Introduction
The permanent (also know as the metanephric) kidney
begins to develop on embryonic day 11 (E11) in mice, and around E35
in humans (1). At this stage, the
metanephric blastema induces the caudal portion of the Wolffian (or
mesonephric) duct to evaginate. An epithelial tubule forms, which
is known as the ureteric bud, and invades the adjacent metanephric
mesenchyme. During development, the branching ureteric bud tips
induce the surrounding nephron progenitors of the metanephric
blastema to proliferate and differentiate into nephrons. Signaling
between the ureteric tip and the surrounding metanephric mesenchyme
regulates the development of structures within the kidney (2,3). The
absorption of the ureteric bud, a process termed renal branching
morphogenesis, ultimately constitutes the mature collecting duct
system and is regulated by this signaling pathway (3). In addition, signaling between these
tissues promotes the transformation of the metanephric mesenchyme
to form the epithelial components, including the glomerulus and the
distal tubule, in a process known as nephrogenesis (2,4).
The morphogen sonic hedgehog-smoothened (SHH-SMO)
signaling pathway serves an important role during mammalian kidney
development. Binding of the SHH ligand to its receptor, patched
(PTC), activates the transmembrane protein SMO, resulting in the
modulation of the glioma-associated oncogene 1 (GLI1), GLI2 and
GLI3, as well as in the processing and binding of GLI activators
and repressors to SHH target genes (5,6).
Antagonists of SMO have been demonstrated to affect downstream
SHH-SMO pathway regulation, and the most clinically advanced SMO
targeting agents compete with cyclopamine, an SHH-SMO receptor
inhibitor (7). SHH gene deletion
mutations in humans have been linked to kidney malformations, such
as hydroureter (8,9). Aberrant SHH signaling is considered
to be associated with VACTERL syndrome, which is characterized by
renal anomalies (10). In mice,
homozygous inactivation of SHH generates a series of defects,
including renal aplasia or dysplasia (11). The presence of renal
hypoplasia/dysplasia in the ureteric bud lineage of SHH-deficient
mice demonstrates a crucial role for SHH signaling during mammalian
renal development (11). However,
the mechanisms by which the SHH protein modulates kidney
development in mice remain to be elucidated. Therefore,
understanding the functional role of the SHH protein in renal
morphogenesis may provide a novel strategy for preventing and
alleviating congenital renal diseases.
Fibroblast growth factors (Fgfs) and their receptors
are also known to serve key roles in kidney morphogenesis. A number
of Fgf ligands, particularly Fgf2, Fgf7, Fgf8 and Fgf10, are
secreted by the mesenchymal and ureteric epithelium in the
developing kidney (12–15). Knockout studies in mice have
demonstrated the importance of Fgf signaling in the embryonic
kidney. Targeted deletion of Fgf7 or Fgf10 (13,14),
or their corresponding receptors (Fgfr1 and Fgfr2, respectively)
(16,17), results in a reduction in the number
of collecting duct branches and loss of the metanephric mesenchyme.
The absence of Fgf8 from the metanephric mesenchyme also leads to a
decrease in kidney size due to the disruption in nephron formation
subsequent to the renal vesicle stage (12,18).
In nonrenal tissues, an increasing number of studies
have been investigating the interaction between SHH and Fgf
proteins during embryonic development (19–22).
However, the functional association between SHH and Fgf proteins in
the kidney has not been elucidated. In the present study, the
effects of exogenous SHH on Fgf8 and Fgf10 expression levels were
investigated using mouse embryonic kidney tissue culture
techniques, in order to further understand the regulation of Fgf
gene expression during the kidney development.
Materials and methods
Animal preparation
A total of 28 virgin female BALB/c mouse (obtained
from and bred at Biomedical Facility of Shandong University, Jinan,
China; 12 h light/dark cycle; temperature, 24°C; weight, 20–35 g;
age, 60 days) were naturally mated overnight and vaginal plugs were
identified the following morning. Plug-positive females were
transferred to single cages and then sacrificed by cervical
dislocation at embryonic day 11.5 (E11.5). Embryonic kidney tissues
were obtained between E11.5 and E14.5 and then randomly divided
into the control [8 female mice; 1% bovine serum albumin (BSA) in
culture medium for 4 days], SHH-treatment (10 female mice; 1.0
µg/ml SHH protein in culture medium for 4 days) and
cyclopamine-treatment groups (10 female mice; 1.0 µg/ml SHH
protein and 10 µM cyclopamine in culture medium for 4 days).
At least 5 fetuses were obtained from 1 pregnant mouse and 2
embryonic kidneys were obtained from each fetal mouse.
