Introduction
Angiogenesis is one of the characteristics of tumor
progression and it has been regarded as essential for tumor growth
and metastasis (1). A number of
proteins are not expressed, or are expressed at low levels, by the
endothelium of normal blood vessels that are upregulated in
angiogenic blood vessels of the tumor, including integrin αvβ3
(2), vascular endothelial growth
factor receptor (3), and
aminopeptidase N (4). Numerous
peptides, such as arginine-glycine-aspartic acid (RGD), glutamic
acid-leucine-arginine and asparagine-glycine-arginine, have been
reported and evaluated (2,5,6).
Lee et al (7) identified a novel peptide sequence
containing serine-aspartic acid-valine (SDV) using the
ProteoChip-based screening system (7). In their subsequent studies, SDV
peptide was demonstrated to bind specifically to the same binding
site (integrin αvβ3) as RGD peptide, and induce upregulation of
tumor protein p53 in human umbilical vein endothelial cells
(8,9). Despite the desirable specific binding
of SDV peptide to integrin αvβ3, there have been no previous
efforts to develop an imaging agent for tumors using the SDV
sequence. Molecular imaging targeted to angiogenesis has potential
for clinical use, and as a research tool in tumor biology and in
the development of therapeutic agents.
Accurate distinguishing of cancer and inflammation
is important for cancer diagnosis. Due to the overlap of
biochemical and imaging features, differentiation of an
inflammation from malignancy using conventional computed tomography
can be difficult (10). The
diagnostic accuracy of F-18 fluorodeoxyglucose (FDG) positron
emission tomography (PET) has been also compromized by acute
inflammatory diseases, including tuberculosis, pneumonia, and
sarcoidosis (11,12). Novel tumor imaging agents
discriminating malignancy from benign inflammation may provide
improved patient management in a clinical setting.
The present study successfully developed
technetium-99m SDV-glutamic acid-cysteine-glycine (ECG) to target
the integrin αvβ3 of tumor cells. Furthermore, the diagnostic
performance of Tc-99m SDV-ECG as a tumor molecular imaging agent
was evaluated in the murine model. Furthermore, the potential of
Tc-99m SDV-ECG in differentiating tumors from inflammatory lesions
was considered.
Materials and methods
1N-HCl, sodium tartrate, ethanol, SnCl2
and Freund's complete adjuvant (FCA) were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Silica
gel-coated thin-layer chromatography plates (ITLC-SG) were
purchased from Gelman Sciences (Ann Arbor, MI, USA). A fresh Tc-99m
pertechnetate solution was eluted from a commercial Mo-99/Tc-99m
generator (Covidien, Ltd., Dublin, Ireland) at Wonkwang University
School of Medicine (Iksan, Korea).
Chemical synthesis of peptides
Peptides were obtained from Peptron, Inc. (Daejeon,
Korea) and the purity of the peptides was >98%. The peptides
were synthesized using Fmoc solid-phase peptide synthesis (SPPS)
with ASP48S (Peptron Inc., Daejeon, Korea). Reverse phase
high-performance liquid chromatography (RP-HPLC) with a Vydac
Everest column (C28, 250×22 mm, 10 µm; W.R. Grace & Co.,
Columbia, MD, USA) was used to purify the synthesized compound.
Gradients used for elution were linear from 0–75% acetonitrile in
water containing 0.1% trifluoroacetic acid. LC/mass spectrometry
(LC/MS) was used to confirm the purified peptide molecular
weights.
Tc-99m radiolabeling study
Radiolabeling of SDV-ECG with Tc-99m was conducted
using a previously described method (13). Briefly, 30 µl of the SDV-ECG
solution (10 mg/ml in nitrogen-purged water) and 30 µl of sodium
tartrate (100 mg/ml in nitrogen-purged water) were added and mixed
in a microcentrifuge tube. In the tartrate solution with SDV-ECG,
100 µl of Tc-99m pertechnetate (~144 MBq) and 30 µl SnCl2 (1 mg/ml
in 0.01 M HCl) were added. The solution was heated to 95°C for 30
min and cooled. A radio-RP-HPLC system was used to purify Tc-99m
SDV-ECG. Gradients used for elution were linear from 30 to 90%
acetonitrile in water containing 0.1% trifluoroacetic acid for 25
min at a flow rate of 2 ml/min. A UV detector (220 nm) and a
γ-radiodetector was used for monitoring.
The stability of the radiolabeling was evaluated by
incubating the Tc-99m SDV-ECG in saline at room temperature.
Following incubation, ITLC-SG with saline and acetone as the mobile
phase was used to measure the radiolabeling efficiency at 15 min,
1, 4 and 8 h after radiolabeling (3 samples).
