Introduction
Flow cytometry is a key approach for immunological
investigations, providing a convenient method to detect cell
differentiation markers, cytokines, transcriptional factors and DNA
content, in a number situations, simultaneously (1). It has also been frequently used for
the analyses of cell function, cytokine expression, cell apoptosis
and proliferation. However, in the majority of cases, flow
cytometric analysis has a strict requirement for fresh samples.
Cell death not only alters the expression level/pattern of cell
surface markers, which is critical for the accuracy of cell
subpopulation determination, but also affects the ex vivo
cell function, which is closest to the in vivo status
experiments are aiming to recreate. This strict time requirement
causes limitations for investigations, particularly clinical
investigations, in two respects. Firstly, it requires that samples
are processed within a short time period without delay. This
problem is worsened if patient surgery or other treatments are
arranged later in the day. Secondly, without an optimal protocol to
preserve the sample, experiments are performed discretely. As a
result, the data collected are of increased variance due to the
unavoidable difference generated from independent experiments. The
inter-assay variance becomes more marked in certain complex
experiments, including cell stimulation in culture followed by
intracellular cytokine measurement.
Clinical trials or approved cell therapy,
particularly immunotherapy, require a large number of viable
functional cells. For example, the availability of large quantities
of functionally effective dendritic cells for immunotherapeutic
trials against infectious diseases is critical for the
effectiveness of cell therapy (2,3). The
infusion of genetically engineered T-cells with chimeric antigen
receptors for cancer therapy involves concentrated cell
preparations (4). Another example
is the storage of cord blood, a valuable source of hematopoietic
stem cells for the treatment of several serious diseases (5).
Therefore, the cryopreservation of immune cells is
indispensable for experimental and clinical use. Different
cryoprotectants, cytomedia additives and freezing procedures are
continuously being assessed to optimize cell cryopreservation for
different purposes (6–9). The primary aim is to protect cells
from the adverse effects of the ice crystals formed, which either
completely destroys cells or eventually affects cell viability and
function.
As an effective cyroprotective formula, 10% dimethyl
sulfoxide (DMSO) in 90% fetal bovine serum (FBS) has been widely
used, particularly for experimental purposes due to the xenogenic
property of FBS to humans and possible zoonotic contamination.
Unlike traditional pooled human serum, which may contain viral or
other bioactive contamination, human serum albumin (HSA) combined
with DMSO is frequently selected as a standard protocol for
clinical use (10).
Lymphocytes in children are different from those of
adults in terms of their subset proportions, cell functions and
their responsiveness to antigens (11,12).
How cryopreservation affects lymphocytes of children remains to be
elucidated. In the present study, alterations in cell viability,
subset proportion, cytokine production and T cell receptor (TCR) Vβ
subfamily distribution were examined following the thawing of cells
from eight cryopreservation methods. The results aimed to provide a
valuable reference for the optimal storage of blood cells from
children for pediatric investigations and clinical
applications.
Materials and methods
Ethics statement
The present study was performed according to the
principles expressed in the Declaration of Helsinki and was
approved by the Ethics Committee of the Beijing Children's
Hospital, Capital Medical University (Beijing, China). Whole blood
samples from 80 children aged 1–6 years old (38 female, 42 male)
were collected in Beijing Children's Hospital following the
provision of written informed consent for its use for experimental
purposes from the children's parents or guardians.
Blood sample collection
Patients at Beijing Children's Hospital were
recruited to the present study between June 2014 and June 2015.
Patients with immune system-associated diseases or diseases
affecting lymphocyte proportion and function were excluded. All
blood samples were collected in EDTA blood collection tubes. The
experiments were performed at least three times to reduce single
operating error and sample variation. Within each cryopreservative
method, comparisons were made for the same sample between data
collected prior to freezing and that collected post-thawing.
