Introduction
Ginkgo biloba has been used as a medicine in
China for >5,000 years. Ginkgo biloba extracts, isolated
from the leaves of the Ginkgo biloba tree, contain flavonoid
and terpenoid substances (1). It
has antioxidant effects, acting as a scavenger of free radicals,
has a relaxing effect on vascular walls, an antagonistic action on
platelet-activating factor, beneficial effects on blood flow and
microcirculation, and it stimulates neurotransmitters (2). A number of studies have demonstrated
that ginkgolides exhibit protective effects on tissue
abnormalities, which include myocardial ischemia reperfusion injury
(3), ischemic brain damage
(4) neuronal apoptosis (5), hypoxia-induced memory deficits and
neuronal DNA damage (6). These
effects are considered to be of benefit in the treatment of
diseases that are associated with the production of free radicals,
including ischemic heart disease, cerebral infarction, chronic
inflammation and aging (7,8). The ginkgolides demonstrated to be
present in the terpenoid extracts can be divided into isotypes A,
B, C, M and J, with ginkgolide B (GB;
C20H24O10) exhibiting the highest
biological activity (9).
The GB ginkgolide is a major constituent of the
terpenoid fraction. A number of studies have shown that GB
possesses neuroprotective effects on various brain injuries,
including permanent focal ischemia, transient focal ischemia and
ischemia-reperfusion injury (10),
by exerting antioxidant effects, reducing brain edema or inhibiting
cell apoptosis (11,12).
To the best of our knowledge, the majority of
previous studies have focused on the neuroprotective effects of GB.
Our previous study (13) indicated
that GB promotes bone marrow-derived endothelial progenitor cell
proliferation, migration and adhesion, and the induction of
angiogenesis through vascular endothelial growth factor (VEGF). As
the effect of GB on osteoblast-like MC3T3-E1 cells has not been
examined previously. The present study aimed to investigate the
effect and underlying mechanism of GB on osteogenic differentiation
in the MC3T3E-1 cell line. The results of the present study
demonstrated that GB promoted osteoblastic differentiation of the
MC3T3-E1 cells through the upregulation of VEGF, and that the p38
mitogen-activated protein (MAP) kinase signaling pathway is
involved in GB-induced expression of VEGF. These findings suggest
GB may be important in the treatment of osteoporosis.
Materials and methods
Cell culture
Murine osteoblastic MC3T3-E1 cells (American Type
Culture Collection, Manassas, VA, USA) were cultured in a 5% CO2
atmosphere at 37°C in α-MEM (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) containing 10% heat-inactivated fetal bovine serum
(FBS; Hyclone; GE Healthcare Life Sciences). On reaching 80%
confluence, the cells were passaged with 0.25% trypsin (BioSharp,
Hefei, China) and transferred to new culture flasks at a ratio of
1:3. To induce osteogenic differentiation, the culture medium was
replaced with differentiation medium (α-MEM containing 10 mM
β-glycerophosphate and 50 µg/ml ascorbic acid).
Chemicals
GB (purity ≥99% by HPLC) was obtained from the
National Institutes for Food and Drug Control (Beijing, China;
http://www.nifdc.org.cn/directory/web/WS02/CL0049/2191.html).
The GB was dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) at a concentration of 100
g/l.
Cell viability assay
The cytotoxic effects of GB on the MC3T3-E1 cells
were evaluated using an MTS assay. The MC3T3-E1 cells were seeded
in a 96-well plate at 10,000 cells per well and cultured for 24 h
at 37°C. Following rinsing with phosphate-buffered saline (PBS),
the cells were treated with various concentrations of GB (0, 1.25,
5, 20, 80 and 160 µg/l) in fresh medium for 48 h. The viable cells
were then treated with newly prepared medium containing 20 µl of 2
mg/ml MTS and 100 µl of α-MEM (10% FBS) in a CO2 incubator for 3 h.
The MTS was transformed by the living cells to a purple formazan
dye, which was dissolved in 100 µl DMSO by shaking at 150 rpm for
10 min on an ELISA shaker (Gentaur, Brussels, Belgium). Finally,
the relative colorimetric intensity of each well was evaluated
using a Varioskan flash multimode reader (Thermo Fisher Scientific
Inc., Waltham, MA, USA) at a 490 nm wavelength.
Measurement of alkaline phosphatase
(ALP) activity
The MC3T3-E1 cells were seeded in a 96-well plate at
10,000 cells per well, and placed in a 5% CO2 incubator at 37°C for
7 and 14 days, respectively. Following being rinsed with PBS, the
MC3T3-E1 cells were treated with 20 µg/ml GB in fresh medium for 4
days. Following removal of the medium, the cells in each well were
incubated with 100 µl of ALP buffer solution for 10 min. The cells
of each well were treated with 100 µl of pNPP at 37°C for 15 min to
determine the ALP activity. Subsequently, 100 µl of 2 M NaOH was
added to terminate the reaction. Finally, the conversion of
p-nitrophenyl phosphate to p-nitrophenol was determined using a
Varioskan flash multimode reader (Thermo Fisher Scientific Inc.) at
a wavelength of 450 nm. The intensity of ALP activity relative to
that of the control group in each well was determined.
