Introduction
Multiple myeloma (MM) is a clonal B-cell malignancy
that primarily affects elderly individuals, accounting for ~1% of
all cancers (1,2). Cancerous plasma cells accumulate in
the bone marrow; the effects of this include hypercalcemia, renal
failure, anemia and osteolytic bone lesions (1,2). MM
may be treated with the novel therapeutic agents, proteasome
inhibitors and immunomodulatory drugs, which may be combined with
conventional chemotherapeutics. However, almost all MM patients
ultimately relapse, even when complete remission is achieved
following initial therapy (2).
The majority of intracellular proteins are degraded
by the ubiquitin-proteasome system (UPS) (3). Abnormal proteasome-dependent protein
degradation is associated with the pathophysiology of multiple
cancer types; therefore, it has been proposed that the selective
inhibition of UPS may provide a novel strategy for the development
of anticancer therapeutics (4–6).
Notably, the proteasome inhibitor bortezomib (Bor) has been
successfully developed for relapsed/refractory MM therapy. Bor has
demonstrated a marked effect in MM patients; however, Bor
resistance and its secondary side effects, including bone growth
impairment, restrict the use of this therapy (7,8).
Adjuvant agents are therefore required to chemosensitize MM cells
to Bor and achieve therapeutic efficacy with limited toxicity.
Bor treatment results in the aggregation of
ubiquitinated proteins, endoplasmic reticulum (ER) stress and
apoptotic cell death, via inhibition of 26S proteasome activity.
The proper folding of proteins prior to exit from the ER is ensured
by quality control mechanisms; ER stress is triggered by improper
protein folding and involves various signaling pathways
collectively referred to as the unfolded protein response (UPR).
Severe or prolonged ER stress promotes apoptotic cell death in the
event that the UPR is unable to resolve the situation (9,10).
Various signaling pathways may modulate ER stress-induced
programmed cell death. To date, three contributing UPR branches
have been identified: Inositol-requiring enzyme 1, protein kinase
RNA-like ER kinase (PERK) and activating transcription factor (ATF)
6 (11–13). PERK signaling induces eukaryotic
initiation factor 2α (eIF2α) phosphorylation, enhancing ATF4
protein synthesis. The pre-apoptotic eIF2α-ATF4 signaling pathway
involves binding protein (BiP), phosphorylated eIF2α, ATF4 and
CCAAT-enhancer binding protein homologous protein (CHOP) activation
(14–16). Heat shock protein 70 (HSP70;
encoded by HSPA8), a member of the 70 kDa HSPs family, is a primary
chaperone involved in ER stress. HSP70 binds to and censors the
folding status of substrate membrane proteins that are synthesized
in the ER and transported to the cell surface via the conventional
ER-to-Golgi secretion pathway (17).
Anacardic acid (AA; also referred to as
6-pentadecylsalicylic acid) is a constituent of the traditional
medicinal plant Amphipterygium adstringens. Previous studies
have revealed that AA exerts anticancer effects in various
carcinomas (18,19). Previous studies by our laboratory
and others have demonstrated that AA induces ER stress (20,21).
In addition, it has been established that the ER stress inducer
fenretinide sensitizes tumor cells to Bor-mediated killing
(22). Therefore, the aim of the
present study was to assess whether AA enhances the anticancer
effects of Bor. AA was observed to significantly increase Bor
activity via enhancing ATF4-dependent ER stress-associated caspase
activation in vitro.
Materials and methods
Materials, reagents and antibodies
AA was manufactured by Sigma-Aldrich (St. Louis, MO,
USA). Bor (Ben Venue Laboratories, Inc.; Boehringer Ingelheim
Pharmaceuticals, Inc., Ridgefield, CT, USA) was used according to
the manufacturer's instructions. Fetal bovine serum (FBS),
RPMI-1640 and antibiotics were produced by Invitrogen; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). Rabbit polyclonal
anti-GAPDH antibody (clone, FL-335; catalog no. sc-25778; 1:500)
was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). The following were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA): Rabbit monoclonal antibodies against
nuclear poly (ADP-ribose) polymerase (PARP; clone, 46D11; catalog
no. 9532; 1:1,000), eIF2α (clone, D7D3; catalog no. 9079; 1:1,000),
phospho-eIF2α (Ser51; clone, D9G8; catalog no. 3398; 1:1,000), BiP
(clone, C50B12; catalog no. 3177; 1:1,000), ATF4 (clone, D4B8;
catalog no. 11,815; 1:1,000), caspase-3 (clone, 8G10; catalog no.
9665; 1:1,000) and caspase-8 (clone, D35G2; catalog no. 4790;
1:1,000); mouse monoclonal antibodies against caspase-9 (clone, C9;
catalog no. 9508; 1:1,000) and CHOP (clone, L63F7; catalog no.