Cell freezing and thawing
In method 1, the whole blood sample was not
pre-treated prior to freezing. In method 2, DMSO (1:10 total blood
volume) was added to the whole blood prior to freezing. In methods
3 and 4, red blood cells (RBCs) were lysed with RBC lysis buffer
(OptiLyse C lysis solution; Beckman Coulter, Miami, FL, USA). In
methods 7 and 8, the RBCs were lysed with ammonium chloride lysing
solution (10X stock) containing 1.5 M NH4Cl, 100 mM
NaHCO3 and 10 mM EDTA-Na2 (pH 7.4). The cells
were spun for 5 min at 500 RCF. The cell pellets were then washed
once with RPMI medium prior to being resuspended with 10% DMSO+90%
FBS or with 10% DMSO+90% HSA, respectively. In methods 5 and 6,
lymphocytes were purified from the whole blood by density gradient
centrifugation (1000 × g, 20 min, room temperature) over lymphocyte
separation medium. The cells were then washed and resuspended with
10% DMSO +90% FBS or with 10% DMSO+90% HSA as cryoprotective
additives, respectively. The cells in the cryovials were first
stored at −80°C for 3 days in a Nalgene cell freezing container
(Thermo Fisher Scientific, Inc., Watlham, MA, USA) filled with
isopropanol, and then moved to a −196°C liquid nitrogen tank for
long-term storage. Cell thawing was performed by removing the
frozen vial from the liquid nitrogen tank and immediately immersing
it into a 37°C water bath for ~5 min with intermittent agitation.
The thawed cells were washed once with PBS and resuspended with
cell staining buffer or cell culture medium for the respective
experiments.
Cell count and viability
assessment
The absolute cell count was determined using a
Millipore Guava Easycyte 8 flow cytometer (EMD Millipore,
Billerica, MA, USA). In brief, with appropriate adjustment of FSC
(47.3 V) and SSC (108 V) voltages, granulocytes, monocytes and
lymphocytes were well separated. Targeting a total of 5,000
lymphocyte-gated events, the flow cytometry recorded a volume of
sample consumed, and the number of lymphocytes (number/µl of loaded
sample) was calculated. The dilution factor (10) was then applied to obtain the total
lymphocyte cell count (cells/ml blood). The sample was stained with
7-amino-actinomycin D (7-AAD) to distinguish between the viable
(7-AAD−) and dead cells (7-AAD+).
Flow cytometric analysis
The fluorochrome-conjugated mouse anti-human
antibodies: Fluorescein isothiocyanate (FITC)-CD8, FITC-CD16,
phycoerythrin (PE)-CD4, PE-interleukin (IL)-2, PEcy5-CD3,
PEcy5-CD56, PEcy5-CD19 and antigen presenting cell-interfron γ
(APC-IFNγ) were purchased from BioLegend, Inc. (San Diego, CA,
USA). Incubation was conducted for at 4°C. For surface staining,
cells prior to freezing and after thawing, and those harvested from
cell culture were washed once with PBS, and stained with antibodies
in cell staining buffer (3% FBS in PBS) in the dark for 25 min. For
intracellular staining, the Cytofix/Cytoperm™
Fixation/Permeabilization kit (BD Biosciences, San Diego, CA, USA)
was used according to the manufacturer's protocol. For determining
the TCR Vβ repertoire, the IO Test Beta Mark Vβ-TCR repertoire kit
(Beckman Coulter) was used. FlowJo software, version 7.6 (Tree
Star, Inc., Ashland, OR, USA) was used for flow cytometric data
analysis.
Cell culture and lymphocyte
activation
A U-bottom 96-well culture plate was coated with 10
µg/ml solution of anti-CD3 in sterile PBS and maintained at 4°C
overnight. The cells (1×106/well) were cultured for 20 h at 37°C
with 5% CO2 in complete RPMI 1640 medium (200 µl/well) supplemented
with 10% FBS, 2 mM L-glutamine, 1% penicillin/streptomycin, 5 ng/ml
IL-2 and anti-CD28 (2.5 µg/ml)-free antibodies. Final lymphocyte
activation was performed by adding PMA (50 ng/ml) and ionomycin (1
µg/ml) in the presence of GolgiStop (monensin; 2.5 µl/ml; BD
Biosciences). The culture was maintained in the incubator for
another 5 h. The cells were then washed twice with staining buffer
prior to surface and intracellular staining with PE-IL-2 and
APC-IFNγ.
Statistical analysis
A paired t-test was performed to compare the
differences between each fresh blood sample and its corresponding
thawed frozen sample in terms of surface marker and intracellular
cytokine expression following activation. GraphPad Prism software,
version 5 (GraphPad, Inc., La Jolla, CA, USA) was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Viability maintenance of lymphocytes
cryopreserved using different methods
In the present study, eight methods for the
cryopreservation of blood cells from children were compared. As
expected, the whole blood cells frozen directly without any
cryoprotective additive exhibited complete loss of the typical
cytometric pattern comprising the three major cell populations of
granulocytes, monocytes and lymphocytes, based on FSC, vs. SSC
values in flow cytometry (Fig. 1).
