Introduction
Cell migration is a crucial cellular function that
contributes to a number of important physiological and pathological
processes, such as vascular disease and chronic inflammatory
diseases including cancer (1–3).
Therefore, understanding the fundamental mechanisms underlying cell
migration may provide novel and effective therapeutic approaches
for the treatment of these diseases, particularly malignant tumors
(4). Cell adhesion and migration
are important factors involved in the transition of tumors from a
non-malignant to a metastatic phenotype (5). An important cellular response to a
migration-promoting agent is the extension of protrusions in the
direction of migration, with the assembly of focal adhesions at the
leading edge and their disassembly at the trailing edge (1). Focal adhesions are large protein
complexes located at the basal surface of cells that mediate the
physical connection between the extracellular matrix (ECM) and the
actin-based cytoskeleton within the cell (6). An important cellular response to a
migration-promoting agent is the extension of protrusions in the
direction of migration, with the assembly of focal adhesions at the
leading edge and their disassembly at the trailing edge (7). Focal adhesions are large protein
complexes located at the basal surface of cells that mediate the
physical connection between the extracellular matrix (ECM) and the
actin-based cytoskeleton within the cell (6). They are speculated to serve critical
roles in numerous cell functions, particularly migration and
adhesion (8). Focal adhesions
consist of heterodimeric transmembrane matrix receptors, known as
integrins, which interact with a complex consisting of
intracellular structural proteins and the actin cytoskeleton
through their cytoplasmic tails. In addition, multiple regulatory
proteins, including paxillin, talin and kindlin, are located within
focal adhesion sites and serve to transduce extracellular signals
inside cells, leading to modulation of adhesion and migration
(9). First described 20 years ago,
paxillin was initially characterized as a 68-kDa focal adhesion
protein that undergoes a significant increase in tyrosine
phosphorylation upon v-src expression, and is one of the
prototypical adaptor proteins involved in integrin signaling
(10,11). Paxillin is a scaffold for the
recruitment of multiple signaling proteins to the plasma membrane,
such as the focal adhesion kinase (FAK) and Src tyrosine kinases.
These proteins are activated through the engagement of integrin
molecules by ECM ligands, such as fibronectin and collagen
(12,13). On the internal plasma membrane,
integrins, cytoskeletal-associated proteins and FAK are connected
to the actin cytoskeleton via talin and kindlin, which are two
structurally and functionally associated protein families that are
essential for integrin activation and integrin-mediated signaling
(14).
The immortal esophageal epithelial cell lines
NE2-hTERT (NE2), NE3 E6E7-hTERT (NE3) and NEcA6-E6E7-hTERT (NEcA6),
which were immortalized by overexpression of the human telomerase
reverse transcriptase (hTERT) and/or human papillomavirus (HPV) 16
E6 and E7 proteins, are valuable models to study the process of
cancer cell immortalization (15–17);
an essential pre-requisite to malignant transformation and an
initial step in carcinogenesis. These immortalized cell lines have
been demonstrated to exhibit a high proliferation rate, but failed
to induce colony formation in soft agar (18–20).
However, there is limited information regarding the cell migration
and adhesion capabilities of these cells. Through a detailed
characterization of NE2, NE3 and NEcA6 cell lines, the aim of the
present study was to investigate the cell migration and adhesion
properties of these cell lines and to identify the molecular
mechanisms that contribute to esophageal carcinogenesis.
Materials and methods
Cell lines and culture conditions
The immortalized human esophageal epithelial cell
lines NE2, NE3, and NEcA6 were donated by Professor Sai-Wah Tsao
(Department of Anatomy, University of Hong Kong, Hong Kong, China).
These cell lines were cultured in a 1:1 mixture of defined
keratinocyte serum-free medium (dKSFM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and EpiLife medium (Cascade
Biologics, Inc., Portland, OR, USA) in 5% CO2 at
37°C.
Characterization of short tandem
repeats (STRs)
In order to characterize STRs in immortalized
esophageal epithelial cell lines, genomic DNA was extracted using
the DNA IQ System kit (Promega Corporation, Madison, WI, USA)
according to the manufacturer's instructions, and subject to
amplification by polymerase chain reaction in a final reaction
volume of 25 µl using the PowerPlex18D System (Promega
Corporation). Collection and analysis of the data were performed
using Data Collection software and GeneMapper software (version
3.2; Applied Biosystems; Thermo Fisher Scientific, Inc.),
respectively. The experiments were performed by Land Huagene
Biosciences Co., Ltd. (Guangzhou, China).
Oligonucleotide transfection
Kindlin-2 small interfering RNA (siRNA) and the
corresponding non-targeting control RNAs were purchased from
Qiagen, Inc. (Valencia, CA, USA). The kindlin-2 siRNA sequence was
5′-CTGGTGGAGAAACTCGATGTA-3′. Oligonucleotide transfections were
performed using Lipofectamine® RNAiMAX Transfection
Reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions.
Soft-agar colony formation assay
Cells were seeded on 24-well plates at a density of
2×104 cells/well in a 1:1 mixture of dKSFM and EpiLife
and 0.7% agar, with a lower solidified base layer consisting of
0.35% agar. Colony formation was visualized and quantified using a
Leica DMI3000B inverted phase contrast microscope (Leica
Microsystems GmbH, Wetzlar, Germany) at 3 weeks after the cells
were seeded. Colonies composed of >16 cells were counted at ×200
magnification, according to the procedures described previously
(20). The mean value was
calculated from data obtained from three independent
experiments.
