Introduction
Pelvic organ prolapse (POP) is a common form of
female pelvic floor dysfunction. Pregnancy, childbirth, obesity and
constipation, which may lead to the increase of abdominal pressure,
are considered to be important risk factors for POP (1–4);
mechanics serve a vital role in the incidence of POP. Further
studies suggest that the occurrence of POP may be associated with
an imbalance of the oxidation-reduction equilibrium in vivo
(5,6). Our previous study demonstrated that
the expression levels of antioxidant enzymes in the pelvic tissues
of patients with POP was significantly decreased, which indicates
that oxidative stress is closely associated with the occurrence of
POP (7). In addition, this study
revealed that a specific range of mechanical forces may cause an
increase in reactive oxygen species (ROS) in human parametrial
ligament fibroblasts (HPLFs) (8).
Therefore, it was hypothesized that mechanical forces may damage
cells by inducing oxidative stress, furthering the development of
POP.
The activity and proliferation of cells, which
reduce when cells are subjected to external injury, is an important
indicator of the cell state (9).
While suffering from external damage, the cytoskeleton may
depolymerize and rearrange to adapt to external stimuli (10). In cells, the mitochondria are the
primary production site of active oxygen, and therefore exhibit the
clearest damage upon increased oxidative stress. Furthermore,
cellular senescence is a process consisting of the repair of DNA
damage, mitochondrial energy metabolism and gene regulation
(11). Therefore, the present
study derived fibroblasts from female parametrial ligaments. Cell
proliferation, cytoskeletal structure, mitochondrial morphology and
cellular senescence were subsequently analyzed to investigate
whether mechanical force may induce damage to the internal
structure of the cell, and to examine the underlying mechanisms of
the effects of mechanical stress on the occurrence of POP.
Materials and methods
Materials
A total of 10 patients with cervical intraepithelial
neoplasia grades II–III from June 2014-June 2015 in Renmin Hospital
of Wuhan University, who had received a total vaginal hysterectomy,
were selected for the present study. Patients who had received
estrin treatment within the past three months were excluded.
Uterosacral ligaments and cardinal ligaments were obtained from
patients following the receipt of informed consent. This study was
approved by the Ethics Committee of Renmin Hospital of Wuhan
University (Wuhan, China).
The present study employed a modified enzyme
digestion method, as previously described (12) to obtain HPLFs. The tissue
(0.5×0.5×0.2 cm) was placed into the Dulbecco's modified Eagle's
medium (DMEM; Hangzhou Gino Biological Pharmaceutical Co., Ltd.,
Hangzhou, China) immediately following surgery, and was incubated
at 4°C within 30 min. The tissue was subsequently washed with
phosphate-buffered saline (PBS) containing 100 KU/ml penicillin G
and 100 mg/ml streptomycin (Hangzhou Gino Biological Pharmaceutical
Co., Ltd.), and sectioned into small pieces. Following this, it was
digested with 1% collagenase-I (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 3 h at 37°C in 5%
CO2, followed by further digestion with 0.25% trypsin
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 5 min.
Fetal bovine serum (FBS; 2 ml; Gibco; Thermo Fisher Scientific,
Inc.) was used to terminate digestion. DMEM containing 15% FBS was
slowly added to the culture flask. The medium was replaced every
two days and the primary HPLF cells were cultured. Cells at passage
2–3 were considered to be relatively pure; these primary cells were
identified as fibroblasts in our previous study (13). HPLFs at passage 4–7 were used for
subsequent experiments.
Mechanical tensile strain
Fibroblasts were loaded with mechanical strain using
a four-point bending device (Chengdu Miracle Technology Co., Ltd.,
Chengdu, China), which was divided into three parts: Mechanical
power systems, a host computer and a strain-loading dish. The
deformation displacement, loading frequency and loading time were
set via the host computer. An engine was used to generate a
mechanical force, which exerted strain onto Petri-dishes containing
cells via a stamping motion. Once the Petri-dish was buckled, a
corresponding force was exerted on the cells in the Petri-dish.
Fibroblasts at passage 4–7 were subjected to the
loading strain. Cells were digested with 0.25% trypsin plus EDTA
(Sigma-Aldrich; Merck Millipore). DMEM containing 15% FBS was
subsequently added to the cell pellet, following centrifugation
(200 × g at room temperature for 8 min), to obtain the cell
suspension. The cell suspension (1.5–2×105 cells/ml, 1.5
ml) was evenly spread onto the rat tail collagen-precoated culture
plate (Sigma-Aldrich; Merck Millipore), which was incubated in 5%
CO2 at 37°C for 24 h. Following this, the adherence of
cells was observed. Once the cell volume was ~80% of whole culture
plate, cells were transferred to a strain-loading dish for
mechanical strain testing.