The addition of DMSO only to the whole blood prior to freezing in
method 2 resulted in the thawed cells exhibiting a similar pattern
to those frozen using method 1. The three major cell populations
were well maintained in the cells cryopreserved with methods 3, 4,
7 and 8, in which the RBCs were lysed first (hemolysin for methods
3 and 4; NH4Cl for methods 7 and 8) followed by the
addition of standard medium (90% FBS+10% DMSO for methods 3 and 7;
90% HSA+10% DMSO for methods 4 and 8) prior to freezing. There was
an appreciable decrease in SSC values for the granulocyte
population. In methods 5 and 6, in which the lymphocytes were
isolated by lymphocyte separation medium prior to being frozen with
10% DMSO+90% FBS or HSA, respectively, no alterations in the FSC or
SSC values of the lymphocyte population were observed, compared
with the same sample fresh following collection.
Measuring the absolute number of viable lymphocytes
in the same sample prior to and following freezing revealed that
methods 1 and 2 resulted in a marked cell death (Fig. 2). Following thawing from method 1,
the remaining viable (7-AAD−) lymphocytes were 40% ± 13
(mean ± standard error of the mean) of the lymphocytes measured in
the fresh sample. Method 2 was the least effective among the
cryopreservative methods in terms of their ability to maintain
lymphocyte viability. The percentage of remaining lymphocytes
relative to the fresh sample was only 25% ± 13. The remaining
methods (methods 3–8) maintained a mean viability of lymphocytes
between 59 and 77%, relative to their respective cell counts
determined prior to freezing. Among these, method 5 provided the
optimal lymphocyte protection, with a viability of 77% ± 10.
However, the lymphocyte counts prior to freezing with methods 5 and
6 decreased by 50%, caused by the lymphocytes isolation procedure,
compared with the lymphocyte count in the same sample measured
without purification. Therefore, a high ratio of thawed viable
lymphocytes to their counterparts prior to freezing does not
necessarily indicate a high absolute lymphocyte count in the thawed
samples.
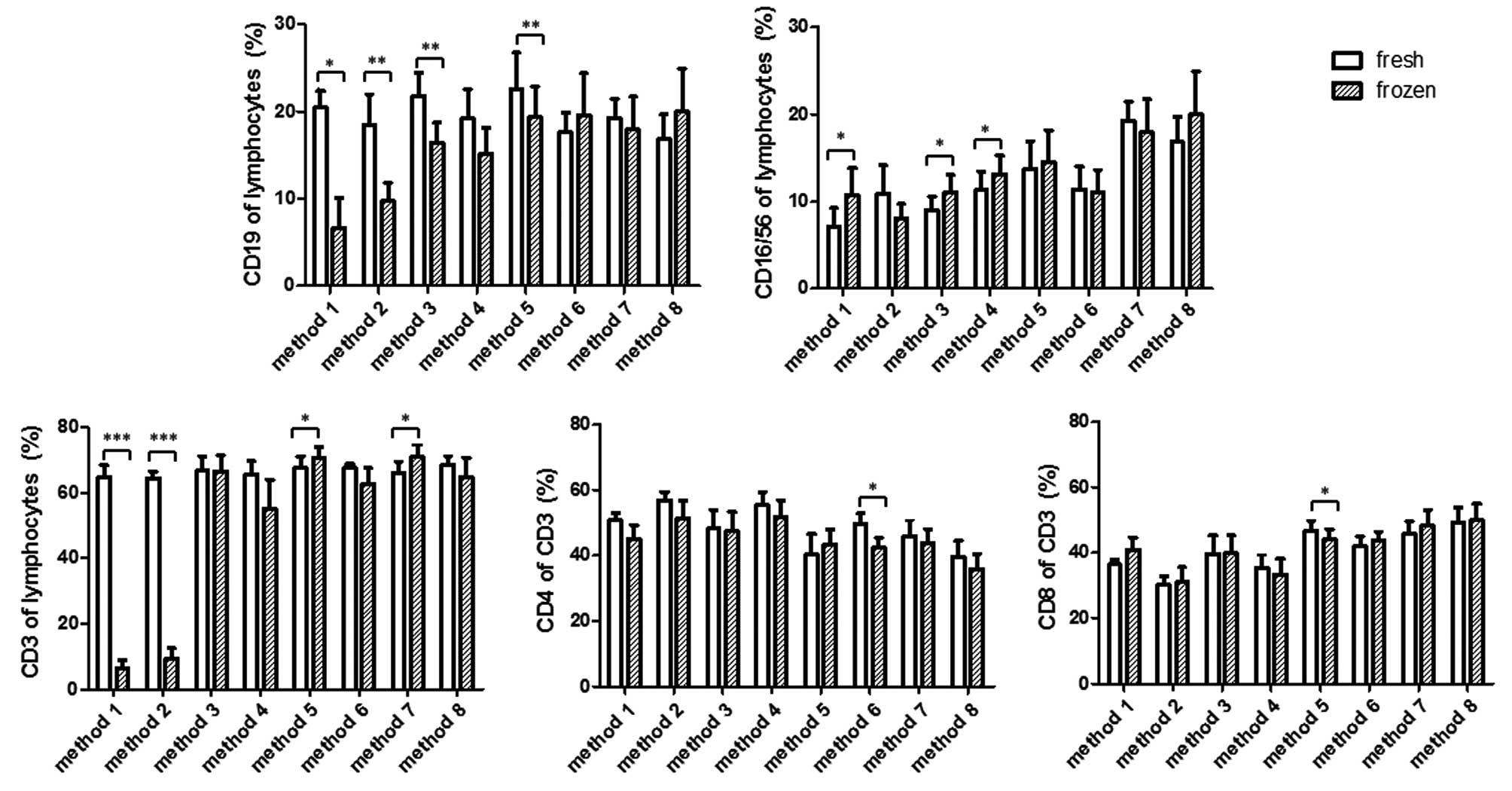
