Introduction
Hepatic fibrosis is a common process that occurs in
the liver in response to diverse chronic injury. Factors associated
with hepatic injury can result in hepatic fibrosis, including viral
hepatitis, steatohepatitis (including alcoholic and nonalcoholic),
autoimmune hepatic disease, Schistosomiasis japonica
infection, toxic or drug-induced hepatic disease, and genetic and
metabolic diseases (1). During the
pathological process of hepatic fibrosis the following symptoms are
observed: Decreased normal liver cells, diffuse liver cell
necrosis, abnormal proliferation of connective tissue, and
disordered hepatic circulation, which can lead to hepatic function
failure and subsequently to hepatic cirrhosis (2). A previous study reported that hepatic
fibrosis is a reversible pathological phenomenon, in which fibrotic
tissue is excessively deposited in the liver during the repair
process that follows hepatic injury. Early prevention or treatment
of hepatic fibrosis has great significance on the treatment of
chronic hepatic diseases (3).
Although there have been some drugs used to treat this pathogenic
process, the efficacy fails to meet necessary requirements. At
present, researchers aim to seek drugs with definite efficacy and
few adverse effects in order to delay, suppress or even reverse the
process of hepatic fibrosis. Several clinical practices and
experimental studies have demonstrated that traditional Chinese
medicine can relieve hepatic fibrosis and hepatic cirrhosis via
multi-component, multi-path, multi-level and multi-target
comprehensive pharmacological effects (4–6). In
order to further investigate the antifibrotic action of traditional
Chinese medicines, several scholars have aimed to research drugs
extracted from medicinal plants and to explore the underlying
antifibrotic mechanisms of action.
Safflower (Carthamus tinctorius L.), which
belongs to the Compositae family, consists of tubular florets and
is distributed in the Mediterranean and Asia. Safflower is a
traditional drug used for activating circulation, dissolving
stasis, activating collaterals and alleviating pain (7). The main components of safflower
include pigment, flavonoids, volatile oils, fatty acids and
phenolic acids (8). The yellow
pigment of safflower is predominantly used for activating blood and
dissolving stasis. Hydroxysafflor yellow A (HSYA) is a
water-soluble monomer that is extracted from safflower and is a
type of chalcone glycoside (molecular formula,
C27H32O16; molecular weight,
612.53 g/mol) (9). Previous
studies have demonstrated that HSYA possesses several functions,
including widening of coronary arteries and ameliorating myocardial
ischemia, anticoagulation, anti-platelet aggregation and relief
from unstable angina pectoris, protecting against brain and spinal
cord ischemia-reperfusion injury, and preventing steroid-induced
avascular necrosis of the femoral head (10,11).
In addition, it has been reported that HSYA is able to eliminate
free radicals, inhibit lipid peroxidation, relieve inflammatory
injury and suppress cell apoptosis (12). HSYA has been demonstrated to reduce
pulmonary fibrosis (13); however,
few studies have investigated the role of HSYA in hepatic
fibrosis.
The present study was performed using a rat model of
hepatic fibrosis induced by carbon tetrachloride (CCl4).
CCl4 is often used to generate a reliable animal model
of hepatic fibrosis (14). This
model simulates the clinical pathological process of hepatic
fibrosis, and provides an ideal animal model for analyzing the
anti-hepatic fibrosis effect of drugs. CCl4 is a
selective liver toxicant that can induce liver cell necrosis.
Treatment with low-dose CCl4 for a long duration can
induce hepatic fibrosis in animals. In addition, the degree of
hepatic fibrosis is positively associated with the dose. Using the
method of intragastric administration can reduce the mortality of
animals. CCl4, together with ethanol and a high-fat
diet, can accelerate liver cell injury, and trigger inflammation
and fibrosis. The rat model of hepatic fibrosis was induced by
CCl4, alongside administration of alcohol and a high-fat
diet, which may accelerate liver cell injury, inflammation and
fibrosis. With the use of the complex model combining carbon
tetrachloride oxidative damage, alcoholism and lipid oxidative
damage with a variety of factors, the formation mechanism of
hepatic fibrosis is closer to that of clinical patients and is
conducive to screening antifibrotic drugs.
The present study aimed to further explore the
mechanism underlying the effects of HSYA on the prevention and
treatment of hepatic fibrosis. In the present study, a rat model of
hepatic fibrosis was established, and the rats were administered
various doses of HSYA. The effects of HSYA on the pathological
alterations of liver tissue in rats with hepatic fibrosis were
observed using hematoxylin-eosin staining and Masson staining. In
order to explore the anti-hepatic fibrosis effects and underlying
mechanisms of HSYA, serum levels, and hepatic function and hepatic
fibrosis indices were measured.
Materials and methods
Reagents
HSYA (purity >98%) was extracted from the herb
Carthamus tinctorius L.; the water-soluble yellow amorphous
powder was provided by Dr. Fenghua Fu (School of Pharmaceutical
Sciences, Yantai University, Yantai, China). CCl4
(purity 99%) was purchased from Tianjin Bodi Chemical Co., Ltd.
(Tianjin, China). Colchicine was purchased from Xishuangbanna
Pharmaceutical Co., Ltd. (Xishuangbanna, China). Total protein (TP)
quantitative assay kit (A045-2), albumin (ALB) assay kit (A028-1),
total cholesterol (TCHO) assay kit (A111-1), triglyceride (TG)
assay kit (A110-1), high-density lipoprotein cholesterol (HDL)
assay kit (A112-1), low-density lipoprotein cholesterol (LDL) assay
kit (A113-1), alanine aminotransferase (ALT) assay kit (C009-1),
aspartate aminotransferase (AST) assay kit (C010-1), alkaline
phosphatase (ALP) assay kit (C059-1), hydroxyproline (HYP) assay
kit (A030-2), malondialdehyde (MDA) assay kit (A003-1) and
superoxide dismutase (SOD) assay kit (A001-1) were obtained from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Hyaluronic acid (HA) radioimmunoassay kit (H141), laminin (LN)
radioimmunoassay kit (H148), procollagen III N-terminal peptide
(pcIII) radioimmunoassay kit (H212) and collagen type IV (cIV)
radioimmunoassay kit (H145) were purchased from Shanghai Haiyan
Pharmaceutical Technology Co., Ltd. (Shanghai, China). Trichrome
stain (Masson) kit was purchased from Fujian Maixin Biotechnology
Development Co., Ltd. (Fuzhou, China).
Animals and group design
The current study was approved by the ethics
committee of Binzhou Medical University (Yantai, China). The
present study was conducted on male Sprague Dawley rats (age, 5–6
weeks; weight, 110–130 g), which were provided by the Experimental
Animal Center of Shandong University [animal permit number: SUXK
(Lu) 20130009; Jinan, China]. The rats were allowed to acclimate
for 1 week, after which the rats that exhibited a normal reaction
to food, water and activity, and no adverse effects, were enrolled
in the study. Rats were maintained in a temperature-controlled room
with an alternating 12-h dark and light cycle. A total of 10 rats
were randomly selected as the normal control group (Group A). The
remaining rats were randomly divided into six groups: Hepatic
fibrosis model group (Group B), HSYA prevention group (Group C),
low dose HSYA group (Group D), moderate dose HSYA group (Group E),
high dose HSYA group (Group F) and the colchicine group (Group G).
