Introduction
Gastric cancer is the fourth most common cancer and
the second leading cause of cancer-associated mortality in
developed countries (1). Surgery
remains the most effective treatment and accordingly, patients with
resectable tumors have a better prognosis. However, most of these
patients suffer a relapse at a later stage. Since early detection
is difficult, gastric cancer is detected at the advanced stage in
approximately two thirds of all patients, survival rates remain low
(2). Surgical treatment alone is
not effective in patients with local and distal recurrence, who are
therefore subjected to radiotherapy (3), which is the current standard
treatment for patients with a high risk of recurrence. The first
radiotherapy techniques developed, however, have limitations due to
destruction of surrounding normal tissue, thereby causing serious
adverse reactions. To overcome this problem, radiotherapy is being
increasingly combined with targeted drugs for the treatment of
human cancer (4).
Evodiamine (EVO) is one of the main constituents of
Tetradium genus and has been shown to have bioactive
properties, including anti-tumor (5,6),
anti-nociceptive (7,8), vasodilatative (9), stimulatory catecholamine secretion
(10) as well as anti-biotic and
anti-inflammatory (11) effects.
Previous studies by our group proved that EVO increased the
radiosensitity of Tca-8113 human oral squamous cell carcinoma cells
in vitro and in vivo and inhibited cell invasion and
metastasis (12,13). However, the role of EVO as a
sensitizer of other cancer types to radiotherapy has remained
elusive. The present study assessed the efficacy of EVO combined
with radiotherapy in the treatment of human gastric cancer in
vitro as well as in vivo. EVO was revealed to enhance
the responses of gastric cancer cells to radiotherapy, suggesting
that it may be applied in the clinic to improve the efficacy of
gastric cancer treatment.
Materials and methods
Cell culture
The BGC-823 human gastric carcinoma cell line was
purchased from Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China) and were cultured in RPMI
1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), supplemented with 10% fetal bovine serum (Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd., Zhejiang, China),
penicillin (100 U/ml; Gibco; Thermo Fisher Scientific, Inc.) and
streptomycin (100 mg/ml; Thermo Fisher Scientific, Inc.) at 37°C in
a humidified atmosphere containing 5% CO2. Cells were
and passaged upon reaching 70–80% confluence.
Reagents and instruments
EVO was purchased from Phytomarker Ltd. (Tianjin,
China) and dissolved in Tween 80 (0.5%; Sigma-Aldrich, St. Louis,
MO, USA) and then diluted with NaCl solution to a final
concentration of 1 mg/ml. Primary antibodies against Bcl-2 (mouse
monoclonal; 1:200; cat. no. ZM-0010) and Bax (mouse monoclonal;
1:150; cat. no. ZA-0611) were obtained from Zhongshan Golden Bridge
Biotechnology Co., Ltd. (Beijing, China). Primary monoclonal
antibodies against Her-2 (rabbit monoclonal; 1:200; cat. no. 2242)
and phosphorylated (p-)Akt (rabbit IgG; 1:200; cat. no. 4060) were
purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
An Elekta linear accelerator (Stockholm, Sweden) was applied for
radiotherapy. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), dimethyl sulfoxide, hematoxylin, eosin and
phosphate-buffered saline (PBS) were purchased from Boster
Bioengineering Co., Ltd. (Wuhan, China)
Cell proliferation assay
Cells (1,000 cells/ml) were seeded in a 96-well
plate and subjected to the indicated treatments. After 24, 48 or 72
h of treatment with EVO (1, 4 or 16 µM) and X-radiation (2,
4, 6 or 8 Gy), cells were stained with MTT (10 µl) and
incubated at 37°C for 4 h. Cells were then lysed in 100 µl
dimethyl sulfoxide and the optical density value was measured at
570 nm using a 96-well plate reader.
Clonogenic cell survival assay
Cells were seeded into 6-well plates and after 24 h
incubation, were incubated with 4 µmol/l EVO for a further
24 h. The cells were then irradiated with various doses (2, 4, 6 or
8 Gy) of X-radiation at a dose rate of 2 Gy/min. The cells were
allowed to form colonies in drug-free medium over 10 days for the
determination of clonogenic cell survival. The cells were then
stained with 0.05% crystal violet in 100% methanol, and colonies
comprising >50 cells were counted. Clonogenic survival curves
were plotted using the 'Multitarget single hit' model.
Cell cycle distribution
Cells (2×106) were seeded in 100-mm
plates and subjected to various treatments [control group (C),
untreated; EVO group (E), treated with (4 µmol/l) EVO;
radiotherapy group (R), treated with 2, 4, 6 or 8 Gy of
X-radiation; and combined therapy group (E+R)]. After 48 h, the
cells were washed with PBS and then fixed in 70% ethanol at −20°C
overnight. Cells were then washed with PBS and re-suspended in
propidium iodide solution (200 µl), incubated in the dark
for 30 min and then analyzed by flow cytometry (Beckman Coulter,
Inc., Brea, CA, USA). The experiment was performed in
triplicate.
