Introduction
Skin photoaging refers to the aging process
resulting from exposure to ultraviolet (UV) light (1), characterized by roughening and
thickening of the skin, increased flaccidity, coarse wrinkles,
local pigmentation, or telangiectasia (2–4). It
may also lead to benign or malignant tumors, including sunlight
keratosis, squamous cell carcinoma and malignant melanoma (5).
Photoaging has a complex mechanism. Altered levels
of reactive oxygen species (ROS), extracellular matrix components,
matrix metalloproteinases and cytokines are linked to photoaging,
particularly increased ROS levels (6). Upon receiving UV energy,
intracellular chromophores interact with oxygen molecules, creating
ROS that cause direct cell damage when levels increase above a
certain threshold (7–9). Elevated ROS levels also activate a
series of signaling pathways through expression of signaling
molecules including extracellular signal-regulated kinases (ERKs),
c-Jun N-terminal kinase (JNK), and p38 in the mitogen-activated
protein kinase (MAPK) pathway. This results in increased matrix
metalloproteinase (MMP) expression, and a reduction in collagen
protein synthesis and secretion (10,11).
Type I collagens are an important component of the extracellular
matrix that are degraded by MMP-1 (12). During the photoaging process MMP-1
is overexpressed, resulting in collagen degradation and
disintegration (12–16).
Physically covering skin is known to minimize UV
damage, but antioxidant and antiinflammatory agents are also
increasingly being used (17,18).
Current clinical treatments to prevent and delay photoaging rely on
photo-dynamic therapy (19) and
antioxidants including polyphenols and β-carotene (20). Previous studies have demonstrated
the positive effects of herbal extracts in preventing skin
photoaging: Sun et al (21)
revealed that wild chrysanthemum extract downregulates ROS levels
within HaCat cells and so reduces MMP-2 and MMP-9 expression by
inhibiting UV-induced ERK1/2 and p38 phosphorylation to prevent
UVB-related acute skin damage and photoaging. Lee et al
(22) demonstrated that
macelignan, an effective antioxidant found in the nutmeg,
Myristica fragans, downregulates MMP-1 expression by
reducing UV exposure-related ROS elevation and MAPK
phosphorylation. In addition, it increases type I collagen
expression by activating transforming growth factor β (TGF-β)/SMAD
signaling to effectively prevent and treat skin photoaging.
The fungus Ganoderma lucidum is a famous
herbal medicine in China, having featured in traditional Chinese
medicine for >1,000 years (23–25).
G. lucidum polysaccharides (GL-PS) display regulatory
abilities toward the immune system, resulting in antitumor and
antioxidation effects (26–28).
GL-PS has been demonstrated to protect murine skeletal muscles from
oxidative stress following exhaustive exercise (29). GL-PS also regulates gene expression
in aging skin cells and induces cytokine secretion, suggesting
potential anti-aging effects (29). Considering the close relation of
photoaging to unbalanced oxidative stress and the antioxidative
effect of GL-PS, the present study hypothesized that GL-PS confers
protection from photoaging on skin cells.
The present study, to the best of our knowledge, is
the first to demonstrate that GL-PS protects fibroblasts from
UVB-induced photoaging. The mechanism underlying this may be the
reduction of UVB-induced ROS levels, inhibiting the MAPK signal
pathway and therefore MMP-1 expression, while promoting type I
collagen expression. This suggests that GL-PS may have clinical
potential in treating photoaging.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) was
obtained from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA), fetal bovine serum (FBS) from HyClone; GE Healthcare Life
Sciences (Logan, UT, USA), 98% purity GL-PS from Shaanxi Ciyuan
Biotech Co., Ltd. (Xi'an, China), senescence-associated
β-galactosidase staining (SA-β-gal) kit from Beyotime Institute of
Biotechnology (Haimen, China), ROS detection kit from Nanjing
KeyGen Biotech Co., Ltd. (Nanjing, China), MMP-1 ELISA detection
kit (cat. no. EK0458) from Boster Systems, Inc. (Wuhan, China),
C-telopeptides of type I collagen (CICP) ELISA detection kit (cat.
no. XY-67851Hu) from Shanghai Xin Yu Biotech Co., Ltd (Shanghai,
China) and UVB radiation apparatus (SS-07) from Shanghai SIGMA High
Tech Co., Ltd. (Shanghai, China).
Fibroblast isolation and culture
Samples were obtained from the donated foreskin of a
healthy man (age, 18 years) following circumcision, with informed
consent. The foreskin was soaked in iodine for 15 min and
subsequently rinsed with PBS. Subcutaneous tissues were removed,
and the remaining tissue was cut into small pieces (<5 mm).