Each group consisted of 15 rats.
Animal model preparation
The rat hepatic fibrosis model was established using
the CCl4 model method. Briefly, CCl4 and
soybean oil were mixed at a ratio of 3:7, in order to obtain a 300
ml/l oil solution, which was administered by gavage at a dose of 3
ml/kg, three times a week. From the 2nd week, 10% ethanol was added
to the drinking water and 20% lard oil was added to the diet. Rats
in Group A were administered 3 ml/kg soybean oil by gavage, three
times a week, and were given ad libitum access to normal
food and water.
Drug treatment
From the first week, rats in Groups B and C were
given drug treatment for 10 weeks via intraperitoneal injection,
five times a week. From the third week, rats in Groups D-G were
given drug treatment for 8 weeks via intraperitoneal injection,
five times a week. The treatments administered were as follows:
Group B, injection of 1 ml/kg normal saline; Group C and E,
injection of 10 mg/kg HSYA water solution; Group D, injection of 5
mg/kg HSYA water solution; Group F, injection of 20 mg/kg HSYA
water solution; Group G, injection of 0.1 mg/kg colchicine. HSYA
and colchicine were dissolved in normal saline for filtration and
sterilization.
Sample collection and detection
Following establishment of the rat model, the growth
conditions of the rats in each group were observed; the rats were
weighed and a weight growth curve was generated. At the
10th week, all rats were fasted for 24 h and were then
administered 3.5% chloral hydrate. After blood samples were
collected via heart puncture, rats were sacrificed via excessive
blood loss. Blood samples were collected via heart puncture, were
maintained at room temperature for 1 h, and were centrifuged at
3,000 r/min for 15 min at 4°C to separate serum, serum levels (TP,
ALB, TCHO, TG, HDL, LDL, ALT, AST and ALP) were detected according
to manufacturer's protocols. GLB content is equal to the total
protein content minus the albumin content (15). The livers were also collected,
separated and weighed to calculate the liver to body weight ratio.
Furthermore, tissue samples were collected from the left lobe of
the liver, fixed with paraformaldehyde, embedded with paraffin and
cut into 4 µm sections. The sections underwent hematoxylin-eosin
(hematoxylin for 10 min and eosin for 3–5 min both at 25°C) and
Masson (5 min at 25°C) staining, and were observed under a
microscope (DM4000B; Leica Microsystems GmbH; Wetzlar, Gernman).
Tissue samples collected from the right lobe of the liver were
weighed, homogenized and detected according to manufacturer's
protocols.
Statistical analysis
Statistical analysis was performed using software
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). All data in the present
study are expressed as × ± s. Data were analyzed using homogeneity
of variances test and intergroup variance analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of HSYA on physiological
state, weight and liver/body ratio in rats with hepatic
fibrosis
A total of 20 rats died during the present study
(one rat in Group A, four in Group B, three in Group C, four in
Group D, three in Group E, three in Group F and two in Group G).
Rats in the normal group were lively and exhibited a good appetite,
with shiny fur and increased weight. After the model was
established, rats exhibited a decreased appetite, little activity,
low spirits, and had dry and lackluster fur. From the third week,
rat weight was significantly decreased in Group B compared with
Group A (P<0.01; Fig. 1A).
Following administration of HSYA and colchicine, food intake, mood
and fur condition were improved compared with in the rats in Group
B. As the duration of HYSA and colchicine administration increased,
the weight of the rats increased compared with in the model group.
After 9 weeks, rat weight in Groups C, E and F was higher than in
Group B (P<0.01). Furthermore, the liver tissue of the normal
rats had a smooth surface, bright reddish color and was soft to the
touch. The liver tissue of rats in Group B had a rough surface,
increased volume, pale color, was hard to the touch, and fine
particles were observed on the surface. In addition, some rats
exhibited hepatic portal vein varices, and the rats that died
exhibited fluid retention in the abdominal cavity. Compared with
Group A, the liver/body ratio in Group B was increased (P<0.01;
Fig. 1B). Liver morphology and
color were slightly ameliorated in the drug-treated rats compared
with in Group B; however, fine particles were still observed.
Compared with Group B, liver/body weight ratio was decreased,
particularly in Groups C and E (P<0.01; Fig. 1B).
 | Figure 1.Effects of HSYA on (A) body weight
and (B) liver/body weight ratio of rats. From the second week, rats
in the model group exhibited naturally decreased body weight, and
liver/body weight ratio was increased compared with in Group A.
**P<0.01 vs. Group A; ∆P<0.05,
∆∆P<0.01 vs. Group B. Group A, normal control; Group
B, hepatic fibrosis model group; Group C, HYSA prevention group;
Group D, low dose HYSA group; Group E, moderate dose HYSA group;
Group F, high dose HYSA group; Group G, colchicine group. HYSA,
hydroxysafflor yellow A; w, weeks. |
Effects of HSYA on pathological
alterations of liver tissue in rats with hepatic fibrosis
Under a light microscope, hematoxylin-eosin staining
demonstrated that hepatic lobules in Group A rats were clear with
an intact structure, the central vein was surrounded by cords of
liver cells that radiated out in all directions, irregular hepatic
sinusoid was seen among the hepatic cords, and liver cells
exhibited no necrosis or inflammatory cell infiltration (Fig. 2A). Parenchymal nodules, varying
from small to large, which are encircled by fibrotic bands were
observed, and these nodules commonly contain proliferating
hepatocytes (Fig. 2B). Compared
with Group B, hepatic lobules in drug-treated rats (Groups C-G)
exhibited reduced structural damage, decreased pseudolobules,
cellular swelling, fatty degeneration, necrosis and inflammatory
cell infiltration, particularly in Group C (Fig. 2C). Masson staining results
demonstrated that the liver calls of rats in Group A were arranged
normally and few blue collagen fibers could be seen in the portal
area (Fig. 2H). Conversely, liver
tissue of rats in Group B exhibited marked collagen fiber
proliferation, predominantly in portal areas and the central vein,
and pseudolobule formation was detected (Fig. 2I). Liver tissue of rats in the
drug-treated group (Groups C-G) exhibited decreased deposition of
collagen fibers, thin fibrous septum and decreased pseudolobule
formation (Fig. 2J)
 | Figure 2.Pathological alterations of liver
tissue, as detected by (A-F) hematoxylin-eosin staining and (G-I)
Masson staining. (A) Normal liver tissue in the control group. (B)
Hepatic lobules of rats in the model group exhibited damage, and
developed a typical false flocculus structure. (C) Compared with
the model group, hepatic lobules in Group D exhibited reduced
structural damage (magnification: ×100). (D) Normal liver tissue in
the control group. (E) Increased proliferation of collagen fibers,
typical pseudolobule formation, hepatic cords, cellular swelling,
fatty degeneration, and necrosis and inflammatory cell infiltration
were observed in model rats. (F) Compared with the model group,
hepatic lobules in Group D exhibited reduced pseudolobule
formation, cellular swelling, fatty degeneration, necrosis and
inflammatory cell infiltration, particularly in the hydroxysafflor
yellow A prevention group (magnification, ×400). (G) Normal liver
tissue in the control group. (H) Liver tissue of rats in the model
group exhibited marked collagen fiber proliferation, predominantly
in portal areas and the central vein, and pseudolobule formation
was observed. (I) Liver tissue of rats in Group D exhibited
decreased deposition of collagen fibers, a thin fibrous septum and
decreased pseudolobule formation (magnification: ×100). |
Effects of HSYA on protein metabolism
in rats with hepatic fibrosis
Compared with Group A, serum TP and ALB levels in
Group B were significantly decreased (P<0.01; Figs. 3A and B); globulin (GLB) was
increased (P<0.01; Fig. 3C) and
the albumin/globulin ratio (A/G) was decreased (P<0.01; Fig. 3D). As compared with Group B, HYSA
and colchicine treatment resulted in an increase in ALB and A/G
ratio. In particular, serum ALB levels were increased in Groups C
and E (P<0.01).