Metanephric explant cultures and surface
area measurements
BALB/c mouse kidney tissues were obtained from E11.5
embryos using a fine needle syringe and cultured for up to 4 days
on polycarbonate filters (0.45 µm; EMD Millipore, Billerica,
MA, USA) and on simple Trowell culture grids, as described in a
previous paper. The tissues were maintained in Dulbecco's modified
Eagle's medium-Ham's F12 nutrient mixture, 10% fetal bovine serum
(FBS; Hyclone; GE Healthcare Life Sciences, Chalfont, UK) and
penicillin/streptomycin solution (100 U/ml penicillin and 0.1 mg/ml
streptomycin; Beyotime, Institute of Biotechnology, Jiangsu,
China). Cyclopamine (cat. no. 239803; EMD Millipore) was dissolved
in dimethyl sulfoxide and added to the culture medium at a final
concentration of 10 µM. SHH protein (cat. no. 461-SH;
R&D Systems, Inc., Minneapolis, MN, USA) was dissolved in 1%
BSA (cat. no. ST023; Beyotime, Institute of Biotechnology, Jiangsu,
China) and added to the culture medium at a final concentration of
1 µg/ml for 96 h. The planar surface area was calculated
from images of the tissues using the Scion Image version 4.03
software (Scion Co., Frederick, MD, USA), and converted to
measurements in mm2 (23). All experimental procedures were
performed in accordance with the Guide for the Care and Use of
Laboratory Animals (publication no. 85–23, revised 1996; US
National Institutes of Health, Bethesda, MD, USA), and were
approved by the Institutional Committee for Use and Care of
Laboratory Animals of Shandong University (Jinan, China).
Histology and immunohistochemistry
(IHC)
Mouse embryonic kidney tissue specimens were fixed
in 4% formaldehyde at 4°C, dehydrated, embedded in paraffin wax and
cut into 5-µm sections. Following hematoxylineosin (HE)
staining, tissue sections were analyzed for histological features.
For IHC and immunofluorescence analyses, the tissue sections were
dewaxed using xylene and rehydrated using a graded ethanol series.
Antigens were retrieved by boiling the tissue sections in citrate
buffer at 98°C for 30 min. The sections were then incubated in 3%
H2O2 for 30 min to inhibit endogenous
peroxidase activity, and blocked with 3% BSA in phosphate-buffered
saline (PBS). Subsequently, sections were incubated overnight at
4°C with polyclonal rabbit anti-Fgf8 (dilution, 1:100; cat. no.
ABIN1107218; Bioss Inc., Woburn, MA, USA) and polyclonal rabbit
anti-Fgf10 (dilution, 1:100; cat. no. ABIN392510; Bioss Inc.)
primary antibodies. Goat anti-rabbit antibodies IgG (dilution,
1:1,000; cat. no. ZDR5209; Zhongshanjinqiao, Beijing, China) were
used as secondary antibodies. The tetramethylrhodamine
(TRITC)-conjugated Dolichos biflorus agglutinin (DBA)-lectin
(dilution, 1:2,000; cat. no. L9658; Sigma-Aldrich, St. Louis, MO,
USA) was used for immunofluorescence analysis. Nuclei were then
counterstained with DAPI.
Calculation of ureteric bud branch points
and the number of nephrons
A total of 20 tissue culture explants were embedded
in paraffin and sectioned at 5 µm. Half of the samples were
stained with HE to determine the number of nephrons according to
the method described by Hoy et al (24), while others were stained with
TRITC-conjugated DBA-lectin to visualize the ureteric buds. The
branch points of the nephric duct were then counted in a
double-blind study (4 pregnant mice were included in each
group).
Microscope and image analysis
Sections of metanephric kidney were visualized and
images were captured using a JVC KY-F70 digital camera (JVC, Wayne,
NJ, USA) attached to a Leitz DMRB microscope (Leica Microsystems,
Wetzlar, Germany), or a Nikon DXM1200 digital camera on a Nikon
SMZ1500 stereoscope (Nikon Corp., Tokyo, Japan). Fgf8 and Fgf10
protein expression levels in metanephric explant tissue sections
were subjected to microscopic analysis. Briefly, following IHC
staining, tissues that were stained purple were selected for
analysis. These regions were visualized and staining intensities
were quantified using the Image-Pro Plus image analysis software
version 7.0 (Media Cybernetics, Inc., Silver Spring, MD, USA). The
mean densitometries of the digital images (magnification, ×400)
were considered to represent the Fgf8/Fgf10 staining intensities,
and were used to quantify the relative protein expression levels.