Parameters were set to a frequency of 0.1 Hz and a
duration of 4 h, and cells were subjected to strains of 0 mm
(control group samples), 1,333 µ (1 mm) or 5,333 µ (4 mm). Except
the degree of mechanical tensile strain, all cells received equal
treatment under identical environmental conditions.
Cell proliferation analysis
Following mechanical strain, cells were washed with
PBS two to three times, wiping the edge of the plate. Cells were
digested with 0.25% trypsin plus EDTA, and DMEM containing 15% FBS
was added to the cell pellet following centrifugation (200 × g at
room temperature for 8 min) to obtain the cell suspension, which
was adjusted to 2 million cells/ml. The cell suspension (100
µl/well) was pipetted into a 96-well plate and subsequently
incubated in 5% CO2 at 37°C for 12–24 h. Following this,
Cell Counting kit-8 (CCK-8) solution (10 µl/well; Beyotime
Institute of Biotechnology, Shanghai, China) was added to each well
and incubated in 5% CO2 at 37°C for ~2 h. Finally, the
optical density was measured at a wavelength of 450 nm using a
microplate reader (Victor 3; Perkin-Elmer, Waltham, MA, USA).
Immunofluorescence imaging
Following mechanical stress, cells were washed with
PBS and digested with 0.25% trypsin plus EDTA. DMEM containing 15%
FBS was added to the cell pellet following centrifugation (200 × g
at room temperature for 8 min) to obtain the cell suspension. The
cell suspension (1 ml) was seeded into a 24-well plate at the
density of 1.5×105 cells/ml and incubated for 8 h in 5%
CO2 at 37°C. Following this, cells were washed three
times with PBS. Paraformaldehyde phosphate buffer (Wuhan Servicebio
Technology Co., Ltd., Wuhan, China) was subsequently used to fix
cells. Cells were again washed three times with PBS, for 5 min each
time. Phalloidin (Wuhan Servicebio Technology Co., Ltd.) was
diluted to a concentration of 5 µg/ml using 1% bovine serum albumin
(Wuhan Servicebio Technology Co., Ltd.), added to each well (100
µl/well) and plates were incubated for 30 min at room temperature.
Following this, cells were washed three times with PBS. DAPI (5
µg/ml, 100 µl/well) was subsequently added to each well and
incubated for 5 min at room temperature for nuclear staining. Cells
were again washed three times with PBS, and a 2.5% glycerol
mounting medium was added. Cell cytoskeletons were imaged using a
fluorescence microscope (CKX31; Olympus Corporation, Tokyo,
Japan).
Mitochondrial morphology
observation
Primary cultured cells (passage 4–7) were seeded
into three plates precoated with rat tail collagen (Sigma-Aldrich;
Merck Millipore), and incubated at 5% CO2 and 37°C for
~24 h. Cells were subjected to a force of 0 mm (control group
samples), 1,333 µ (1 mm) or 5,333 µ (4 mm) at a fixed frequency of
0.1 Hz for 4 h. Following this, cells were washed with PBS and
transferred into a clean dish for treatment. A mixture of 2.5%
glutaraldehyde (Servicebio of Technology) and DMEM (ratio, 1:1; 2
ml) was added to cells for 2 min. Cells were subsequently
centrifuged at 157 × g for 8 min at room temperature. The
supernatant was discarded and 2.5% glutaraldehyde solution (2 ml)
was added to the cell pellet for fixation.
Following this, PBS was used to wash the cells and
1% osmium tetroxide (Wuhan Servicebio Technology Co., Ltd.) was
added for fixation. Cells were dehydrated in ethanol and embedded
in epoxy resin (Wuhan Servicebio Technology Co., Ltd.) for
sectioning. Sections were stained with lead citrate and uranyl
acetate. Alterations in mitochondrial morphology were imaged
(magnification, ×5,000) using a Hitachi transmission electron
microscope (HT7700; Hitachi, Ltd., Tokyo, Japan).
Cell senescence
Cell senescence was assessed using the Senescence
β-Galactosidase Staining kit (Beyotime Institute of Biotechnology).