 | Figure 3.Effects of HSYA on (A) TP, (B) ALB,
(C) GLB and (D) A/G ratio in rats with hepatic fibrosis. Compared
with in Group A, serum TP, ALB and A/G ratio were significantly
decreased in in Group B, and GLB was increased. As compared with
Group B, ALB and A/G ratio were increased in drug-treated groups.
Serum ALB was particularly increased in Groups C and E. **P<0.01
vs. Group A; ∆P<0.05, ∆∆P<0.01 vs.
Group B. TP, total protein, ALB, albumin; GLB, globulin; A/G,
ALB/GLB; Group A, normal control; Group B, hepatic fibrosis model
group; Group C, HYSA prevention group; Group D, low dose HYSA
group; Group E, moderate dose HYSA group; Group F, high dose HYSA
group; Group G, colchicine group. HYSA, hydroxysafflor yellow
A. |
Effects of HSYA on lipid metabolism in
rats with hepatic fibrosis
Compared with Group A, serum TCHO, TG, HDL and LDL
levels were significantly increased in Group B (P<0.01; Fig. 4). As compared with Group B,
following HSYA and colchicine treatment, TCHO and TG were
significantly decreased (P<0.05), and HDL and LDL had the
tendency to decrease; however, no significant differences were
detected in HDL levels (P>0.05).
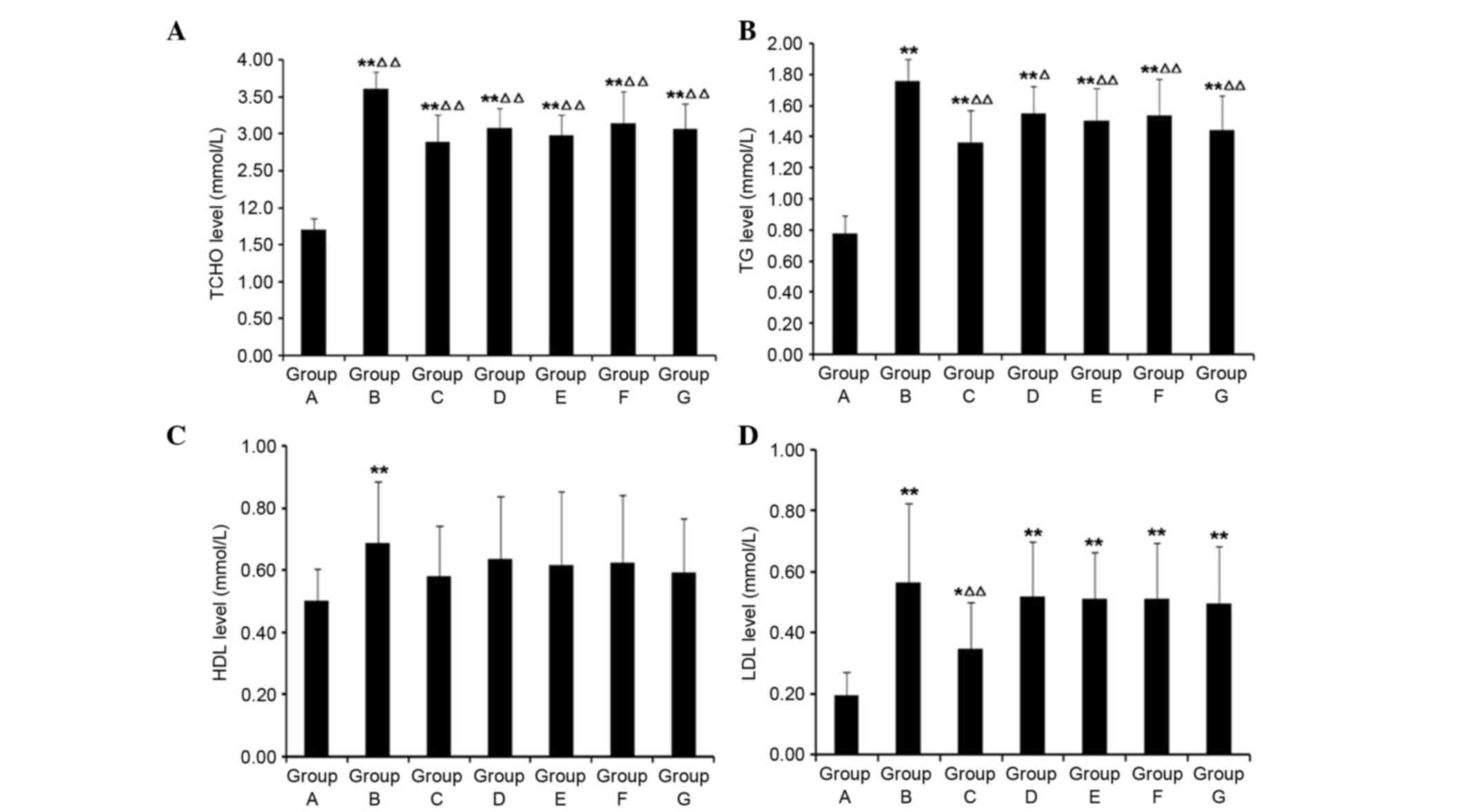 | Figure 4.Effects of HSYA on (A) TCHO, (B) TG,
(C) HDL and (D) LDL in rats with hepatic fibrosis. Compared with
Group A, serum TCHO, TG, HDL and LDL levels were significantly
increased in Group B. Compared with in Group B, following HSYA and
colchicine treatment, TCHO and TG were decreased, and HDL and LDL
had the tendency to decrease. *P<0.05, **P<0.01 vs. Group A;
∆P<0.05, ∆∆P<0.01 vs. Group B. TCHO,
total cholesterol; TG, triglycerides; HDL, high-density
lipoprotein; LDL, low-density lipoprotein; Group A, normal control;
Group B, hepatic fibrosis model group; Group C, HYSA prevention
group; Group D, low dose HYSA group; Group E, moderate dose HYSA
group; Group F, high dose HYSA group; Group G, colchicine group.