Changes in the percentage of the
lymphocyte subpopulations following cryopreservation
The present study then aimed to determine how the
different methods of cryopreservation affected the percentages of
lymphocyte subpopulations. As shown in Fig. 3, methods 1 and 2 resulted in a
significant decrease in the percentages of CD3+ T-cells
and CD19+ B-cells in the thawed cells, in the context of
a marked decline in viable lymphocytes. The percentage of
CD16+CD56+ NK cells in method 1 was increased
in lymphocyte gates, whereas the average percentage of NK cells in
method 2 decreased, but not significantly. Method 3 resulted in a
significant decrease in the percentage of CD19+ B cells,
but not CD3+ T cells, compared with the measurements in
the fresh sample. Method 4 exhibited a decrease in the percentages
of B and T cells, however, this was not significant. By contrast,
methods 3 and 4 led to a statistically significant increase in the
percentage of NK cells. Method 5 also led to a significant
alteration in the percentage of B and T cells, however, this was in
the opposite direction. The percentage of B cells decreased, but
that of T cells increased, compared with their respective values
measured prior to freezing. By contrast, the percentage of NK cells
was not altered by method 5. Methods 6–8 maintained a broad
spectrum of lymphocyte subpopulations, as they did not affect the
percentage of B cells, NK cells or T cells, with the exception of
method 7, which showed a significant increase in T cells,
resembling that observed in method 5.
The present study then investigated whether CD4 and
CD8 T cells are differentially affected by cryopreservation, which
may lead to a percentage change in the CD3+ T cell gate.
The data indicated that CD4 and CD8, in the majority of the
methods, were proportionally affected. As shown in the lower panel
of Fig. 3, the relative CD4 or CD8
percentage in the CD3+ T cell population remained
unaltered in methods 1, 2, 3, 4, 7 and 8. Significant alterations
existed in methods 5 and 6, of which the former caused a decrease
in CD8 cells and the latter caused a decrease in CD4 T cells.