The staining intensities of tissue areas from 10 randomly-selected
fields of view were counted blindly and subjected to statistical
analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Mouse embryonic kidneys were harvested between E11.5
and E14.5, and tissues were collected for culturing. Total RNA was
extracted using the RNAiso Plus Reagent (cat. no. 9108; Takara Bio,
Inc., Tokyo, Japan). A total of 500 ng RNA was reverse transcribed
into first strand cDNA using the Primescript RT reagent kit (cat.
no. DRR037A; Takara Bio, Inc.). SYBR Premix Ex Taq (10 µl;
cat. no. DRR041A; Takara Bio, Inc.) was added to the qPCR reaction
mixture, including 0.4 µl forward primer (10 µM;
Takara Bio Inc.), 0.4 µl reverse primer (10 µM;
Takara Bio Inc.), 2 µl cDNA and 7.2 µl distilled
H2O, at a final volume of 20 µl. The primers used
to detect SHH, Fgf8 and Fgf10 mRNA expression levels are listed in
Table I. Thermal cycling was
performed using the Roche LightCycler 480 system (Roche, Basel,
Switzerland). The mRNA expression levels of each target gene were
determined using a calibration curve of standards, and expressed
relative to GAPDH expression levels.
 | Table IPrimer sequences used for polymerase
chain reaction. |
Table I
Primer sequences used for polymerase
chain reaction.
| Gene | Forward
(5′→3′) | Reverse
(5′→3′) |
|---|
| SHH |
AGCAGACCGGCTGATGACTC |
TCACTCCAGGCCACTGGTTC |
| Fgf8 |
CATCAACGCCATGGCAGAA |
TCTCCAGCACGATCTCTGTGAATAC |
| Fgf10 |
CTGACACATGACCATGGACCAC |
TCCAACGTCTGCACTATTTGCTG |
| GAPDH |
AAATGGTGAAGGTCGGTGTGAAC |
CAACAATCTCCACTTTGCCACTG |
Western blot analysis
Mouse metanephric kidney tissue samples were
homogenized and protein expression levels were determined using
western blot analysis, as previously described (7). Briefly, frozen (−80°C) kidney tissues
were washed with PBS and lysed with radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, China)
containing 50 mM Tris (pH 7.4), 150 mM sodium chloride, 1% Triton
X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS)
and 100 mM phenylmethanesulfonyl fluoride. Protein concentrations
were determined using a bicinchoninic acid protein assay (Thermo
Fisher Scientific, Inc., Rockford, IL, USA), with BSA as the
protein standards. Equal amounts of 50 µg total protein were
subjected to 12% SDS-polyacrylamide gel electrophoresis and
electrotransferred to polyvinylidene fluoride membranes (EMD
Millipore). The membranes were the blocked using 5% skimmed milk
and incubated with primary antibodies at 4°C overnight.
Subsequently, the membranes were washed three times with
Tris-buffered saline and Tween 20 for 10 min, then treated with
horseradish peroxidase-conjugated secondary antibodies for 60 min
at 37°C and visualized using an electrochemical-luminescence method
(Thermo Fisher Scientific, Inc.). Antibodies used for western blot
analysis included the following: polyclonal rabbit anti-Fgf8
(dilution, 1:1,000; cat. no. ABIN1107218; Bioss Inc.), polyclonal
rabbit anti-Fgf10 (dilution, 1:1,000; cat. no. ab71794; Abcam,
Cambridge, MA, USA), mouse monoclonal anti-β-actin (dilution,
1:5,000; cat. no. A5441; Sigma-Aldrich) primary antibodies, and
secondary goat anti-rabbit IgG (dilution, 1:2,000; cat. no.
SA00001-2; ProteinTech Group, Inc., Chicago, IL, USA) or goat
anti-mouse IgG antibodies (dilution, 1:2,000; cat. no. SA00001-1;
ProteinTech Group, Inc.).
Statistical analysis
Statistically significant differences between the
experimental and control groups were calculated using the Student's
t-test or one-way analysis of variance followed by pair-wise
multiple comparisons with the least-significant difference method
using SPSS software (version 13.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Planar surface areas of metanephric
explants
Embryonic metanephric explants were obtained under
sterile conditions from pregnant BALB/c mice at the E11.5
developmental stage. Between E11.5 and 14.5, the planar surface
area of metanephric explants, which is a valid approximation of
kidney size, increased in a time-dependent manner (P=0.012;
Fig. 1). The increase in the
planar surface area of the explants was used to monitor growth due
to its correlation with kidney volume and ureteric bud
branching.