HYSA, hydroxysafflor yellow A. |
Effects of HSYA on serum ALT, AST and
ALP levels in rats with hepatic fibrosis
Compared with Group A, serum ALT, AST and ALP levels
in Group B were significantly increased (P<0.01) and the DeRitis
value (AST/ALT) was decreased (P<0.01; Fig. 5). Compared with Group B, following
treatment with HSYA and colchicine, serum ALT and ALP levels were
decreased, particularly in Groups C, E and G (P<0.05). Serum AST
had the tendency to decrease; however, no significant differences
were detected (P>0.05). In addition, the DeRitis value was
significantly increased in Groups C and E (P<0.05).
 | Figure 5.Effects of HSYA on (A) ALT, (B) AST,
(C) DeRitis and (D) ALP in rats with hepatic fibrosis. Compared
with Group A, ALT, AST and ALP levels were significantly increased
in Group B, and the DeRitis value was decreased. Compared with
Group B, following treatment with HSYA and colchicine, ALT and ALP
levels were decreased, and AST had the tendency to decrease. The
DeRitis value was significantly increased in Groups C and E.
*P<0.05, **P<0.01 vs. Group A; ∆P<0.05,
∆∆P<0.01 vs. Group B. ALT, alanine aminotransferase;
AST, aspartate aminotransferase; ALP, alkaline phosphatase; Group
A, normal control; Group B, hepatic fibrosis model group; Group C,
HYSA prevention group; Group D, low dose HYSA group; Group E,
moderate dose HYSA group; Group F, high dose HYSA group; Group G,
colchicine group. HYSA, hydroxysafflor yellow A. |
Effects of HSYA on serum hepatic
fibrosis index in rats with hepatic fibrosis
Compared with Group A, serum HA, LN, pcIII and cIV
levels in Group B were significantly increased (P<0.01; Fig. 6). Compared with Group B, following
treatment with HSYA and colchicine, serum HA and LN levels were
decreased (P<0.05). pcIII levels in Group C, E and G were
significantly decreased (P<0.05). Only in Groups C and G were
serum cIV levels decreased (P<0.05).
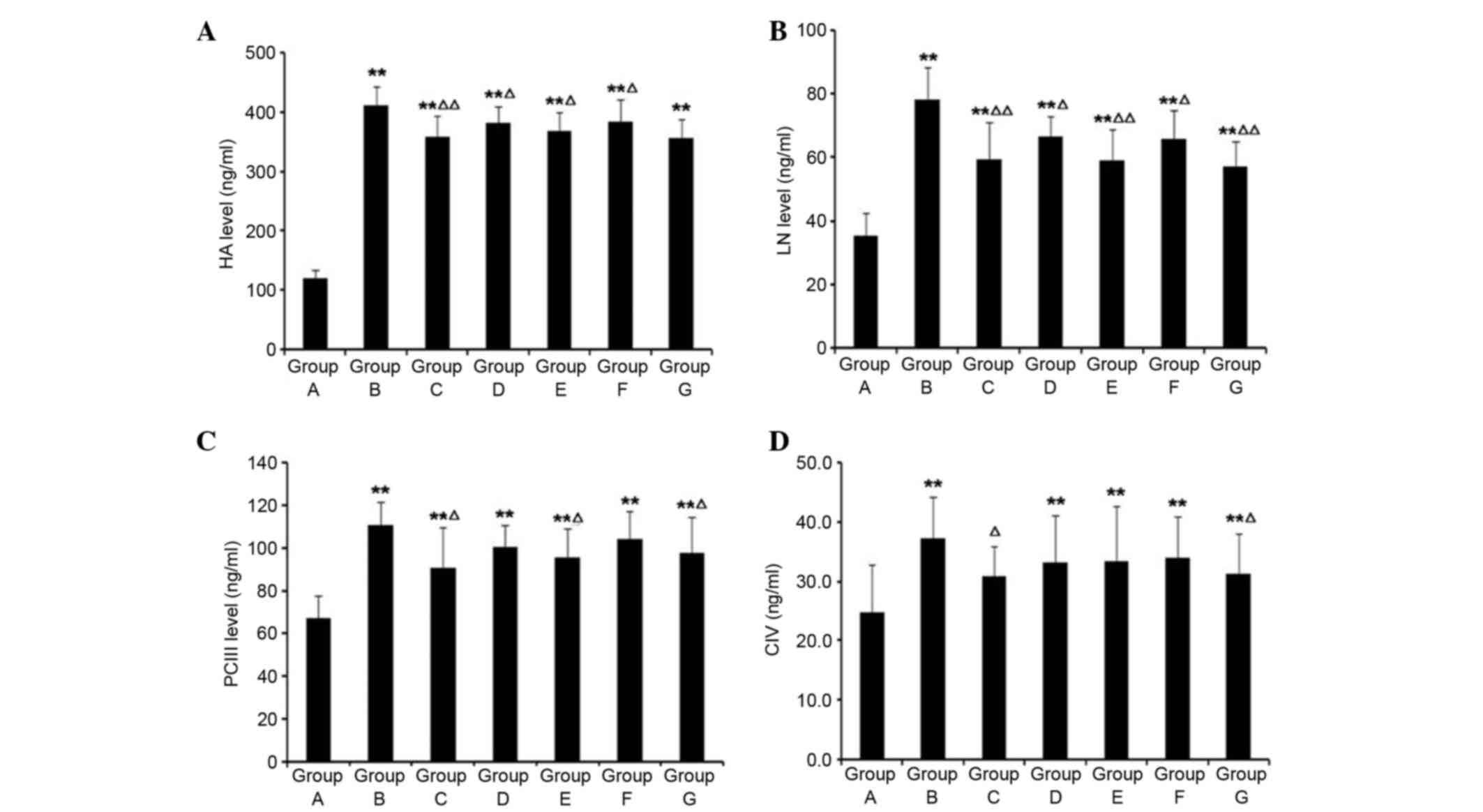 | Figure 6.Effects of HSYA on (A) HA, (B) LN,
(C) pcIII and (D) cIV in rats with hepatic fibrosis. Compared with
Group A, HA, LN, pcIII and cIV levels in Group B were significantly
increased. Compared with in Group B, following treatment with HSYA
and colchicine, HA and LN levels in each group were decreased.
pcIII levels in Groups C, E and G were significantly decreased.