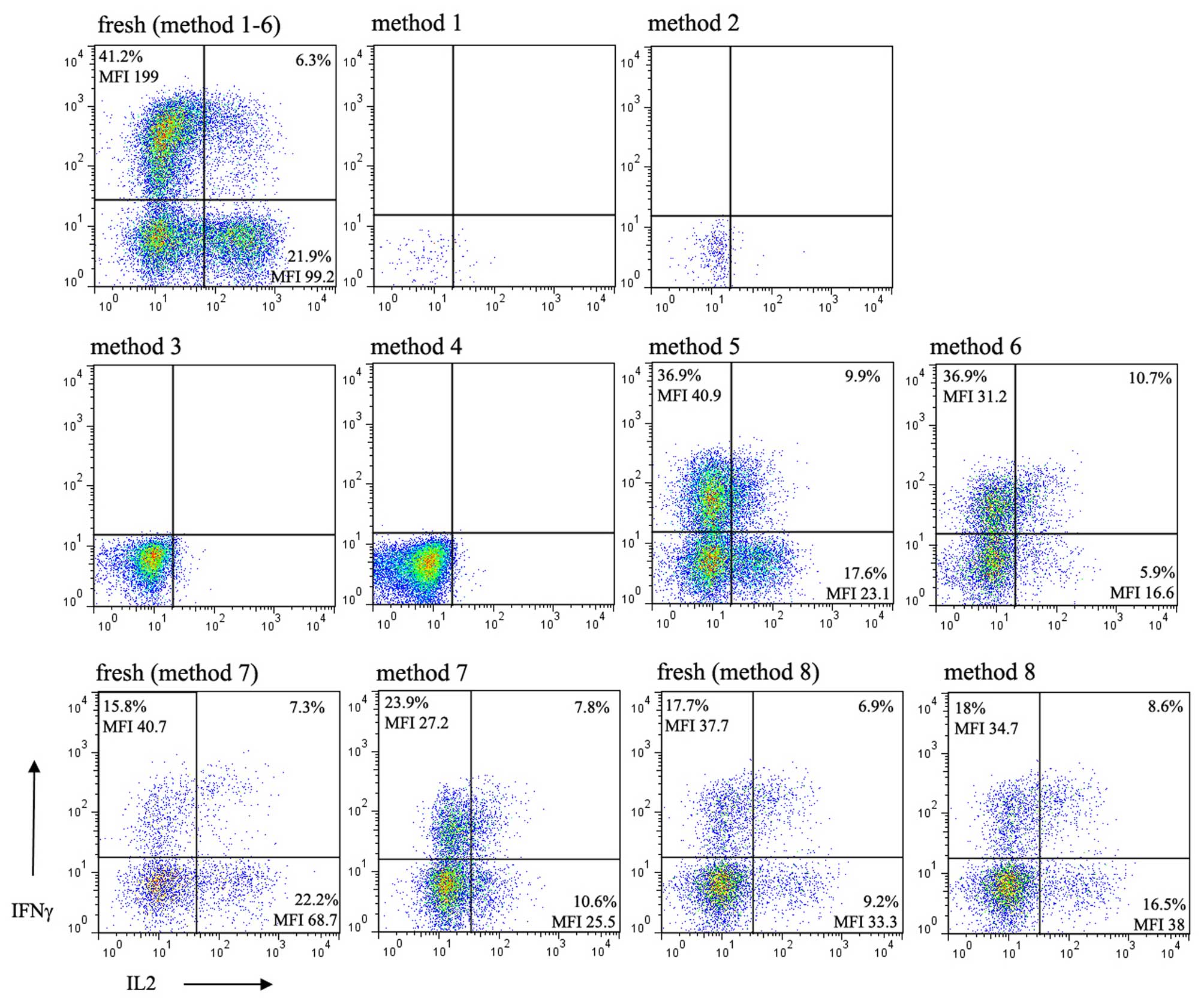
Functional changes of lymphocytes
following cryopreservation
To determine whether there is an optimal freezing
method for the maximum maintenance of function of lymphocytes from
children, the present study determined the intracellular expression
of IFNγ and IL-2 in the lymphocytes of children being
cryopreserved, the expression of which is the hallmark of activated
lymphocyte function. Following stimulation of the thawed cells with
anti-CD3/CD28 in the presence of PMA and ionomycin in culture, no
intracellular expression of IFNγ or IL-2 were detected in the cells
cryopreserved using methods 1 and 2 due to extensive cell death.