Measurement of tumor cell binding
affinity
The binding affinity of Tc-99m ECG (control for
non-specific uptake) and Tc-99m SDV-ECG in tumor cells was measured
by saturation binding studies as previously described (14). The integrin αvβ3-positive HT-1080
human fibrosarcoma cells and the integrin αvβ3-negative HT-29 human
colorectal carcinoma cells were obtained from the Korean Cell Line
Bank (Seoul, Korea). The required cell densities of HT-1080 and
HT-29 cells (1×105 cells/plate) were obtained by plating cells
uniformly and incubating for 24 h. The cells were washed twice
using ice-cold binding buffer [25 mM HEPES and 1% bovine serum
albumin (Amresco, LLC, Solon, OH, USA)] and incubated for 1 h at
37°C with different concentrations (0–800 nM) of Tc-99m ECG and
Tc-99m SDV-ECG. Subsequently, the cells were washed with ice-cold
binding buffer three times, and lysed using lysis buffer. Using a
γ-counter (1480 Wizard 3; PerkinElmer, Inc., Waltham, MA, USA), the
radioactivity was measured. The maximum number of binding sites
(Bmax) and apparent dissociation constant (Kd) was determined using
non-linear regression models in GraphPad Prism software (version
5.03; GraphPad Software, Inc., La Jolla, CA, USA).
Murine models with tumor and
inflammation
Female athymic nude mice (BALB/c nu/nu; age, 6
weeks; weight, 16–18 g) were purchased from Damul Science (Daejeon,
Korea). Mice were housed at a density of 5 mice per cage and
maintained in a specific pathogen-free unit with room temperature
and humidity regulated (21±2°C; 55±10%) and a 12/12 h light/dark
cycle. Mice were given water and alfalfa-free breeding diet).
Suspended HT-1080 (1×105/0.1 ml) and HT-29 (1×107/0.1 ml) cells
were subcutaneously inoculated on each side of the anterior chest
region to produce an orthotopic cancer model in nude mice (n=10).
The γ-camera imaging and biodistribution studies were performed
when the tumor diameter reached 10 mm (~10 days after inoculation).
Intramuscular injection of FCA (25 µl) into the right thigh region
was used to induce inflammation in mice (n=5). Saline (25 µl) was
injected into the left thigh region as a control. The γ-camera
imaging was performed at 12 days after FCA injection.
Imaging with γ-camera
The in vivo imaging of the murine models was
performed using a γ-camera (Vertex; ADAC Laboratories, Inc.,
Milpitas, CA, USA) following intravenous injection of 18.5 MBq
Tc-99m SDV-ECG in tumor-bearing mice and inflammatory model mice.
The in vivo γ-camera images were captured at 30 min, 1, 2
and 4 h after injection (n=5), with acquisition times of 120 sec.
The images were stored at 512×512 matrix size. In the tumor mice
models, regions of interest (ROIs; 15×15 pixel sized) were drawn at
the sites of the tumors on the chest walls (n=5). Additional ROIs
were drawn at the left arm muscle for normal muscle uptake
measurement (n=5). In the mouse model with FCA-induced
inflammation, ROIs were drawn at the right thigh (inflamed, n=5),
and at the left thigh (normal muscle, n=5). The mean counts per
pixel within the ROIs were measured and target-to-non-target ratios
were calculated.
In vivo competition (inhibition) study
with free SDV peptide
An in vivo competition study was performed to
evaluate whether Tc-99m SDV-ECG specifically binds to the tumor and
compete with free SDV. Excess SDV (10 mM) and Tc-99m SDV-ECG (0.1
mM) was administered intravenously via the tail vein to mice that
previously been subcutaneously inoculated with HT-1080 cells as
described (n=5). Serial imaging was conducted using the same manner
as described above at 30 min, 1, 2, and 4 h after injection.
Biodistribution studies
For the biodistribution study, animals were
sacrificed by cervical dislocation and each organ was dissected at
1 and 4 h after injection (n=5, per organ). Animals were dissected
and selected organs and tissues were collected into pre-weighed
γ-counter tubes. The radioactivity of weighed tissues was
determined in a γ-counter. The counts per minute were
decay-corrected, and results were expressed as a percentage
injected dose per gram of wet tissue (%ID/g). The total activities
injected per animal were calculated by analyzing the difference
between the initial syringe counts and the remained syringe counts
following administration.
Statistical analysis
Quantitative variables were expressed as mean ±
standard deviation. One-way analysis of variance and appropriate
post hoc tests were applied to compare the
target-to-non-target ratios obtained from the in vivo
imaging and competition studies. The data were analyzed by SPSS
18.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Ethical considerations
All animal experiments were performed in accordance
with guidelines of the Wonkwang University School of Medicine
Committee in order to reduce the pain and sacrifice of the
animals.