Only in Groups C and E were serum cIV levels significantly
decreased. **P<0.01 vs. Group A; ∆P<0.05,
∆∆P<0.01 vs. Group B. HA, hyaluronic acid; LN,
laminin; pcIII, procollagen III N-terminal peptide; cIV, collagen
type IV; Group A, normal control; Group B, hepatic fibrosis model
group; Group C, HYSA prevention group; Group D, low dose HYSA
group; Group E, moderate dose HYSA group; Group F, high dose HYSA
group; Group G, colchicine group. HYSA, hydroxysafflor yellow
A. |
Effects of HSYA on HYP, MDA and SOD in
the liver tissue homogenate of rats with hepatic fibrosis
Compared with Group A, HYP and MDA levels in rat
liver tissue homogenates from Group B were significantly increased
(P<0.01; Fig. 7A and B),
whereas SOD levels were decreased (P<0.01; Fig. 7C). Compared with Group B, following
treatment with HSYA and colchicine, MDA levels in rat liver tissue
homogenates were significantly decreased in the treatment groups
(P<0.05), except for Group D. HYP levels in rat liver tissue
homogenates were significantly decreased in the treatment groups
(P<0.05). SOD levels were significantly increased in the
treatment groups compared with in Group B (P<0.05), except for
Group D.
 | Figure 7.Effects of HSYA on (A) HYP, (B) MDA
and (C) SOD levels in rats with hepatic fibrosis. Compared with
Group A, HYP and MDA levels were significantly increased in rat
liver tissue homogenates in Group B, whereas SOD levels were
decreased. Compared with Group B, following treatment with HSYA and
colchicine, MDA levels in rat liver tissue homogenates were
significantly decreased in each group. HYP levels in rat liver
tissue homogenates were significantly decreased in the other
groups, except for Group D. SOD levels were significantly increased
in the other groups compared with Group B, except for Group D.
**P<0.01 vs. Group A; ∆P<0.05,
∆∆P<0.01 vs. Group B. HYP, hydroxyproline; MDA,
malondialdehyde; SOD, superoxide dismutase; Group A, normal
control; Group B, hepatic fibrosis model group; Group C, HYSA
prevention group; Group D, low dose HYSA group; Group E, moderate
dose HYSA group; Group F, high dose HYSA group; Group G, colchicine
group. HYSA, hydroxysafflor yellow A. |
Discussion
Hepatic fibrosis, which is commonly seen in hepatic
injury and inflammation, and is caused by several pathogenic
factors, is a significant process in the development and
deterioration of liver cirrhosis and cancer. Hepatic fibrosis is
considered an important health concern worldwide, and accounts for
50% of the mortality rate of patients with liver cancer (16). Despite the high incidence of
hepatic fibrosis, no effective therapeutic method is available for
clinical treatment. Diversified drugs in Western medicine have been
developed for the treatment of hepatic fibrosis, predominantly
those used for antiviral therapy and liver protection. These drugs
can be effective in treating hepatic fibrosis through inhibiting
inflammation, decreasing extracellular matrix proliferation,
promoting extracellular matrix degradation, improving
microcirculation and decreasing complications. Although the
efficacy of these drugs has been demonstrated in clinical research,
some problems remain that require resolving (17,18).
Traditional Chinese medicine has efficacy for the treatment of
complicated diseases and related concerns. The majority of
traditional Chinese medicines are used in combination for several
targets, including transforming growth factor-SMAD, extracellular
signal-related kinase/mitogen-activated protein kinase and the
nuclear factor κB signaling pathway, which can inhibit the
progression of hepatic fibrosis and liver cirrhosis via multi-path
and multi-level action (19–21).
In Chinese medicine, hepatic fibrosis belongs to the
category of hypochondriac pain, accumulation and scar, and the main
pathogenesis is considered the deficiency of Qi and blood stasis
(22). At present, the majority of
studies have focused on promoting circulation and removing stasis
(23). Studies regarding the
pharmacology of traditional Chinese medicine have demonstrated that
promoting circulation and removing stasis results in regulation of
hemorheological properties, expands the circumference of blood
vessels, increases blood volume of organs and improves
microcirculation (24,25). In addition, these drugs can be used
to reduce inflammation, induce analgesia and depress hematic fat,
enhance the tolerance of cells to hypoxia, increase SOD activity
and reduce tissue damage resulting from ischemia-reperfusion injury
(26,27). HSYA is a water-soluble monomer
extracted from safflower. The present study established a rat model
of hepatic fibrosis, and administered HSYA during the process of
hepatic fibrosis. Subsequently, the inhibitory effects of HSYA on
hepatic fibrosis, and the effects of HSYA on the changes to serum
and liver tissue homogenate indices were determined. In particular,
the present study explored the mechanism of action of HSYA on
hepatic fibrosis.
CCl4-induced hepatic fibrosis is a
typical hepatic fibrosis animal model, which is similar to human
hepatic fibrosis in morphology and pathophysiology. This model is
widely used for studying the pathogenetic mechanism of hepatic
fibrosis and for the evaluation of anti-hepatic fibrosis drugs
(28). CCl4 is a
selective toxicant in the liver, which can produce -CCl3
and -OOCl3 via metabolism of P450, strengthen lipid
peroxidation, destroy the membranous structure of liver cells, and
induce liver cell necrosis and disorders in protein synthesis and
energy metabolism (29).
Furthermore, it can induce activation of hepatic stellate cells and
lead to excess deposition of extracellular matrix, finally
resulting in hepatic fibrosis. In the present study, rats were
simultaneously given ethanol and a high-fat diet. Ethanol can
produce toxic metabolites via alcohol dehydrogenase and alcohol
oxidase resulting in the loss of liver cells, and can induce P450
activation, accelerate liver cell necrosis and shorten the time of
model establishment. High-fat diet can result in increased levels
of free fatty acid in the liver. Excess free fatty acid in liver
cells can lead to fatty degeneration, activate relevant cytokines,
stimulate hepatic stellate cells and result in the development of
hepatic fibrosis (30). During the
process of the present study, rat mortality increased with
extension of the model. However, following treatment with HSYA and
colchicine, health of the rats in the treatment groups
improved.
The results of the present study demonstrated that
HSYA resulted in resistance to CCl4-induced weight loss,
and improved the general condition of the rats with hepatic
fibrosis. In addition, compared with in Group B, liver samples
exhibited improved color and size, and had a smooth surface
following treatment, thus suggesting that HSYA intervention during
the process of hepatic fibrosis can improve the quality of life of
rats. Hematoxylin-eosin and Masson staining demonstrated that HSYA
intervention was able to relieve cellular swelling, fatty
degeneration, necrosis, inflammatory cell infiltration and
fibroblast proliferation, thus suggesting that HSYA could protect
against liver damage, and reduce the deposition of collagen and
injury of liver cells.