Upon stimulation of the cells thawed from methods 3 and 4, which
were confirmed to have considerable viability, did not express
intracellular IFNγ or IL-2. By contrast, cytokines were
successfully detected in the purified lymphocytes thawed from
methods 5 and 6, and those cryopreserved using methods 7 and 8 in
which RBCs were lysed with NH4Cl (Fig. 4). In methods 5–8, although the
percentage of IFNγ relative to its respective value in the fresh
sample varied between experiments, the mean fluorescence index
(MFI) was invariably reduced in all independent experiments. By
contrast, the percentage and MFI of IL-2 were consistently
decreased following cryopreservation in every independent
experiment, with method 8 as an exception. The percentage and MFI
of the intracellular staining of IL-2 in method 8 were
bidirectionally variable between experiments.
Altered TCR Vβ subfamily distribution
following cryopreservation
The measurement of bias-usage or diversity changes
of the TCR Vβ repertoire as an immune characteristic has been
documented in several diseases and malignant conditions (13–16).
The results of the present study revealed that freezing
differentially affected cell death in different subsets, which
caused percentage changes in the lymphocyte subpopulations. The
present study then investigated whether any of the cryopreservative
methods supported the TCR Vβ staining without causing a
differential change in the 24 Vβ subfamilies. The preferential
alteration of particular subfamilies of Vβ by cryopreservation
results in a change in Vβ subfamily distribution, which leads to an
error in assessing the TCR repertoire (data not shown). To assess
this, the present study compared the percentages of 24 Vβ
subfamilies in the blood samples prior to freezing, and these
values were monitored following cryopreserved of the cells using
methods 3–8. The cells directly preserved without cryoprotective
additives or with the addition of DMSO only in methods 1 and 2,
respectively were found to cause extensive cell death. Therefore,
the antibody staining of TCR Vβ stored in cells in these two
methods was omitted. The data collected from methods 3–8, as shown
in Fig. 5, were classified into
three categories based on the change in Vβ subfamilies following
cyropreservation. Firstly, the percentage of Vβ subfamilies were
decreased by all cryopreservative methods. These Vβ subfamilies
included Vβ 5.2, Vβ 9 and Vβ 22 (Fig.
5A). Secondly, changes in the percentages of certain Vβ
subfamilies were variable in a method-dependent manner. These
included Vβ 5.1, 13.2, 14, 16, 18, 20 and Vβ 23 (Fig. 5B). Thirdly, the percentages of
certain Vβ subfamilies were not affected by any of the
cryopreservative methods (Fig.
5C). These subfamilies included Vβ 1, 2, 3, 4, 5.3, 7.1, 7.2,
8, 11, 12, 13.1, 13.6, 17 and 21.3.
Discussion
The ability to store lymphocytes, and maintain
sufficient viability and function has been an important issue for
investigations and clinical applications. The present study
compared several commonly used cryopreservation methods for the
efficient storage of lymphocytes of children, which differs from
that of adults with regard to the cell function and, possibly,
susceptibility to freezing. The data based on methods 1 and 2
showed that direct freezing of whole blood samples resulted in
extensive cell death. As shown in the FSC, vs. SSC flow plots in
Fig. 1, adding DMSO as a
cyroprotective additive to the whole blood exacerbated the cell
death in cryopreservation. It is likely that salt and/or other
unknown small molecules in the plasma of the whole blood
contributed to the dominant detrimental effect in the freeze-thaw
cycle. The direct addition of 10% DMSO in the context of
insufficient endogenous human serum, in which the endogenous human
serum was <90% of the total volume as suggested in the frozen
medium formula, provided no beneficial effect, and was toxic to
cells. When the leukocytes in the whole blood were washed with PBS
and resuspended with 10% DMSO+90% FBS or HSA in methods 3,4,7 and
8, the thawed cells largely maintained their viability (Fig. 2). Of note, an appreciable decrease
in the granulocyte SSC values in methods 3, 4, 7 and 8 (Fig. 1) suggested a change in the internal
complexity of granulocytes as a result of freezing.