Alizarin red staining
On days 14 and 21, the cultured cells were fixed for
the detection of Alizarin Red staining (Sigma-Aldrich; Merck
Millipore). Briefly, the cells were fixed with ice-cold 70% ethanol
for 60 min, washed three times with PBS, and stained with Alizarin
Red S (40 mM; pH 4.2) for 10 min, followed by rinsing with PBS
three times. Images of the stained nodules were captured using a
digital camera (Nikon C-SHG; Nikon Corporation, Tokyo, Japan).
Subsequently, 50 µl 10% cetylpyridinium chloride was added to each
well, cultured at room temperature for 20 min and the relative
colorimetric intensity of each well was evaluated using a Varioskan
Flash Multimode Reader (Thermo Fisher Scientific, Inc.) at a
wavelength of 560 nm. Finally, according to the standard curve, the
optical density value was converted to concentration.
Reverse-transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA of the MC3T3-E1 cells was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Following subsequent DNase digestion (Invitrogen; Thermo Fisher
Scientific, Inc.), 500 ng of RNA was used to synthesize 10 µl of
cDNA using an PrimeScript® RT Master Mix kit (Takara
Biotechnology, Co., Ltd., Dalian, China). The qPCR reactions were
performed in a CFX ConnectTM real time system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using All-in-One™ qPCR mix
(GeneCopoeia, Guangzhou, China). SYBR Green Master mix
(GeneCopoeia) was used for all PCR experiments. Thermocycling
conditions were as follows: 95°C for 20 sec for pre-denaturation;
and 40 cycles of 95°C for 3 sec and 60°C for 30 sec. The expression
in each sample was evaluated in three technical replicates. The
specificity of primer pairs and absence of primer dimers was
validated by analysis of the dissociation curves and agarose gel
electrophoresis. The comparative Cq method (14) was used for data analysis. The level
of expression in each sample was normalized to the mRNA level of
glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The mean
expression from three experiments was calculated. The sequences of
the primers used in the present study were as follows: VEGF,
forward 5′GGACCCTGGCTTTACTGCTGTACC-3′ and reverse
5′TCACCGCCTTGGCTTGTCACA-3′; Osx, forward 5′-GCAAGGCTTCGCATCTGAA-3
and reverse 5′-TAGCAGGTTGCTCTGCTC-3′; GAPDH, forward
5′-ACCACAGTCCATGCCATCAC-3′ and reverse
5′-TCCACCACCCTGTTGCTGTA-3′.
Western blot analysis
The proteins were isolated from MC3T3-E1 cells using
RIPA lysis buffer (Google Biological Technology Co., Ltd., Wuhan,
China) and concentration was determined by the BCA Protein assay
kit (Beyotime Institute of Biotechnology, Haimen, China) according
to the manufacturer's protocols. The equal samples of proteins (30
µg) were separated on 10% SDS-PAGE gels and transferred onto a PVDF
membrane followed by western blot analysis. Briefly, 5% milk in TBS
containing 0.1% Tween-20 was used to block non-specific binding.
The membrane was incubated with anti-VEGF rabbit polyclonal
antibody (Merck Millipore; cat. no. AB1876), P-38 polyclonal
antibody (Cell Signaling Technology, Inc., Danvers, MA, USA; cat.
no. 9228), phosphorylated (phospho)-specific P-38 polyclonal
antibody (Cell Signaling Technology, Inc.; cat. no. 4092),
phospho-specific p44/p42 (Cell Signaling Technology, Inc.; cat. no.
4695), or p44/p42 MAP kinase antibodies (Cell Signaling Technology,
Inc.; 9106) overnight at 4°C at a dilution of 1:1,000, followed by
extensive washing with PBS and incubation for 1 h at 37°C with
horseradish peroxidase-conjugated secondary antibody (1:20,000;
Cell Signaling Technology, Inc.; cat. no.). Following antibody
incubation and washes with PBS, an ECL kit (GE Healthcare Life
Sciences, Chalfont, UK) was used for detection.
Enzyme-linked immunosorbent assay
(ELISA)
The MC3T3-E1 cells were cultivated and treated with
GB, as described for the cell viability assay. Following incubation
with GB for 24 and 48 h respectively, the supernatants were
harvested and used for the ELISA assay (eBioscience, Inc., San
Diego, CA, USA), according to the manufacturer's protocol.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS software
(version 13.0; SPSS, Inc., Chicago, IL, USA). All data were
analyzed using one-way analysis of variance and Student's t test.