Tumorigenicity assays in nude
mice
All animal experiments were conducted with the
approval of the Institutional Animal Care and Use Committee of
Shantou University (Shantou, China). A total of 14 male Nu/Nu mice
(age, 4–5 weeks) were purchased from Beijing Weitong Lihua
Experimental Animal Technical Co., Ltd. (Beijing, China). Animals
were maintained at a constant temperature (26°C) under a light/dark
cycle (14/10 h), and had ad libitum access to food and
water. Cells (1×106 cells/inoculation) were diluted in
100 µl PBS and injected subcutaneously into the left and right side
of the hip areas of nine Nu/Nu mice. Three groups of three mice
were inoculated with NE2, NE3 or NEcA6 cells. Animals were palpated
twice a week for the appearance of tumors. Mice were sacrificed by
ether inhalation at 6 weeks following inoculation, and all tumors
were excised, fixed in formalin, embedded in paraffin and cut into
sections. Tumors formed by NEcA6 cells were subject to
hematoxylin-eosin and immunohistochemical staining.
Immunohistochemical staining was performed as described previously
(21), using the mouse
anti-pan-cytokeratin AE1/AE3 antibody (1:200; cat. no. ZM-0069;
ZSGB-Bio, Beijing, China). The tumorigenicity of NEcA6 cells was
confirmed by injection of 1×106 cells into the front,
and the left and right sides of the hip areas of five additional
male Nu/Nu mice. Each mouse had four injection points.
Cell proliferation assay
Cell proliferation was assessed using the
xCELLigence Real-Time Cell Analysis (RTCA) DP instrument (Roche
Applied Science, Mannheim, Germany), which was placed in a
humidified incubator at 37°C and 5% CO2. The cell
proliferation assay was performed as described previously (22). Briefly, 1×104 cells were
seeded in 16-well microtiter E-plates (Roche Applied Science). Cell
impedance was monitored every 15 min for ~48 h by measuring the
cells adhered to the gold metallic plates. After plotting the
impedence (cell index) against time (h), the rate of cell impedance
(slope) was calculated using the following calculation: Slope =
impedance (Ω) / time (h). The median ± standard deviation rate of
cell impedance for each cell line was calculated from duplicate
wells. Experiments were performed in duplicate.
Cell adhesion assay
The NE2, NE3 and NEcA6 cell adhesion assays were
performed as described previously (23). Briefly, the wells of 16-well
E-Plates were coated with or without 10 µg/ml fibronectin
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and seeded
with 1×104 cells. Kindlin-2 in NE2 and NEcA6 cells were
specifically knocked down by siRNA, and after 48 h, plates were
coated with 10 µg/ml fibronectin and seeded with 1×104
cells. Cells were monitored every 5 min using the Real Time-Cell
Electronic Sensing system (Roche Applied Science) for a period of 5
h. After plotting cell impedance against time, the rate of cell
impedance (slope) was calculated using the following formula: Slope
= cell impedance (Ω) / time (h). The median ± standard deviation
rate of cell impedance for each cell line was calculated from
duplicate wells and the experiment repeated twice.
Cell migration assay
The cell migration assay was performed as described
previously (22). Briefly,
2×105 cells were washed once in serum-free medium, and
seeded into the upper chambers of 16-well C-plates. Cell impedance
was monitored as described earlier every 30 min for ~48 h. After
plotting the cell impedance against time, the rate of cell
impedance (slope) was calculated using the following formula: Slope
= cell impedance (Ω) / time (h). The median ± standard deviation
rate of cell impedance for each cell line was calculated from
duplicate wells. Experiments were performed in duplicate.
Immunocytochemical analysis
Cells (1×105) were seeded on coverslips
and incubated for 24 h, before they were fixed in PBS containing 4%
paraformaldehyde for 10 min. After rinsing cells with PBS, they
were permeabilized in 0.1% Triton X-100 for 10 min before washing
again with PBS. Non-specific binding was inhibited by incubating
cells with 5% normal donkey serum (cat. no. 017-000-12; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) diluted in
PBS for 1 h. The primary antibodies used in the present study are
summarized in Table I. Cells were
probed with an Alexa Fluor 488-conjugated Affinipure donkey
anti-mouse secondary antibody (cat. no. 705-515-147; Jackson
ImmunoResearch Laboratories, Inc.), and simultaneously incubated
with 100 nM Acti-stain 555 phalloidin (cat. no. PHDH1;
Cytoskeleton, Inc., Denver, CO, USA), followed by counterstaining
with 0.1 µg/ml 4′,6-diamidino-2-phenylindole (cat. no. P4170;
Sigma-Aldrich; Merck Millipore). Cells were analyzed using an
Olympus FV1000 confocal microscope (Olympus Corporation, Tokyo,
Japan).
 | Table I.Primary antibodies used in the
present study. |
Table I.
Primary antibodies used in the
present study.
| Name | Dilution | Catalog number | Clone number | Source |
|---|
| Mouse anti-FAK | 1:500 | 610088 | 77 | BD Biosciences,
Franklin Lakes, NJ, USA |
| Mouse
anti-paxillin | 1:50 | 610620 | 165 | BD Biosciences |
| Mouse
anti-talin | 1:500 | T3287 | 8d4 | Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany |
| Rabbit anti-FAK
(pY397) | 1:1,000 | 44-625G | 141-9 | Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA |
| Goat anti-ERM | 1:1,000 | sc6407 | C19 | Santa Cruz
Biotechnology, Inc., Dallas, TX, USA |
| Mouse
anti-γ-catenin | 1:200 | sc8415 | H-1 | Santa Cruz
Biotechnology |
| Mouse
anti-β-actin | 1:2,000 | sc47778 | C4 | Santa Cruz
Biotechnology |
| Rabbit
anti-E-cadherin | 1:300 | sc7870 | H108 | Santa Cruz
Biotechnology |
| Mouse
anti-kindlin-2 | 1:200 | MAB2617 | 3A3 | EMD Millipore,
Billerica, MA, USA |
| Rabbit
anti-ERM[ezrin(Thr567)/radixin(Thr564)/moesin(Thr558)] | 1:1,000 | AB3832 | THR558 | EMD Millipore |
| Mouse
anti-fascin | 1:100 | M3567 | 55K-2 | Dako, Glostrup,
Denmark |
| Mouse
anti-vimentin | 1:500 | M0725 | V9 | Dako |
Western blot analysis
Protein expression levels were analyzed by western
blot analysis as described previously (17). Briefly, total protein cell lysates
from 1×105 cells were prepared in Laemmli sample buffer
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins (30 µg)
were separated by 10% SDS-PAGE and transferred to 0.45 µm
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% non-fat milk powder in PBS
containing 0.1% Tween-20 for 1 h at room temperature and
subsequently incubated at 4°C overnight with primary antibodies
(Table I). Membranes were then
incubated with a horseradish peroxidase-conjugated goat anti-mouse
secondary antibody (cat. no. sc-2005; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) diluted in TBS containing 0.1% Tween-20 for
1 h at room temperature. Immunoreactive bands were visualized using
luminol reagent (Santa Cruz Biotechnology, CA).