Following mechanical strain, cells were washed with PBS and
transferred to a clean dish. β-galactosidase dye fixing solution (1
ml) was added to cells for 15 min at room temperature. Following
this, cells were washed three times with PBS, and 1 ml working
fluid dye (10 µl β-galactosidase staining solution A, 10 µl
β-galactosidase staining solution B, 930 µl β-galactosidase
staining solution C and 50 µl X-Gal solution) was added. Cells were
incubated at 37°C overnight. Images were obtained using a light
microscope (BX51, Olympus Corporation, Tokyo, Japan).
Statistical analysis
Statistical analyses were performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA), and data are presented here as the
mean ± SD, and groups were compared using one-way analysis of
variance. Differences between two groups were determined using
Student's t-test, and multiple comparison tests were performed
using Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Primary culture and identification of
HPLFs
A previous study by this group identified primary
cultured HPLFs as fibroblasts by immunohistochemical staining
(13). The purity of fibroblasts
may reach >90% at passage 2–3. The fibroblasts primarily
appeared to possess long spindles and were closely connected when
observed by light microscopy. Under mechanical stress conditions,
cell morphology was altered and cell connections appeared weakened
(8).
Mechanical stress reduces cell
viability
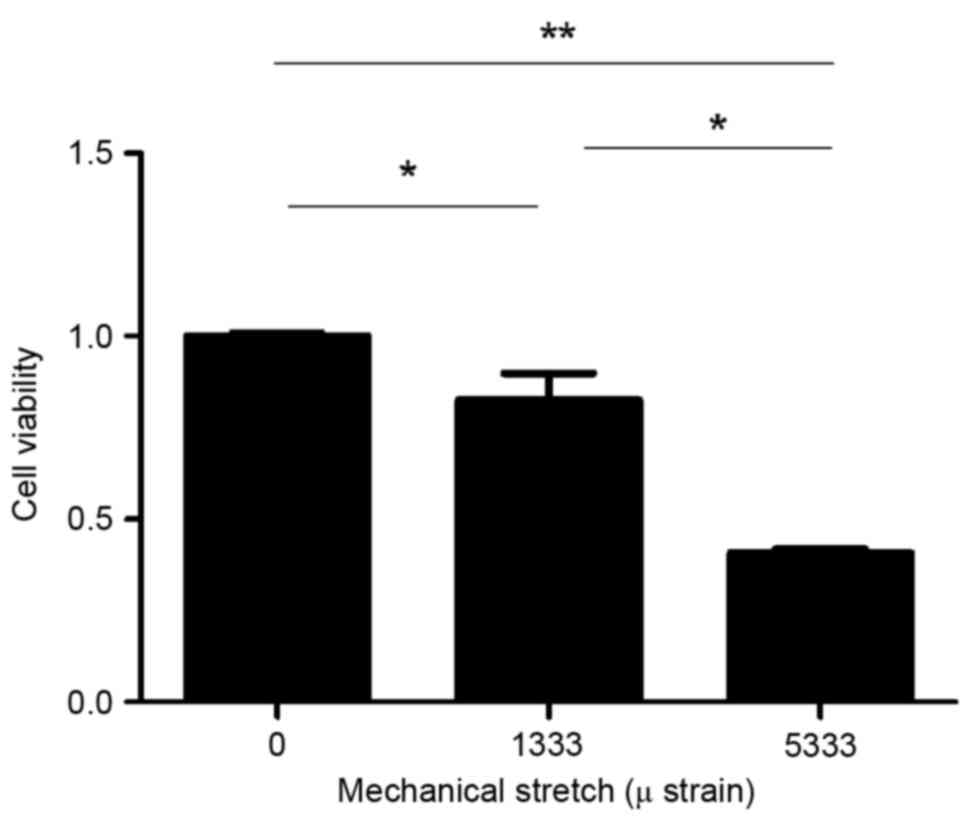
The present study used CCK-8 to detect the activity
and proliferation of fibroblasts. Cell viability decreased when
subjected to mechanical force (Fig.
1). The optical density values following exposure to strains of
0, 1,333 or 5,333 µ were 1.112±0.12, 0.88±0.09 and 0.46±0.02,
respectively. The 1,333 and 5,333 µ groups demonstrated
significantly reduced cell viability compared with the control
group (P=0.02 and P=0.000089, respectively; Fig. 1). In addition, cell viability was
reduced in the 5,333 µ group compared with the 1,333 µ group
(P=0.001; Fig. 1), indicating that
cell viability decreased with increasing mechanical force.