P<0.05 was considered to indicate a statistically significant
difference. All experiments were performed independently at least
three times.
Results
Effects of GB on the viability of
MC3T3E1 cells
To determine the effects of various concentrations
of GB on the cell viability of MC3T3E1 cells, which are have the
potential to differentiate into osteoblast cells on culture in
osteoblastic differentiation medium, an MTS assay was performed.
The cells were treated with different concentrations of GB for 48 h
and cell viability was assessed using the MTS assay. As shown in
Fig. 1, GB marginally increased
the viability of the MC3T3-E1 cells within a 1.25–160 µg/ml dose
range, with 20 µg/ml GB showing the maximal effect.
Effects of GB on osteoblastic
differentiation in MC3T3-E1 cells
ALP is a well-recognized marker for the
differentiation of osteoblasts, and to determine the effects of GB
on the activities of ALP in MC3T3 cells, ALP activity assays were
performed. The MC3T3-E1 cells were cultured in α-MEM (control), GB
(20 µg/ml), osteogenic medium and osteogenic medium supplemented
with 20 µg/ml GB for 1 and 2 weeks, respectively. The ALP activity
in the cells treated with osteogenic medium supplemented with GB
was significantly higher, compared with those in the control cells
and cells treated with osteogenic medium alone at 1 and 2 weeks,
respectively (Fig. 2A). These
results indicated that GB promoted ALP activity in the MC3T3-E1
cells at the early stage.
 | Figure 2.Effect of GB on the osteoblastic
differentiation of MC3T3-E1 cells. (A) Measurements of ALP activity
in MC3T3-E1 cells. The cells were cultured in α-MEM, GB, osteogenic
medium and osteogenic medium supplemented with 20 µg/ml GB,
respectively. Following treatment of 1 and 2 weeks, the cells were
subjected to an ALP activity assay. The data are presented as the
mean + standard deviation (*P<0.05, vs. Con;
#P<0.05 vs. OS. n=3). (B) Quantitative analysis of
mineralization, based on the results of the Alizarin Red staining.
The data are presented as the mean + standard deviation (*P<0.05
and **P<0.01, vs. Con; #P<0.05, vs. OS; n=3). (C)
Images in the top line show Alizarin Red S staining, with calcium
deposition stained bright orange/red in the osteocytes at 2 weeks
in the (a) Con, (b) GB, (c) OS and (d) OSGB groups. Images below
show Alizarin Red S staining with calcium deposition stained bright
orange/red in the osteocytes at 3 weeks in the (e) Con, (f) GB, (g)
OS and (h) OSGB groups. Magnification, ×100. GB, ginkgolide B; OS,
osteogenic medium; OSGB, OS supplemented with GB; Con, control;
ALP, alkaline phosphatase; OD, optical density. |
To determine the effect of GB on calcium deposition
in the MC3T3-E1 cells, mineralization was examined using Alizarin
Red staining, based on the results of the staining, quantification
of the mineralization was performed. The MC3T3-E1 cells were
examined following culture for 2 and 3 weeks in osteogenic medium
with or without 20 µg/ml GB. GB significantly increased
osteoblastic mineralization in the cells following treatment for 2
and 3 weeks, as visualized by the Alizarin Red S staining (Fig. 2B and C). There was an increase in
the concentration of mineralized nodules in the group treated with
osteogenic medium supplemented with GB, compared with the control
group following culture for 2 and 3 weeks, respectively (Fig. 2B and C).
Effect of GB on the secretion of VEGF
in MC3T3-E1 cells
To examine the effect of GB on the expression of
VEGF in MC3T3-E1 cells, the present study used RT-qPCR analysis and
ELISA assays to evaluate the mRNA expression and secretion levels
of VEGF in the MC3T3-E1 cells. Over an incubation period of 24 h
with GB, the mRNA expression of VEGF was significantly increased in
the MC3T3-E1 cells treated with GB at 20 µg/ml, compared with the
untreated MC3T3-E1 cells (P<0.05; Fig. 3A). However, the mRNA expression of
VEGF decreased following incubation for 48 h with 20 µg/ml GB
(Fig. 3A). Consistently, the
concentration of VEGF in the supernatant was significantly
increased in the MC3T3-E1 cells treated with 20 µg/ml GB, compared
with the untreated MC3T3-E1 cells at 24 and 48 h, respectively
(P<0.05; Fig. 3B).
Effects of SB203580 and PD98059 on the
GB-induced synthesis of VEGF in MC3T3-E1 cells
To determine whether p38 MAP kinase or p44/p42 MAP
kinase are involved in the GB-induced VEGF synthesis, the present
study examined the effects of SB203580, which specifically inhibits
p38 MAP kinase, and PD98059, which inhibits the upstream kinase
that activating p44/p42 MAP kinase, on the synthesis of VEGF
induced by GB. SB203580, which by itself had minimal effect on the
synthesis of VEGF, significantly reduced the GB-stimulated mRNA
expression (Fig. 4A) and synthesis
(Fig. 4B) of VEGF at a
concentration of 30 µM. By contrast, PD98059 had no significant
effect on the mRNA expression of VEGF (Fig. 4C) or GB-stimulated secretion of
VEGF (Fig. 4D).