Statistical analysis
Statistical analyses were performed in SPSS software
version 13.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation. Statistical significance was
determined using the Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of STRs in NE2, NE3,
and NEcA6 cells
To the best of our knowledge, the present study is
the first to characterize STRs in the NE2, NE3, and NEcA6
esophageal epithelial cell lines (Table II), which were immortalized by
transfection with hTERT and/or human HPV 16 E6/E7. The detailed
differences in genetic characterization are presented in Table II.
 | Table II.Characteristics of STRs in NE2, NE3,
and NEcA6 cell lines. |
Table II.
Characteristics of STRs in NE2, NE3,
and NEcA6 cell lines.
|
| Cell line |
|---|
|
|
|
|---|
| STR | NE2 | NE3 | NEcA6 |
|---|
| D3S1358 | – | 15 | 16,18 |
| TH01 | 6,7 | 7,9 | 9 |
| D21S11 | 30,31 | 32,32.2 | 28,32.2 |
| D18S51 | 18 | 14,15 | 15,16 |
| Penta E | 12,20 | 12,17 | 5,11 |
| D5S818 | 12 | 12,13 | 11 |
| D13S317 | 9,12 | 8,11 | 10 |
| D7S820 | 12 | 8,12 | 11,12 |
| D16S539 | 9,11 | 10,12 | 9,12 |
| CSF1PO | 10,11 | 12 | 10,11 |
| Penta D | 9,12 | 8,12 | 10,14 |
| Amelogenin | – | – | XY |
| vWA | 14,15 | 14 | 17,19 |
| D8S1179 | – | 12,14 | 12,15 |
| TPOX | – | 11 | 7,8 |
| FGA | 21,23 | 22,25.2 | 23,25 |
| D19S433 | – | – | 14,14.2 |
| D2S1338 | – | – | 23,24 |
NEcA6 cells were transformed and
exhibited a more aggressive tumorigenic phenotype in vitro and in
vivo when compared with NE2 and NE3 cells
Previous studies have reported that NE2, NE3 and
NEcA6 immortalized cell lines were highly proliferative, but failed
to induce colony formation in soft agar (19,20,24).
These observations were verified using the xCELLigence RTCA system
and soft agar colony formation assays in the present study. As
shown in Fig. 1A, the
proliferation rate of NE3 cells and NEcA6 cells was significantly
higher when compared with NE2 cells during the 40-h period after
cell seeding (P=0.043). NE2 cells and NE3 cells were unable to grow
in soft agar, whereas NEcA6 cells formed small colonies (Fig. 1B), indicating that the NEcA6 cells
may have acquired the capacity for anchorage-independent growth
during the passaging process. To further test the tumorigenicity of
these cell lines in vivo, they were injected subcutaneously
into nude mice. As shown in Fig.
1C, NE2 cells and NE3 cells failed to form tumors, whereas two
out of six sites injected with NEcA6 cells formed tumors (~5
mm3 in volume) 35 days after xenografting.
Immunohistochemical staining demonstrated that cytokeratins, which
are markers for epithelial tissues, were expressed in
NEcA6-generated xenografts (Fig.
1C). These results were confirmed using an additional five
Nu/Nu mice, of which 10 out of 20 NEcA6 implanted sites formed
tumors (~5 mm3 in volume; data not shown). These data
suggest that NEcA6 were transformed and exhibit a more aggressive
tumorigenic phenotype in vitro and in vivo compared
with NE2 and NE3 cells.
 | Figure 1.Proliferation and tumorigenic
phenotype of immortalized esophageal epithelial cell lines NE2, NE3
and NEcA6 in vitro and in vivo. (A) The proliferation
profile of NE2, NE3 and NEcA6 cells. Real-time detection of
impedance response/cell index curves determined over 48 h. The bar
chart shows the slope [cell impedance (Ω)/time (h)] of the cell
proliferation profiles. *P<0.05 vs. NE2 cells. (B) Soft-agar
colony formation assay of NE2, NE3 and NEcA6 cells over the course
of 3 weeks (magnification, ×40; scale bars, 40 µm). Black arrows
indicate the cells in agar. The bar chart shows the number of
colonies formed for each cell line. Data are presented as the mean
± standard deviation (n=3). (C) Tumorigenicity assays in nude mice.