Expression levels of SHH, Fgf8 and Fgf10
mRNA between E11.5 and E14.5
As shown in Fig. 2,
the expression levels of SHH, Fgf8 and Fgf10 mRNA in mouse
embryonic metanephric explants increased between E11.5 and E14.5,
as determined by RT-qPCR. At E11.5, the expression levels of all
three genes were relatively low. As demonstrated in Fig. 2A, the expression of SHH increased
at E12.5, however, this did not reach statistical significance when
compared with other developmental time points (P=0.140; Fig. 2A). By contrast, at E13.5 and E14.5,
SHH expression increased significantly compared with the E11.5 time
points (P=0.041 and P=0.0008, respectively; Fig. 2A). Similarly, the expression of
Fgf8 increased at E12.5, but with no statistically significant
difference observed (P=0.067; Fig.
2B). By contrast, at E13.5 and E14.5, the expression levels of
Fgf8 increased significantly compared with those at E11.5 or E12.5
(P=0.032 and P=0.006, respectively; Fig. 2B). Furthermore, the expression
levels of Fgf10 mRNA at E12.5, E13.5 and E14.5 were significantly
increased compared with the level at E11.5 (P=0.0008, P=0.006 and
P=0.0009, respectively; Fig. 2C);
however, no significant diference in Fgf10 expression was observed
between the E12.5 and E14.5 time points (P=0.110; Fig. 2C).
Effect of exogenous SHH on ureteric bud
branching morphogenesis and nephrogenesis
Compared with the control tissues, mouse embryonic
kidney explants treated with exogenous SHH protein demonstrated a
significant increase in the number of ureteric bud branches
(Fig. 3A–C) and the number of
nephrons (Fig. 3D–F). The number
of formed ureteric bud branch points increased by 25% following
treatment with SHH (P=0.032; Fig.
3C). In addition, histological analysis of the embryonic kidney
sections revealed that SHH protein treatment significantly
increased the number of nephrons by 35% (P=0.022; Fig. 3F). These results suggest that
SHH-SMO signaling serves a crucial role during ureteric bud
branching morphogenesis and nephrogenesis.
Effect of exogenous SHH on Fgf8 and Fgf10
mRNA expression levels
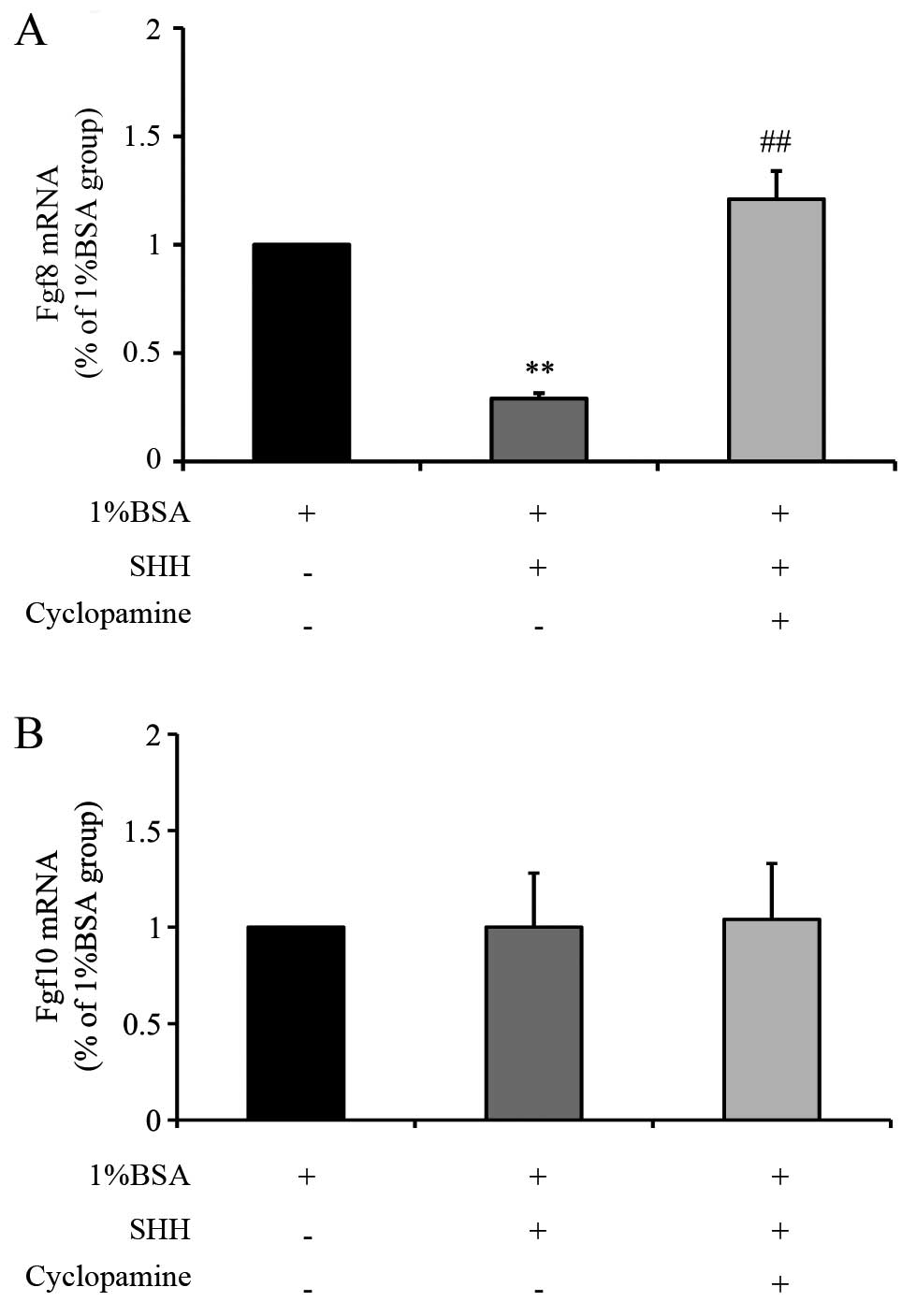
Compared with the control tissues, treatment of
embryonic kidney explants with exogenous SHH protein significantly
reduced the Fgf8 mRNA expression by 71% (P=0.007; Fig. 4A). By contrast, exposure to
cyclopamine was associated with a significant increase in Fgf8 mRNA
expression by 417% (P=0.009; Fig.
4A) compared with the SHH-group. However, no significant
alterations in the expression levels of Fgf10 mRNA were observed
following the addition of SHH protein alone or in combination with
cyclopamine (P=0.31 and P=0.27, respectively; Fig. 4B). These results indicate that
exogenous SHH protein reduced Fgf8 mRNA expression but had little
effect on Fgf10 expression.
Effect of exogenous SHH on Fgf8 and Fgf10
protein expression levels
IHC staining demonstrated positive Fhg8 expression
primarily in the nephrons and regions of the renal tubules of mouse
embryonic kidney tissue explants (Fig.
5A). Compared with control group, the integral optical density
(IOD) values of Fgf8 staining decreased by 24% in the SHH-treated