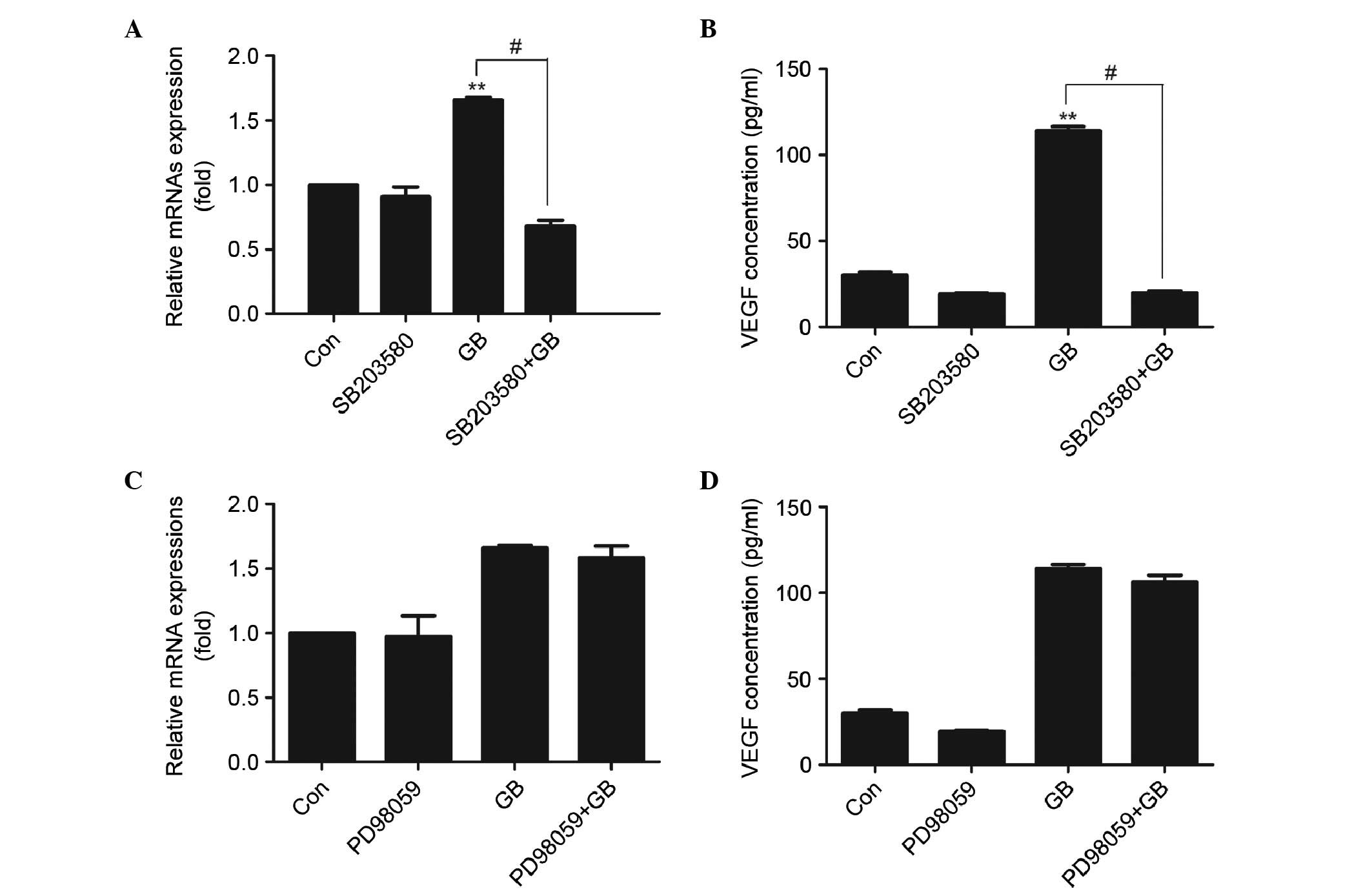 | Figure 4.Effect of SB203580 and PD98059 on the
expression of VEGF in MC3T3E1 cells. (A) Cells were pretreated with
30 µM SB203580 for 1 h, following which the cells were treated with
20 µg/ml GB for 24 h. The RNA was extracted and mRNA expression
levels of VEGF were determined using RT-qPCR analysis. Results are
expressed as the mean + standard deviation (**P<0.01, vs. Con;
#P<0.01; n=3) (B) Cells were pretreated with 30 µM
SB203580 for 1 h, followed by treatment with 20 µg/ml GB for 24 h.