NE2, NE3, and NEcA6 cells were injected into Nu/Nu mice
(1×106 cells/flank). At 35 days following injection,
tumors were removed (upper panel). HE staining and
immunohistochemical staining of cytokeratin in the NEcA6
xenografted tumors (lower panel; scale bars, 50 µm). HE,
hematoxylin-eosin. |
NEcA6 cells migrated further than NE2
and NE3 cells
Cancer cell migration is known to serve a major role
in the progression of malignant tumors. Therefore, the migration
capabilities of NE2, NE3 and NEcA6 cell lines were examined using
the xCELLigence RTCA system. As shown in Fig. 2A, the migration capabilities of
NEcA6 cells were significantly higher when compared to that of the
NE2 and NE3 cells, which was consistent with the results of the
tumorigenicity assay. In order to investigate the mechanisms
underlying the prominent migration ability of NEcA6 cells, the
expression levels of fascin and ezrin-radixin-moesin (ERM), as well
as their activated forms, which have been demonstrated to serve
important roles in the migration of esophageal carcinoma cells
(25,26), were examined. As shown in Fig. 2B, no significant differences in the
protein expression levels of fascin and ERM proteins were observed
among the three cell lines. In addition, no significant differences
in the protein expression levels of FAK and phosphorylated (p)-FAK
(Tyr397) were observed among the three cell lines (Fig. 2B). FAK and p-FAK are known to
regulate several signaling pathways that lead to cell
proliferation, migration, and adhesion (27). The protein expression levels of
epithelial and mesenchymal biomarkers vimentin, γ-catenin and
E-cadherin in these cells were then examined. Notably, high
expression levels of vimentin and γ-catenin were detected in all
cell lines, however, NE2 cells lacked detectable levels of
E-cadherin (Fig. 2B). This
indicates that NE2 cells may have undergone an
epithelial-mesenchymal transition (EMT)-like process, which is
consistent with results of a previous study (24).
Immunocytochemical staining of
kindlin, talin and paxillin reveals typical dot-like focal
adhesions located at the distal ends of stress fibers in NEcA6
cells
Cell proliferation, migration and adhesion depend on
cell-to-cell and cell-to-ECM interactions, and the focal adhesion
receptors kindlin, talin and paxillin serve essential roles in
these processes (9,14). Therefore, the expression and
subcellular localization of these focal adhesion markers, as well
as their co-localization with F-actin were examined in NE2, NE3 and
NEcA6 cells using immunofluorescence assays (Fig. 3). Kindlin-2 was localized to the
nucleus in all cell lines (Fig.
3A). Kindlin-2 was also present in focal adhesions in NEcA6
cells, where it was co-localized with the termini of actin stress
fibers (Fig. 3A). By contrast, NE2
and NE3 cells exhibited reduced kindlin-2 expression at the distal
ends of stress fibers (Fig. 3A).
Talin was present in the typical punctate structures of focal
adhesions and distributed in the protrusions of NEcA6 cells,
whereas talin staining in NE2 cells and NE3 cells was diffuse
(Fig. 3B). Low talin expression
was observed in the perinuclear region of NE3 cells (Fig. 3B). As presented in Fig. 3C, paxillin was dispersed in the
cytoplasm of NE2 cells, and was present at the edge of NE3 cells.
By contrast no detectable co-localization of paxilin and F-actin
was observed in all cell lines examined (Fig. 3C). Paxillin was observed in the
typical focal adhesions of NEcA6 cells, as indicated by strong
staining at the termini of thick stress fibers. In addition,
paxillin was localized at the nucleus and perinuclear regions in
NEcA6 cells (Fig. 3C). Notably,
kindlin-2, talin and paxillin were present at sites of focal
adhesion and localized at the two distal terminals of stress fibers
in NEcA6 cells, suggesting that NEcA6 cells form a greater number
of mature focal adhesions compared with the other two cell lines
(Fig. 3). These features may be
associated with increased cell proliferation and migration, which
may lead to a more aggressive tumorigenic phenotype in NEcA6 cells,
compared with NE2 and NE3 cells.
 | Figure 3.Localization of kindlin, talin, and
paxillin in NE2, NE3 and NEcA6 immortalized esophageal epithelial
cell lines. NE2, NE3, and NEcA6 cells were cultured on slides for
24 h, fixed and stained for the predominant components of focal
adhesions (A) kindlin, (B) talin, and (C) paxillin (green
fluorescent signals), as well as for actin microfilaments (red
fluorescent signals). Merged images show the co-localization of
focal adhesions and actin microfilaments (yellow fluorescent
signals), as indicated by white arrows. Images obtains by confocal
microscopy (FV1000; Olympus) equipped with a 60x, 1.42NA, Plan oil
objective (Olympus, Japan) Scale bar, 20 µm. DAPI,
4′,6-diamidino-2-phenylindole. |
NEcA6 cells exhibit increased adhesion
capabilities when compared with NE2 and NE3 cells
Cells adhere to the ECM via focal contacts, which
mature into focal adhesions containing kindlin, talin, and paxillin
(13,14,28).
Cell adhesion depends on ECM interactions (29–31).
The integrin protein family and focal adhesion proteins serve
essential roles during this process by interacting with ECM
proteins, such as fibronectin and the actin-based cytoskeleton
(29,30,32).
It was therefore hypothesized that the larger number of mature
focal contacts in NEcA6 cells may lead to abnormal cell adhesion,
proliferation and migration. In order to test this hypothesis, the
degree of cell adhesion and migration of all cell lines with and
without fibronectin was examined using the xCELLigence RTCA system.