TP, ALB and GLB are important indices that reflect
hepatic function, which are predominantly used to detect chronic
hepatic injury and reflect the function of storing hepatocytes
(31). The present study revealed
that TP and ALB levels were significantly decreased in Group B; and
the inversion of the A/G ratio suggested the induction of hepatic
function injury. Following treatment with HSYA and colchicine, ALB
in Groups C and E was increased (P<0.01), suggesting that ALB
content was positively associated with liver cell number and
function. Following model establishment, ALB levels were decreased,
which indicated severe liver cell necrosis; following HSYA and
colchicine treatment, increased ALB levels indicated effective
treatment.
Liver cell injury can result in aberrant lipid
metabolism, which is predominantly manifested as lecithin
cholesterol acyltransferase deficiency and decreased activation of
lipoprotein lipase (32). The
present study demonstrated that TCHO, TG, HDL and LDL were
increased in Group B compared with in Group A (P<0.01),
suggesting the presence of aberrant lipid metabolism caused by
liver cell injury. Following treatment with HSYA and colchicine,
TCHO and TG levels were reduced, suggesting that HSYA may improve
lipid metabolism and promote the recovery of liver cell
function.
ALT and AST in the cytoplasm release into the plasma
when liver cells are damaged and permeability of liver membranes is
increased, thus leading to increased ALT and AST activation
(33). ALT predominantly exists
outside of the mitochondria, whereas ~80% AST exists in the
mitochondria. Moderate liver cell injury leads to an increased
leakage of ALT, more so than AST. Severe liver cell injury results
in mitochondrial membrane impairment and the release of AST, thus
resulting in an increased DeRitis ratio. The majority of ALP found
in the serum is from the liver and skeleton; therefore, serum ALP
levels can serve as an examination index for liver diseases
(34). The present study
demonstrated that serum ALT, AST and ALP levels were increased in
Group B (P<0.01). Higher serum ALT levels, rather than AST
levels, and a DeRitis ratio <1 indicate a high degree of hepatic
fibrosis. Following drug treatment in the present study, the
results demonstrated that HSYA was able to reduce the levels of ALT
and AST in rats with hepatic fibrosis, particularly in Groups C and
E (P<0.05). These results suggested that HSYA can regulate
CCl4-induced liver cell injury and improve hepatic
function.
Serum HA, LN, pcIII and cIV are degradation products
of collagen tissue, which reflects the change of extracellular
matrix during the process of liver fibrosis. They are commonly used
to diagnose hepatic fibrosis in clinical practice (35). HA is a type of proteoglycan, which
is formed due to the covalent binding between protein and
glycosaminoglycan. It is synthesized by interstitial cells and is
the main component of the extracellular matrix. HA is absorbed into
the blood through the lymph, is degraded following uptake by
hepatic sinusoidal endothelial cells, and is finally discharged via
the liver. The synthesis and discharge of HA is relatively stable,
and the serum content of HA is usually very low. HA is
significantly synthesized in chronic hepatic disease, particularly
in hepatic fibrosis. Due to endothelial cell damage, the uptake and
degradation of HA is decreased, resulting in significantly
increased levels of serum HA (36,37).
LN is a macromolecular noncollagenous glycoprotein
that is present in the extracellular matrix of the liver. LN is
present in the stratum lucidum of the basement membrane, and the
content is usually very low in the serum. LN settles in hepatic
sinusoids during fibrosis, and LN content is related to hepatic
fibrosis and the degree of inflammatory cell infiltration,
promoting hepatic sinusoid capillarization. LN is a key factor in
the induction of portal hypertension (38).
pcIII is an amino-terminal polypeptide formed by
collagen III decomposition via amino-terminal peptidase prior to
the secretion of collagen III outside the liver for settlement,
which can directly reflect the metabolic status of collagen III.
During the early stage of hepatic fibrosis, collagen III levels are
increased; therefore, pcIII is considered a sensitive indicator for
early hepatic fibrosis. Serum pcIII levels are closely associated
with the degree of hepatic fibrosis, and its increase is associated
with inflammation and necrosis (39).
cIV is combined with LN, and co-exists in the
basement membrane of blood vessels, adjusting the adhesion, growth
and differentiation of cells. There is no significant cIV
settlement in normal hepatic sinusoids; however, during liver
fibrosis, cIV synthesis increases, there is a large amount of
deposition in the liver blood sinus endothelial cells, alongside
LN, which is involved in the formation of liver fibrosis (40). The main pathological alteration
associated with hepatic fibrosis is an excessive deposition of
several types of extracellular matrix proteins, including collagen,
proteoglycan and fibronectin, resulting in hepatic fibrosis, or
even cirrhosis. Hepatic fibrosis can be diagnosed by the joint
detection of serum HA, LN, pcIII and cIV. Their levels are related
to the severity of chronic hepatic disease (41). This study indicated that HA, LN,
pcIII and cIV were reduced following treatment with HSYA, which
implied an inhibiting effect on synthesis and deposition of rat's
extracellular matrix during fibrosis, and then decreased hepatic
fibrosis degree. However, further studies regarding the mechanism
of action are required.
HYP is a specific amino acid and is a major
component of collagenous fiber. HYP content in liver tissue can
reflect collagenous fiber content relatively accurately (42). The present study demonstrated that
HYP in liver tissue was significantly increased following induction
of hepatic fibrosis by CCl4, which indicated active
collagen hyperplasia. This result was in accordance with those of
previous reports (43,44), thus indicating generation of a
successful model. Conversely, HYP content was significantly
decreased following treatment with various doses of HSYA. The
results from Groups C and E were particularly obvious, thus
indicating a good preventative effect of HYSA on the degree of
hepatic fibrosis in experimental rats.
It has been indicated that single liver cell damage
is not enough to induce hepatic fibrosis, however, lipid
peroxidation is one of the main links during hepatic fibrosis
caused by various factors (45).
MDA is the final decomposition product following lipid
peroxidation, which is caused by free radicals attacking the
polyunsaturated fatty acids in biological membranes; MDA content
can be used to reflect the degree of lipid peroxidation in cells
(46). SOD is a principal
antioxidant, which catalyzes the dismutation of the superoxide
anion free radical, resulting in elimination of the superoxide
anion free radical and protection of cells from damage (47). In the present study, MDA and SOD
levels were detected, indirectly reflecting the degree of lipid
peroxidation damage to liver tissue. The results demonstrated that
MDA levels in the liver tissue homogenates of Group B rats were
significantly increased (P<0.01), whereas SOD levels were
markedly decreased (P<0.01), indicating severe liver cell damage
induced by hepatic fibrosis. Conversely, HSYA could enhance SOD
activity and reduce MDA levels, thus inhibiting lipid peroxidation
caused by free radicals, inducing protection of liver cell
structure and strengthening antioxidant functions. These results
indicated that HSYA may be involved in the repair of damaged liver
cells.