Cell supernatants were then collected and used for enzyme-linked
immunosorbent assay analysis. The results are expressed as the mean
+ standard deviation (**P<0.01, vs. Con; #P<0.01;
n=3). (C) Cells were pretreated with 50 µM PD98059 for 1 h,
followed by treatment with 20 µg/ml GB for 24 h. The RNA was
extracted and the mRNA expression levels of VEGF were determined
using RT-qPCR analysis. The results are expressed as the mean +
standard deviation. (D) Cells were pretreated with 50 µM PD98059
for 1 h, followed by treatment with 20 µg/ml GB for 24 h. Cell
supernatants were then collected and used for enzyme-linked
immunosorbent assay analysis. Results are expressed as the mean +
standard deviation. GB, ginkgolide B; VEGF, vascular endothelial
growth factor; Con, control; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Effects of GB on the phosphorylation
of p44/p42 MAP kinase and p38 MAP kinase in MC3T3-E1 cells
In order to determine whether GB activates p44/p42
MAP kinase and/or p38 MAP kinase, the present study investigated
the effects of GB on these MAP kinases and, specifically, their
levels of phosphorylation. Treatment with GB led to an increase in
the phosphorylation of p44/p42 MAP kinase and p38 MAP kinase in a
time-dependent manner (Fig. 5).
The significant stimulatory effects of GB on the phosphorylation of
p44/p42 MAP kinase and p38 MAP kinase were observed 5 min
post-stimulation (Fig. 5).
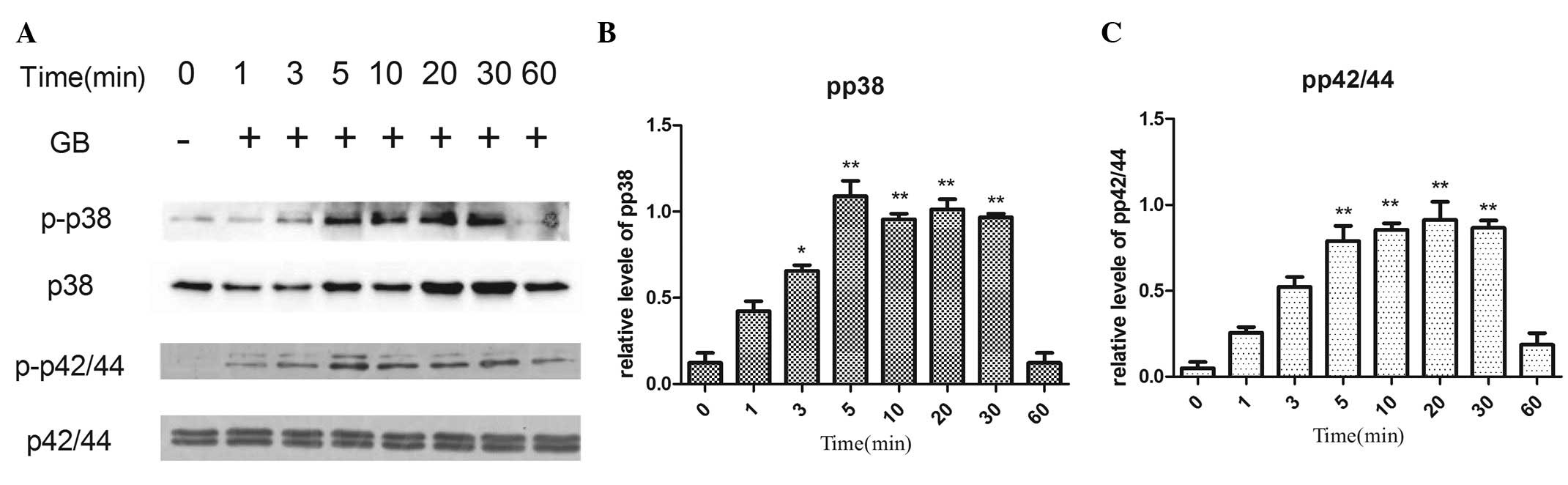 | Figure 5.Effects of GB on the phosphorylation
of p38 and p42/44 MAP kinase in MC3T3-E1 cells. The cultured cells
were stimulated with 20 µg/ml GB for 1, 3, 5, 10, 20, 30 and 60
min, respectively. (A) The extracts of cells were subjected to
SDS-PAGE and subsequent western blot analysis with antibodies
against p-p38 or p42/44 MAP kinase and p38 or p42/44MAP kinase. (B
and C) Quantification of the levels of p-p38 and p42/44 be
densitometry. *P<0.05, **P<0.01 vs. 0 min GB, ginkgolide B;
MAP, mitogen-activated protein; p-, phosphorylated. |
Effects of SB203580 and PD98059 on the
GB-induced phosphorylation of p38 MAP kinase and p44/p42 MAP kinase
in MC3T3-E1 cells
To investigate whether p38 or p44/p42 MAP kinase is
involved in the phosphorylation induced by GB in the MC3T3-E1
cells, The MC3T3-E1 cells were pretreated with 30 µM SB203580 or 50
µM PD98059 prior to treatment with 20 µg/ml GB. As shown in
Fig. 6, PD98059 had minimal effect
on the GB-induced phosphorylation of p44/p42 MAP kinase (Fig. 6A). SB203580 alone had minimal
effect on the phosphorylation of p38 MAP kinase; however, it
significantly decreased the GB-induced phosphorylation of p38 MAP
kinase (Fig. 6B).
