863. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Wang WR, Lin R, Zhang JY, Ji QL,
Lin QQ and Yang LN: Buyang Huanwu decoction ameliorates coronary
heart disease with Qi deficiency and blood stasis syndrome by
reducing CRP and CD40 in rats. J Ethnopharmacol. 130:98–102. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen DK, Zhang HQ and Zhang JH:
Intervening effect of naoxintong on anti-platelet treatment with
aspirin. Zhongguo Zhong Xi Yi Jie He Za Zhi. 28:843–846. 2008.In
Chinese. PubMed/NCBI
|
|
17
|
Grundy SM, Cleeman JI, Daniels SR, Donato
KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith
SC Jr, et al: Diagnosis and management of the metabolic syndrome:
An American heart association/national heart, lung and blood
institute scientific statement. Circulation. 112:2735–2752. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alberti KG, Zimmet P and Shaw J; IDF
Epidemiology Task Force Consensus Group: The metabolic syndrome-a
new worldwide definition. Lancet. 366:1059–1062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang B, Li G, Guo YF, Wang SS, Liu F, Xu
HY and Yang HJ: Study on absorption location of four components
from Naoxintong capsule. Zhongguo Zhong Yao Za Zhi. 38:889–893.
2013.PubMed/NCBI
|
|
20
|
Songsong W, Haiyu X, Yan M, Xuguang W,
Yang S, Bin H, Shihuan T, Yi Z, Defeng L, Rixin L, et al:
Characterization and rapid identification of chemical constituents
of NaoXinTong capsules by UHPLC-linear ion trap/Orbitrap mass
spectrometry. J Pharm Biomed Anal. 111:104–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chung BH, Wilkinson T, Geer JC and Segrest
JP: Preparative and quantitative isolation of plasma lipoproteins:
Rapid, single discontinuous density gradient ultracentrifugation in
a vertical rotor. J Lipid Res. 21:284–291. 1980.PubMed/NCBI
|
|
22
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miura S, Fujino M, Matsuo Y, Kawamura A,
Tanigawa H, Nishikawa H and Saku K: High density
lipoprotein-induced angiogenesis requires the activation of Ras/MAP
kinase in human coronary artery endothelial cells. Arterioscler
Thromb Vasc Biol. 23:802–808. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katakami N, Kaneto H and Shimomura I:
Carotid ultrasonography: A potent tool for better clinical practice
in diagnosis of atherosclerosis in diabetic patients. J Diabetes
Investig. 5:3–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seetharam D, Mineo C, Gormley AK, Gibson
LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens
RL, Marce YL, et al: High-density lipoprotein promotes endothelial
cell migration and reendothelialization via scavenger receptor-B
type I. Circ Res. 98:63–72. 2006. View Article : Google Scholar
|
|
26
|
Zhu W, Saddar S, Seetharam D, Chambliss
KL, Longoria C, Silver DL, Yuhanna IS, Shaul PW and Mineo C: The
scavenger receptor class B type I adaptor protein PDZK1 maintains
endothelial monolayer integrity. Circ Res. 102:480–487. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ansell BJ, Navab M, Hama S, Kamranpour N,
Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST and
Fogelman AM: Inflammatory/antiinflammatory properties of
high-density lipoprotein distinguish patients from control subjects
better than high-density lipoprotein cholesterol levels and are
favorably affected by simvastatin treatment. Circulation.
108:2751–2756. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan B, Yu B, Ren H, Willard B, Pan L, Zu
L, Shen X, Ma Y, Li X, Niu C, et al: High-density lipoprotein
nitration and chlorination catalyzed by myeloperoxidase impair its
effect of promoting endothelial repair. Free Radic Biol Med.
60:272–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Persegol L, Verges B, Foissac M, Gambert P
and Duvillard L: Inability of HDL from type 2 diabetic patients to
counteract the inhibitory effect of oxidised LDL on
endothelium-dependent vasorelaxation. Diabetologia. 49:1380–1386.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Biesbroeck RC, Albers JJ, Wahl PW,
Weinberg CR, Bassett ML and Bierman EL: Abnormal composition of
high-density lipoproteins in non-insulin-dependent diabetics.
Diabetes. 31:126–131. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taskinen MR: Quantitative and qualitative
lipoprotein abnormalities in diabetes mellitus. Diabetes. 41(Suppl
2): S12–S17. 1992. View Article : Google Scholar
|
|
32
|
Nobécourt E, Zeng J, Davies MJ, Brown BE,
Yadav S, Barter PJ and Rye KA: Effects of cross-link breakers,
glycation inhibitors and insulin sensitisers on HDL function and
the non-enzymatic glycation of apolipoprotein A-I. Diabetologia.
51:1008–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tong X, Lv P, Mathew AV, Liu D, Niu C,
Wang Y, Ji L, Li J, Fu Z, Pan B, et al: The compensatory enrichment
of sphingosine -1-phosphate harbored on glycated high-density
lipoprotein restores endothelial protective function in type 2
diabetes mellitus. Cardiovasc Diabetol. 13:822014. View Article : Google Scholar
|
|
34
|
Hai-Yan Z, Yong-Hong G, Zhi-Yao W, Bing X,
Ai-Ming W, Yan-Wei X, Bei L, Li-Xia and Li-Xin C: Astragalus
polysaccharide suppresses the expression of adhesion molecules
through the regulation of the p38 MAPK signaling pathway in human
cardiac microvascular endothelial cells after ischemia-reperfusion
injury. Evid Based Complement Alternat Med. 2013:2804932013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chan JY, Koon JC, Leung PC, Che CT and
Fung KP: Suppression of low-density lipoprotein oxidation, vascular
smooth muscle cell proliferation and migration by a herbal extract
of Radix Astragali, Radix Codonopsis and Cortex Lycii. BMC
Complement Altern Med. 11:322011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao J, Zhu H, Wang S, Ma X, Liu X, Wang
C, Zhao H, Fan S, Jin X, Zhao B, et al: Naoxintong protects against
atherosclerosis through lipid-lowering and inhibiting maturation of
dendritic cells in LDL receptor knockout mice fed a high-fat diet.
Curr Pharm Des. 19:5891–5896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shin HY, Shin TY, An NH, Kim HR, Chae HJ,
Kim YK, Um JY, Hong SH and Kim HM: The immunosuppressive effect of
Buchang-tang through inhibition of mitogen-activated protein kinase
and nuclear factor activation in MOLT-4 cells. J Ethnopharmacol.
102:95–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen H, Yu G, Sun H, Wu X and Wang H:
Comparison of adjunctive naoxintong versus clopidogrel in
volunteers with the CYP2C19*2 gene mutation accompanied with qi
deficiency and blood stasis constitution. Evid Based Complement
Alternat Med. 2011:2070342011. View Article : Google Scholar
|
|
39
|
Chen H, Zhang Y, Wu X, Li C and Wang H: In
Vitro assessment of cytochrome P450 2C19 potential of naoxintong.
Evid Based Complement Alternat Med. 2012:4302622012. View Article : Google Scholar : PubMed/NCBI
|



