In vivo tumor xenograft model
Sixteen male BALB/c mice (3–4 weeks old; weighing
15–18 g) raised under specific pathogen-free conditions were
purchased from Vital River Laboratory Animal Technology Co. Ltd
(Beijing, China). The present study was approved by the Medical
Ethics Committee of Lanzhou University (Lanzhou, China). The
feeding room was maintained at a constant temperature of 26–28°C
with normal ventilation and a 12 h light/dark cycles (relative
humidity, 50±5%). The mice had unlimited access to a standard mouse
chow diet and water. To establish the gastric carcinoma xenografts,
106 BGC-823 cells were suspended in 100 µl PBS
and then subcutaneously inoculated into the left flank of each
mouse. When the tumor size had reached a mean diameter of ~7 mm
after two weeks, the mice were randomly divided into four groups.
The control group and the radiotherapy group was treated with
placebo (0.5% Tween 80 and 0.9% sodium chloride via oral gavage)
for five consecutive days. The EVO group and the combined group
received EVO orally (10 mg/kg daily via oral gavage) for five days
and then underwent mock irradiation. The tumors of the radiotherapy
group were exposed to radiation at a dosage totaling 5 Gy (2
Gy/min) at 2 h after the final placebo treatment. The mice of the
combined treatment group were treated with irradiation (5 Gy) after
EVO treatment for five days. Mice were then maintained under
specific pathogen-free conditions for 24 days. The volume of the
sub-cutaneous tumors was measured every two days using a Vernier
caliper and tumor volumes were calculated using the formula
(LxW2)/2. At the end of the experiments, all of the mice
were sacrificed by cervical dislocation. Tumors were recovered and
weighed, and the tumor growth inhibition rate was caculated using
the following formula (14): Tumor
inhibition rate (%) = (1-average tumor weight in treated
group/average tumor weight in control) × 100%.
Morphological changes
The tumors were fixed in 10% formalin for 24 h at
room temperature, dehydrated and then embedded in paraffin.
Sections (4 µm) were prepared, stained with hematoxylin and
eosin and observed by light microscopy (Olympus, Tokyo, Japan).
Furthermore, morphological changes of BGC-823 xenografts
characteristic of apoptosis were assessed using transmission
electron microscopy (TEM; Philips, Eindhoven, The Netherlands).
Tumors were cut into 1×1×1-mm specimens. Subsequently, the samples
were fixed in 4% glutaraldehyde overnight at 4°C using 0.1 mol/l
sodium cacodylate buffer and incubated in 1% osmium tetroxide
(OsO4) at room temperature for 1 h. Fixed samples were
washed and dehydrated using a graded ethanol series followed by
100% acetone. Tissues were then embedded in epoxy resin and
ultrathin sections (70 nm) were prepared, which were observed by
TEM.
Immunohistochemical detection of Her-2,
p-Akt, Bcl-2 and Bax
Tissue sections (4 µm) were de-paraffinized
in xylene, hydrated and treated with 0.3%
H2O2 for 10 min. Antigen retrieval was
performed by standard microwave heating (92–98°C) for 10 min in
citrate buffer (pH 6.0). Slides were then probed with primary
antibodies against Her-2, p-Akt, Bcl-2 and Bax overnight at 4°C.
Subsequently, slides were incubated with specific secondary
antibodies (cat. no. PV-6000-D; Zhong Shan Golden Bridge Co., Ltd.,
Beijing, China) at 37°C for 1 h. Diaminobenzidine was used for
color development according to manufacturer's instructions. The
proportion and distribution of immunoreactive cells was assessed
using an optical microscope (Olympus). Semi-quantitative analysis
was performed using Image J v.1.45 software (National Institutes of
Health, Bethesda, MD, USA). Negative controls were obtained by
excluding the primary antibody.
Statistical analysis
All analyses were performed using the SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA). Values are
expressed as the mean ± standard deviation. The data were
subsequently analyzed using Student's t-test when only two groups
were present, or assessed by one-way analysis of variance when
>2 groups were compared. P<0.05 was considered to indicate a
statistically significant difference.