Discussion
Traditional Chinese medicines have long been used
among the Chinese population for the treatment of several diseases
and conditions. Several in vitro and in vivo studies
have demonstrated that Chinese herbs have positive effects on bone
formation, which occur by promoting osteoblastic proliferation and
inhibiting osteoclastic formation (15). Li et al (16) found that Naringin, a Chinese herbal
medicine, promotes the proliferation and differentiation of bone
marrow mesenchymal stem cells and effectively reverses
ovariectomy-induced osteoporosis in rats. In a study by Huh et
al (17), it was found that
Puerariae radix is vital in osteoblastic bone formation and
that this may be associated with the angiogenic property of the
herb via the increased mRNA expression of VEGF.
GB, a herbal extract from the leaves of the
Ginkgo biloba tree, has been shown to inhibit
platelet-activating factor (18).
Its suggested biological effects include the scavenging of free
radicals, reducing oxidative stress, neural damage and platelet
aggregation, and possessing anti-inflammatory, antitumor and
anti-aging activities (19). Our
previous study demonstrated that GB increases the number and
functional activities of EPCs, with involvement of Akt/endothelial
nitric oxide synthase and MAPK/p38 signaling pathways. However, to
the best of our knowledge, whether GB induces osteogenic activity
in mouse osteoblast-like MC3T3-E1 cells has not been reported. In
the present study, it was found that GB promoted osteoblastic
differentiation of MC3T3-E1 cells through the upregulation of VEGF,
and the p38 MAP kinase signaling pathways was involved in the
GB-induced synthesis of VEGF.
A number of previous reports have demonstrated a
close association between angiogenesis, fracture healing and bone
growth. It is known that, during bone formation, angiogenesis and
osteogenesis are coupled spatially and temporally (20). The critical step in endochondral
ossification is the introduction of highly vascularized bone to
replace the avascular cartilage. The mechanism underlying
angiogenesis involves in the action of VEGF, which is a potent
angiogenic peptide and has mitogenic and chemotactic effects on
endothelial cells (21,22).
There are an increasing number of reports indicating
that VEGF has a positive effect in regulating osteoblast activity.
D'Alimonte et al (23)
demonstrated that VEGF enhances the proliferation and osteogenic
differentiation of human dental pulp stem cells in vitro.
Hah et al (24)
demonstrated that VEGF stimulates the differentiation of cultured
human periosteal-derived cells into the osteoblastic and that VEGF
may act as an autocrine growth factor for the osteoblastic
differentiation of cultured human periosteal-derived cells. Tan
et al (25) reported that
VEGF promotes bone remodeling by direct effects on osteoblasts via
regulating the gene expression of ALP, osteocalcin and
osteoprotegerin through the VEGF receptor 2 signaling pathway. In
the processes of bone formation and fracture healing, a study by
Deckers et al (26)
demonstrated that the VEGF produced by osteoblast-like cells
enhances osteoblastic differentiation by stimulating endothelial
cells to secrete growth factors and cytokines, which induce
mesenchymal cells into osteogenic differentiation. In the present
study, it was found that GB significantly increased ALP activity
and osteoblastic mineralization in MC3T3-E1 cells. In addition, GB
significantly increased the mRNA expression and secretion levels of
VEGF in the MC3T3-E1 cells. These findings suggested that GB
promoted the osteogenic differentiation of MC3T3-E1 cells through
the upregulation of VEGF.
A previous study demonstrated that p38 MAP kinase,
but not p42/p44 MAP kinase, is involved in prostaglandin E1-induced
VEGF synthesis in MC3T3-E1 cells (27). Kozawa et al (28) showed that endothelin-1 stimulates
VEGF synthesis via endothelin A receptor in osteoblasts, and that
p38 MAP kinase is involved in endothelin-1-induced VEGF synthesis.