2895; 1:1,000); and a rat monoclonal antibody against HSP70 (clone
6B3; catalog no. 4873; 1:1,000). Rabbit polyclonal antibodies
against active caspase-3 (catalog no. BS7004; 1:1,000), caspase-8
(catalog no. AP0358; 1:1,000) and caspase-9 (catalog no. BS7070;
1:1,000) were manufactured by Bioworld Technology, Inc. (St. Louis
Park, NM, USA). Horseradish peroxidase (HRP)-conjugated goat
anti-mouse IgG (catalog no. sc-395,763; 1:5,000), HRP-conjugated
goat anti-rabbit IgG (catalog no. sc-2004; 1:5,000) and
HRP-conjugated goat anti-rat IgG (catalog no. sc-2006; 1:5,000)
were purchased from Santa Cruz Biotechnology, Inc. The enhanced
chemiluminescence (ECL) kit was obtained from GE Healthcare Life
Sciences (Chalfont, UK). Propidium iodide (PI) and Caspase-3
Activity and Annexin V-fluorescein isothiocyanate (FITC) Apoptosis
Detection kits were manufactured by Nanjing Keygen Biotech Co.,
Ltd. (Nanjing, China).
Cell culture
U266 human myeloma cells were obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
as previously described (21). AA
and Bor were dissolved in dimethyl sulfoxide (DMSO) to a stock
concentration of 50 mM, aliquoted and stored at −80°C. Prior to
use, AA was diluted to 10, 20 and 30 mM; Bor was diluted to 25, 50
and 75 µM. During the treatment of each group, the
corresponding drugs were diluted 1:1,000 in medium, added to the
wells or plates and cultured at 37°C and 5% CO2 for the
indicated time.
3-(4,5-dimethylthiazol-2-yl-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay
Cytotoxicity was assessed by the MTS assay as
described previously (21,23). Exponentially growing cells were
seeded into 96-well plates (2,500/well) and incubated with drugs
for 48 h prior to assessment with MTS.
Flow cytometric analysis of cell
apoptosis
Exponentially growing cells were seeded into 6-well
plates (5×104/well) and incubated with drugs for 24 h.
Apoptosis was quantified in cells using Annexin V-FITC and PI
double staining as previously described (24). Stained U266 cells were assessed by
flow cytometry within 30 min. The data was analyzed using FACSDiva
software version 6.1.3 (BD Biosciences, Franklin Lakes, NJ,
USA).
Caspase-3 activity evaluation
Exponentially growing cells were seeded into 6-cm
dishes (1×106/well) and incubated with drugs for 24 h.
Caspase-3 activity was determined in U266 cell lysates using a
specific colorimetric assay kit according to the manufacturer's
instructions. Following drug treatment, 1×106 cells were
lysed with lysis buffer (Nanjing Keygen Biotech Co., Ltd.) and
submitted to centrifugation (10,000 × g, 4°C, 1 min). The
supernatants were harvested and the enzyme-specific substrate was
added at 37°C for 4 h. The resulting product was quantified on a
microplate reader at 405 nm.
RNA interference
CHOP or ATF-4 genes were silenced using small
interfering RNA (siRNA) technology as described previously
(21). CHOP/GADD153 siRNA (catalog
no. sc-35437), ATF4/CREB-2 siRNA (catalog no. sc-35112) and control
siRNA (catalog no. sc-37007), purchased from Santa Cruz
Biotechnology, Inc., were transfected separately into cells using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Western blot analysis
Exponentially growing cells were seeded into 6-cm
dishes (1×106/well) and incubated with drugs for 24 h.
Protein expression levels were determined as previously described
(25,26). Briefly, total protein extracts (40
µg) from U266 cell lysates were resolved by 12% SDS-PAGE
(100 V for 90 min) and transferred onto polyvinylidene difluoride
membranes. Membranes were blocked with 5% milk, and following
sequential incubations with primary and secondary antibodies, an
ECL kit was used for protein detection. Blots were quantified with
Image-Pro Plus software version 5.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Combination index assessment
The effects of AA and Bor were assessed by
evaluating the combination index (CI) using the Chou-Talalay
method, as described previously (26,27).
A CI of <1, 1 or >1 indicated synergistic, additive or
antagonistic effects, respectively.
Statistical analysis
Data are presented as the mean ± standard deviation.
One-way analysis of variance was utilized to compare groups, with
the least significant difference test being performed as a post
hoc test. Statistical analyses were performed using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
AA and Bor induce human myeloma U266 cell
killing in a synergistic fashion
To assess if AA alone causes myeloma cell death, the
effects of AA at various concentrations on MM cell viability were
assessed. Cell viability was inhibited <48% in U266 cells
treated with 30 µM AA as a monotherapy compared with DMSO
treatment (P=0.001; Fig. 1A).