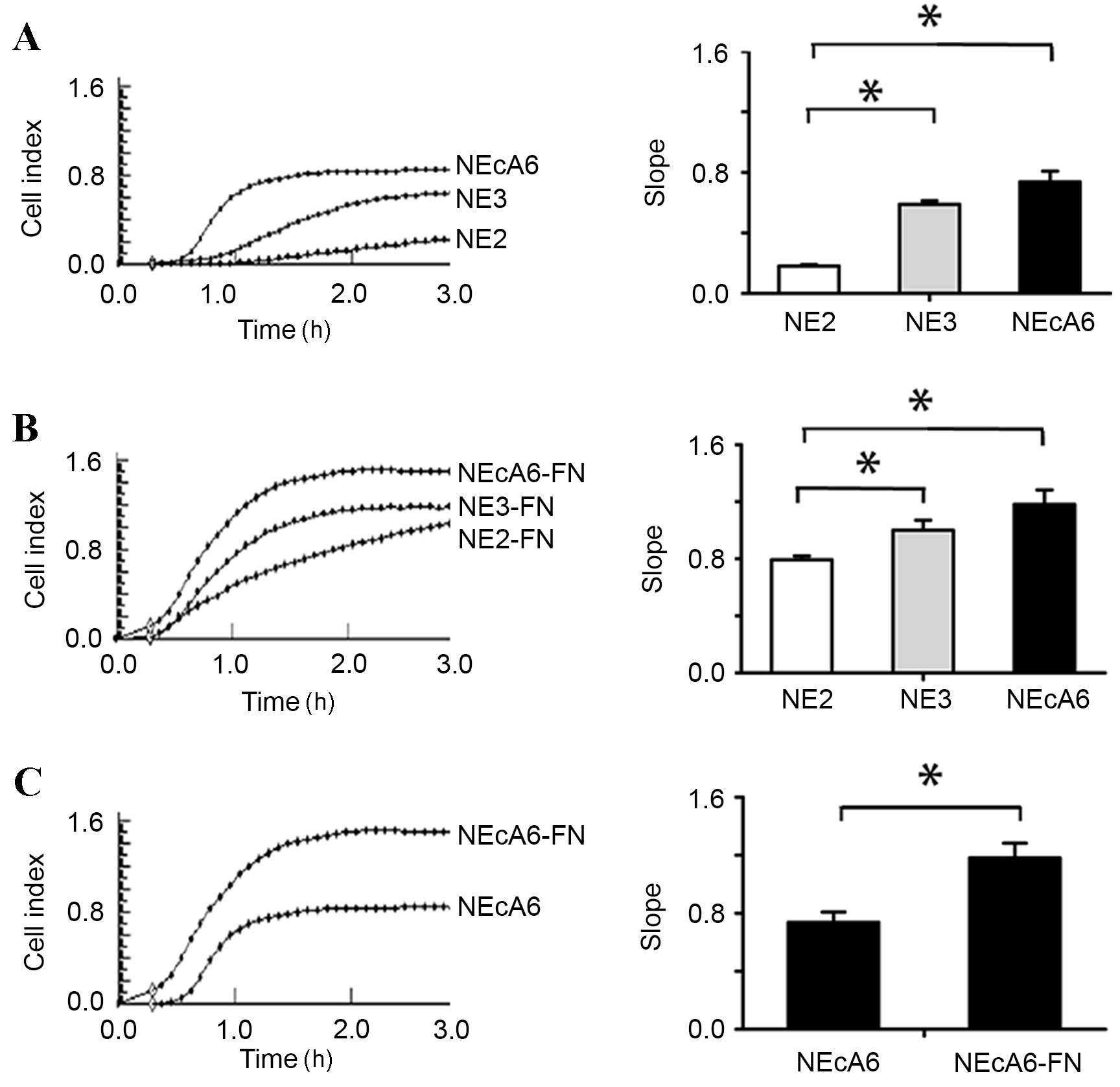
As shown in Fig. 4, NEcA6 cells
exhibited significantly higher (P=0.002) adhesion capabilities when
compared with NE2 and NE3 cell lines in the presence and absence of
fibronectin. Cell adhesion was maximal at 2 h after the cells were
seeded (Fig. 4). The seeding of
all cell lines onto fibronectin-coated wells led to a marked
increase in the cell index (indicative of increased cell adhesion)
when compared with cells seeded on plates without fibronectin
(Fig. 4).
siRNA-mediated depletion of kindlin-2
leads to a greater decrease in cell adhesion capabilities in NEcA6
cells than NE3 cells when compared with non-targeting controls
The above results demonstrate that the focal
adhesion receptors kindlin, talin, and paxillin exhibited typical
focal adhesion morphology in NEcA6 cells, which was different from
the other two cell lines. These alterations may be responsible for
the observed alterations in cell migration, adhesion and
proliferation among the cell lines. In order to investigate this
further, the effect of siRNA-mediated depletion of kindlin-2, which
has been demonstrated to regulate cell-to-ECM interactions, induce
the formation of focal adhesions and promote cancer cell adhesion
(23,25), was examined in NE3 and NEcA6 cells.
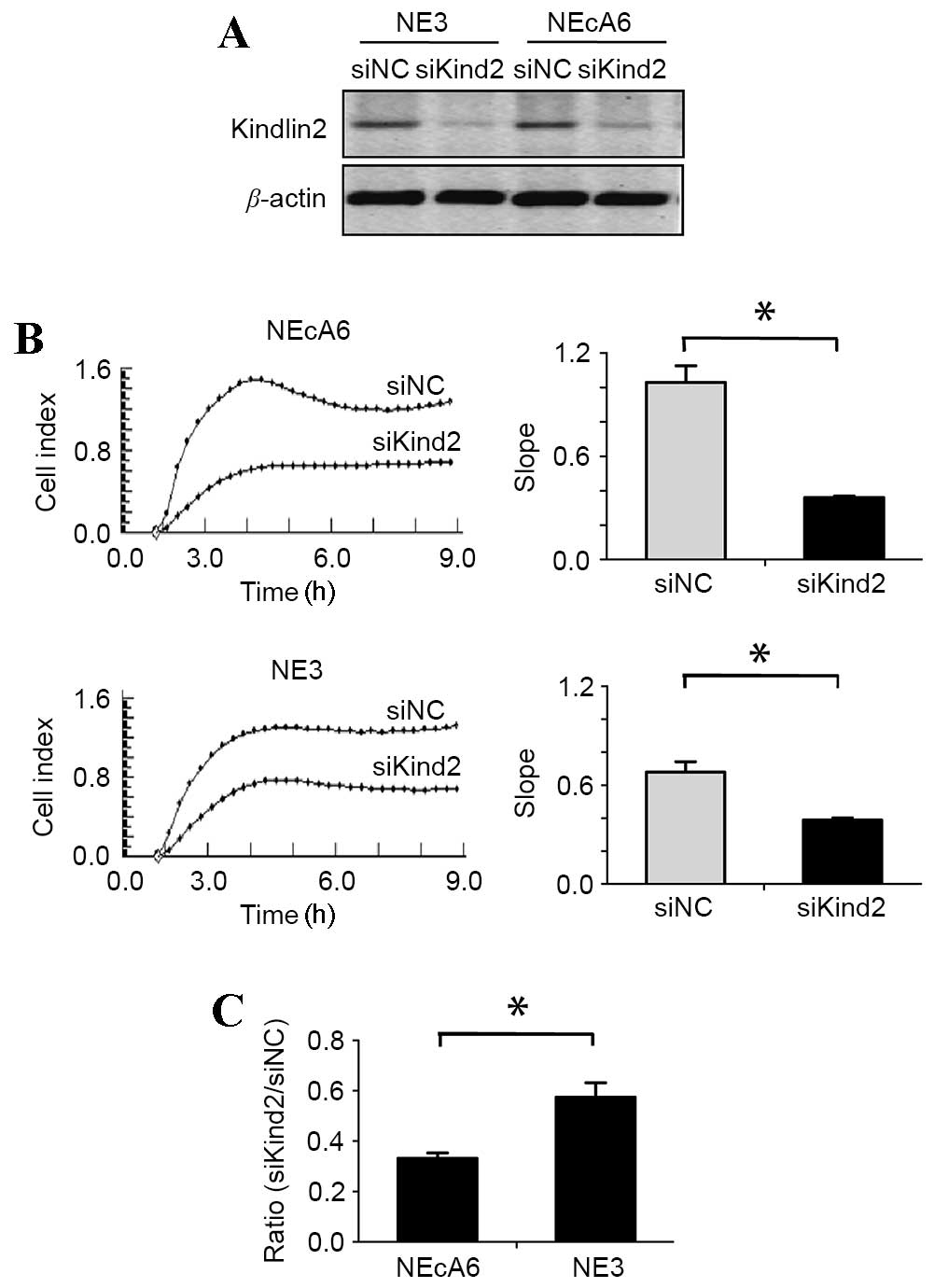
Western blotting results revealed that kindlin-2 siRNA-treated
cells demonstrated a marked reduction in kindlin-2 protein
expression levels when compared with the negative control cells
(Fig. 5A). The effect of kindlin-2
depletion on cell adhesion and migration was then evaluated using
the xCELLigence RTCA system. siRNA-treated NE3 and NEcA6 cells
exhibited a significant reduction (P=0.003) in cell adhesion when
compared with negative control cells (Fig. 5B). Notably, the adhesion rates of
siRNA-treated NE3 and NEcA6 cells were reduced by 42 and 66%,
respectively, when compared with the negative controls. In
addition, the adhesion rate of NEcA6 cells was decreased by 24%
compared with that of NE3 cells (Fig.
5B and C). These results indicate that the effect of kindlin-2
knockdown on cell adhesion capabilities was greater in NEcA6 cells
when compared with that of NE3 cells. Therefore, the authors
hypothesize that kindlin-2 may contribute to the carcinogenesis of
NEcA6 cells.
Discussion
Immortalized esophageal epithelial cell lines
derived from Chinese patients are currently scarce (18,24,33).
To the best of our knowledge, the present study is the first to
characterize the STRs of NE2, NE3 and NEcA6 immortalized esophageal
epithelial cell lines. This characterization may serve as the
foundation for the genetic identification of these cell lines in
future studies. Unexpectedly, our in vitro and in