Tokuda et al (29) reported
that p44/p42 MAP kinase and p38 MAP kinase are involved in
transforming growth factor-β-stimulated VEGF synthesis in
osteoblasts. In the present study, in order to detect the role of
p44/p42 and p38 MAP kinase in the GB-induced the expression of VEGF
in MC3T3-E1 cells, the effects of GB on the phosphorylation of
these MAP kinases were examined. It was found that GB induced the
phosphorylation of p44/p42 and p38 MAP kinase in a time-dependent
manner. Furthermore, SB203580, a specific inhibitor of p38 MAP
kinase, markedly suppressed the GB-induced p38 kinase
phosphorylation and GB-stimulated VEGF synthesis. PD98059, an
inhibitor of the upstream kinase, which activates p44/p42 MAP
kinase, had minimal effect on the GB-induced phosphorylation of
p44/p42 MAP kinase or GB-induced VEGF synthesis. These data
indicated that the p38, but not the p44/p42, MAP kinase signaling
pathway is involved in the GB-induced expression of VEGF.
A series of studies have examined the role of MAP
kinases in modulating osteogenic differentiation. Greenblatt et
al demonstrated that p38 promoted skeleton formation and bone
homeostasis through runt-related transcription factor 2, which is a
key transcription factor associated with osteoblast differentiation
(30,31). A study by Hu et al confirmed
that p38 has a positive effect in the early and late osteogenesis
of osteoblasts, bone marrow osteoprogenitor cells and the MC3T3-E1
osteoblast line (32,33).
In conclusion, the results obtained in the present
study demonstrated that GB enhanced the osteoblastic
differentiation of MC3T3-E1 cells through VEGF, and that the p38
MAP kinase signaling pathway was involved in the GB-induced
expression of VEGF. These findings suggest that GB is a potential
candidate target for treating or preventing osteoporosis.
Acknowledgements
The present study was supported by the Key Program
of National Natural Science of Foundation of China (grant no.
31430030), and the National Program on Key Basic Research Project
(973 Program; grant no. 2012CB619105).
References
|
1
|
Liang L: The history of cultivation
techniques of Ginkgo biloba in China. Agricultural Archaeology.
1:259–261. 2002.
|
|
2
|
Dias MA, Sampaio AL, Venosa AR, Meneses EA
and Oliveira CA: The chemopreventive effect of Ginkgo biloba
extract 761 against cisplatin ototoxicity: A pilot study. Int
Tinnitus J. 19:12–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen J, Wang J, Zhao B, Hou J, Gao T and
Xin W: Effects of EGb 761 on nitric oxide and oxygen free radicals,
myocardial damage and arrhythmia in ischemia-reperfusion injury in
vivo. Biochim Biophys Acta. 1406:228–236. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang WR, Hayashi T, Kitagawa H, Sasaki C,
Sakai K, Warita H, Wang JM, Shiro Y, Uchida M and Abe K: Protective
effect of ginkgo extract on rat brain with transient middle
cerebral artery occlusion. Neurol Res. 22:517–521. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahlemeyer B, Mowes A and Krieglstein J:
Inhibition of serum deprivation- and staurosporine-induced neuronal
apoptosis by Ginkgo biloba extract and some of its constituents.
Eur J Pharmacol. 367:423–430. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdel-Wahab BA and Abd El-Aziz SM: Ginkgo
biloba protects against intermittent hypoxia-induced memory
deficits and hippocampal DNA damage in rats. Phytomedicine.
19:444–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nash KM and Shah ZA: Current perspectives
on the beneficial role of Ginkgo biloba in neurological and
cerebrovascular disorders. Integr Med Insights. 10:1–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nabavi SM, Habtemariam S, Daglia M, Braidy
N, Loizzo MR, Tundis R and Navabi SF: Neuroprotective effects of
Ginkgolide B against ischemic stroke: A review of current
literature. Curr Top Med Chem. 15:2222–2232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li R, Chen B, Wu W, Bao L, Li J and Qi R:
Ginkgolide B suppresses intercellular adhesion molecule-1
expression via blocking nuclear factor-kappaB activation in human
vascular endothelial cells stimulated by oxidized low-density
lipoprotein. J Pharmacol Sci. 110:362–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang M, Qian Y, Guan T, Huang L, Tang X
and Li Y: Different neuroprotective responses of Ginkgolide B and
bilobalide, The Two Ginkgo Components Hyperglycemia. Eur J
Pharmacol. 677:71–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang JYSJMS: Protective effects of
ginkgolide B on cerebral ischemia reperfusion injury in rats. Chin
Pharmacol Bull. 269–272. 2008.
|
|
12
|
Botao Y, Ma J, Xiao W, Xiang Q, Fan K, Hou
J, Wu J and Jing W: Protective effect of ginkgolide B on high
altitude cerebral edema of rats. High Alt Med Biol. 14:61–64. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang Y, Huang B, Sun L, Peng X, Chen X and
Zou X: Ginkgolide B promotes proliferation and functional
activities of bone marrow-derived endothelial progenitor cells:
Involvement of Akt/eNOS and MAPK/p38 signaling pathways. Eur Cell
Mater. 21:459–469. 2011.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Y, Chin A, Zhang L, Lu J and Wong RW:
The role of traditional Chinese medicines in osteogenesis and
angiogenesis. Phytother Res. 28:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li N, Jiang Y, Wooley PH, Xu Z and Yang
SY: Naringin promotes osteoblast differentiation and effectively
reverses ovariectomy-associated osteoporosis. J Orthop Sci.