Trypsin was applied to isolate fibroblasts, and all cells were
subsequently collected, washed and cultured in DMEM containing 10%
FBS. When cells were 80–90% confluent, they were passaged at a
ratio of 1:3. Exponential growth phase cells from passages 4–8 were
used for subsequent experiments.
Groups, treatment and UVB
exposure
Cells were either exposed to UVB, exposed to UVB and
treated with GL-PS, or non-exposed and untreated. GL-PS 1 mg/ml was
reconstituted in 1X PBS and was stored at 4°C. Solutions of
different GL-PS concentration were made by diluting in FBS-free
DMEM prior to use. All cells were cultured in FBS-free media for 24
h prior to UVB exposure. Immediately prior to exposure, all media
was vacuumed, and cell layers were rinsed 3 times with sterile PBS.
Following UVB exposure, matched media was added immediately.
Cell viability assay (MTT assay)
Cells were seeded in a 96-well plate
(1×104 cells/well), with a volume of 200 µl per well.
Following attachment to the dish, cells were starved with FBS-free
media for 24 h. All media was vacuumed prior to UVB exposure. When
exposure was completed, cells were cultured in serum-free media for
24 h, with the specified concentrations of GL-PS where appropriate.
MTT was then added (20 µl, 5 mg/ml) and the cells cultured for a
further 4 h. Supernatant was then discarded and 150 µl dimethyl
sulfoxide added. Absorbances were measured at 490 nm with a
microplate reader.
Detection of aging cells using an
SA-β-gal kit
Following UVB exposure and treatment with the
specified concentrations of GL-PS, an SA-β-gal kit was used to
stain aging cells according to the manufacturer's instructions.
Samples were checked using a light microscope under ×200
magnification. For each sample, >200 cells from 15 randomly