Results
Chemical synthesis of peptides and
Tc-99m radiolabeling study
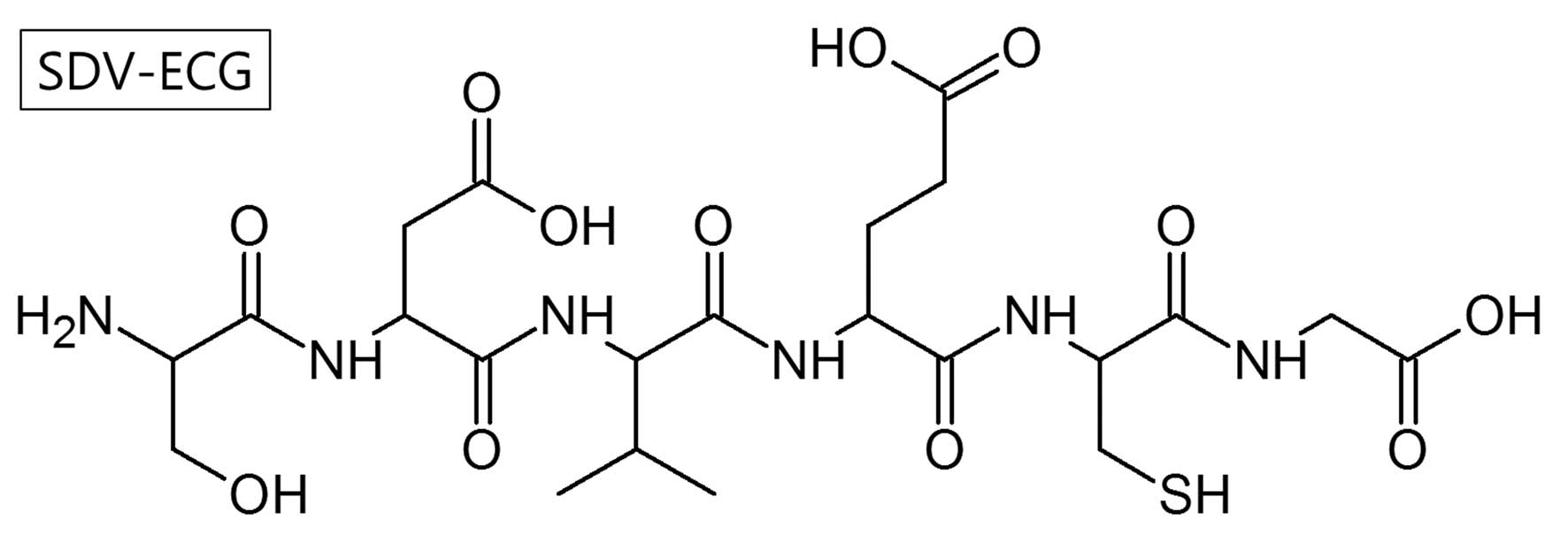
Using Fmoc SPSS, a hexapeptide, SDV-ECG
(C22H36N6O12S1;
molecular weight, 608.21 Da) was synthesized (Fig. 1). A single radio-peptide species
was obtained by radio-RP-HPLC (retention time= 10.8 min) following
Tc-99m radiolabeling. The Tc-99m SDV-ECG complex was prepared at a
high yield (>97%) and it demonstrated high stability at room
temperatures. The intact percentages of Tc-99m SDV-ECG measured by
ITLC-SG were 97.6±0.2, 97.4±0.3, 96.1±0.2 and 93.7±3.9 at 15 min,
1, 4, and 8 h, respectively.