Results
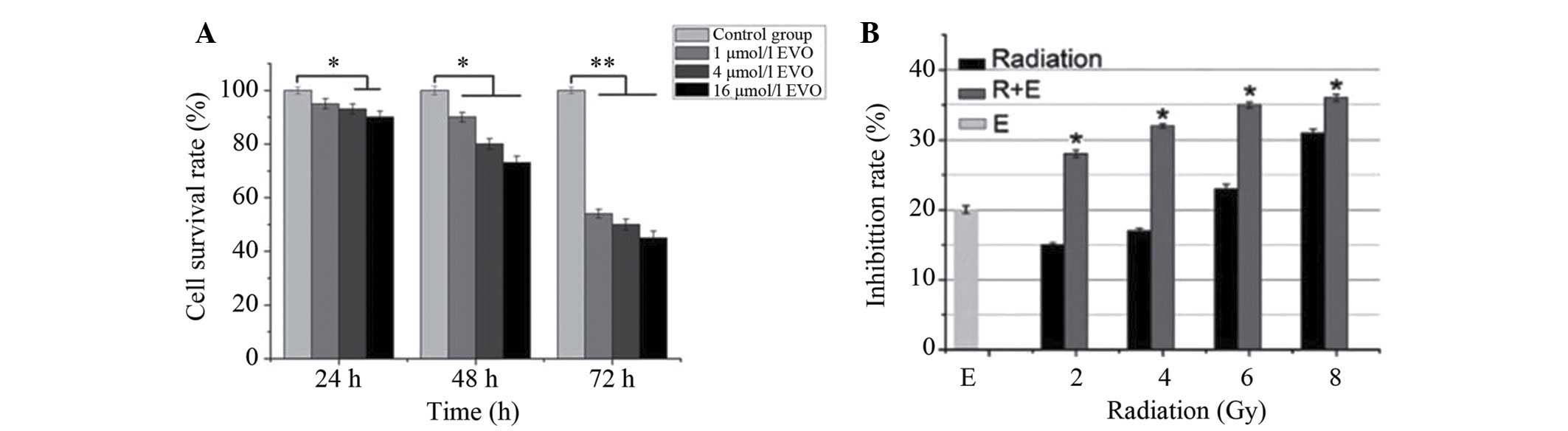
EVO reduces the viability of BGC-823
cells and enhances the efficacy of radiation in vitro
As shown in Fig.
1A, EVO time-dependently reduced the number of viabile BGC-823
cells. From the inhibition curves, the IC20 value of EVO
was determined to be 4 µmol/l for the 48 h time-point, which
was then used for combined treatment with radiation. Compared with
radiation or EVO treatment alone, combined treatment demonstrated a
significantly higher inhibitory effect on BGC-823 cells (P<0.05;
Fig. 1B) and that low-dose
combination therapy is superior to the high-dose group.
EVO enhances the inhibitory effects of
radiation on the clonogenic survival and cell cycle progression of
BGC823 cells
As shown in Fig. 2,
EVO significantly enhanced the radio-sensitivity of BGC823 cells in
terms of reducing clonogenic cell survival. The clonogenic survival
curves (Fig. 2A) were plotted
using the 'Multitarget single hit' model. The radio-biological
parameters (D0=3.9 Gy, Dq=0.8 Gy, SF2=0.5) of the combination group
were lower than those in the radiation only group (D0=5.0 Gy,
Dq=1.2 Gy, SF2=0.8) (P<0.05), which indicated that combination
therapy enhanced the radiosensitivity of BGC823 cells.
The effects of EVO and radiation on the cell cycle
distribution of BGC823 cells were also assessed. The ability of
radiation to dose-dependently induce G2/M phase arrest in BGC823
cells was significantly enhanced by EVO (P<0.05) (Fig. 2B). Therefore, it was indicated that
EVO enhanced the effect of radiation in BGC823 cells (15).
Pre-treatment with EVO sensitizes BGC-823
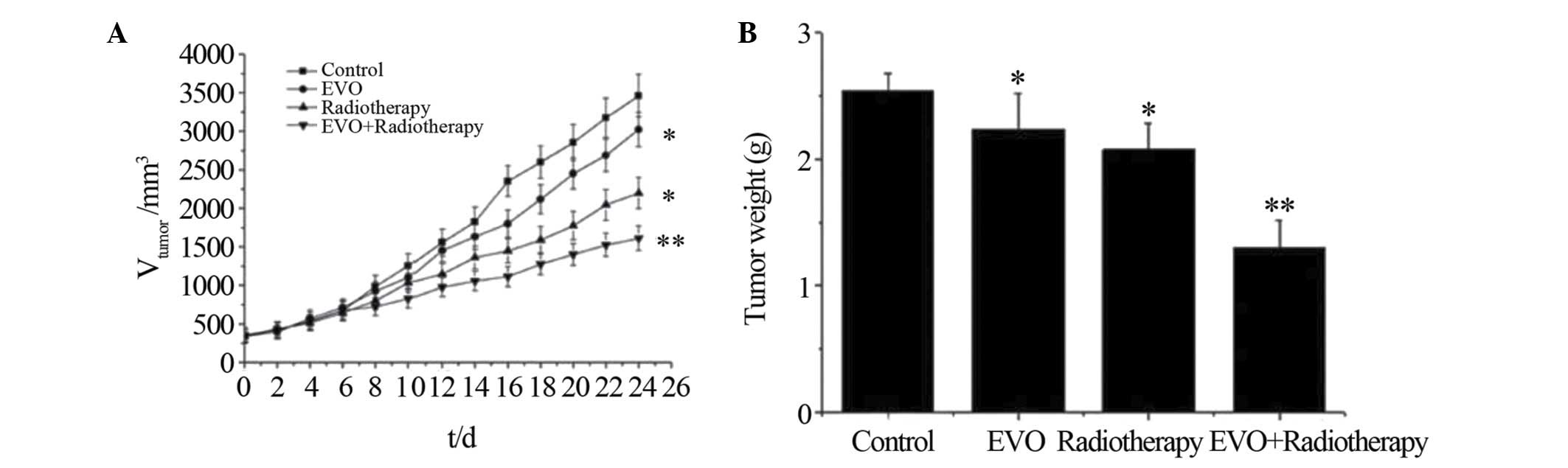
cell-derived xenograft tumors to radiation
To evaluate the enhanced efficacy of combined EVO
and radiotherapy treatment in vivo, a murine xenograft model
of human gastric carcinoma was used. Model animals were treated
with EVO or placebo, followed by optional radiation therapy.