Based on these findings, 10, 20 and 30 µM AA were
co-administered for 48 h with 25, 50 and 75 nM Bor. All CI results
were <0.8, except one CI value of 0.809 (Fig. 1B), indicating synergy between these
two agents in U266 cells. Doses of 20 µM AA and 50 nM Bor
were chosen for subsequent experiments, as these doses were
effective at reducing cell viability, but not to the extent that
further analysis would be impossible.
 | Figure 1Effects of AA, Bor and combination
therapy on cell viability. (A) U266 cells were incubated with Bor
(25, 50 or 75 nM) and AA (10, 20, or 30 µM) as monotherapy
or in combination for 48 h. Cell viability was assessed using the
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
assay. Cell viability was reduced by AA or Bor monotherapy, and by
AA and Bor in combination, in a dose-dependent manner. Data are
presented as the mean ± standard deviation from three independent
experiments. *P<0.05 vs. DMSO; #P<0.01
vs. respective AA monotherapy. (B) Combination index values were
determined, and revealed that AA and Bor act synergistically in
U266 cells. AA, anacardic acid; Bor, bortezomib; DMSO, dimethyl
sulfoxide. |
AA sensitizes U266 cells to Bor-mediated
caspase-dependent apoptosis
To investigate whether AA- and/or Bor-induced
cytotoxicity correlated with cell death, myeloma cells were
incubated with AA and/or BOR, and cell death was assessed using
Annexin V/PI double staining. Co-administration of Bor and AA
resulted in a significant increase in Annexin V and PI positive
cells compared with monotherapy (P<0.001; Fig. 2A and B), indicating that increased
cell death was the result of Bor and AA combination therapy. The
effects of combination therapy on cleavage of the apoptosis
mediators, caspase and PARP, were investigated by western blotting.
As presented in Fig. 2C, AA/Bor
co-administration resulted in markedly enhanced cleavage of
caspase-3, -8 and -9, as well as PARP, compared with monotherapies.