Mechanical stress causes cytoskeleton
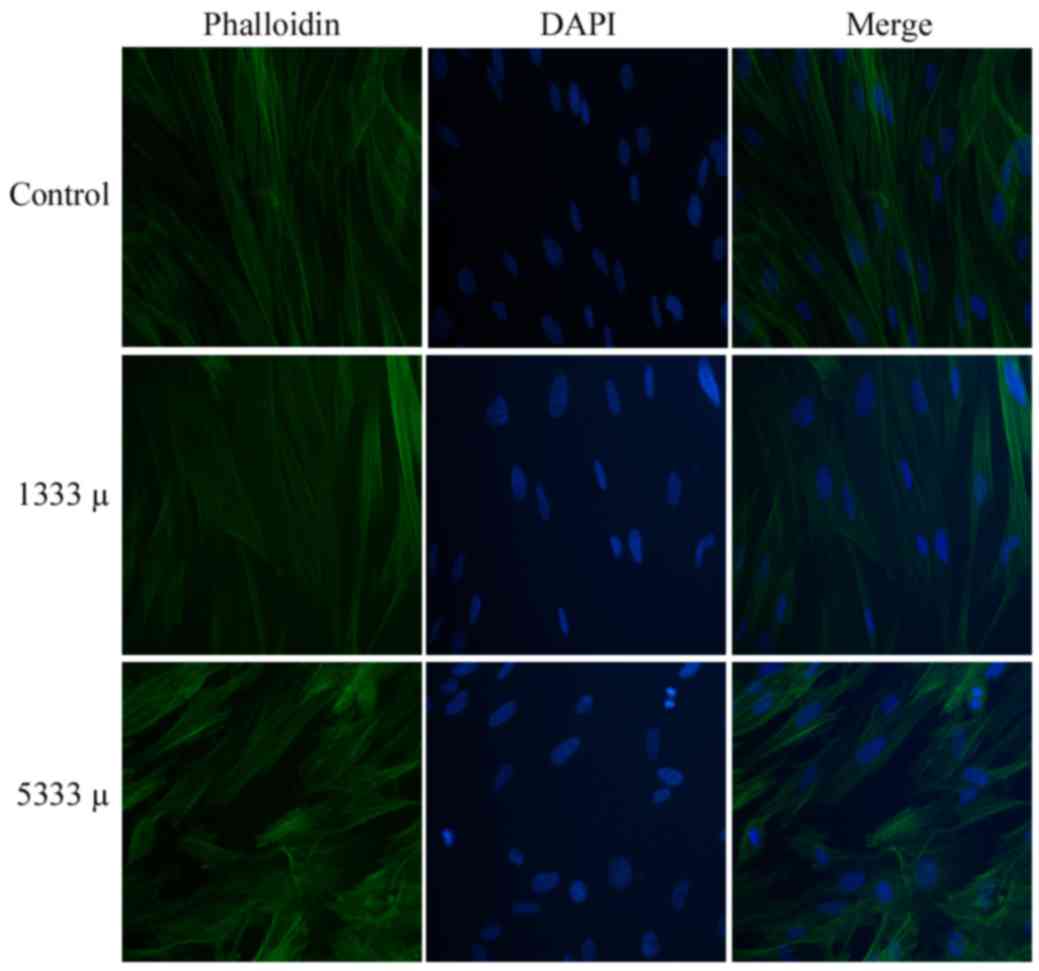
rearrangement
In the control group, cells typically possessed long
spindles, close connections to each other and uniformly distributed
green fluorescence (Fig. 2, top
row). Following mechanical stress loading of 1,333 (Fig. 2, middle row) or 5,333 µ (Fig. 2, bottom row), the cells
demonstrated a weaker green fluorescence signal, sparser, thinner
and maldistributed fasciculi, and a shrunken cell morphology. The
cell cytoskeleton revealed depolymerization and rearrangement as
mechanical stress increased, and cells subjected to the greatest
strain (5,333 µ) demonstrated a more disordered structure.