selected fields were checked to calculate the percentage of aging
cells (blue stained cell number/total cell number ×100).
Measurement of intracellular ROS by
flow cytometry
Cells were prepared according to the manufacturer's
instructions for use with the ROS detection kit 24 h after UVB
exposure and treatment with GL-PS. Images were captured using a
fluorescence microscope, and fluorescence intensity was detected by
flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA) with BD CellQuest Pro software (version 6.0; BD
Biosciences).
Detection of MMP-1 and CICP expression
by ELISA
Cell supernatants were collected 24 h following UVB
exposure and treatment with GL-PS, and centrifuged at 1,000 ×
g for 10 min. ELISA kits were used to detect MMP-1 and CICP
concentrations according to the manufacturer's instructions. CICP
content was used to represent the Type I collagen content.
Statistical analysis
All experiments were repeated independently at least
in triplicate. Experimental data were analyzed with SPSS 19.0 (IBM
SPSS, Armonk, NY, USA). Unpaired Students t-tests were
applied to analyze differences between groups. All data are
presented as the mean + standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Fibroblast viability is reduced
dose-dependently by UVB exposure
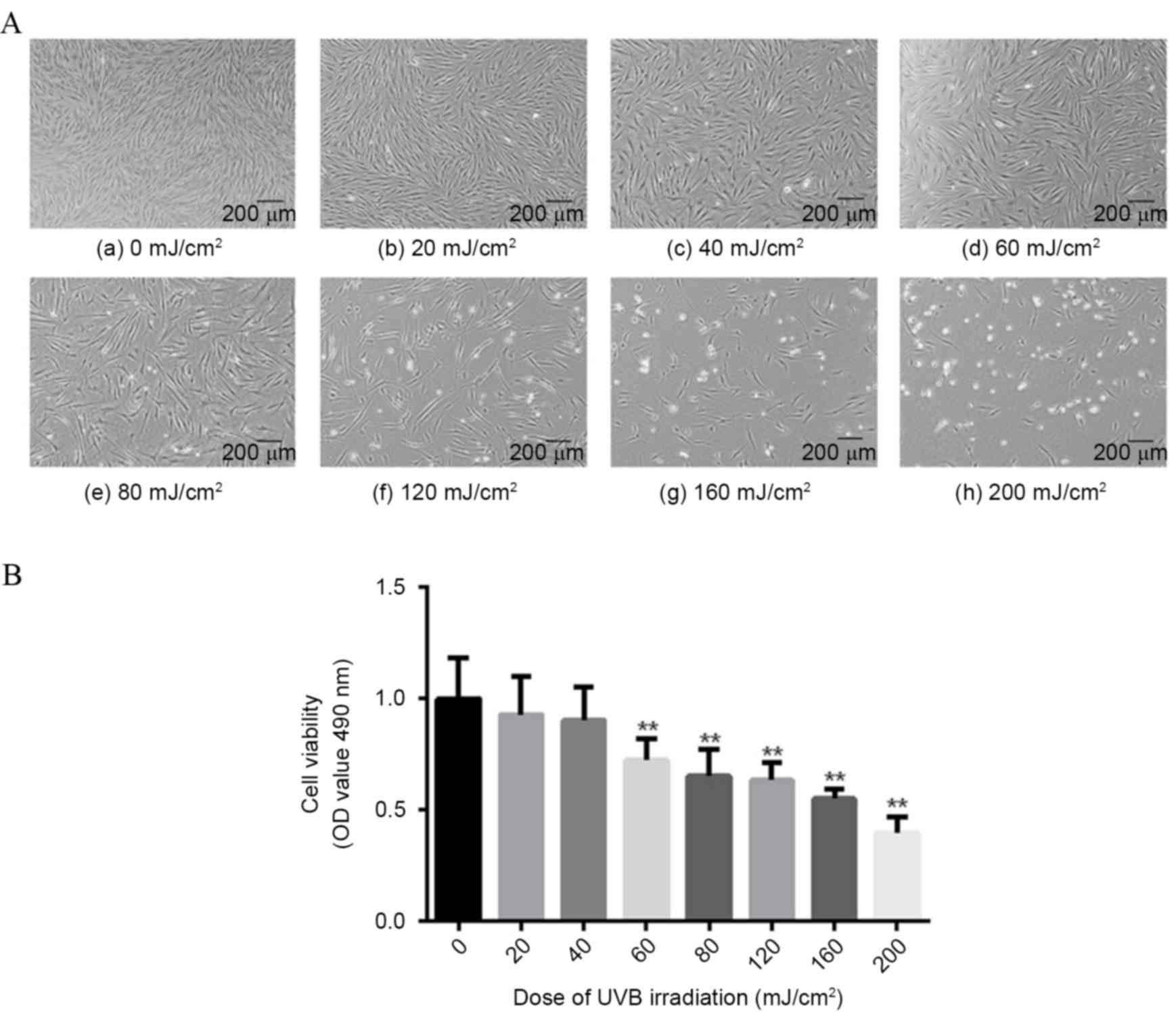
Fibroblasts were exposed to a range of UVB doses (0,
20, 40, 60, 80, 120, 160 and 200 mJ/cm2), then cellular
density and changes in condition were observed, and cell viability
assessed by MTT assay. Following UVB exposure, fibroblast density
decreased in a dose-dependent manner (Fig. 1A). In addition, when UVB dosage
exceeded 80 mJ/cm2, a noticeable increase in dead cells
was observed (Fig. 1A). A
dose-dependent decrease in cellular viability following UVB