According to the tumor growth curves (Fig. 3A), combined therapy with EVO and
radiation showed an enhanced inhibitory effect on the tumor volume
compared with that of all other treatments over 24 days
(P<0.05). Furthermore, the tumor weight at the end of the
experiment was significantly lower in the combination treatment
group compared to that in the other groups, with an inhibition rate
of 48.8±2.6% compared to 12.1±2.8 and 17.1±2.4% in the EVO and
radiotherapy group, respectively.
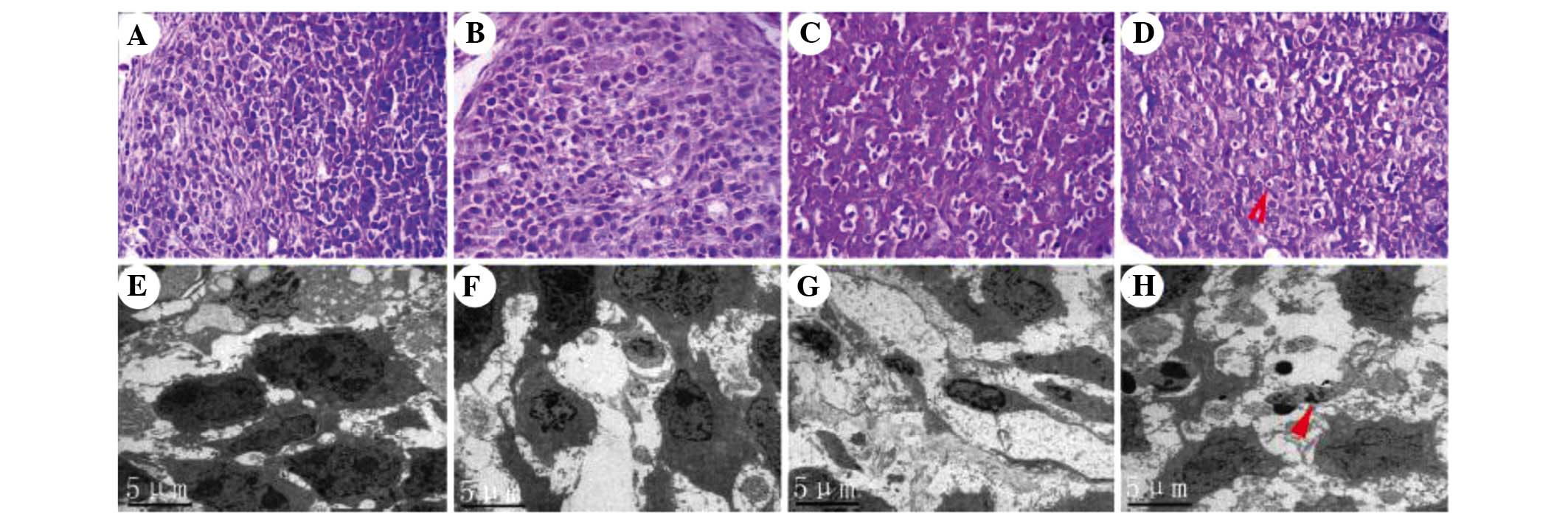
Morphological changes in xenograft
tumors
Microscopic observation of histological slides of
the tumors indicated that in the control group, the cells showed
dense, large and deeply stained nuclei with minimal cytoplasm, and
the tissue structure was in order (Fig. 4A). In the EVO group, the nuclei
were distorted and shrunk, while chromatin was partly dissolved and
had migrated to the cell edges (Fig.
4B). Pathological karyokinesis was markedly reduced. Cell
mitosis was decreased compared with the control group. In the
radiotherapy group, various degrees of cell shrinkage were present
and most cells appeared necrotic (Fig.