vivo investigations revealed that NEcA6 cells formed small
dense colonies in soft agar and small tumors in nude mice,
indicating that these cells may have undergone transformation.
Compared with NE2 and NE3 cells, NEcA6 cells exhibited a more
aggressive tumorigenic phenotype in vitro and in
vivo. Based on these results, it is formally possible that
cytogenetic aberrations occurred during the progressive passaging
of these cells (18). However, the
molecular/epigenetic events underlying the more aggressive
tumorigenic phenotype in NEcA6 cells remain unknown. In addition,
NEcA6 cells demonstrated increased migration capabilities when
compared with NE2 and NE3 cells. However, no significant
alterations in the expression levels of migration-related proteins
fascin, ERM, and p-FAK (Tyr397) were observed among the cell lines.
Furthermore, NE2 cells appeared to have undergone an EMT-like
process, whereas NE3 cells and NEcA6 cells expressed both
E-cadherin and vimentin. These findings suggest that additional
mechanisms may have been responsible for the high migration ability
of NEcA6 cells.
The expression and subcellular localization of
kindlin-2, talin, and paxillin was investigated further using
immunofluorescence techniques. Notably, these focal adhesion
components exhibited a typical morphology and were located at the
two distal terminals of stress fibers in NEcA6 cells, but not in
NE2 or NE3 cells. The focal adhesion protein paxillin functions as
a multi-domain adapter molecule, and is a target of many oncogenes
such as v-Src and the human papillomavirus E6 protein (19,34).
In addition, paxillin recruits actin to its C-terminus at sites of
cell adhesion to the ECM, and undergoes extensive phosphorylation
during integrin-mediated cell adhesion (35,36).
Therefore, paxillin is considered to function at the crossroads of
cell adhesion and cell migration (13,37).
Similarly, talin and kindlin, which are two protein families
consisting of four point one, ezrin, radixin, moesin-domain
proteins, are components of focal adhesions that link the ECM to
the actin cytoskeleton by binding directly to the cytoplasmic tails
of β-integrin subunits (9). These
proteins also serve a key role in modulating integrin activity, and
transduce signals primarily through the inside-out activation of
integrin signaling pathways (14,38).
The detailed mechanisms underlying the effects of kindlin proteins
on integrin activation remain unclear, however, it is clear that
these proteins cooperate with talin to activate integrin by binding
to the cytoplasmic tails of β1 and β3 integrins (34,39).
In the present study, these three crucial components of focal
adhesions exhibited a typical dot-like staining in NEcA6 cells,
indicative of the formation of a large number of focal contacts.