To confirm these results, caspase-3 activity in cell lysates was
assessed. AA/Bor combination therapy significantly increased
caspase-3 activity compared with monotherapies (P<0.001;
Fig. 2D). These results suggested
that AA sensitized U266 cells to Bor via caspase-dependent
apoptotic cell death.
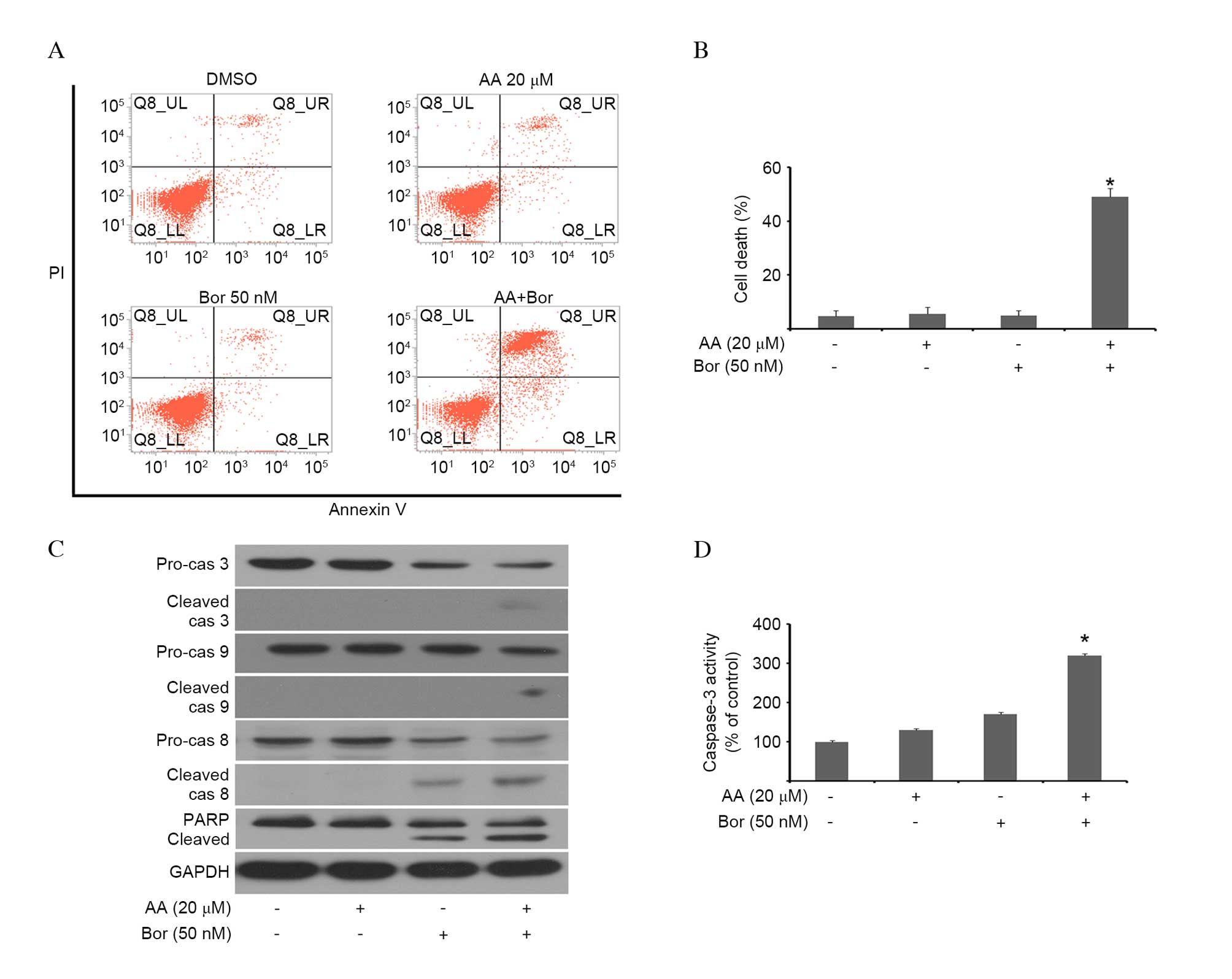 | Figure 2AA sensitizes U266 cells to
Bor-induced cytotoxicity. U266 cells were incubated with AA (20
µM), Bor (50 nM) or combination therapy for 24 h. (A) Cells
were stained with Annexin V and propidium iodide. Representative
flow cytograms are presented. Apoptotic cells were defined as those
in the upper left, upper right and lower right quadrants.
Co-administration of Bor and AA resulted in a significant increase
in Annexin V and PI positive cells compared with monotherapy. (B)
Flow cytometric analysis of (A), presented as the mean ± SD (n=3).
*P<0.01 vs. monotherapy. (C) Western blotting was
performed to assess the expression levels of various proteins, with
GAPDH serving as a loading control. Cleavage of caspase-3, -8 and
-9, and PARP, was increased following AA/Bor co-administration. (D)
Caspase-3 activity was assessed in U266 cells by colorimetric
assay, and was significantly increased upon AA/Bor combination
therapy. Data are presented as the mean ± SD (n=3).
*P<0.01 vs. monotherapy. AA, anacardic acid; Bor,
bortezomib; PI, propidium iodide; SD, standard deviation; cas,
caspase; PARP, poly (ADP-ribose) polymerase. |
AA/Bor combination therapy amplifies ER
stress
The effects of combination therapy on the UPR
signaling pathway in U266 cells were analyzed by western blotting
(Fig. 3). The expression levels of
HSP70 (P=0.027) and BiP (P=0.001) were significantly increased by
24 h compared with Bor monotherapy. Combination therapy induced
increased protein expression levels of CHOP, phospho-eIF2α and
ATF4. These findings suggest that ER stress is involved in AA/Bor
combination therapy-induced cell death.