4C). In the combination group, a large number of BGC-823 cells
appeared necrotic, and the tumor volume was reduced and atrophic
(Fig. 4D; arrow). Numerous BGC823
cells showed obvious chromatin condensation, nuclear condensation,
and the cellular structure was dissolved. Typical radiation-induced
changes in the microstructure of BGC-823 cells were assessed by
TEM. In the control group, cell structure was clear with prominent
nucleoli (Fig. 4E). No significant
apoptosis was observed. In the EVO group, certain cells presented a
morphology characteristic of apoptosis, such as accumulation of
chromatin on the cell edges (Fig.
4F). In the radiotherapy group, a number of cells showed
chromatic agglutination and protoplasm concentration, and had
formed apoptotic bodies (Fig. 4G),
a typical phenomenon observed in cells undergoing radiation-induced
apoptosis. Combination treatment was found to strongly promote
apoptosis (Fig. 4H; arrow). The
number of cells was markedly decreased. Nuclear condensation,
chromatin fragmentation and cell fragmentation into apoptotic
bodies were frequently observed.
EVO combined with radiotherapy reduces
the levels of Her-2, p-Akt and Bcl-2 and increases the expression
of Bax in BGC-823 cells
The effects of the various treatments on the levels
of certain proteins were assessed by immunohistochemistry followed
by optical density analysis (Fig.
5). Positivity for Her-2 was indicated by brown granules
located on the cell membrane. The expression levels of Her-2 in the
combination group were significantly descreased compared with those
in the mono treatment groups (P<0.05). p-Akt (activated Akt) can
be found in the cytoplasm and the nuclei. The levels of p-Akt were
significantly decreased in the combination group compared with
those in the mono treatment groups (P<0.05). Positive staining
for Bcl-2 and Bax was mainly located in the cytoplasm. The
expression levels of Bax in the combination group were
significantly higher, while those of Bcl-2 were significantly lower
than those in the mono treatment groups (P<0.05 for both).
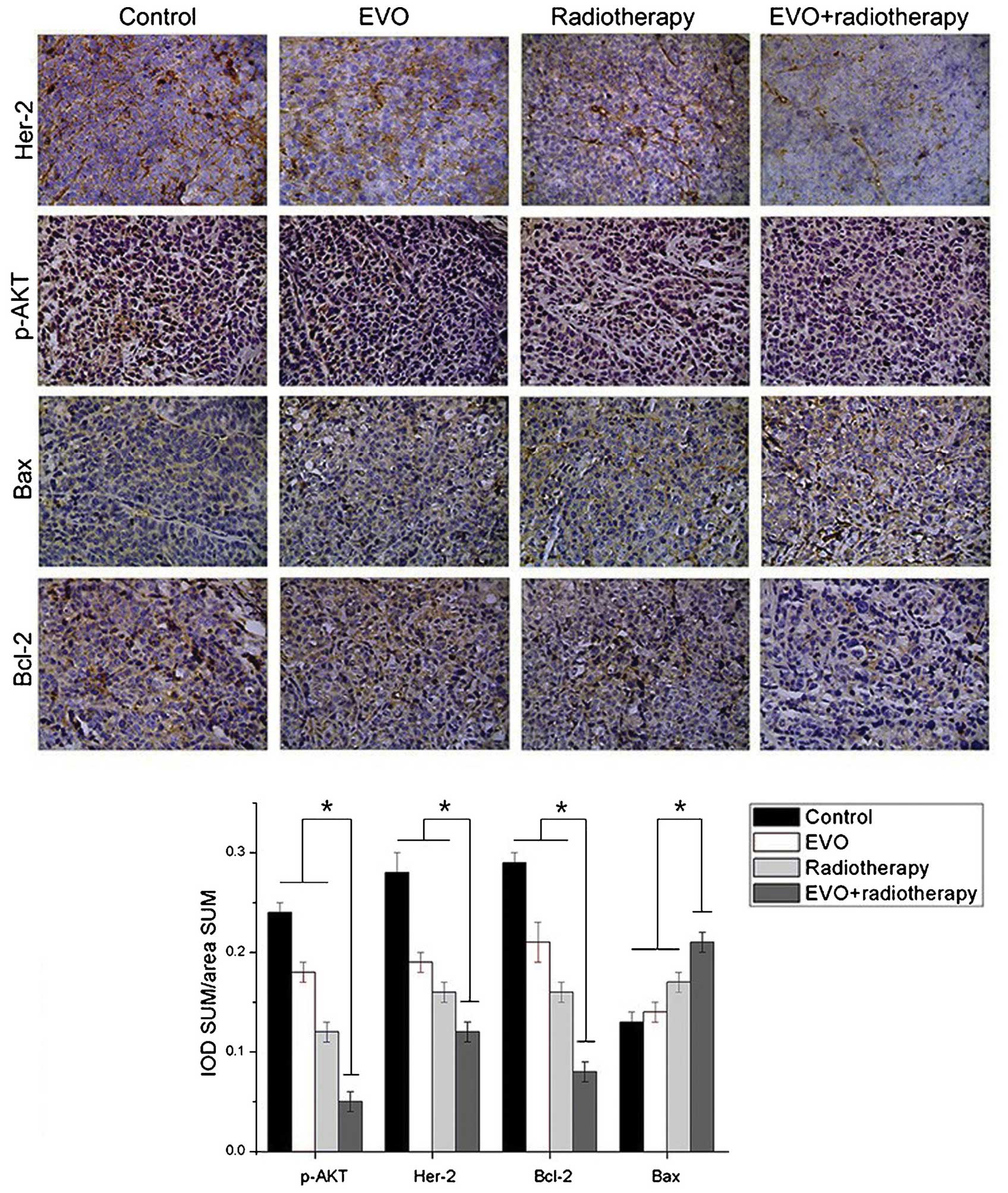 | Figure 5Expression of Her-2, p-Akt, Bcl-2 and
Bax in xenograft tumors derived from BGC-823 cells as detected by
immunohistochemistry (magnification, ×200). Positivity for Her-2
was indicated by brown granules located on the cell membrane. p-Akt
was present in the cytoplasm and the nuclei, while Bcl-2 and Bax
were located in the cytoplasm. Values are expressed as the mean ±
standard deviation. *P<0.05 for EVO+radiotherapy
group vs. all other groups. EVO, evodiamine; p-Akt, phosphorylated
Akt; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein;
IOD, integrated optical density; SUM area, the total area of
positive cells. |
Discussion
Radiotherapy serves an important role in treatment
of gastric cancer at the advanced stage. In localized advanced
tumors, radiotherapy has been combined with systemic agents,
including chemotherapeutic drugs and radiosensitizers, to improve
patient survival and outcome of the treatment (16–18).
However, most radiosensitizers currently used in the clinic have
toxic side effects (19).