group (P=0.028; Fig. 5B), while
the IOD values were increased by 46% in the SHH +
cyclopamine-treated group (P=0.013; Fig. 5B). In contrast to Fgf8, Fgf10
protein expression was detected primarily in the renal tubules
(Fig. 5A). However, no significant
difference in the IOD values for Fgf10 was observed between the
control and treatment groups (Fig.
5B). Western blot analysis demonstrated that SHH treatment was
associated with a significant reduction in Fgf8 protein expression
levels by 40% compared with the control group (P=0.006; Fig. 5C and D), whereas the addition of
cyclopamine significantly increased the Fgf8 protein expression
levels compared with the group treated with SHH alone (P=0.005;
Fig. 5C and D). However, no
significant alterations in the protein expression levels of Fgf10
were observed in the groups treated with SHH alone or with SHH +
cyclopamine compared with the control group (P=0.093; Fig. 5C and D).
Discussion
In the present study, it was demonstrated that
exogneous SHH protein treatment of mouse embryonic kidney explants
was associated with a significant increase in the number of the
ureteric bud branches and the number of nephrons. In addition,
exogenous SHH protein treatment significantly reduced the Fgf8 mRNA
and protein expression levels, but had no significant effect on the
Fgf10 levels. These results demonstrated that SHH is involved in
the modulation of renal morphogenesis and Fgf8 expression in BALB/c
mouse metanephric explant cultures, thus suggesting that SHH is an
important developmental regulator of renal morphogenesis.
A previous study has demostrated that kidney
development and gene expression patterns in ex vivo organ
culture recapitulates early development in vivo (25). The ureteric bud grows and branches
as part of a process known as branching morphogenesis, which
establishes the collecting duct network of the kidney. A reciprocal
signaling network exists between the ureteric bud and the
metanephric mesenchymal tissues, which is responsible for
establishing the final structure of the kidney and forming the
majority of nephrons. Mouse metanephric explant models have been
used extensively for the analysis of branching morphogenesis and
nephron formation in response to exogenous factors that can be
directly added to the culture medium, such as growth factors,
agonists or inhibitors (23,25).
SHH is an inhibitory ligand for the PTC receptor,
which constituitively inhibits SMO. Following activation by SHH
binding, SMO inhibits the processing of full-length GLI3 to a
smaller protein product that functions to repress target gene
transcription, and stimulates translocation of GLI1 and GLI2 to the
nucleus. In the nucleus, GLIs activate transcription of multiple
downstream effectors, such as Pax2 and Sall1 (1). These factors are required for nephron
development, and loss of their function in the developing
metanephric mesenchyme results in renal agenesis or hypoplasia
(26,27). According to the results of the
present study, SHH gene expression levels were low at the E11.5 and
E12.5 developmental stages, and increased at E13.5 and at E14.5.