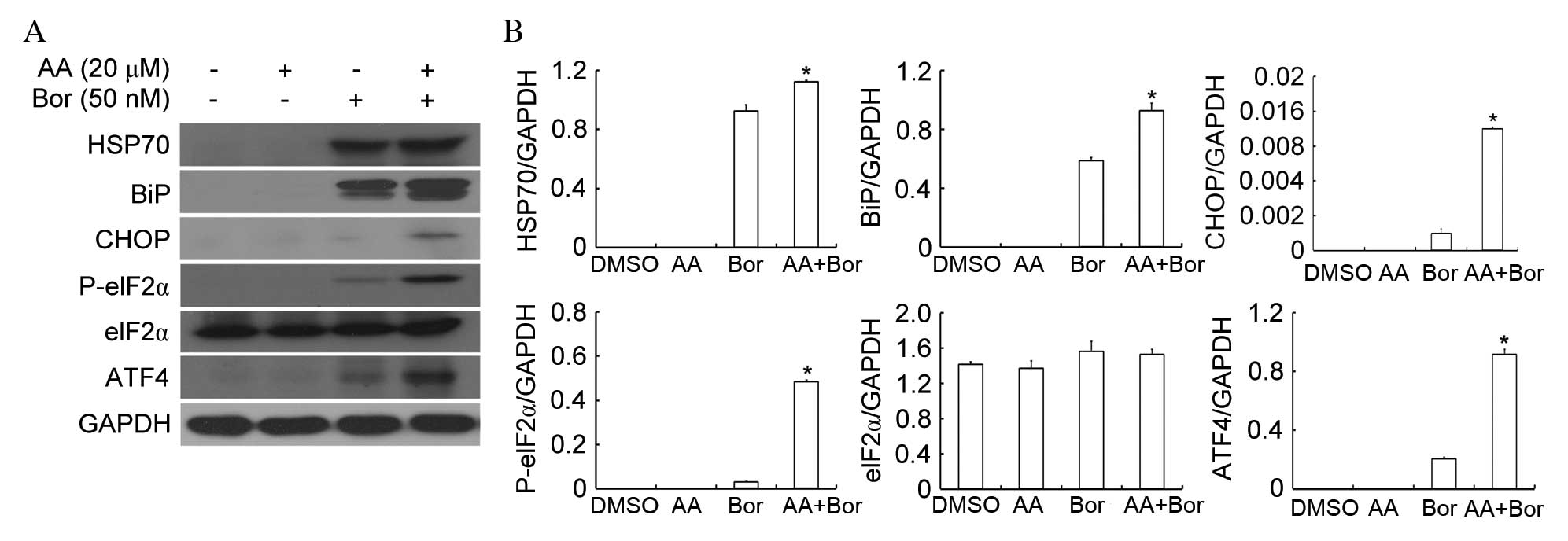 | Figure 3AA enhances Bor-induced ER stress.
U266 cells were incubated with AA (20 µM), Bor (50 nM) or
combination therapy for 24 h. (A) Western blotting was performed to
analyze protein expression levels of HSP70, BiP, CHOP, P-eIF2α,
eIF2α, ATF4 and GAPDH. (B) Protein bands were quantified and
normalized to GAPDH. The protein expression levels of HSP70, BiP,
CHOP, P-eIF2α and ATF4 were significantly increased by AA/Bor
combination therapy. *P<0.05 vs. monotherapy. Data
are presented as the mean ± standard deviation (n=3). AA, anacardic
acid; Bor, bortezomib; ER, endoplasmic reticulum; HSP70, heat shock
protein 70; BiP, binding protein; CHOP, CCAAT-enhancer binding
protein homologous protein; eIF2α, eukaryotic initiation factor 2α;
P, phosphorylated; ATF4, activating transcription factor 4; DMSO,
dimethyl sulfoxide. |
Role of ER stress in AA/Bor combination
therapy-mediated cytotoxicity
To identify UPR effectors involved in AA/Bor
combination therapy-mediated cell death, CHOP was silenced in U266
cells. Cells were then incubated for 24 h in the presence or
absence of AA/Bor combination therapy. CHOP siRNA inhibited CHOP
protein expression levels and slightly increased PARP cleavage in
U266 cells incubated with combination therapy, compared to cells
that received scrambled siRNA (Fig.
4A). In addition, CHOP silencing significantly increased the
cytotoxicity of combination therapy compared with scrambled siRNA
(P=0.008; Fig. 4B). These findings
suggested that CHOP was not the primary UPR signaling pathway
branch involved in U266 cell death mediated by AA/Bor combination
therapy.
 | Figure 4AA enhances Bor-mediated cytotoxicity
involving ATF4 but not CHOP. CHOP-silenced U266 cells were
incubated with AA (20 µM), Bor (50 nM) or combination
therapy for 24 h. (A) Western blotting was performed to assess the
protein expression levels of CHOP and PARP, with GAPDH serving as a
loading control. (B) Apoptotic cell death was assessed by flow
cytometry; CHOP silencing increased the cytotoxicity of combination
therapy. ATF4-silenced U266 cells were incubated with AA (20
µM), Bor (50 nM) or combination therapy for 24 h. (C)
Western blotting was performed to assess the protein levels of ATF4
and PARP, with GAPDH serving as a loading control. (D) Apoptotic
cell death was assessed by flow cytometry; ATF4 silencing decreased
the cytotoxicity of combination therapy. Data are presented as the
mean ± standard deviation (n=3). *P<0.05 vs.
scrambled siRNA. AA, anacardic acid; Bor, bortezomib; CHOP,
CCAAT-enhancer binding protein homologous protein; ATF4, activating
transcription factor 4; PARP, poly (ADP-ribose) polymerase; siRNA,
small interfering RNA; DMSO, dimethyl sulfoxide. |
The role of ATF4 in AA/Bor combination
therapy-mediated cell death was subsequently assessed. In contrast
to CHOP repression, ATF4 silencing decreased PARP cleavage
(Fig. 4C) and partially attenuated
AA/Bor combination therapy-mediated cytotoxicity compared with
scrambled siRNA (P=0.002; Fig.