Formation of these adhesion contacts depends on FAK and integrin,
and these adhesions stabilize the lamellipodia by mediating
attachment to the ECM and the rearrangement of actin, thereby
contributing to efficient migration (32). In addition to the modulation of
integrin-mediated cell migration, these three focal adhesion
markers have been demonstrated to regulate cell adhesion by
affecting the recruitment of focal adhesion complex components
(14,37). Adhesion of fibroblasts to
fibronectin stimulates the phosphorylation of tyrosine in paxillin
by FAK, which is a signal transduction mechanism associated with
cell adhesion and cytoskeletal reorganization (37). Therefore, the authors hypothesize
that NEcA6 cells not only possess prominent migration ability, but
also display high adhesion ability. Indeed NEcA6 cells demonstrated
the highest adhesion capabilities in the absence of ECM and in
fibronectin-coated wells among all cell lines examined.
An increasing number of studies have demonstrated
the importance of kindlin-2 in the malignant progression of tumors
(40–43). A recent study reported that
kindlin-2 is highly expressed in the invasive edge of tumors, and
its overexpression promotes cell migration/invasion (34,44).
However, the expression and biological significance of kindlin-2 in
immortalized esophageal epithelial cells is unclear. The results of
the present study revealed that kindlin-2 was expressed in NE3 and
NEcA6 cells, and siRNA-mediated knockdown of kindlin-2 reduced the
adhesion capabilities of these cell lines. Compared with NE3 cells,
knockdown of kindlin-2 in NEcA6 cells was associated with a greater
reduction in cell adhesion, which suggests that kindlin-2 may serve
a role in the tumorigenic phenotype displayed by these cells.
However, kindlin-2 knockdown did not completely inhibit cell
adhesion in these cell lines, which suggests that additional
regulatory mechanisms may be involved in mediating carcinogenesis.
Further studies are required to validate this mechanism in the cell
lines.
In conclusion, the findings of the present study
reveal the migration and adhesion characteristics of NE2, NE3 and
NEcA6 immortalized esophageal epithelial cell lines, and
demonstrate the distribution of kindlin-2, talin and paxillin. The
results suggest that these cell lines may be valuable models for
future studies investigating the processes of cell migration and
adhesion, as well as the molecular mechanisms that contribute to
esophageal carcinogenesis. Of particular note, the transformed
NEcA6 cells may be useful in future studies involving the
investigation of cytogenetic alterations that occur in the initial
stages of carcinogenesis in vitro.
Acknowledgments
The authors would like to thank Professor Sai-Wah
Tsao (Department of Anatomy, University of Hong Kong, Hong, Kong,
China) for supplying the NE2, NE3, and NEcA6 cells used for the
purposes of this study. In addition, the authors would like to
thank Dr Wei-Jiang Zhao, (Neural Science Center, Shantou University
Medical College, Shantou, China) for assisting with the completion
of the confocal microscopy analysis. The present study was
supported by grants from the Natural Science Foundation of
China-Guangdong Joint Fund (grant nos. U1301227 and U0932001) and
the National Natural Science Foundation of China (grant nos.
81172264 and 81472613).
References
|
1
|
Vicente-Manzanares M, Webb DJ and Horwitz
AR: Cell migration at a glance. J Cell Sci. 118:4917–4919. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Axelrad JE, Lichtiger S and Yajnik V:
Inflammatory bowel disease and cancer: The role of inflammation,
immunosuppression, and cancer treatment. World J Gastroenterol.
22:4794–4801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mayor R and Etienne-Manneville S: The
front and rear of collective cell migration. Nat Rev Mol Cell Biol.
17:97–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: Integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Etienne-Manneville S: Adherens junctions
during cell migration. Subcell Biochem. 60:225–249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turner CE: Paxillin. Int J Biochem Cell
Biol. 30:955–959. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim DH and Wirtz D: Focal adhesion size
uniquely predicts cell migration. FASEB J. 27:1351–1361. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clark EA and Brugge JS: Integrins and
signal transduction pathways: The road taken. Science. 268:233–239.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turner CE, Glenney JR Jr and Burridge K:
Paxillin: A new vinculin-binding protein present in focal
adhesions. J Cell Biol. 111:1059–1068. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown MC and Turner CE: Paxillin: Adapting
to change. Physiol Rev. 84:1315–1339. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheppard D: In vivo functions of
integrins: Lessons from null mutations in mice. Matrix Biol.
19:203–209. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turner CE: Paxillin and focal adhesion
signalling. Nat Cell Biol. 2:E231–E236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calderwood DA, Campbell ID and Critchley
DR: Talins and kindlins: Partners in integrin-mediated adhesion.
Nat Rev Mol Cell Biol. 14:503–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Spillare EA, Wang QS, Sabourin CLK
and Stoner GD: p53-independent down-regulation of cyclin D1 and
p21Waf1 in the process of immortalization of human esophageal
epithelial cells. Int J Oncol. 12:325–328. 1998.PubMed/NCBI
|
|
16
|
Shen Z, Cen S, Shen J, Cai W, Xu J, Teng
Z, Hu Z and Zeng Y: Study of immortalization and malignant
transformation of human embryonic esophageal epithelial cells
induced by HPV18 E6E7. J Cancer Res Clin Oncol. 126:589–594. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sashiyama H, Shino Y, Kawamata Y, Tomita
Y, Ogawa N, Shimada H, Kobayashi S, Asano T, Ochiai T and Shirasawa
H: Immortalization of human esophageal keratinocytes by E6 and E7
of human papillomavirus type 16. Int J Oncol. 19:97–103.