18:478–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huh JE, Yang HR, Park DS, Choi DY, Baek
YH, Cho EM, Cho YJ, Kang-Il K, Kim DY and Lee JD: Puerariae radix
promotes differentiation and mineralization in human
osteoblast-like SaOS-2 cells. J Ethnopharmacol. 104:345–350. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lamant V, Mauco G, Braquet P, Chap H and
Douste-Blazy L: Inhibition of the metabolism of platelet activating
factor (PAF-acether) by three specific antagonists from Ginkgo
biloba. Biochem Pharmacol. 36:2749–2752. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan PC, Xia Q and Fu PP: Ginkgo biloba
leave extract: Biological, medicinal and toxicological effects. J
Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 25:211–244.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L,
Li C, Xie L, Crane J, Wan M, et al: PDGF-BB secreted by
preosteoclasts induces angiogenesis during coupling with
osteogenesis. Nat Med. 20:1270–1278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Street J, Winter D, Wang JH, Wakai A,
McGuinness A and Redmond HP: Is human fracture hematoma inherently
angiogenic? Clin Orthop Relat Res. 224–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Geiger F, Bertram H, Berger I, Lorenz H,
Wall O, Eckhardt C, Simank HG and Richter W: Vascular endothelial
growth factor gene-activated matrix (VEGF165-GAM) enhances
osteogenesis and angiogenesis in large segmental bone defects. J
Bone Miner Res. 20:2028–2035. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Alimonte I, Nargi E, Mastrangelo F,
Falco G, Lanuti P, Marchisio M, Miscia S, Robuffo I, Capogreco M,
Buccella S, et al: Vascular endothelial growth factor enhances in
vitro proliferation and osteogenic differentiation of human dental
pulp stem cells. J Biol Regul Homeost Agents. 25:57–69.
2011.PubMed/NCBI
|
|
24
|
Hah YS, Jun JS, Lee SG, Park BW, Kim DR,
Kim UK, Kim JR and Byun JH: Vascular endothelial growth factor
stimulates osteoblastic differentiation of cultured human
periosteal-derived cells expressing vascular endothelial growth
factor receptors. Mol Biol Rep. 38:1443–1450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan YY, Yang YQ, Chai L, Wong RW and Rabie
AB: Effects of vascular endothelial growth factor (VEGF) on
MC3T3-E1. Orthod Craniofac Res. 13:223–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deckers MM, Karperien M, van der Bent C,
Yamashita T, Papapoulos SE and Löwik CW: Expression of vascular
endothelial growth factors and their receptors during osteoblast
differentiation. Endocrinology. 141:1667–1674. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tokuda H, Kozawa O, Miwa M and Uematsu T:
p38 mitogen-activated protein (MAP) kinase but not p44 p42 MAP
kinase is involved in prostaglandin E1-induced vascular endothelial
growth factor synthesis in osteoblasts. J Endocrinol. 170:629–638.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kozawa O, Kawamura H, Hatakeyama D,
Matsuno H and Uematsu T: Endothelin-1 induces vascular endothelial
growth factor synthesis in osteoblasts involvement of p38
mitogen-activated protein kinase. Cellular Signal. 12:375–380.
2000. View Article : Google Scholar
|
|
29
|
Tokuda H, Hatakeyama D, Akamatsu S, Tanabe
K, Yoshida M, Shibata T and Kozawa O: Involvement of MAP kinases in
TGF-beta-stimulated vascular endothelial growth factor synthesis in
osteoblasts. Arch Biochem Biophys. 415:117–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen M, Chen PM, Dong QR, Huang Q, She C
and Xu W: p38 signaling in titanium particle-induced MMP-2
secretion and activation in differentiating MC3T3-E1 cells. J
Biomed Mater Res A. 102:2824–2832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Greenblatt MB, Shim JH, Zou W, Sitara D,
Schweitzer M, Hu D, Lotinun S, Sano Y, Baron R, Park JM, et al: The
p38 MAPK pathway is essential for skeletogenesis and bone
homeostasis in mice. J Clin Invest. 120:2457–2473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu Y, Chan E, Wang SX and Li B: Activation
of p38 mitogen-activated protein kinase is required for osteoblast
differentiation. Endocrinology. 144:2068–2074. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thouverey C and Caverzasio J: The p38α
MAPK positively regulates osteoblast function and postnatal bone
acquisition. Cell Mol Life Sci. 69:3115–3125. 2012. View Article : Google Scholar : PubMed/NCBI
|




