exposure was also demonstrated (Fig.
1B). The lowest dose to demonstrate significantly reduced cell
viability compared with non-exposed cells was 60 mJ/cm2
(−27.71%; P=0.0083 Fig. 1B),
therefore this dose was used for subsequent experiments.
GL-PS confers protection against
UVB-induced cell death to fibroblasts
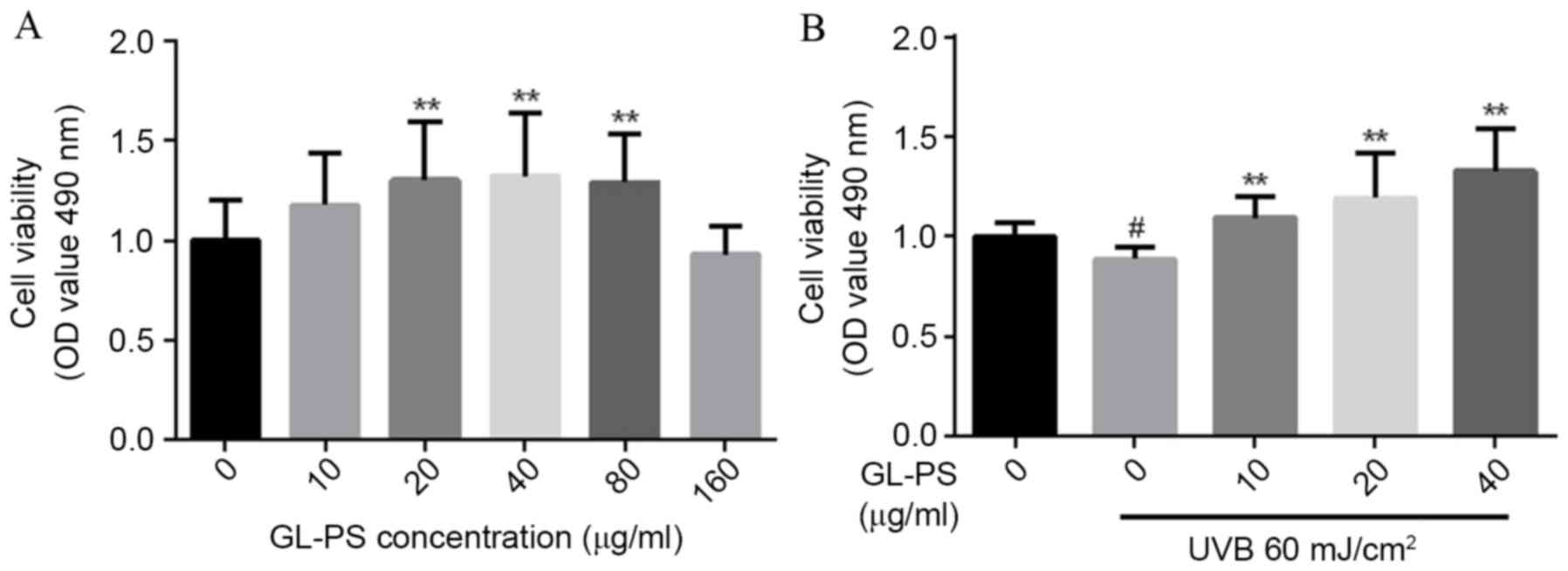
GL-PS toxicity towards fibroblasts was examined by
analysis of cell viability following treatment with different GL-PS
concentrations. Compared with untreated cells, increased cell
viability was observed in groups treated with 20, 40 and 80 µg/ml
GL-PS (P=0.0069, P=0.0074 and P=0.0037, respectively; Fig. 2A), but cells treated with 160 µg/ml
GL-PS demonstrated no significant difference in viability compared
with untreated control (Fig. 2A).
Therefore, GL-PS promotes fibroblast viability, but only within a
certain range.
Following exposure to 60 mJ/cm2 UVB,
cells treated with 10, 20 and 40 µg/ml GL-PS demonstrated increased
viability compared with untreated cells (P=0.0025, P=0.0096 and
P=0.0016, respectively; Fig. 2B),
suggesting that GL-PS confers protective effects towards
fibroblasts from UVB damage.
GL-PS inhibits UVB-induced fibroblast
aging
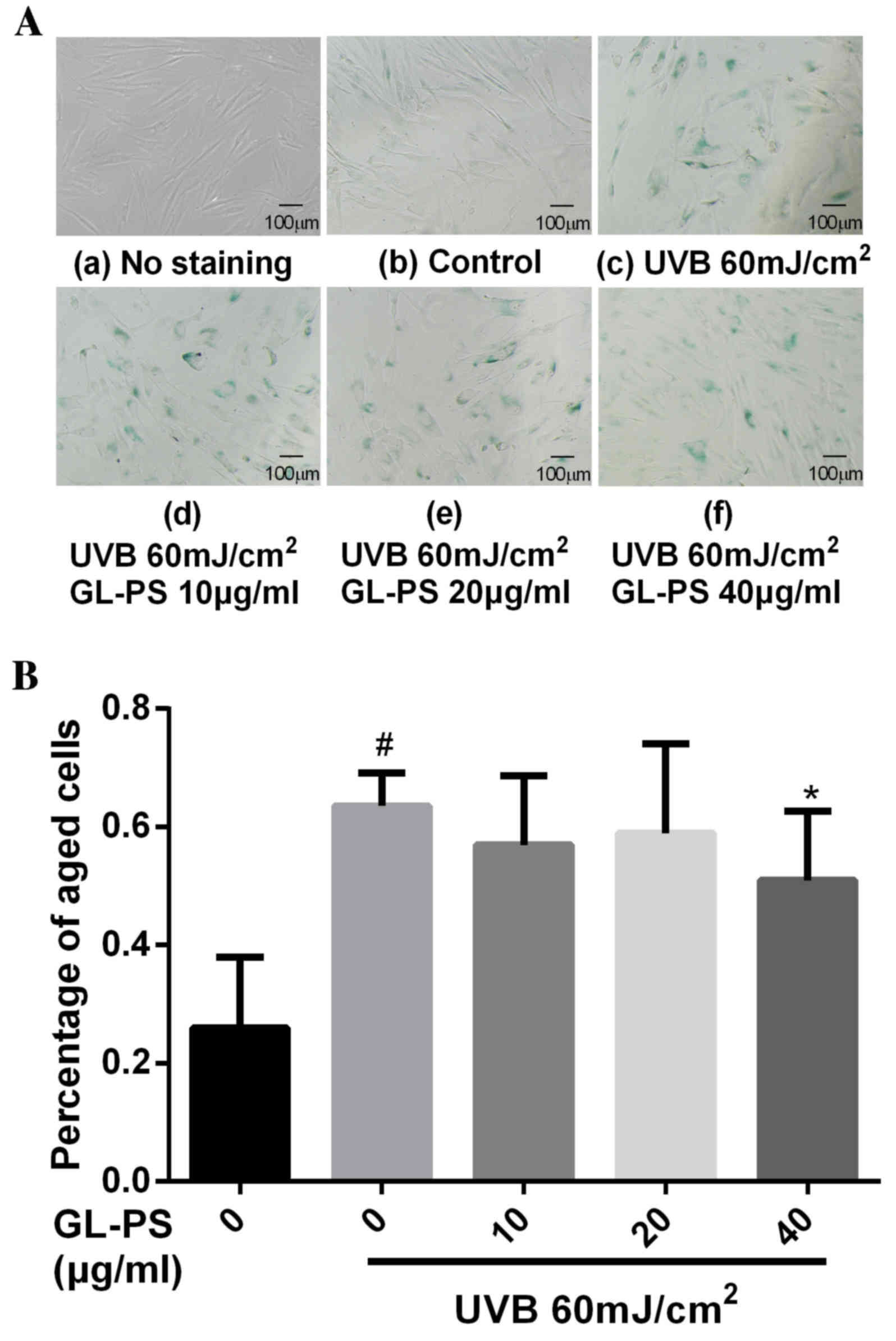
Aging cells were peri-nuclearly stained blue, using
the SA-β-gal kit (Fig. 3A). The
percentage of stained cells was 25.95% in the UVB non-exposed/GL-PS
untreated group compared with 63.53% in the UVB exposed/GL-PS
untreated group (P=0.0001; Fig.
3B). No significant difference in staining was observed in UVB
exposed cells treated with 10 (57.00%) and 20 µg/ml (58.98%) GL-PS
compared with UVB exposed/GL-PS untreated cells (Fig. 3B). However, for UVB exposed cells
treated with 40 µg/ml GL-PS, there was a statistically significant