4D). These data indicate that ATF4-dependent ER stress
contributed, at least partially, to AA/Bor combination
therapy-mediated cytotoxicity.
Discussion
Various novel natural compounds have been reported
to have synergistic anti-cancer cytotoxic effects when administered
in combination with Bor (26,28,29).
Our previous study demonstrated that AA is a potent inducer of ER
stress (21). Based on previous
findings that the ER stress inducer fenretinide sensitizes tumor
cells to killing by Bor (22), the
effect of AA/Bor combination therapy on U266 cells in vitro
was investigated, to examine the potential clinical application of
AA.
Inhibition of cell growth and promotion of apoptosis
constitute the primary mechanisms underlying the cytotoxicity of
cancer chemotherapeutics; therefore, the present study assessed
these effects. AA or Bor alone inhibited cell viability in a
dose-dependent manner. Notably, the combined inhibitory effects of
AA and Bor on cell viability were markedly greater compared with
those observed following AA and Bor monotherapies in vitro,
with CI values <0.8. In addition, Bor and AA combination therapy
significantly increased cancer cell apoptosis compared with AA or
Bor treatment alone. Proteasome inhibition by Bor induces caspase
activation; this constitutes an important mechanism underlying
Bor-induced cell death (30–32).
In the present study, combined treatment with AA and Bor activated
caspase-3, -8 and -9, and induced PARP cleavage in U266 cells.
AA/Bor co-administration promoted U266 apoptotic cell death via
intrinsic (mitochondria-mediated; associated with caspase-9) and
extrinsic (death receptor-mediated; associated with caspase-8)
pathways, reflected by increased activation of caspase-3, -8 and
-9, alongside PARP cleavage.
Certain studies have demonstrated that Bor activates
HSPs, including HSP90, HSP70 and HSP25, which are associated with
Bor resistance (33,34). Qi et al (35) reported that inhibition of inducible
HSP70 increases Bor-induced human bladder cancer cell cytotoxicity.
In the present study, AA/Bor combination therapy in U266 cells was
associated with increased HSP70 induction. These results support
the notion that enhancing Bor-mediated HSP70 induction represents
an attractive means of enhancing its activity.
Protein synthesis, folding and trafficking occurs
primarily in the ER; thus, intensive ER stress results in cell
death (9,10). AA and Bor are ER stress inducers
(21,22); therefore, it was investigated
whether combination therapy induced UPR signaling. BiP, CHOP,
phospho-eIF2α and ATF4 were all induced in U266 cells treated with
AA and Bor. A previous study revealed that the ER stress-induced
transcription factor ATF4 is a key mediator of Bor-induced
cytotoxicity in neuroectodermal tumor cells, while CHOP is
dispensable (14). Beck et
al (36) reported that
vemurafenib-induced melanoma cell death is associated with ATF4-
but not CHOP-dependent ER stress, in agreement with our previous
report (21). The effects of CHOP
and ATF4 in promoting apoptosis were investigated in the present
study. Consistent with previous reports, CHOP silencing failed to
reduce the cytotoxic activity of combination therapy, and instead
moderately enhanced this effect. However, ATF4 knockdown
significantly reduced the cytotoxic effects of AA/Bor combination
therapy. These findings demonstrate that ATF4 and CHOP are pro- and
anti-apoptotic, respectively, in AA/Bor combination
therapy-mediated cytotoxicity. However, future studies are required
to reveal the mechanisms underlying these effects.
In conclusion, the present study demonstrated that
AA sensitizes MM cells to Bor-mediated growth inhibition and
apoptotic cell death in vitro. Therefore, AA may have
potential applications as a chemosensitizer in human cancer
treatment. Future in-depth studies, including in vivo
experiments, are required to confirm the efficacy of AA in
combination with Bor for MM treatment.
Acknowledgments
The present study was supported by: The National
High Technology Research and Development Program of China (grant
no. 2006AA02Z4B5) and the National Natural Science Foundation of
China (grant nos. 81272451/H1609 and 81472762/H1609), awarded to
J.L.; and the National Natural Science Foundation of China (grant
no. 81472390/H1619), General Project from Guangzhou Education
Commission (grant no. 1201410188), the Science and Technology
Program of Guangzhou (grant no. 201510010127) and the Science and
Technology Planning Project of Guangdong Province (grant no.
2014A020212691), awarded to H.H. The authors thank Guangdong
Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene
Regulation, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University
(Guangzhou, China) for assistance with flow cytometry.