Measurement of tumor cell binding
affinity
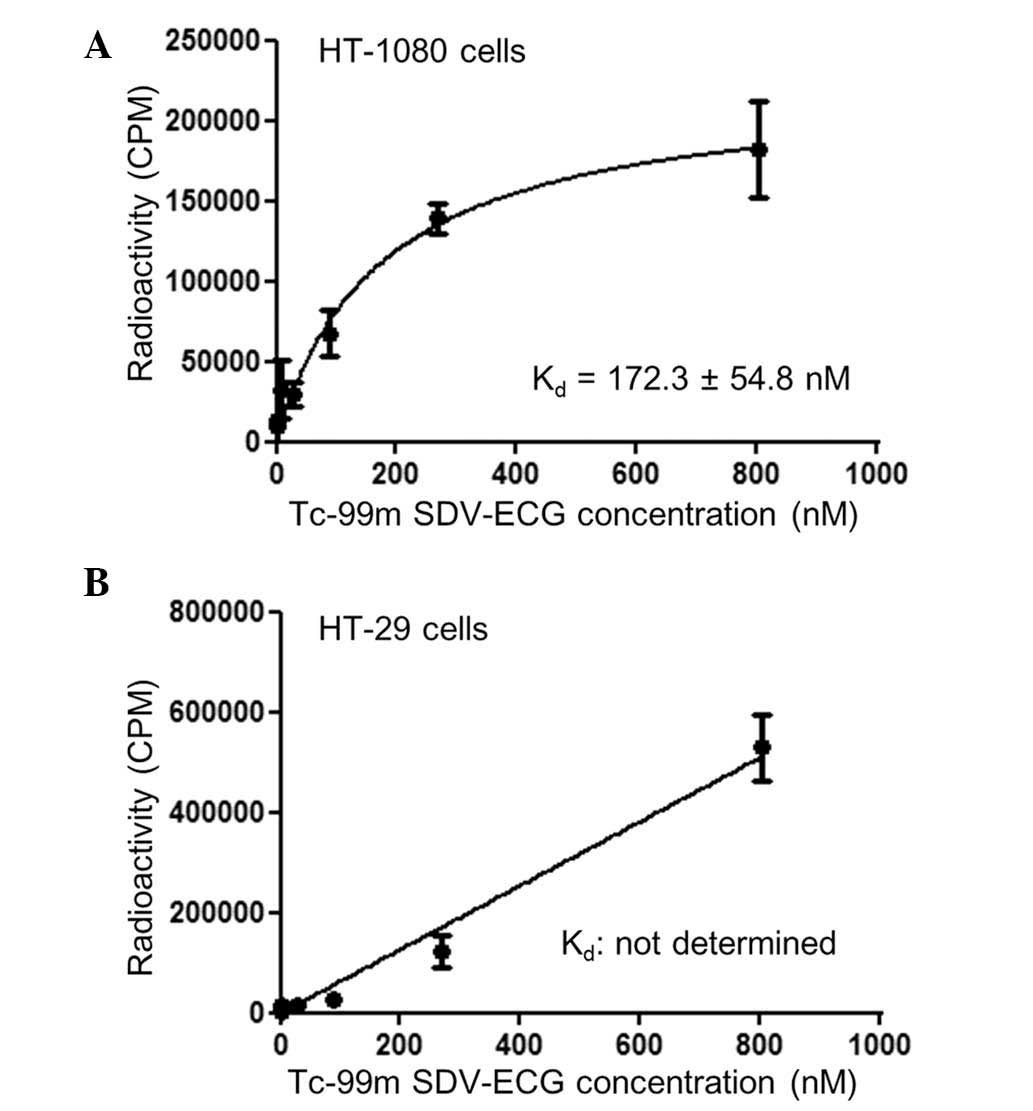
The cell binding affinity study demonstrated that
Tc-99m SDV-ECG was saturated within the integrin αvβ3-positive
HT-1080 tumor cells. The Kd of Tc-99m SDV-ECG within the HT-1080
tumor cells determined by saturation binding was 172.3±54.8 nM
(Fig. 2A). By contrast, Tc-99m
SDV-ECG was not saturated within the integrin αvβ3-negative HT-29
tumor cells, and the Kd value could not be determined by saturation
binding (Fig. 2B).
γ-camera imaging
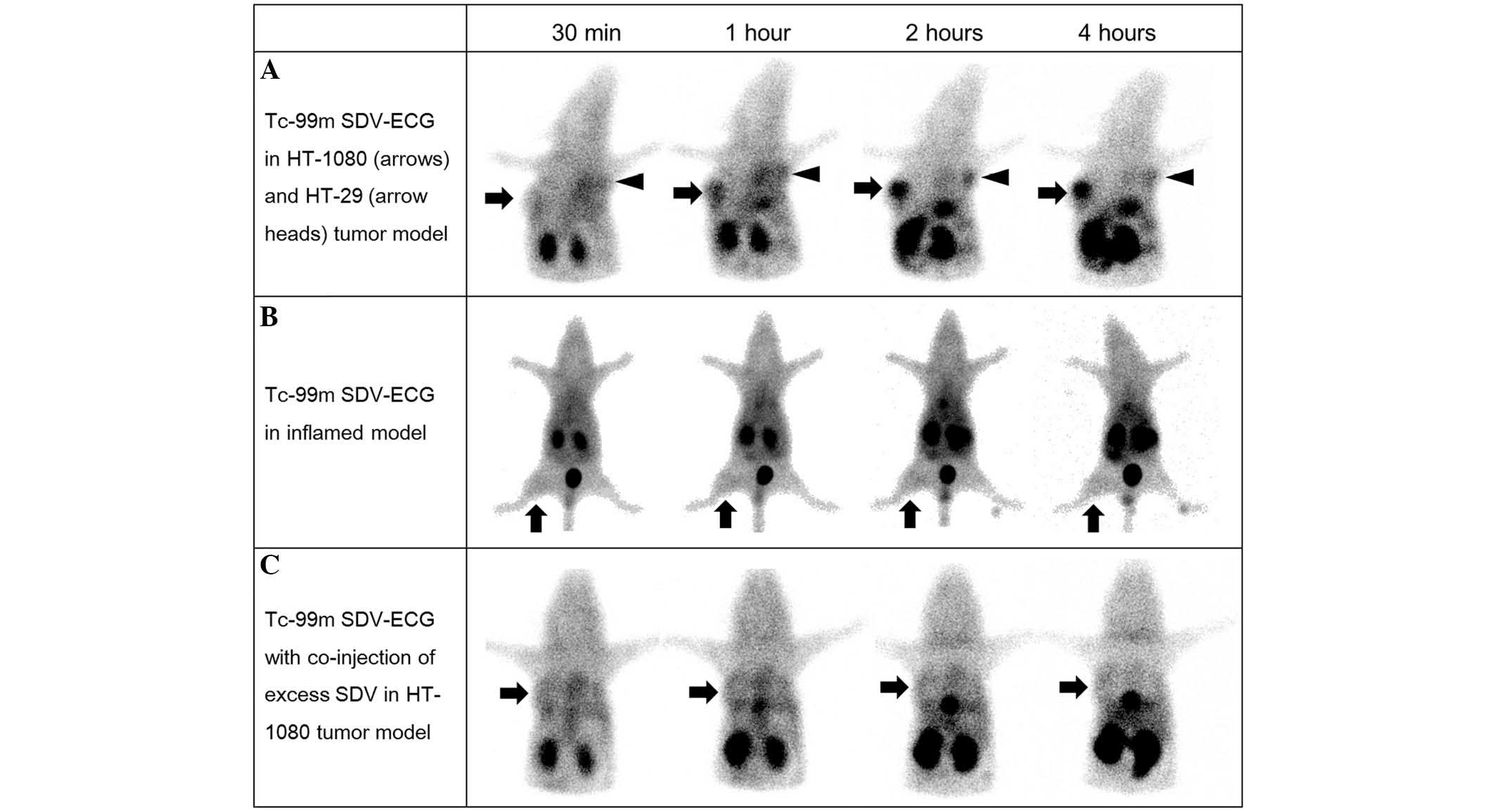
In the in vivo γ-camera imaging, intense
activity was observed in the urinary bladder, gall bladder and
bowel, suggesting Tc-99m SDV-ECG was excreted via the genitourinary
and hepatobiliary systems. In the in vivo imaging of the
mouse tumor model (Fig. 3), Tc-99m
SDV-ECG was notably accumulated in the HT-1080 tumors, however, not
in the HT-29 tumors (Fig. 3A). The
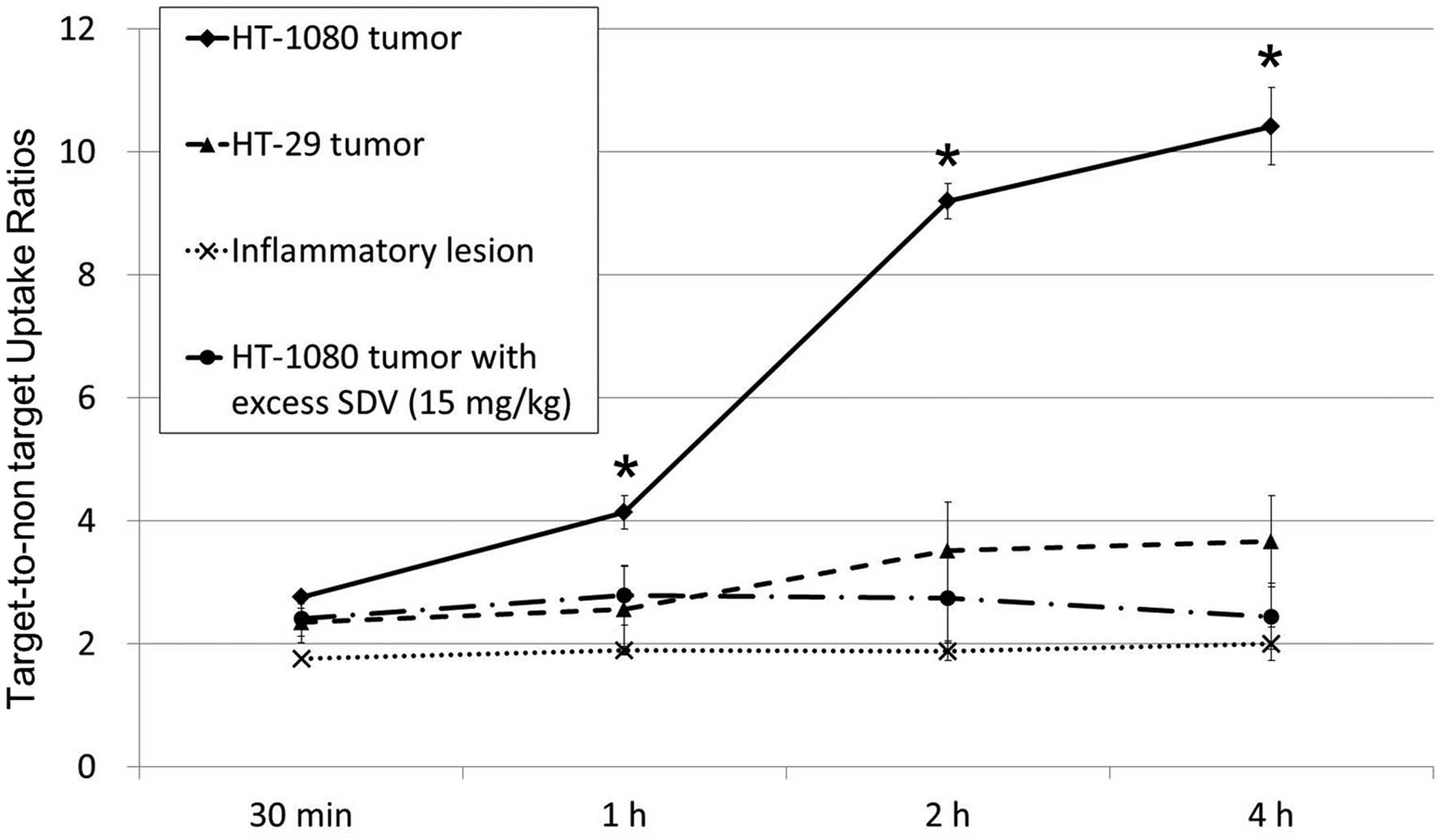