decrease in staining compared with UVB exposed/GL-PS untreated
cells, with 50.96% of cells stained (P=0.0322; Fig. 3B).
GL-PS inhibits UVB-induced protein
expression of MMP-1 in fibroblasts, and increases protein
expression of CICP
MMP-1 protein expression in fibroblasts was
significantly increased following UVB exposure compared with
non-exposed cells (P=0.0097; Fig.
4A). UVB exposed cells expressed significantly lower levels of
MMP-1 protein following treatment with 10, 20 and 40 µg/ml GL-PS
compared with untreated cells (P=0.0043, P=0.0007 and P=0.0090,
respectively; Fig. 4A). CICP
protein expression levels decreased in fibroblasts following UVB
exposure compared with non-exposed cells (P=0.0362 Fig. 4B), but CICP protein expression
levels increased dose-dependently in UVB exposed cells following
GL-PS-treatment, with a statistically significant difference
observed in the 40 µg/ml treatment group compared with untreated
cells (P=0.0074; Fig. 4B).
GL-PS inhibits UVB-induced ROS in
fibroblasts
UVB exposed cells exhibited increased fluorescence
compared with non-exposed control cells, indicating increased ROS
production (P=0.0032; 2.3 fold difference; Fig. 5A). Fluorescence decreased in UVB
exposed cells as GL-PS concentration increased (Fig. 5A). The flow cytometry results
demonstrated a similar trend, with a non-significant decrease in
ROS in UVB exposed cells treated with 10 µg/ml GL-PS compared with
UVB exposed/GL-PS untreated cells (P=0.313; Fig. 5B) and significant decreases in UVB
exposed cells treated with 20 and 40 µg/ml GL-PS compared with UVB
exposed/GL-PS untreated cells (P=0.0406 and P=0.0172, respectively;
Fig. 5B). This suggests that above
a threshold concentration, GL-PS reduces UVB-induced ROS
production.