References
|
1
|
Sultan S, Irfan SM, Parveen S, Ali H and
Basharat M: Multiple Myeloma: A retrospective analysis of 61
patients from a tertiary care center. Asian Pac J Cancer Prev.
17:1833–1835. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu JD, Sun CY, Tang L, Wu YY, Wang QY, Hu
B and Hu Y: Efficacy and safety of panobinostat in relapsed or/and
refractory multiple myeloma: Meta analyses of clinical trials and
systematic review. Sci Rep. 6:273612016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hershko A and Ciechanover A: The ubiquitin
system. Annu Rev Biochem. 67:425–479. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adams J: The development of proteasome
inhibitors as anticancer drugs. Cancer Cell. 5:417–421. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adams J, Palombella VJ, Sausville EA,
Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S and
Elliott PJ: Proteasome inhibitors: A novel class of potent and
effective antitumor agents. Cancer Res. 59:2615–2622.
1999.PubMed/NCBI
|
|
6
|
Orlowski RZ and Dees EC: The role of the
ubiquiti-nation-proteasome pathway in breast cancer: Applying drugs
that affect the ubiquitin-proteasome pathway to the therapy of
breast cancer. Breast Cancer Res. 5:1–7. 2003. View Article : Google Scholar
|
|
7
|
Jagannathan S, Abdel-Malek MA, Malek E,
Vad N, Latif T, Anderson KC and Driscoll JJ: Pharmacologic screens
reveal metformin that suppresses GRP78-dependent autophagy to
enhance the anti-myeloma effect of bortezomib. Leukemia.
29:2184–2191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eriksson E, Wickström M, Perup LS, Johnsen
JI, Eksborg S, Kogner P and Sävendahl L: Protective role of humanin
on bortezomib-induced bone growth impairment in anticancer
treatment. J Natl Cancer Inst. 106:djt4592014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang WC, Lin YS, Chen CL, Wang CY, Chiu
WH and Lin CF: Glycogen synthase kinase-3beta mediates endoplasmic
reticulum stress-induced lysosomal apoptosis in leukemia. J
Pharmacol Exp Ther. 329:524–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang C, Zhang S, Liu H, Zeng Q, Xia T,
Chen Y, Kuang G, Zhao G, Wu X, Zhang X, et al: The role of the IRE1
pathway in PBDE-47-induced toxicity in human neuroblastoma SH-SY5Y
cells in vitro. Toxicol Lett. 211:325–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Armstrong JL, Flockhart R, Veal GJ, Lovat
PE and Redfern CP: Regulation of endoplasmic reticulum
stress-induced cell death by ATF4 in neuroectodermal tumor cells. J
Biol Chem. 285:6091–6100. 2010. View Article : Google Scholar :
|
|
15
|
Jiang HY and Wek RC: Phosphorylation of
the alpha-subunit of the eukaryotic initiation factor-2 (eIF2alpha)
reduces protein synthesis and enhances apoptosis in response to
proteasome inhibition. J Biol Chem. 280:14189–14202. 2005.
View Article : Google Scholar
|
|
16
|
Qing G, Li B, Vu A, Skuli N, Walton ZE,
Liu X, Mayes PA, Wise DR, Thompson CB, Maris JM, et al: ATF4
regulates MYC-mediated neuroblastoma cell death upon glutamine
deprivation. Cancer Cell. 22:631–644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung J, Kim J, Roh SH, Jun I, Sampson RD,
Gee HY, Choi JY and Lee MG: The HSP70 co-chaperone DNAJC14 targets
misfolded pendrin for unconventional protein secretion. Nat Commun.
7:113862016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, He L, Zhang L, Chen J, Yi Z, Zhang
J, Liu M and Pang X: Anacardic acid (6-pentadecylsalicylic acid)
inhibits tumor angiogenesis by targeting Src/FAK/Rho GTPases
signaling pathway. J Pharmacol Exp Ther. 339:403–411. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seong YA, Shin PG and Kim GD: Anacardic
acid induces mitochondrial-mediated apoptosis in the A549 human
lung adenocarcinoma cells. Int J Oncol. 42:1045–1051.
2013.PubMed/NCBI
|
|
20
|
Seong YA, Shin PG, Yoon JS, Yadunandam AK
and Kim GD: Induction of the endoplasmic reticulum stress and
autophagy in human lung carcinoma A549 cells by anacardic acid.