In conclusion, HSYA serves certain roles in
improving hepatic function and alleviating hepatic fibrosis. Its
underlying mechanism may be associated with liver cell protection,
metabolic adjustment of extracellular matrix molecules, and
inhibition of lipid peroxidation. These results provide the
experimental basis for the use of HSYA in clinical application for
hepatic fibrosis treatment. The cause and pathogenesis of hepatic
fibrosis are complex; therefore, it remains unclear the underlying
mechanisms of HSYA anti-hepatic fibrosis effects. More
comprehensive studies regarding the effects of HYSA at the cell,
sub-cell, protein and molecular level are required.
Acknowledgements
The present study was supported by a grant from the
Natural Science Foundation of Shandong Province (grant no.
ZR2011HL063), the Natural Science Foundation of Shandong Province
(grant no. ZR2011HM073) and the Natural Science Foundation of
Shandong Province (grant no. ZR2013HM047). The authors would like
to thank Dr. Fenghua Fu (School of Pharmaceutical Sciences, Yantai
University, Yantai, China) for providing HSYA, and would like to
thank the reviewers for their valuable comments on how to improve
the quality of this paper.
References
|
1
|
Zois C, Baltayiannis G, Karayiannis P and
Tsianos EV: Systematic review: Hepatic fibrosis-regression with
therapy. Aliment Pharmacol Ther. 28:1175–1187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bralet MP: Image in pathology. Congenital
hepatic fibrosis. Ann Pathol. 24:2842004.(In French). PubMed/NCBI
|
|
3
|
Cogliati B, Da Silva TC, Aloia TP, Chaible
LM, Real-Lima MA, Sanches DS, Matsuzaki P, Hernandez-Blazquez FJ
and Dagli ML: Morphological and molecular pathology of
CCL4-induced hepatic fibrosis in connexin43-deficient
mice. Microsc Res Tech. 74:421–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Cheng D, Wang H, Di L, Zhou X, Xu
T, Yang X and Liu Y: The hepatoprotective and antifibrotic effects
of Saururus chinensis against carbon tetrachloride induced hepatic
fibrosis in rats. J Ethnopharmacol. 126:487–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu P, Hu YY, Liu C, Xu LM, Liu CH, Sun
KW, Hu DC, Yin YK, Zhou XQ, Wan MB, et al: Multicentre clinical
study On Fuzhenghuayu capsule against liver fibrosis due to chronic
hepatitis B. World J Gastroenterol. 11:2892–2899. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ru QJ, Tang ZM, Zhang ZE and Zhu Q:
Clinical observation on effect of xuefu zhuyu decoction in treating
patients with liver fibrosis caused by chronic hepatitis B. Chin J
Integr Trad Western Med. 24:983–985. 2004.
|
|
7
|
Lu C, Shen Q, Yang J, Wang B and Song C:
The complete chloroplast genome sequence of Safflower (Carthamus
tinctorius L.). Mitochondrial DNA A DNA Mapp Seq Anal.
27:3351–3353. 2016.PubMed/NCBI
|
|
8
|
Zhao JF, Liu J, Guo Y, Liu Q, Dai Z, Ma SC
and Lin RC: Chemical constituents from safflower injection and
their bioactivity. Zhongguo Zhong Yao Za Zhi. 39:3102–3106.
2014.(In Chinese). PubMed/NCBI
|
|
9
|
Li Y, Chen Y, Wang L, Chen X, Liu X, Sun C
and Yan W: Research on technological process of two-pot
countercurrent extraction of hydroxysafflor yellow A. Zhongguo
Zhong Yao Za Zhi. 34:2743–2747. 2009.PubMed/NCBI
|
|
10
|
Bie XD, Han J and Dai HB: Effects of
hydroxysafflor yellow A on the experimental traumatic brain injury
in rats. J Asian Nat Prod Res. 12:239–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu SX, Zhang Y, Wang YF, Li XC, Xiang MX,
Bian C and Chen P: Upregulation of heme oxygenase-1 expression by
hydroxysafflor yellow A conferring protection from
anoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes. Int
J Cardiol. 160:95–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu HJ, Wang LJ, Wang XQ, Pan H, Li NS,
Yang HB, Jin M, Zang BX and Gong FY: Hormone-sensitive lipase is
involved in the action of hydroxysafflor yellow A (HYSA) inhibiting
adipogenesis of 3T3-L1cells. Fitoterapia. 93:182–188. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Huang Q, Wang C, Zhu X, Duan Y,
Yuan S and Bai X: Hydroxysafflor yellow A suppresses oleic
acid-induced acute lung injury via protein kinase A. Toxicol Appl
Pharmacol. 272:895–904. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chobert MN, Couchie D, Fourcot A, Zafrani
ES, Laperche Y, Mavier P and Brouillet A: Liver precursor cells
increase hepatic fibrosis induced by chronic carbon tetrachloride
intoxication in rats. Lab Invest. 92:135–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang XH, Lu XF, Liu CY, Hu SJ, Kang XX and
Yang J: Diagnostics (8th). People's Health Publishing House.
3522015.(In Chinese).
|
|
16
|
Friedman SL: Hepatic fibrosis-overview.
Toxicology. 254:120–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo J and Friedman SL: Hepatic
fibmgenesis. Semin Liver Dis. 27:413–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spengler U: Hepatic microcirculation: A
critical but neglected factor for the outcome of viral hepatitis. J
Hcpatol. 50:631–633. 2009.
|
|
19
|
Liu C, Wang G, Chen G, Mu Y, Zhang L, Hu
X, Sun M, Liu C and Liu P: Huangqi decoction inhibils apoptosis and
fibrosis, but promotes Kupffer cell activation in
dimefhyllnitrosamine-induced rat liver fibrosis. BMC Complement
Altern Med. 12:512012.PubMed/NCBI
|
|
20
|
Wang Q, Wen R, Lin Q, Wang N, Lu P and Zhu
X: Wogonoside shows antifibrotic effects in an experimental
regression model of hepatic fibrosis. Dig Dis Sci. 60:3329–3339.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang F, Li J, Zhu J, Wang D, Chen S and
Bai X: Hydroxysafflor yellow A inhibits angiogenesis of
hepatocellular carcinoma via blocking ERK/MAPK and NF-κB signaling
pathway in H22 tumor-bearing mice. Eur J Pharmacol. 754:105–114.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu C, Wang G, Chen G, Mu Y, Zhang L, Hu
X, Sun M, Liu C and Liu P: Huangqi decoction inhibits apoptosis and
fibrosis, but promotes Kupffer cell activation in
dimethylnitrosamine-induced rat liver fibrosis. BMC Complement
Altern Med. 12:512012.PubMed/NCBI
|
|
23
|
Yang XH, Wang DD and Zhu Y: Recent
research progress on hydroxysafflor yellow A. J Trad Chin Med Univ
Hunan. 33:102–106. 2013.(In Chinese).
|
|
24
|
Liu SY, Zhang YQ, Liu YL, Guo P and Zhou
CM: Intervention of chronic hepatitis B liver fibrosis patients in
different stages by syndrome typing and different activating blood
removing stasis methods: A clinical study. Zhongguo Zhong Xi Yi Jie
He Za Zhi. 33:1457–1461. 2013.(In Chinese). PubMed/NCBI
|
|
25
|
Shi XF, Xu M and Liu Q: The effects of
total saponins of panax notoginseng on I, III-type collage and TGF
in liver of cirrhosis rats. Zhongyao Yaoli Yu Linchuang. 17:7–8.