HT-1080 tumor-to-normal muscle uptake ratio of Tc-99m SDV-ECG
reached 10.4±0.6 at 4 h (2.8±0.1, 4.1±0.3, 9.2±0.3 and 10.4±0.6 at
30 min, 1, 2 and 4 h, respectively). The HT-29 tumor-to-normal
muscle uptake ratios of Tc-99m SDV-ECG (2.4±0.2, 2.6±0.7, 3.5±0.8
and 3.7±0.7 at 30 min, 1, 2 and 4 h, respectively) were
significantly lower than those of HT-1080 tumor. Furthermore, the
inflamed lesions demonstrated low uptake of Tc-99m SDV-ECG. The
inflammatory muscle-to-normal muscle ratios were 1.8±0.1, 1.9±0.1,
1.9±0.2, and 2.0±0.3 at 30 min, 1, 2, and 4 h, respectively
(Fig. 3B). The
target-to-non-target ratio of the HT-1080 tumors was markedly
higher than those of the HT-29 tumors and inflamed lesions at 1, 2
and 4 h after injection, respectively (P<0.05; Fig. 4).
In vivo competition (inhibition) study
with free SDV peptide
Co-injection of excess SDV and Tc-99m SDV-ECG was
used for the competition study, it was demonstrated that the
tumor-to-normal muscle uptake ratios of Tc-99m SDV-ECG was
decreased (2.4±0.4, 2.8±0.5, 2.8±0.7 and 2.4±0.5 at 30 min, 1, 2
and 4 h, respectively; Fig. 3C).
It was suggested that the co-injection of excess concentration SDV
blocked the uptake of Tc-99m SDV-ECG into the HT-1080 tumors
(P<0.05; Fig. 4).
Biodistribution studies. The %ID/g
values of the biodistribution of Tc-99m SDV-ECG were summarized in
Table I
At 1 and 4 h after injection, the kidney
demonstrated the highest activity (9.81±7.58 and 6.60±2.89 at 1 and
4 h, respectively). At 1 h, the blood and the lung indicated
relatively high activity (3.86±0.98 and 1.85±0.57, respectively).
At 4 h after injection, the majority of the organs, excluding the
kidneys, indicated low activities. The %ID/g of the HT-1080 tumor
was 0.76±0.24 and 0.39±0.06 at 1 and 4 h, respectively. The %ID/g
of HT-29 tumor was lower than that of HT-1080 tumor (0.65±0.25 and
0.19±0.06 at 1 and 4 h, respectively). HT-1080 tumor-to-normal
muscle ratios of %ID/g were 1.5 and 4.6 at 1 and 4 h,
respectively.