Discussion
Ganoderma, a popular traditional drug in
Asia, is a member of the Polyporaceae family and is endemic to
tropical areas (24). Previous
research had revealed multiple pharmaceutical properties of
Ganoderma, including antitumor effects (30), immune regulation (31), antioxidative effects (32), hepatoprotective effect against
CCl4-induced liver injury (33),
and the ability to lower blood sugar (34). A major active ingredient in
Ganoderma is GL-PS, which is formed from three single
strands of monosaccharide chains, including glucose, galactose,
mannose and arabinose, which form a helical three dimensional
configuration (35). In
traditional Chinese medicine, Ganoderma is used as an
anti-aging drug, and modern research has previously demonstrated
the anti-aging effects of Ganoderma (26–28).
Photoaging refers to accelerated aging of the skin induced by UV
light exposure, usually with the involvement of ROS (6). It was therefore hypothesized that the
anti-aging effects of GL-PS may derive from inhibition of ROS
production; however, to the best of our knowledge, no previous
studies had investigated the effects of GL-PS on UVB-induced
photoaging. Therefore, in the present study, the protective effect
of GL-PS on UVB-induced fibroblast aging was examined.
A UVB toxicity study on fibroblasts revealed that
fibroblast cell viability decreased dose-dependently as the UVB
dose increased; 60 mJ/cm2 UVB was the lowest dose
resulting in significantly decreased cell viability, so was
selected to create the photoaging fibroblast model. The effect of
GL-PS on cell viability was subsequently explored. Treatment of
cells that had not been exposed to UVB with 20, 40 and 80 µg/ml
GL-PS was demonstrated to promote fibroblast cell viability in
excess of untreated cells. Treatment of cells exposed to UVB with
10, 20 and 40 µg/ml GL-PS also increased cell viability in excess
of UVB exposed cells. GL-PS was reported to prevent apoptosis of
pancreatic β-cells (36). Thus,
the ability to inhibit apoptosis of GL-PS may be the possible
reason for the increased the cell viability of GL-PS treated
fibroblasts. Furthermore, 40 µg/ml GL-PS treatment also
significantly decreased the percentage of aged cells, suggesting
that GL-PS protects fibroblasts from photoaging.
Photoaging includes epidermal photoaging and dermal
photoaging, with dermal photoaging more important to the overall
photoaging process (2). Skin
wrinkles, induced by decreased extra-cellular matrix components,
such as collagens, are a major product of photoaging (37). Collagens are primarily produced by
fibroblasts and are a major component of the extra-cellular matrix
(16). Previous studies have
demonstrated that type I collagen, the most abundant collagen, is
degraded by MMP-1 (14,16,38).
When degradation of normal collagen and accumulation of abnormal
collagen fiber fragments increases, the proliferation of
fibroblasts is inhibited, resulting in decreased collagen synthesis
(15,16). UVB exposure results in upregulated
MMP-1 protein expression and decreased collagen expression
(19), which the present study
also confirmed. In addition, the present study demonstrated the
ability of GL-PS to inhibit UVB-induced MMP-1 protein expression
and promote CICP protein expression in fibroblasts. Therefore,
GL-PS may be involved in the inhibition of extra-cellular matrix
degradation, resulting in protection of skin against
photoaging.
Major mechanisms of photoaging include UV-associated
DNA damage, and UV-induced increases in cellular ROS. Intracellular
chromophores absorb UVB energy and interact with oxygen molecules
to produce ROS, resulting in induction of the oxidative stress
response and subsequent damage to cells (7–9).
Zhao et al (29)
demonstrated that GL-PS treatment protects murine skeletal muscles
from exhaustive-exercise-induced oxidative stress. In addition, Kao
et al (39) isolated low
molecular weight β-1,3-glucan from Ganoderma and
demonstrated that this reduces H2O2-induced
intracellular ROS production, and so inhibits inflammation-induced
oxidative stress. In the present study, ROS levels were
demonstrated to increase significantly in photoaging fibroblasts
and, for the first time, GL-PS treatment was demonstrated to
inhibit ROS production following UVB treatment.
During the photoaging process, increased ROS levels
also activate growth cytokines and their receptors in fibroblasts
and keratinocytes, leading to activation of ERK, p38, and JNK in
the MAPK pathway (29,40). This promotes MMP expression and
decreases collagen levels (10,11).
The results of the present study demonstrate the antiphotoaging
effect of GL-PS in vitro, however, the effects of GL-PS on
the MAPK and other signaling pathways were not investigated. In
addition, the efficacy of GL-PS on photoaging in vivo remain
to be elucidated. Further studies are required to verify the
results of the present study and to assess the underlying mechanism
of GL-PS on photoaging.
In conclusion, the present study demonstrates that
GL-PS protects fibroblasts from photoaging via its antioxidant
ability. This indicates GL-PS treatment may serve as a novel
strategy for anti-photoaging.