Therefore, large research efforts have been made to discover
radiosensitizers with low toxicity and high efficiency. EVO is a
compound extracted from the Tetradium genus of plants and has shown
efficacy against various tumor types, including liver cancer
(20), breast cancer (21) and prostate cancer (22). However, the use of EVO as a
radiosensitizer has been rarely reported. Previous studies by our
group indicated that EVO has radiosensitizing effects on Tca-8113
cells in vitro and in vivo (12,13).
Based on these findings, the present study investigated the
efficacy of EVO in combination with radiotherapy in the treatment
of gastric carcinoma. In vitro viability and clonogenic
assays indicated that combination treatment had greater inhibitory
effects on the BGC-823 gastric cancer cell line compared with mono
treatments. Furthermore, flow cytometric cell cycle distribution
analysis revealed that radiation dose-dependently induced cell
cycle arrest in G2/M phase, which was aggravated by EVO.
The present study further demonstrated that EVO in
combination with radiotherapy resulted in significantly enhanced
tumor growth inhibition compared with that in the other groups
in vivo. Cell morphological observation using TEM revealed
that in the combination group, DNA condensation, chromatic
agglutination, which are characteristics of radiation-induced
apoptosis, were present. Furthermore, histological analysis
revealed the presence of necrotic cells. Apoptosis induced by
combined treatment with EVO and radiation is therefore a mechanism
responsible for the enhanced anti-tumor effects on human gastric
carcinoma. The combination group increased the inhibition of the
G2/M phase. It reduced the capacity of the cells for self repair
following radiation damage. EVO may have enhanced the
radiation-induced decreases of cell survival by reducing the
capacity of the cells for self-repair following radiation
damage.
The present study further hypothesized that
apoptosis induced by the combination treatment was mediated through
Her-2 as well as deactivation of AKT and downregulation of Bcl-2
(23). Immunohistochemical
analysis revealed that treatment with EVO or radiation led to the
downregulation of Bcl-2 and upregulation of Bax in BGC-823 cells,
which is indicative of apoptosis (24,25).
As the combination of EVO and radiotherapy significantly increased
the Bax/Bcl-2 ratio in BGC823 cells compared with mono treatment,
is was further evidenced that EVO enhanced the efficacy of
radiotherapy.
Her-2 is a prognostic indicator of breast cancer and
expression in gastric carcinoma. It also has a significant
association with the prognosis of gastric cancer, and more and more
individuals are researching Her-2 in gastric cancer. The
proto-oncogene Her-2 has been demonstrated to be expressed in
gastric cancer tissues (26). It
can enhance kinase activity, cell proliferation and cell
transformation. The two signaling transduction pathways activated
by Her-2 are the mitogen-activated protein kinase and the
phosphoinositide-3 kinase (PI3K)/AKT pathways. Numerous studies
suggested that Her-2 also modulates cell survival through
activation of the PI3K/AKT pathway (27,28).