The changes in the percentage of lymphocyte
subpopulations in the present study suggested that, in certain
situations (Fig. 3; methods 3 and
5), CD19+ B cells were more susceptible to freezing
damage, compared with CD3+ T cells. The fact that the
percentage of NK cells increased in methods 3 and 4 is possibly a
reflection of relative decreases in B and T cells in the lymphocyte
population. It is likely that the increase of NK cells in method 1
was a reflection of the substantial reduction of B and T cells due
to extensive cell death. However, the possibility that the change
in NK cells may have been a stochastic event cannot be excluded;
compared with other cells, the percentage of NK cells exhibited the
highest variability in different individuals. This is complicated
further when age-dependent NK change is considered in children
(17). With the exception of
methods 1 and 2, the observation that cryopreservation induced a
percentage change in CD3+ T cells in lymphocytes or
CD4+/CD8+ single positive T cells in total
CD3+ T cells may be the result of insufficient sampling
as these changes are marginal.
Isolating lymphocytes using separation medium prior
to freezing with standard cryoprotective medium (methods 5 and 6),
conferred protection of cell viability regardless of whether HSA or
FBS was used. The data revealed that the cells thawed from these
two methods expressed intracellular IFNγ and IL-2 (Fig. 4), but not those from methods 3 and
4, which has been confirmed to maintain cell viability and surface
marker expression. Commonly used hemolysin RBC lysis buffer
(Optilyse C lysis solution) contains formaldehyde as a fixative. It
is likely that the lymphocytes were partially fixed and that
activation of the lymphocytes was inhibited by hemolysin treatment.
This result provided experimental evidence that, when cell
functional analysis is planned, the hemolysin lysis method is not
suitable. When NH4Cl was used instead of hemolysin RBC
lysis buffer in methods 7 and 8, the intracellular expression of
IL-2 and IFNγ recovered, compared with those in methods 3 and 4. Of
note, as the majority of monocytes and granulocytes were lost in
methods 5 and 6, these two methods are not suggested for use if
cells other than lymphocytes are the focus of interest (Fig. 1). In addition, ~50% of total
lymphocytes were lost by cell isolation, which indicated this
method is not suitable for experiments or clinical preparations
requiring large quantities of cells.
The cryopreservative method with the optimal
performance among the methods assessed in the present study was
method 8, in terms of the viability maintenance, surface marker
expression and cell function of activated lymphocytes. Considering
the HSA used in method 8 is cheaper than FBS and does not contain
zoogenic substances, method 8 may be a cost-effective and safer
cryopreservative approach for clinical applications in addition to
experimental investigations.
The TCR repertoire, determined by specific
antibodies recognizing different TCR subfamilies with variable Vβ
chains, is a useful index for antigen-specific responses, including
infection, immunodeficiency or autoimmune diseases (18–20).
However, in the present study, none of the cryopreservative methods
were found to maintain the unbiased percentages of all the 24 Vβ
subfamilies simultaneously. Therefore, method-dependent alterations
in the percentages of particular Vβ subfamilies were found, which
are misleading in forming conclusions on the TCR Vβ repertoire.
Accordingly, accurate evaluation of the TCR repertoire may be
attained either by using fresh lymphocyte samples or using
sequencing technology from DNA material (21,22).
Acknowledgements
This study was supported by a startup research fund
to Professor Jingang Gui from the Beijing Children's Hospital, a
teaching hospital affiliated with Capital Medical University
(Beijing, China).
References
|
1
|
Chattopadhyay PK and Roederer M:
Cytometry: Today's technology and tomorrow's horizons. Methods.
57:251–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buhl T, Legler TJ, Rosenberger A, Schardt
A, Schön MP and Haenssle HA: Controlled-rate freezer
cryopreservation of highly concentrated peripheral blood
mononuclear cells results in higher cell yields and superior
autologous T-cell stimulation for dendritic cell-based
immunotherapy. Cancer Immunol Immunother. 61:2021–2031. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finn OJ: Cancer Immunology. N Engl J Med.
358:2704–2715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Curran KJ, Pegram HJ and Brentjens RJ:
Chimeric antigen receptors for T cell immunotherapy: Current
understanding and future directions. J Gene Med. 14:405–415. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antoniewicz-Papis J, Lachert E, Woźniak J,
Janik K and Łętowska M: Methods of freezing cord blood
hematopoietic stem cells. Transfusion. 54:194–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stevens VL, Patel AV, Feigelson HS,
Rodriguez C, Thun MJ and Calle EE: Cryopreservation of whole blood
samples collected in the field for a large epidemiologic study.