2001.
|
|
26
|
Wynn TA: Cellular and molecular mechanisms
on fibrosis. J Pathl. 214:199–210. 2008. View Article : Google Scholar
|
|
27
|
Friedman SL, Rockey DC and Bissell DM:
Hepatic fibrosis 2006: Report of the third SSALD single topic
conference. Hepatology. 45:242–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krählenbühl S, Reichen J, Zimmermann A,
Gehr P and Stucki J: Mitochondrial structure and function in
CCl4-induced cirrhosis in the rat. Hepatology.
12:526–532. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Domitrović R, Rashed K, Cvijanović O,
Vladimir-Knežević S, Škoda M and Višnić A: Myricitrin exhibits
antioxidant, anti-inflammatory and antifibrotic activity in carbon
tetrachloride-intoxicated mice. Chem Biol Interact. 230:21–29.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang ZB, Huang ZM, Wang JJ, Wu JM, Chen
XR, Wu JS and Zhang QY: Inducing rat liver cirrhosis by adjusting
the dosage of CCl4 according to body weight changes.
Zhonghua Gan Zang Bing Za Zhi. 16:234–235. 2008.(In Chinese).
PubMed/NCBI
|
|
31
|
Gonzalez M, Sealls W, Jesch ED, Brosnan
MJ, Ladunga I, Ding X, Black PN and DiRusso CC: Defining a
relationship between dietary fatty acids and the cytochrome P450
system in a mouse model of fatty liver disease. Physiol Genomics.
43:121–135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang CC, Lim LY, Deubner H, Tapia K, Lau
AW, Manansala J, Krows M, Shuhart MC and Kowdley KV: Factors
predictive of significant hepatic fibrosis in adults with chronic
hepatitis B and normal serum ALT. J Clin Gastroenterol. 42:820–826.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park JH, Park CK, Kim ES, Park SY, Jo CM,
Tak WY, Kweon YO, Kim SK and Choi YW: The diagnostic value of serum
hyaluronic acid, 7S domain of type IV collagen and AST/ALT ratio as
markers of hepatic fibrosis in chronic hepatitis B and cirrhosis
patients. Taehan Kan Hakhoe Chi. 9:79–88. 2003.PubMed/NCBI
|
|
34
|
Li CH, Piao DM, Xu WX, Yin ZR, Jin JS and
Shen ZS: Morphological and serum hyaluronic acid, laminin and type
IV collagen changes in dimethylnitrosamine-induced hepatic fibrosis
of rats. World J Gastroenterol. 11:7620–7624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khan JA, Khan FA, Dilawar M, Ijaz A, Khan
NA and Mehmood T: Serum hyaluronic acid as a marker of hepatic
fibrosis. J Coll Physicians Surg Pak. 17:323–326. 2007.PubMed/NCBI
|
|
36
|
Geramizadeh B, Janfeshan K and
Saberfiroozi M: Serum hyaluronic acid as a noninvasive marker of
hepatic fibrosis in chronic hepatitis B. Saudi J Gastroenterol.
14:174–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang Z, Li Q and Wang Z: Observation on
dynamic changes of serum procollagen III, hyaluronic acid and
laminin in rats with hepatic fibrosis treated with Hujin pill.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 20:447–449. 2000.(In Chinese).
PubMed/NCBI
|
|
38
|
Tsutsumi M, Takase S, Urashima S, Ueshima
Y, Kawahara H and Takada A: Serum markers for hepatic fibrosis in
alcoholic liver disease: Which is the best marker, type III
procollagen, type IV collagen, laminin, tissue inhibitor of
metalloproteinase, or prolyl hydroxylase? Alcohol Clin Exp Res.
20:1512–1517. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Iushchuk ND, Znoĭko OO, Safiullina NKh,
Dudina KR, Kelli EI, Klimova EA, Kashirin VI, Braginskiĭ DM,
Kushlinskiĭ NE, Liubimova NV, et al: Diagnostic significance of
type IV collagen and hyaluronic acid in the serum of patients with
chronic hepatitis C for staging hepatic fibrosis. Ter Arkh.
77:50–55. 2005.(In Russian).
|
|
40
|
Toyoki Y, Sasaki M, Narumi S, Yoshihara S,
Morita T and Konn M: Semiquantitative evaluation of hepatic
fibrosis by measuring tissue hydroxyproline.
Hepatogastroenterology. 45:2261–2264. 1998.PubMed/NCBI
|
|
41
|
Liu J, Tan H, Sun Y, Zhou S, Cao J and
Wang F: The preventive effects of heparin-superoxide dismutase on
carbon tetrachloride-induced acute liver failure and hepatic
fibrosis in mice. Mol Cell Biochem. 327:219–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang Q, Li Y, Zhang S, Huang R, Zheng L,
Wei L, He M, Liao M, Li L, Zhuo L and Lin X: Effect and mechanism
of methyl helicterate isolated from Helicteres angustifolia
(Sterculiaceae) on hepatic fibrosis induced by carbon tetrachloride
in rats. J Ethnopharmacol. 143:889–895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kanno K, Tazuma S and Chayama K:
AT1A-deficient mice show less severe progression of liver fibrosis
induced by CCl(4). Biochem Biophys Res Commun. 308:177–183. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Smyth R, Munday MR, York MJ, Clarke CJ,
Dare T and Turton JA: Comprehensive characterization of serum
clinical chemistry parameters and the identification of urinary
superoxide dismutase in a carbon tetrachloride-induced model of
hepatic fibrosis in the female Hanover Wistar rat. Int J Exp
Pathol. 88:361–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu J, Wang Y, Qian H, Zhao Y, Liu B and Fu
C: Polyprenols from Taxus chinensis var. mairei prevent the
development of CCl4-induced liver fibrosis in rats. J
Ethnopharmacol. 142:151–160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu J, Tan H, Sun Y, Zhou S, Cao J and
Wang F: The preventive effects of heparin-superoxide dismutase on
carbon tetrachloride-induced acute liver failure and hepatic
fibrosis in mice. Mol Cell Biochem. 327:219–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ljubuncic P, Abu-Salach O and Bomzon A:
Ursodeoxycholic acid and superoxide anion. World J Gastroenterol.
11:4875–4878. 2005. View Article : Google Scholar : PubMed/NCBI
|





