2001.PubMed/NCBI
|
|
18
|
Zhang H, Jin Y, Chen X, Jin C, Law S, Tsao
SW and Kwong YL: Cytogenetic aberrations in immortalization of
esophageal epithelial cells. Cancer Genet Cytogenet. 165:25–35.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu L, Qin YR, Xie D, Hu L, Kwong DL,
Srivastava G, Tsao SW and Guan XY: Characterization of a novel
tumor-suppressor gene PLC delta 1 at 3p22 in esophageal squamous
cell carcinoma. Cancer Res. 67:10720–10726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhuang ZH, Tsao SW, Deng W, Wang JD, Xia
HH, He H, Feng HC, Wang LD, Gu Q, Lam SK, et al: Early upregulation
of cyclooxygenase-2 in human papillomavirus type 16 and
telomerase-induced immortalization of human esophageal epithelial
cells. J Gastroenterol Hepatol. 23:1613–1620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Q, Shen JH, Shen ZY, Wu ZY, Xu XE,
Xie JJ, Wu JY, Huang Q, Lu XF, Li EM and Xu LY: Phosphorylation of
fascin decreases the risk of poor survival in patients with
esophageal squamous cell carcinoma. J Histochem Cytochem.
58:979–988. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Limame R, Wouters A, Pauwels B, Fransen E,
Peeters M, Lardon F, De Wever O and Pauwels P: Comparative analysis
of dynamic cell viability, migration and invasion assessments by
novel real-time technology and classic endpoint assays. PLoS One.
7:e465362012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Atienza JM, Zhu J, Wang X, Xu X and Abassi
Y: Dynamic monitoring of cell adhesion and spreading on
microelectronic sensor arrays. J Biomol Screen. 10:795–805. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheung PY, Deng W, Man C, Tse WW,
Srivastava G, Law S, Tsao SW and Cheung AL: Genetic alterations in
a telomerase-immortalized human esophageal epithelial cell line:
Implications for carcinogenesis. Cancer Lett. 293:41–51. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie JJ, Xu LY, Zhang HH, Cai WJ, Mai RQ,
Xie YM, Yang ZM, Niu YD, Shen ZY and Li EM: Role of fascin in the
proliferation and invasiveness of esophageal carcinoma cells.
Biochem Biophys Res Commun. 337:355–362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie JJ, Xu LY, Xie YM, Zhang HH, Cai WJ,
Zhou F, Shen ZY and Li EM: Roles of ezrin in the growth and
invasiveness of esophageal squamous carcinoma cells. Int J Cancer.
124:2549–2558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: In command and control of cell motility. Nat
Rev Mol Cell Biol. 6:56–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rondas D, Tomas A and Halban PA: Focal
adhesion remodeling is crucial for glucose-stimulated insulin
secretion and involves activation of focal adhesion kinase and
paxillin. Diabetes. 60:1146–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baumann K: Cell adhesion: Extracellular
bonds. Nat Rev Mol Cell Biol. 14:4042013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wrighton KH: Cell adhesion: The ‘ins’ and
‘outs’ of integrin signalling. Nat Rev Mol Cell Biol. 14:7522013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Multhaupt HA, Leitinger B, Gullberg D and
Couchman JR: Extracellular matrix component signaling in cancer.
Adv Drug Deliv Rev. 97:28–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carragher NO and Frame MC: Focal adhesion
and actin dynamics: A place where kinases and proteases meet to
promote invasion. Trends Cell Biol. 14:241–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu HP, Xu SQ, Liu L, Shi LY, Cai XK, Lu
WH, Lu B, Su YH and Li YY: Cyclooxygenase-2 expression in squamous
dysplasia and squamous cell carcinoma of the esophagus. Cancer
Lett. 198:193–201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
An Z, Dobra K, Lock JG, Strömblad S,
Hjerpe A and Zhang H: Kindlin-2 is expressed in malignant
mesothelioma and is required for tumor cell adhesion and migration.
Int J Cancer. 127:1999–2008. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deakin NO and Turner CE: Paxillin comes of
age. J Cell Sci. 121:2435–2444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lawson C and Schlaepfer DD: Integrin
adhesions: Who's on first? What's on second? Connections between
FAK and talin. Cell Adh Migr. 6:302–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schaller MD: Paxillin: A focal
adhesion-associated adaptor protein. Oncogene. 20:6459–6472. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karaköse E, Schiller HB and Fässler R: The
kindlins at a glance. J Cell Sci. 123:2353–2356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liao Z, Kato H, Pandey M, Cantor JM,
Ablooglu AJ, Ginsberg MH and Shattil SJ: Interaction of kindlin-2
with integrin β3 promotes outside-in signaling responses by the
αVβ3 vitronectin receptor. Blood. 125:1995–2004. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen Z, Ye Y, Kauttu T, Seppänen H,
Vainionpää S, Wang S, Mustonen H and Puolakkainen P: Novel focal
adhesion protein kindlin-2 promotes the invasion of gastric cancer
cells through phosphorylation of integrin β1 and β3. J Surg Oncol.
108:106–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang HF, Zhang K, Liao LD, Li LY, Du ZP,
Wu BL, Wu JY, Xu XE, Zeng FM, Chen B, et al: miR-200b suppresses
invasiveness and modulates the cytoskeletal and adhesive machinery
in esophageal squamous cell carcinoma cells via targeting
Kindlin-2. Carcinogenesis. 35:292–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ge YS, Liu D, Jia WD, Li JS, Ma JL, Yu JH
and Xu GL: Kindlin-2: A novel prognostic biomarker for patients
with hepatocellular carcinoma. Pathol Res Pract. 211:198–202. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ren Y, Jin H, Xue Z, Xu Q, Wang S, Zhao G,
Huang J and Huang H: Kindlin-2 inhibited the growth and migration
of colorectal cancer cells. Tumour Biol. 36:4107–4114. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu Y, Wu J, Wang Y, Zhao T, Ma B, Liu Y,
Fang W, Zhu WG and Zhang H: Kindlin 2 forms a transcriptional
complex with β-catenin and TCF4 to enhance Wnt signalling. EMBO
Rep. 13:750–758. 2012. View Article : Google Scholar : PubMed/NCBI
|