Acknowledgements
The present study was supported by The New Xiangya
Talent Projects of the Third Xiangya Hospital of Central South
University (grant no. JY201623).
References
|
1
|
Han A, Chien AL and Kang S: Photoaging.
Dermatol Clin. 32291–299. (vii)2014.PubMed/NCBI
|
|
2
|
Fisher GJ, Kang S, Varani J, Bata-Csorgo
Z, Wan Y, Datta S and Voorhees JJ: Mechanisms of photoaging and
chronological skin aging. Arch Dermatol. 138:1462–1470.
2002.PubMed/NCBI
|
|
3
|
Sjerobabski Masnec I and Poduje S:
Photoaging. Coll Antropol. 32:(Suppl 2). S177–S180. 2008.
|
|
4
|
Hwang E, Park SY, Lee HJ, Lee TY, Sun ZW
and Yi TH: Gallic acid regulates skin photoaging in UVB-exposed
fibroblast and hairless mice. Phytother Res. 28:1778–1788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Williams JD, Bermudez Y, Park SL, Stratton
SP, Uchida K, Hurst CA and Wondrak GT: Malondialdehyde-derived
epitopes in human skin result from acute exposure to solar UV and
occur in nonmelanoma skin cancer tissue. J Photochem Photobiol B.
132:56–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rittié L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valencia A and Kochevar IE: Nox1-based
NADPH oxidase is the major source of UVA-induced reactive oxygen
species in human keratinocytes. J Invest Dermatol. 128:214–222.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Emri G, Horkay I and Remenyik E: The role
of free radicals in the UV-induced skin damage. Photo-aging. Orv
Hetil. 147:731–735. 2006.(In Hungarian).
|
|
9
|
Van Laethem A, Nys K, Van Kelst S,
Claerhout S, Ichijo H, Vandenheede JR, Garmyn M and Agostinis P:
Apoptosis signal regulating kinase-1 connects reactive oxygen
species to p38 MAPK-induced mitochondrial apoptosis in
UVB-irradiated human keratinocytes. Free Radic Biol Med.
41:1361–1371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watanabe H, Shimizu T, Nishihira J, Abe R,
Nakayama T, Taniguchi M, Sabe H, Ishibashi T and Shimizu H:
Ultraviolet A-induced production of matrix metalloproteinase-1 is
mediated by macrophage migration inhibitory factor (MIF) in human
dermal fibroblasts. J Biol Chem. 279:1676–1683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quan T, He T, Voorhees JJ and Fisher GJ:
Ultraviolet irradiation induces Smad7 via induction of
transcription factor AP-1 in human skin fibroblasts. J Biol Chem.
280:8079–8085. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brennan M, Bhatti H, Nerusu KC,
Bhagavathula N, Kang S, Fisher GJ, Varani J and Voorhees JJ: Matrix
metalloproteinase-1 is the major collagenolytic enzyme responsible
for collagen damage in UV-irradiated human skin. Photochem
Photobiol. 78:43–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gambichler T, Skrygan M, Tomi NS, Breuksch
S, Altmeyer P and Kreuter A: Significant downregulation of
transforming growth factor-beta signal transducers in human skin
following ultraviolet-A1 irradiation. Br J Dermatol. 156:951–956.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imokawa G: Recent advances in
characterizing biological mechanisms underlying UV-induced
wrinkles: A pivotal role of fibrobrast-derived elastase. Arch
Dermatol Res. 300:(Suppl 1). S7–S20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ
and Fisher GJ: Matrix-degrading metalloproteinases in photoaging. J
Investig Dermatol Symp Proc. 14:20–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Afaq F and Mukhtar H: Botanical
antioxidants in the prevention of photocarcinogenesis and
photoaging. Exp Dermatol. 15:678–684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katiyar SK: UV-induced immune suppression
and photocarcinogenesis: Chemoprevention by dietary botanical
agents. Cancer Lett. 255:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Luo X, Lu J, Chen J, Zuo C, Xiang
Y, Yang S, Tan L, Kang J and Bi Z: IPL irradiation rejuvenates skin
collagen via the bidirectional regulation of MMP-1 and TGF-β1
mediated by MAPKs in fibroblasts. Lasers Med Sci. 26:381–387. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsui MS, Hsia A, Miller JD, Hanneman K,
Scull H, Cooper KD and Baron E: Non-sunscreen photoprotection:
Antioxidants add value to a sunscreen. J Investig Dermatol Symp
Proc. 14:56–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun S, Jiang P, Su W, Xiang Y, Li J, Zeng
L and Yang S: Wild chrysanthemum extract prevents UVB
radiation-induced acute cell death and photoaging. Cytotechnology.