Cancer Epidemiol Biomarkers Prev. 16:2160–2163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Germann A, Schulz JC, Kemp-Kamke B,
Zimmermann H and von Briesen H: Standardized serum-free cryomedia
maintain peripheral blood mononuclear cell viability, recovery, and
antigen-specific T-cell response compared to fetal calf serum-based
medium. Biopreserv Biobank. 9:229–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dijkstra-Tiekstra MJ, Setroikromo AC,
Kraan M, Gkoumassi E and de Wildt-Eggen J: Optimization of the
freezing process for hematopoietic progenitor cells: Effect of
precooling, initial dimethyl sulfoxide concentration, freezing
program, and storage in vapor-phase or liquid nitrogen on in vitro
white blood cell quality. Transfusion. 54:3155–3163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petrenko YA, Rogulska OY, Mutsenko VV and
Petrenko AY: A sugar pretreatment as a new approach to the Me2SO-
and xeno-free cryopreservation of human mesenchymal stromal cells.
Cryo Letters. 35:239–246. 2014.PubMed/NCBI
|
|
10
|
Hreinsson J, Zhang P, Swahn ML, Hultenby K
and Hovatta O: Cryopreservation of follicles in human ovarian
cortical tissue. Comparison of serum and human serum albumin in the
cryoprotectant solutions. Hum Reprod. 18:2420–2428. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiegering V, Eyrich M, Wunder C, Günther
H, Schlegel PG and Winkler B: Age-relate changes in intracellular
cytokine expression in healthy children. Eur Cytokine Netw.
20:75–80. 2009.PubMed/NCBI
|
|
12
|
Bunders M, Cortina-Borja M and Newell ML:
European Collaborative Study: Age-related standards for total
lymphocyte, CD4+ and CD8+ T cell counts in children born in Europe.
Pediatr Infect Dis J. 24:595–600. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J, Liu D, Tu W, Song W and Zhao X:
T-cell receptor diversity is selectively skewed in T-cell
populations of patients with Wiskott-Aldrich syndrome. J Allergy
Clin Immunol. 135:209–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tzifi F, Kanariou M, Tzanoudaki M, Mihas
C, Paschali E, Chrousos G and Kanaka-Gantenbein C: Flow cytometric
analysis of the CD4+ TCR Vβ repertoire in the peripheral blood of
children with type 1 diabetes mellitus, systemic lupus
erythematosus and age-matched healthy controls. BMC Immunol.
14:332013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Attaf M, Huseby E and Sewell AK: αβ T cell
receptors as predictors of health and disease. Cell Mol Immunol.
12:391–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gil A, Yassai M, Naumov Y and Selin L:
Narrowing of human influenza A virus-specific T cell receptor α and
β repertoires with increasing age. J Virol. 89:4102–4116. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Osugi Y, Hara J, Kurahashi H, Sakata N,
Inoue M, Yumura-Yagi K, Kawa-Ha K, Okada S and Tawa A: Age-related
changes in surface antigens on peripheral lymphocytes of healthy
children. Clin Exp Immunol. 100:543–548. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Ndhlovu ZM, Liu D, Porter LC, Fang
JW, Darko S, Brockman MA, Miura T, Brumme ZL, Schneidewind A, et
al: TCR clonotypes modulate the protective effect of HLA class I
molecules in HIV-1infection. Nat Immunol. 13:691–700. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aleman K, Noordzij JG, de Groot R, van
Drogen JJ and Hartwig NG: Reviewing Omenn syndrome. Eur J Pediatr.
160:718–725. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Somma P, Ristori G, Battistini L, Cannoni
S, Borsellino G, Diamantini A, Salvetti M, Sorrentino R and
Fiorillo MT: Characterization of CD81 T cell repertoire in
identical twins discordant and concordant for multiple sclerosis. J
Leukoc Biol. 81:696–710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang H, Yamaguchi R, Liu X, Daigo Y, Yew
PY, Tanikawa C, Matsuda K, Imoto S, Miyano S and Nakamura Y:
Quantitative T cell repertoire analysis by deep cDNA sequencing of
T cell receptor α and β chains using next-generation sequencing
(NGS). Oncoimmunology. 3:e9684672015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao F and Wang K: Ligation-anchored PCR
unveils immune repertoire of TCR-beta from whole blood. BMC
Biotechnol. 15:392015. View Article : Google Scholar : PubMed/NCBI
|