Discussion
The present study produced an SDV-containing
hexapeptide, SDV-ECG, for use as a tumor imaging agent. A
substantial uptake of Tc-99m SDV-ECG into orthotopic HT-1080 tumor
(integrin αvβ3-positive) and low uptake of Tc-99m SDV-ECG in HT-29
tumor (integrin αvβ3-negative) was demonstrated. A competition
study demonstrated that HT-1080 tumor uptake was effectively
blocked by the co-injection of excess concentration of SDV. These
results support that Tc-99m SDV-ECG is a good surrogate for tumor
imaging and Tc-99m SDV-ECG and free SDV may be internalized into
tumors via the same pathways and mechanisms. Furthermore, Tc-99m
SDV-ECG barely accumulated in the FCA-induced inflamed lesions.
These results suggest Tc-99m SDV-ECG may be a novel molecular
imaging agent to differentiate cancer from inflamed disease.
Integrins are heterodimeric glycoproteins that
mediate transmembrane signals between extracellular signal
molecules and an intracellular cytoskeleton (15,16).
They are understood to be crucial targets for therapeutic agents
due to their associations with different conditions and diseases,
including inflammation, osteoporosis, angiogenesis, and tumor
metastasis (17,18). Thus, the molecular imaging agents
for an integrin receptor have been thoroughly developed (19). Short linear peptide sequences, such
as RGD and leucine-aspartic acid-valine (LDV), served as a
targeting motif for tumors (20,21).
Thus, the SDV sequence identified and evaluated by Lee et al
(7–9), may be an additional and effective
targeting motif for tumor imaging agents. Despite this SDV peptide
binding specifically to integrin αvβ3, there was no previous effort
to develop a molecular imaging agent for tumors using SDV sequence.
Thus, this is the first study, to the best of our knowledge, to
develop an SDV-containing molecular imaging agent for tumor.
The Tc-99m labeled imaging agent includes two
essential parts. One is the targeting section enabling the specific
function of the agent, and the other is the chelating section
binding with Tc-99m. In our previous study, the ECG tripeptide,
including multiple nitrogen and one sulfur atoms demonstrated
strong and stable chelation with Tc-99m, and is considered a good
candidate for the Tc-99m chelating section of the imaging agent
(6). Using a small peptide, such
as SDV-ECG, as a Tc-99m-based molecular imaging agent has a number
of advantages. Firstly, small peptides can be easily synthesized
using Fmoc SPSS. Secondly, numerous small peptides may provide
stable and strong chelating sequences for complexing with Tc-99m
(22).
Several efforts have been reported to develop novel
tumor imaging agents, indicating low accumulation in inflamed
tissues. For example, van Waarde et al (23) and Sugae et al (24) have developed a positron emitter
based imaging agent for the selective detection of tumors over
inflammatory lesion. F-18 FDG has been demonstrated to have a
marked impact on cancer patient management, thus, research efforts
have been focused on the development of molecular imaging agents
based on the positron emitters (25,26).
However, positron emitters are rarely available in institutions
without a cyclotron and an expensive PET imaging system is
essential (27). By contrast,
Tc-99m is the most widely used radioisotopes in nuclear medicine
and has excellent physical characteristics (140.5 keV emission of
89% abundance, decay by isomeric transition) for in vivo
imaging. While the research developing positron emitter based
imaging agent is developing, the development of Tc-99m based tumor
imaging agent remains important when increasing global demand of
Tc-99m is considered (28).
In conclusion, Tc-99m SDV-ECG was developed as a
novel Tc-99m agent for tumor imaging. Tc-99m SDV-ECG demonstrated a
substantial uptake in HT-1080 tumors, and it is a good candidate
for integrin αvβ3-positive tumor imaging. Furthermore, Tc-99m
SDV-ECG effectively distinguished between malignant and inflamed
lesions in mice.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (2013R1A1A2059262) and a
NRF grant funded by the Korean government (MSIP: Ministry of
Science, ICT and Future Planning) (2014M2B2A9030059).
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gladson CL and Cheresh DA: Glioblastoma
expression of vitronectin and the alpha v beta 3 integrin. Adhesion
mechanism for transformed glial cells. J Clin Invest. 88:1924–1932.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Veikkola T, Karkkainen M, Claesson-Welsh L
and Alitalo K: Regulation of angiogenesis via vascular endothelial
growth factor receptors. Cancer Res. 60:203–212. 2000.PubMed/NCBI
|
|
4
|
Bhagwat SV, Lahdenranta J, Giordano R,
Arap W, Pasqualini R and Shapiro LH: CD13/APN is activated by
angiogenic signals and is essential for capillary tube formation.
Blood. 97:652–659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang RE, Niu Y, Wu H, Amin MN and Cai J:
Development of NGR peptide-based agents for tumor imaging. Am J
Nucl Med Mol Imaging. 1:36–46. 2011.PubMed/NCBI
|
|
6
|
Kim DW, Kim WH, Kim MH and Kim CG: Novel
Tc-99m labeled ELR-containing 6-mer peptides for tumor imaging in
epidermoid carcinoma xenografts model: A pilot study. Ann Nucl Med.