68:229–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee KE, Mun S, Pyun HB, Kim MS and Hwang
JK: Effects of macelignan isolated from Myristica fragrans (Nutmeg)
on expression of matrix metalloproteinase-1 and type I procollagen
in UVB-irradiated human skin fibroblasts. Biol Pharm Bull.
35:1669–1675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yun TK: Update from Asia. Asian studies on
cancer chemoprevention. Ann N Y Acad Sci. 889:157–192. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sliva D: Cellular and physiological
effects of Ganoderma lucidum (Reishi). Mini Rev Med Chem.
4:873–879. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dudhgaonkar S, Thyagarajan A and Sliva D:
Suppression of the inflammatory response by triterpenes isolated
from the mushroom Ganoderma lucidum. Int Immunopharmacol.
9:1272–1280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
XiaoPing C, Yan C, ShuiBing L, YouGuo C,
JianYun L and LanPing L: Free radical scavenging of Ganoderma
lucidum polysaccharides and its effect on antioxidant enzymes and
immunity activities in cervical carcinoma rats. Carbohydr Polym.
77:389–393. 2009. View Article : Google Scholar
|
|
27
|
Xu Z, Chen X, Zhong Z, Chen L and Wang Y:
Ganoderma lucidum polysaccharides: Immunomodulation and potential
anti-tumor activities. Am J Chin Med. 39:15–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi M, Zhang Z and Yang Y: Antioxidant and
immunoregulatory activity of Ganoderma lucidum polysaccharide
(GLP). Carbohydr Polym. 95:200–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Z, Zheng X and Fang F: Ganoderma
lucidum polysaccharides supplementation attenuates exercise-induced
oxidative stress in skeletal muscle of mice. Saudi J Biol Sci.
21:119–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuen JW and Gohel MD: Anticancer effects
of Ganoderma lucidum: A review of scientific evidence. Nutr Cancer.
53:11–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin ZB and Zhang HN: Anti-tumor and
immunoregulatory activities of Ganoderma lucidum and its possible
mechanisms. Acta Pharmacol Sin. 25:1387–1395. 2004.PubMed/NCBI
|
|
32
|
Yen GC and Wu JY: Antioxidant and radical
scavenging properties of extracts from Ganoderma tsugae. Food Chem.
65:375–379. 1999. View Article : Google Scholar
|
|
33
|
Kim DH, Shim SB, Kim NJ and Jang IS:
Beta-glucuronidase-inhibitory activity and hepatoprotective effect
of Ganoderma lucidum. Biol Pharm Bull. 22:162–164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seto SW, Lam TY, Tam HL, Au AL, Chan SW,
Wu JH, Yu PH, Leung GP, Ngai SM, Yeung JH, et al: Novel
hypoglycemic effects of Ganoderma lucidum water-extract in
obese/diabetic (+db/+db) mice. Phytomedicine. 16:426–436. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nie S, Zhang H, Li W and Xie M: Current
development of polysaccharides from Ganoderma: Isolation, structure
and bioactivities. Bioactive Carbohydrates and Dietary Fibre.
1:10–20. 2013. View Article : Google Scholar
|
|
36
|
Zheng J, Yang B, Yu Y, Chen Q, Huang T and
Li D: Ganoderma lucidum polysaccharides exert anti-hyperglycemic
effect on streptozotocin-induced diabetic rats through affecting
β-cells. Comb Chem High Throughput Screen. 15:542–550. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gilchrest BA: Photoaging. J Invest
Dermatol. 133:E2–E6. 2013. View Article : Google Scholar
|
|
38
|
Wang XY and Bi ZG: UVB-irradiated human
keratinocytes and interleukin-1alpha indirectly increase MAP
kinase/AP-1 activation and MMP-1 production in UVA-irradiated
dermal fibroblasts. Chin Med J (Engl). 119:827–831. 2006.PubMed/NCBI
|
|
39
|
Kao PF, Wang SH, Hung WT, Liao YH, Lin CM
and Yang WB: Structural characterization and antioxidative activity
of low-molecular-weights beta-1,3-glucan from the residue of
extracted Ganoderma lucidum fruiting bodies. J Biomed Biotechnol.
2012:6737642012.PubMed/NCBI
|
|
40
|
Chen CC, Chiang AN, Liu HN and Chang YT:
EGb-761 prevents ultraviolet B-induced photoaging via inactivation
of mitogen-activated protein kinases and proinflammatory cytokine
expression. J Dermatol Sci. 75:55–62. 2014. View Article : Google Scholar : PubMed/NCBI
|