PI3K catalyzes the phosphorylation of phosphatidyl inositol, which
concomitantly activates downstream targets, including AKT (29). The PI3K/AKT pathway is considered
to be a survival pathway and can protect cells from various
stresses. AKT activation promotes cell survival by modulating its
downstream elements, such as Bcl-2-associated death promoter (BAD)
(30,31), which stimulates the expression of
Bcl-2 which exerts its anti-apoptotic effects (32–34).
The Bax/Bcl-2 ratio as a molecular switch keeps a balance between
proliferation and apoptosis. Therefore, the decreased expression of
Her-2 may have contributed to the increase in the Bax/Bcl-2 ratio
observed in present study, which subsequently caused apoptosis.
In conclusion, the present study suggested that EVO
is able to sensitize gastric cancer cells to radiotherapy. It is
likely that DNA damage-associated signaling may have triggered
apoptosis, and that EVO may have facilitated this process.
Therefore, that this requires further investigation in the future.
Combined application of EVO and radiotherapy may therefore
represent a suitable regimen with enhanced efficacy for the
treatment of gastric cancer.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81372893) and the
Natural Science Foundation of Gansu Province (no. 1208RJZA193).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. Ca-Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohtsu A, Shah MA, Van Cutsem E, Rha SY,
Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al:
Bevacizumab in combination with chemotherapy as first-line therapy
in advanced gastric cancer: A randomized, double-blind,
placebo-controlled phase III study. J Clin Oncol. 29:3968–3976.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maier-Hauff K, Ulrich F, Nestler D,
Niehoff H, Wust P, Thiesen B, Orawa H, Budach V and Jordan A:
Efficacy and safety of intratumoral thermotherapy using magnetic
iron-oxide nanoparticles combined with external beam radiotherapy
on patients with recurrent glioblastoma multiforme. J Neurooncol.
103:317–324. 2011. View Article : Google Scholar :
|
|
4
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li-Weber M: Targeting apoptosis pathways
in cancer by Chinese medicine. Cancer Lett. 332:304–312. 2013.
View Article : Google Scholar
|
|
6
|
Shu L, Cheung KL, Khor TO, Chen C and Kong
AN: Phytochemicals: Cancer chemoprevention and suppression of tumor
onset and metastasis. Cancer Metast Rev. 29:483–502. 2010.
View Article : Google Scholar
|
|
7
|
Yuan SM, Gao K, Wang DM, Quan XZ, Liu JN,
Ma CM, Qin C and Zhang LF: Evodiamine improves congnitive abilities
in SAMP8 and APP(swe)/PS1(ΔE9) transgenic mouse models of
Alzheimer's disease. Acta Pharmacol Sin. 32:295–302. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu H, Jin H, Gong W, Wang Z and Liang H:
Pharmacological actions of multi-target-directed evodiamine.
Molecules. 18:1826–1843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia YY, Xu HY, Cai YY, Si DY and Liu CX:
Simultaneous determination of evodiamine and evodine in Beagle dog
plasma using liquid chromatography tandem mass spectrometry. J
Asian Nat Prod Res. 15:235–243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwarz NA, Spillane M, La Bounty P,
Grandjean PW, Leutholtz B and Willoughby DS: Capsaicin and
evodiamine ingestion does not augment energy expenditure and fat
oxidation at rest or after moderately-intense exercise. Nutr Res.
33:1034–1042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao JF, Chiou WF, Shen YC, Wang GJ and
Chen CF: Anti-inflammatory and anti-infectious effects of Evodia
rutaecarpa (Wuzhuyu) and its major bioactive components. Chin Med.
6:62011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han YY: The Radio Sensitizing Effects of
EVO on Human Gastric Cancer Cells. Lanzhou University; 2014, In
Chinese.
|
|
13
|
Li J, Zhang KL, Hu CQ, et al: Inhibitory
effect of evodiamine combined with radiotherapy on the growth of
xenografts of human tongue squamous-cell carcinoma Tca-8113 cells
in nude mice. Tumor. 2:108–112. 2014.In Chinese.
|
|
14
|
Ueno S, Yamazaki R, Ikeda T, Yaegashi T
and Matsuzaki T: Antitumor effect of a novel phenanthroindolizidine
alkaloid derivative through inhibition of protein synthesis.
Anticancer Res. 34:3391–3397. 2014.PubMed/NCBI
|
|
15
|
Sun H, Hou H, Lu P, et al: Isocorydine
inhibits cell proliferation in hepatocellular carcinoma cell lines
by inducing G2/M cell cycle arrest and apoptosis. PloS one.