27:892–897. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee Y, Kang DK, Chang SI, Han MH and Kang
IC: High-throughput screening of novel peptide inhibitors of an
integrin receptor from the hexapeptide library by using a protein
microarray chip. J Biomol Screen. 9:687–694. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi Y, Kim E, Lee Y, Han MH and Kang IC:
Site-specific inhibition of integrin alpha v beta 3-vitronectin
association by a ser-asp-val sequence through an
Arg-Gly-Asp-binding site of the integrin. Proteomics. 10:72–80.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bang JY, Kim EY, Kang DK, Chang SI, Han
MH, Baek KH and Kang IC: Pharmacoproteomic analysis of a novel
cell-permeable peptide inhibitor of tumor-induced angiogenesis. Mol
Cell Proteomics. 10:M110.005264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dutta AK and Chacko A: Head mass in
chronic pancreatitis: Inflammatory or malignant. World J
Gastrointest Endosc. 7:258–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takamochi K, Yoshida J, Murakami K, Niho
S, Ishii G, Nishimura M, Nishiwaki Y, Suzuki K and Nagai K:
Pitfalls in lymph node staging with positron emission tomography in
non-small cell lung cancer patients. Lung Cancer. 47:235–242. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kubota R, Kubota K, Yamada S, Tada M, Ido
T and Tamahashi N: Microautoradiographic study for the
differentiation of intratumoral macrophages, granulation tissues
and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose
uptake. J Nucl Med. 35:104–112. 1994.PubMed/NCBI
|
|
13
|
Kim DW, Kim WH, Kim MH and Kim CG:
Synthesis and evaluation of novel Tc-99m labeled NGR-containing
hexapeptides as tumor imaging agents. J Labelled Comp Radiopharm.
58:30–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu C, Wei J, Gao K and Wang Y:
Dibenzothiazoles as novel amyloid-imaging agents. Bioorg Med Chem.
15:2789–2796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Albelda SM and Buck CA: Integrins and
other cell adhesion molecules. FASEB J. 4:2868–2880.
1990.PubMed/NCBI
|
|
16
|
Humphries MJ: Integrin structure. Biochem
Soc Trans. 28:311–339. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mousa SA: Anti-integrin as novel
drug-discovery targets: Potential therapeutic and diagnostic
implications. Curr Opin Chem Biol. 6:534–541. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tucker GC: Inhibitors of integrins. Curr
Opin Pharmacol. 2:394–402. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carman CV: Overview: Imaging in the study
of integrins. Methods Mol Biol. 757:159–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gaertner FC, Kessler H, Wester HJ,
Schwaiger M and Beer AJ: Radiolabelled RGD peptides for imaging and
therapy. Eur J Nucl Med Mol Imaging 39 (Suppl 1). 126–138. 2012.
View Article : Google Scholar
|
|
21
|
Shi P, Chen H, Cho MR and Stroscio MA:
Peptide-directed binding of quantum dots to integrins in human
fibroblast. IEEE Trans Nanobioscience. 5:15–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu S and Edwards DS: 99mTc-Labeled small
peptides as diagnostic radiopharmaceuticals. Chem Rev.
99:2235–2268. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Waarde A, Jager PL, Ishiwata K,
Dierckx RA and Elsinga PH: Comparison of sigma-ligands and
metabolic PET tracers for differentiating tumor from inflammation.
J Nucl Med. 47:150–154. 2006.PubMed/NCBI
|
|
24
|
Sugae S, Suzuki A, Takahashi N, Minamimoto
R, Cheng C, Theeraladanon C, Endo I, Togo S, Inoue T and Shimada H:
Fluorine-18-labeled 5-fluorouracil is a useful radiotracer for
differentiation of malignant tumors from inflammatory lesions. Ann
Nucl Med. 22:65–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fazaeli Y, Jalilian A, Amini M, Ardaneh K,
Rahiminejad A, Bolourinovin F, Moradkhani S and Majdabadi A:
Development of a (68)Ga-fluorinated porphyrin complex as a possible
PET imaging agent. Nucl Med Mol Imaging. 46:20–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin KH, Park SA, Kim SY, Lee SJ, Oh SJ
and Kim JS: Effect of animal condition and fluvoxamine on the
result of [18F]N-3-Fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)
Nortropane ([18F]FP-CIT) PET study in mice. Nucl Med Mol Imaging.
46:27–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berger M, Gould MK and Barnett PG: The
cost of positron emission tomography in six United States veterans
affairs hospitals and two academic medical centers. AJR Am J
Roentgenol. 181:359–365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matthews KM, Bowyer TW, Saey PR and Payne
RF: The workshop on signatures of medical and industrial isotope
production-WOSMIP; Strassoldo, Italy, 1–3 July 2009. J Environ
Radioact. 110:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|