This suggests that SHH serves an important role during the
formation of ureteric bud branches and nephrons, but may not be
required for the development of mesonephros and its derivatives.
This is consistent with the findings of previous in vivo
studies (11,28). The number of ureteric bud branches
has been demonstrated to affect the number of formed nephrons
(2–4). Following addition of SHH protein into
the culture, there was an increase in ureteric bud formation, which
may have led to the observed increase in nephron formation. Based
on the DBA and HE staining results of the present study, the
addition of SHH protein to the culture of explanted mouse embryonic
kidney tissues increased the number of ureteric bud branches and
enhanced the formation of nephrons. Consistent with these
observations, the results of the in vitro experiments
suggested that enhancing SHH signaling may promote kidney
development.
Fgf8 is expressed in the early metanephric
mesenchyme, and is therefore an attractive candidate ligand for
regulating nephron development. A previous study demonstrated that
Fgf8 promotes renal mesenchymal-epithelial transformation and
condensation; however, early kidney development is not dependent on
Fgf8 signaling (12). In the
absence of Fgf8, mouse embryonic kidney development is normal until
the vesicle formation stage (12).
According to the results of the present study, there was no
significant difference in Fgf8 mRNA expression between E11.5 and
E12.5. However, the expression of Fgf8 mRNA increased significantly
at E13.5 and E14.5, which is consistent with its established
function in nephron formation (12). In a previous study, Fgf8 was
demonstrated to be expressed in metanephric mesenchymal tissues
around the ureteric bud at E11.5, in the tubule precursor at E12.0,
and in the small tubule precursor condensates, comma-shaped and
s-shaped bodies, and glomerular epithelial cells at E14.5 (29). Knockout of Fgf8
(Fgf8−/−) in mice has been shown to lead to
prenatal lethality, and to be associated with the development of
small kidneys and abnormal nephron formation (30). By contrast, Fgf10 is widely
expressed in mesenchymal and epithelial tissues, which suggests
that Fgf10 may function as an epithelial-mesenchymal signaling
molecule (13,31). Global targeting of Fgf10 led to
perinatal lethality caused by severe dysgenesis/agenesis of the
lungs and limbs (32).
Furthermore, Fgf10 knockout mice were observed to develop smaller
kidneys and fewer collecting ducts (13). As demonstrated in the present
study, Fgf10 mRNA expression levels were relatively low at E11.5;
however, they increased and remained high between E12.5 and E14.5.
Consistent with a previous study (33), this suggests that Fgf10 primarily
functions to regulate the formation of ureteric buds but not
nephron formation. In addition, during the process of limb
induction and heart formation, Fgf8 and Fgf10 have reciprocal
regulatory functions, which emphasizes the critical role of Fgf
proteins in organ development (34,35).
Therefore, the results of the present study provide evidence
demonstrating that Fgf8 and Fgf10 serve key functional roles at
distinct stages of kidney development.
The effect of exogenous SHH protein on Fgf8 and
Fgf10 protein and mRNA expression levels in mouse embryonic kidney
tissue explants were investigated in the present study. The culture
medium of tissue explants was supplemented with cyclopamine (an SMO
receptor inhibitor) to block SHH signal transduction, and a mouse
recombinant SHH protein to stimulate SHH-SMO signaling. The results
demonstrated that treatment with SHH protein was associated with a
significant decrease in Fgf8 mRNA and protein expression levels,
whereas treatment with SHH in combination with cyclopamine was
associated with no significant alterations in these expression
levels compared with the control group. This suggests that SHH may
negatively regulate Fgf8 expression levels, which is consistent
with the results presented by Urban et al (36), demonstrating that ectopic SHH
treatment inhibited the Fgf8 expression in aquatic larvae. Reduced
Fgf8 signals may increase nephron precursor cell apoptosis, thereby
decreasing the number of nephrons during the advanced stages of
kidney development (36).
In conclusion, the results of the present study
suggested that the SHH protein serves a crucial role in regulating
ureteric branching and nephron formation in metanephric explant
tissues from BALB/c mice. In addition, SHH may inhibit Fgf8
expression in the developing kidney. This signaling interaction may
explain, in part, the regulatory effects of SHH protein expression
on Fgf signaling in vitro (37). The results of the present study
also suggest that SHH may serve an indirect functional role during
nephrogenesis, similar to its indirect role during head ectoderm
development (21). Despite the
fact that the association between SHH and Fgf signaling in
vivo remains unclear, the present study demonstrated the
presence of an SHH-Fgf signaling pathway that functions to regulate
embryonic kidney development. These observations may provide
valuable information for the prevention and therapeutic targeting
of congenital kidney malformations.