7:e368082012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lawrence YR, Vikram B, Dignam JJ,
Chakravarti A, Machtay M, Freidlin B, Takebe N, Curran WJ Jr,
Bentzen SM, Okunieff P, et al: NCI-RTOG translational program
strategic guidelines for the early-stage development of
radiosensitizers. J Natl Cancer Inst. 105:11–24. 2013. View Article : Google Scholar
|
|
17
|
Ma S, Jiao B and Liu X, Yi H, Kong D, Gao
L, Zhao G, Yang Y and Liu X: Approach to radiation therapy in
hepatocellular carcinoma. Cancer Treat Rev. 36:157–163. 2010.
View Article : Google Scholar
|
|
18
|
Morris ZS and Harari PM: Interaction of
radiation therapy with molecular targeted agents. J Clin Onocl.
32:2886–2893. 2014. View Article : Google Scholar
|
|
19
|
Waseem M and Parvez S: Mitochondrial
dysfunction mediated cisplatin induced toxicity: Modulatory role of
curcumin. Food Chem Toxicol. 53:334–342. 2013. View Article : Google Scholar
|
|
20
|
Yang J, Cai X, Lu W, Hu C, Xu X, Yu Q and
Cao P: Evodiamine inhibits STAT3 signaling by inducing phosphatase
shatterproof 1 in hepatocellular carcinoma cells. Cancer Lett.
328:243–251. 2013. View Article : Google Scholar
|
|
21
|
Wang KL, Hsia SM, Yeh JY, Cheng SC, Wang
PS and Wang SW: Anti-proliferative effects of evodiamine on human
breast cancer cells. PLoS One. 8:e672972013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kan SF, Huang WJ, Lin LC and Wang PS:
Inhibitory effects of evodiamine on the growth of human prostate
cancer cell line LNCaP. Int J Cancer. 110:641–651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sukawa Y, Yamamoto H, Nosho K, et al: HER2
expression and PI3K-Akt pathway alterations in gastric cancer.
Digestion. 89:12–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi L, Chen J, Yang J, Pan T, Zhang S and
Wang Z: MiR-21 protected human glioblastoma U87MG cells from
chemotherapeutic drug temozolomide induced apoptosis by decreasing
Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 1352:255–264.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schwartzberg LS, Franco SX, Florance A,
O'Rourke L, Maltzman J and Johnston S: Lapatinib plus letrozole as
first-line therapy for HER-2+ hormone receptor-positive metastatic
breast cancer. Oncologist. 15:122–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: a new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dave B, Migliaccio I, Gutierrez MC, Wu MF,
Chamness GC, Wong H, Narasanna A, Chakrabarty A, Hilsenbeck SG,
Huang J, et al: Loss of phosphatase and tensin homolog or
phosphoinositol-3 kinase activation and response to trastuzumab or
lapatinib in human epidermal growth factor receptor
2-overexpressing locally advanced breast cancers. J Clin Oncol.
29:166–173. 2011. View Article : Google Scholar
|
|
28
|
Bacus SS, Altomare DA, Lyass L, Spohn B,
Bartholomeusz G, Yan DH and Hung MC: AKT2 is frequently upregulated
in HER-2/neu-positive breast cancers and may contribute to tumor
aggressiveness by enhancing cell survival. Oncogene. 21:3532–3540.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong KK, Engelman JA and Cantley LC:
Targeting the PI3K signaling pathway in cancer. Curr Opin Genet
Dev. 20:87–90. 2010. View Article : Google Scholar :
|
|
30
|
Chen KC, Hsieh CL, Peng CC and Peng RY:
Exercise rescued chronic kidney disease by attenuating cardiac
hypertrophy through the cardiotrophin-1 - LIFR/gp 130 - JAK/STAT3
pathway. Eur J Prev Cardiol. 21:507–520. 2014. View Article : Google Scholar
|
|
31
|
Ridnour LA, Barasch KM, Windhausen AN,
Dorsey TH, Lizardo MM, Yfantis HG, Lee DH, Switzer CH, Cheng RY,
Heinecke JL, et al: Nitric oxide synthase and breast cancer: Role
of TIMP-1 in NO-mediated Akt activation. PLoS One. 7:e440812012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zeng KW, Wang XM, Ko H, Kwon HC, Cha JW
and Yang HO: Hyperoside protects primary rat cortical neurons from
neurotoxicity induced by amyloid β-protein via the
PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur
J Pharmacol. 672:45–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sakamaki J, Daitoku H, Ueno K, Hagiwara A,
Yamagata K and Fukamizu A: Arginine methylation of BCL-2 antagonist
of cell death (BAD) counteracts its phosphorylation and
inactivation by Akt. Proc Natl Acad Sci USA. 108:6085–6090. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao A, Zeng Q, Xie X, Zhou J, Yue W, Li Y
and Pei X: MicroRNA-125b induces cancer cell apoptosis through
suppression of Bcl-2 expression. J Genet Genomics. 39:29–35. 2012.
View Article : Google Scholar : PubMed/NCBI
|