Cell Biochem Biophys. 68:369–377. 2014. View Article : Google Scholar
|
|
21
|
Huang H, Hua X, Liu N, Li X, Liu S, Chen
X, Zhao C, Lan X, Yang C, Dou QP, et al: Anacardic acid induces
cell apoptosis associated with induction of ATF4-dependent
endoplasmic reticulum stress. Toxicol Lett. 228:170–178. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hill DS, Martin S, Armstrong JL, Flockhart
R, Tonison JJ, Simpson DG, Birch-Machin MA, Redfern CP and Lovat
PE: Combining the endoplasmic reticulum stress-inducing agents
bortezomib and fenretinide as a novel therapeutic strategy for
metastatic melanoma. Clin Cancer Res. 15:1192–1198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang H, Liu N, Guo H, Liao S, Li X, Yang
C, Liu S, Song W, Liu C, Guan L, et al: L-carnitine is an
endogenous HDAC inhibitor selectively inhibiting cancer cell growth
in vivo and in vitro. PLoS One. 7:e490622012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang H, Zhang X, Li S, Liu N, Lian W,
McDowell E, Zhou P, Zhao C, Guo H, Zhang C, et al: Physiological
levels of ATP negatively regulate proteasome function. Cell Res.
20:1372–1385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu N, Li X, Huang H, Zhao C, Liao S, Yang
C, Liu S, Song W, Lu X, Lan X, et al: Clinically used antirheumatic
agent auranofin is a proteasomal deubiquitinase inhibitor and
inhibits tumor growth. Oncotarget. 5:5453–5471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang H, Chen D, Li S, Li X, Liu N, Lu X,
Liu S, Zhao K, Zhao C, Guo H, et al: Gambogic acid enhances
proteasome inhibitor-induced anticancer activity. Cancer Lett.
301:221–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Q, Li J, Gu J, Huang B, Zhao Y, Zheng
D, Ding Y and Zeng L: Potentiation of
(−)-epigallocatechin-3-gallate-induced apoptosis by bortezomib in
multiple myeloma cells. Acta Biochim Biophys Sin (Shanghai).
41:1018–1026. 2009. View Article : Google Scholar
|
|
29
|
Ma C, Mandrekar SJ, Alberts SR, Croghan
GA, Jatoi A, Reid JM, Hanson LJ, Bruzek L, Tan AD, Pitot HC, et al:
A phase I and pharmacologic study of sequences of the proteasome
inhibitor, bortezomib (PS-341, Velcade), in combination with
paclitaxel and carboplatin in patients with advanced malignancies.
Cancer Chemother Pharmacol. 59:207–215. 2007. View Article : Google Scholar
|
|
30
|
Fribley A, Zeng Q and Wang CY: Proteasome
inhibitor PS-341 induces apoptosis through induction of endoplasmic
reticulum stress-reactive oxygen species in head and neck squamous
cell carcinoma cells. Mol Cell Biol. 24:9695–9704. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nawrocki ST, Carew JS, Dunner K Jr, Boise
LH, Chiao PJ, Huang P, Abbruzzese JL and McConkey DJ: Bortezomib
inhibits PKR-like endoplasmic reticulum (ER) kinase and induces
apoptosis via ER stress in human pancreatic cancer cells. Cancer
Res. 65:11510–11519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao X, Qiu W, Kung J, Zhao X, Peng X,
Yegappan M, Yen-Lieberman B and Hsi ED: Bortezomib induces
caspase-dependent apoptosis in Hodgkin lymphoma cell lines and is
associated with reduced c-FLIP expression: A gene expression
profiling study with implications for potential combination
therapies. Leuk Res. 32:275–285. 2008. View Article : Google Scholar
|
|
33
|
Shringarpure R, Catley L, Bhole D, Burger
R, Podar K, Tai YT, Kessler B, Galardy P, Ploegh H, Tassone P, et
al: Gene expression analysis of B-lymphoma cells resistant and
sensitive to bortezomib. Br J Haematol. 134:145–156. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Calvaruso G, Giuliano M, Portanova P,
Pellerito O, Vento R and Tesoriere G: Hsp72 controls
bortezomib-induced HepG2 cell death via interaction with
pro-apoptotic factors. Oncol Rep. 18:447–450. 2007.PubMed/NCBI
|
|
35
|
Qi W, White MC, Choi W, Guo C, Dinney C,
McConkey DJ and Siefker-Radtke A: Inhibition of inducible heat
shock protein-70 (hsp72) enhances bortezomib-induced cell death in
human bladder cancer cells. PLoS One. 8:e695092013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beck D, Niessner H, Smalley KS, Flaherty
K, Paraiso KH, Busch C, Sinnberg T, Vasseur S, Iovanna JL, Driessen
S, et al: Vemurafenib potently induces endoplasmic reticulum
stress-mediated apoptosis in BRAFV600E melanoma cells. Sci Signal.
6:ra72013. View Article : Google Scholar : PubMed/NCBI
|


