Acknowledgments
The present study was financially supported by the
Scientific and Technological Projects of Shandong Province (grant
no. 2008-01-03-41-01).
References
|
1
|
Gill PS and Rosenblum ND: Control of
murine kidney development by sonic hedgehog and its GLI effectors.
Cell cycle. 5:1426–1430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah MM, Sampogna RV, Sakurai H, Bush KT
and Nigam SK: Branching morphogenesis and kidney disease.
Development. 131:1449–1462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reidy KJ and Rosenblum ND: Cell and
molecular biology of kidney development. Semin Nephrol. 29:321–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piscione TD and Rosenblum ND: The
molecular control of renal branching morphogenesis: Current
knowledge and emerging insights. Differentiation. 70:227–246. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bai CB, Auerbach W, Lee JS, Stephen D and
Joyner AL: Gli2, but not Gli1, is required for initial Shh
signaling and ectopic activation of the Shh pathway. Development.
129:4753–4761. 2002.PubMed/NCBI
|
|
6
|
Park HL, Bai C, Platt KA, Matise MP,
Beeghly A, Hui CC, Nakashima M and Joyner AL: Mouse Gli1 mutants
are viable but have defects in SHH signaling in combination with a
Gli2 mutation. Development. 127:1593–1605. 2000.PubMed/NCBI
|
|
7
|
Ding H, Zhou D, Hao S, Zhou L, He W, Nie
J, Hou FF and Liu Y: Sonic hedgehog signaling mediates
epithelial-mesenchymal communication and promotes renal fibrosis. J
Am Soc Nephrol. 23:801–813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lurie IW, Ilyina HG, Podleschuk LV,
Gorelik LB and Zaletajev DV: Chromosome 7 abnormalities in parents
of children with holoprosencephaly and hydronephrosis. Am J Med
Genet. 35:286–288. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nowaczyk MJ, Huggins MJ, Tomkins DJ, Rossi
E, Ramsay JA, Woulfe J, Scherer SW and Belloni E:
Holoprosencephaly, sacral anomalies, and situs ambiguus in an
infant with partial monosomy 7q/trisomy 2p and SHH and HLXB9
haploinsufficiency. Clin Genet. 57:388–393. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim PC, Mo R and Hui Cc C: Murine models
of VACTERL syndrome: Role of sonic hedgehog signaling pathway. J
Pediatr Surg. 36:381–384. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Carroll TJ and McMahon AP: Sonic
hedgehog regulates proliferation and differentiation of mesenchymal
cells in the mouse metanephric kidney. Development. 129:5301–5312.
2002.PubMed/NCBI
|
|
12
|
Grieshammer U, Cebrián C, Ilagan R, Meyers
E, Herzlinger D and Martin GR: FGF8 is required for cell survival
at distinct stages of nephrogenesis and for regulation of gene
expression in nascent nephrons. Development. 132:3847–3857. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohuchi H, Hori Y, Yamasaki M, Harada H,
Sekine K, Kato S and Itoh N: FGF10 acts as a major ligand for FGF
receptor 2 IIIb in mouse multi-organ development. Biochem Biophys
Res Commun. 277:643–649. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiao J, Uzzo R, Obara-Ishihara T,
Degenstein L, Fuchs E and Herzlinger D: FGF-7 modulates ureteric
bud growth and nephron number in the developing kidney.
Development. 126:547–554. 1999.PubMed/NCBI
|
|
15
|
Barasch J, Qiao J, McWilliams G, Chen D,
Oliver JA and Herzlinger D: Ureteric bud cells secrete multiple
factors, including bFGF, which rescue renal progenitors from
apoptosis. Am J Physiol. 273:F757–F767. 1997.PubMed/NCBI
|
|
16
|
Poladia DP, Kish K, Kutay B, Hains D, Kegg
H, Zhao H and Bates CM: Role of fibroblast growth factor receptors
1 and 2 in the metanephric mesenchyme. Dev Biol. 291:325–339. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao H, Kegg H, Grady S, Truong HT,
Robinson ML, Baum M and Bates CM: Role of fibroblast growth factor
receptors 1 and 2 in the ureteric bud. Dev Biol. 276:403–415. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perantoni AO, Timofeeva O, Naillat F,
Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF and
Lewandoski M: Inactivation of FGF8 in early mesoderm reveals an
essential role in kidney development. Development. 132:3859–3871.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
White AC, Xu J, Yin Y, Smith C, Schmid G
and Ornitz DM: FGF9 and SHH signaling coordinate lung growth and
development through regulation of distinct mesenchymal domains.
Development. 133:1507–1517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Z, Verheyden JM, Hassell JA and Sun
X: FGF-regulated Etv genes are essential for repressing Shh
expression in mouse limb buds. Dev Cell. 16:607–613. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mukhopadhyay A, Krishnaswami SR,
Cowing-Zitron C, Hung NJ, Reilly-Rhoten H, Burns J and Yu BD:
Negative regulation of Shh levels by Kras and Fgfr2 during hair
follicle development. Devel Biol. 373:373–382. 2013. View Article : Google Scholar
|
|
22
|
Haworth KE, Wilson JM, Grevellec A,
Cobourne MT, Healy C, Helms JA, Sharpe PT and Tucker AS: Sonic
hedgehog in the pharyngeal endoderm controls arch pattern via
regulation of Fgf8 in head ectoderm. Dev Biol. 303:244–258. 2007.
View Article : Google Scholar
|
|
23
|
Gupta IR, Lapointe M and Yu OH:
Morphogenesis during mouse embryonic kidney explant culture. Kidney
Int. 63:365–376. 2003. View Article : Google Scholar
|
|
24
|
Hoy WE, Douglas-Denton RN, Hughson MD,
Cass A, Johnson K and Bertram JF: A stereological study of
glomerular number and volume: Preliminary findings in a multiracial
study of kidneys at autopsy. Kidney Int Suppl. S31–S37. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barak H and Boyle SC: Organ culture and
immunostaining of mouse embryonic kidneys. Cold Spring Harb Protoc.
2011:pdb.prot55582011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Narlis M, Grote D, Gaitan Y, Boualia SK
and Bouchard M: Pax2 and pax8 regulate branching morphogenesis and
nephron differentiation in the developing kidney. J Am Soc Nephrol.
18:1121–1129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chai L, Yang J, Di C, Cui W, Kawakami K,
Lai R and Ma Y: Transcriptional activation of the SALL1 by the
human SIX1 homeodomain during kidney development. J Biol Chem.
281:18918–18926. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murashima A, Akita H, Okazawa M, Kishigami
S, Nakagata N, Nishinakamura R and Yamada G: Midline-derived Shh
regulates mesonephric tubule formation through the paraxial
mesoderm. Dev Biol. 386:216–226. 2014. View Article : Google Scholar :
|
|
29
|
Pedersen A, Skjong C and Shawlot W: Lim 1
is required for nephric duct extension and ureteric bud
morphogenesis. Dev Biol. 288:571–581. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun X, Meyers EN, Lewandoski M and Martin
GR: Targeted disruption of Fgf8 causes failure of cell migration in
the gastrulating mouse embryo. Genes Dev. 13:1834–1846. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Igarashi M, Finch PW and Aaronson SA:
Characterization of recombinant human fibroblast growth factor
(FGF)-10 reveals functional similarities with keratinocyte growth
factor (FGF-7). J Biol Chem. 273:13230–13235. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sekine K, Ohuchi H, Fujiwara M, Yamasaki
M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N and
Kato S: Fgf10 is essential for limb and lung formation. Nat Genet.
21:138–141. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Michos O, Cebrian C, Hyink D, Grieshammer
U, Williams L, D'Agati V, Licht JD, Martin GR and Costantini F:
Kidney development in the absence of Gdnf and Spry1 requires Fgf10.
PLoS Genet. 6:e10008092010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu X, Weinstein M, Li C, Naski M, Cohen
RI, Ornitz DM, Leder P and Deng C: Fibroblast growth factor
receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8
and FGF10 is essential for limb induction. Development.
125:753–765. 1998.PubMed/NCBI
|
|
35
|
Watanabe Y, Miyagawa-Tomita S, Vincent SD,
Kelly RG, Moon AM and Buckingham ME: Role of mesodermal FGF8 and
FGF10 overlaps in the development of the arterial pole of the heart
and pharyngeal arch arteries. Circ Res. 106:495–503. 2010.
View Article : Google Scholar :
|
|
36
|
Urban AE, Zhou X, Ungos JM, Raible DW,
Altmann CR and Vize PD: FGF is essential for both condensation and
mesenchymal-epithelial transition stages of pronephric kidney
tubule development. Dev Biol. 297:103–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo W, Yi X, Ren F, Liu L, Wu S and Yang
J: Activation of SHH signaling pathway promotes vasculogenesis in
post-myocardial ischemic-reperfusion injury. Int J Clin Exp Pathol.
8:12464–12472. 2015.
|