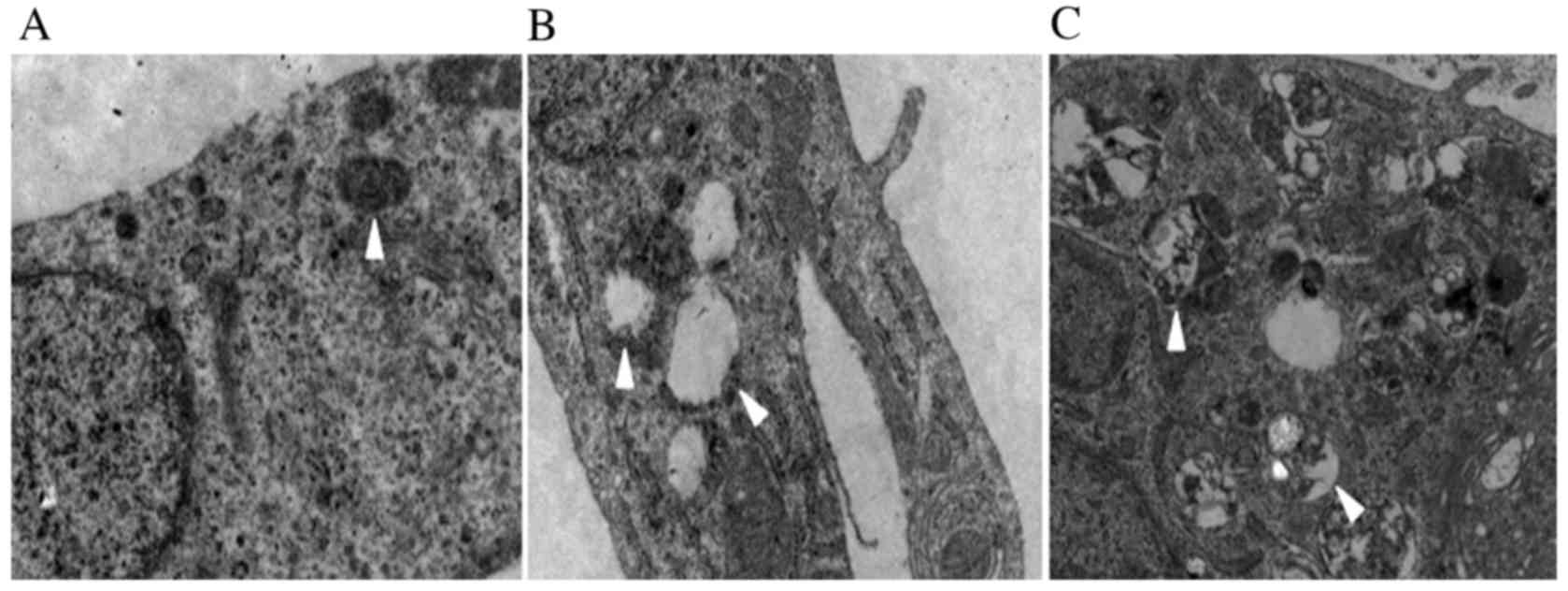
Mechanical stress alters mitochondrial
morphology
The electron microscopy images revealed that
mechanical force caused vacuolization of the fibroblast
mitochondria (Fig. 3). Compared
with the control group, the 1,333 µ group demonstrated
disappearance of mitochondrial crista and the appearance of
mitochondrial vacuoles (Fig. 3B).
When the mechanical stress increased to 5,333 µ, the complete
structure of the cell was destroyed, and apoptotic bodies were
observed (Fig. 3C).
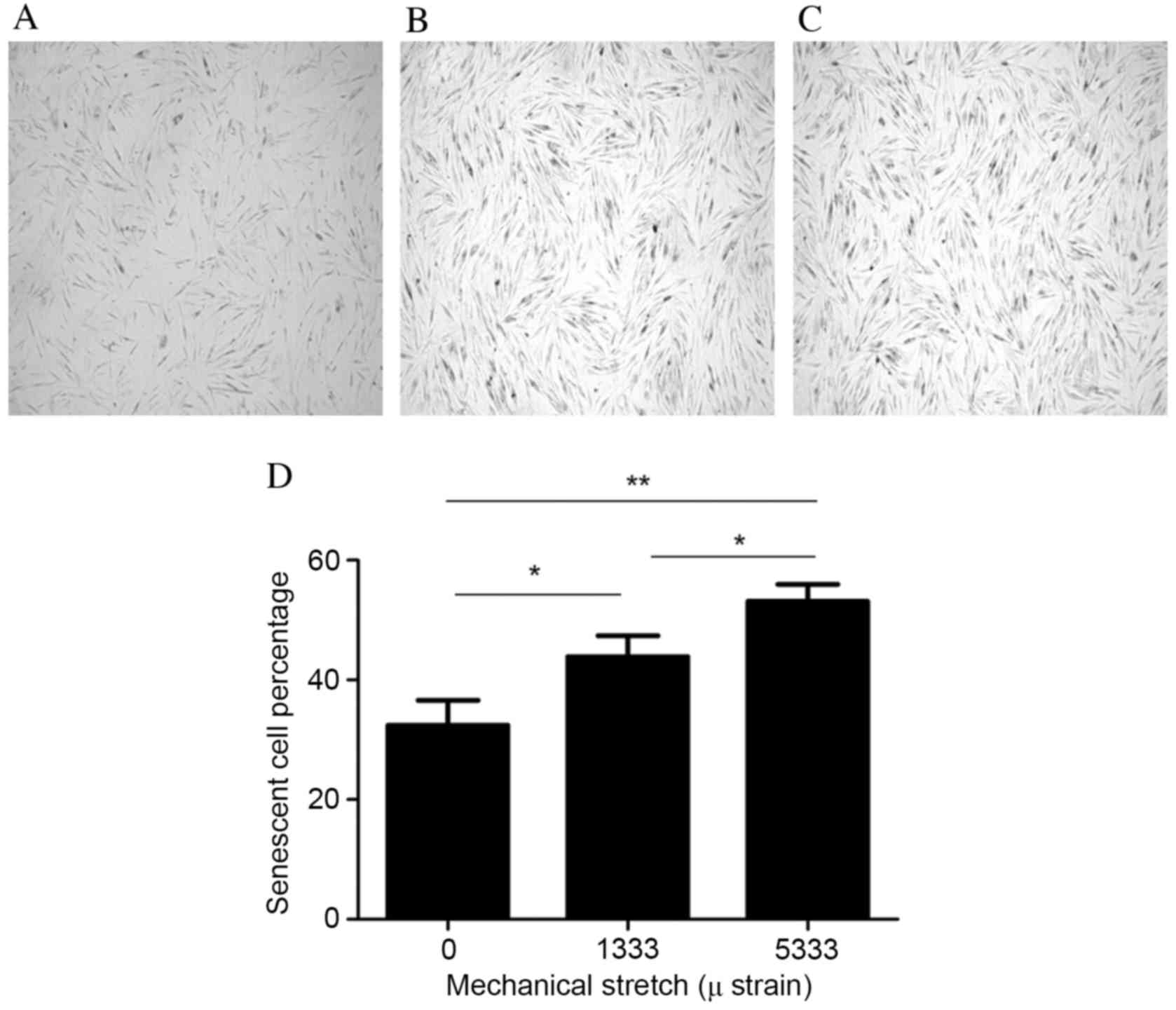
Mechanical stress increases cell
senescence
The present study used the β-Galactosidase Staining
kit to detect the cell senescence of fibroblasts. Blue staining
indicated cell senescence, with the number of stained cells
increasing as the mechanical force increased (Fig. 4A-C). Cells were imaged using a
light microscope (magnification, ×40) and the number of senescent
cells/300 cells was counted and the percentage calculated. The
senescent cell percentages following exposure to strains of 0, 1333
and 5,333 µ were 32.41±4.17, 43.89±3.47 and 53.14±2.85%,
respectively (Fig. 4D). The
percentage of senescent cells therefore increased with increasing
mechanical force. The 1,333 and 5,333 µ groups demonstrated greater
cell senescence compared with the control group (P=0.0017 and
P=0.001, respectively; Fig. 4D).
In addition, there was a significantly greater percentage of
senescent cells in the 5,333 µ group compared with the 1,333 µ
group (P=0.042; Fig. 4D).
Discussion
POP is a common disease in middle-aged and elderly
women, which may seriously affect the physical health, mental
wellbeing and quality of life of patients (14). The human parametrial ligament is
the primary ligament used to maintain the normal position of the
uterus, with the tissue comprising of cells and the extracellular
matrix. A decrease in the mechanical properties of the human
parametrial ligament may lead to POP. HPLFs respond to mechanical
stimulation by synthesizing and secreting fluid into the
extracellular matrix. Therefore, the present study examined
cytomechanics by subjecting cells to external mechanical loading to
simulate the internal environment. Whether mechanical stress
influences cell proliferation, the cytoskeleton, mitochondrial
alterations and cell senescence in HPLFs was investigated by
inducing oxidative stress, a factor hypothesized to contribute to
the development of POP. Based on our previous findings, the
parameters of mechanical strain loading were set to a frequency of
0.1 Hz for 4 h, and cells were subjected to strains of 0 mm, 1,333
µ (1 mm) and 5,333 µ (4 mm), to investigate the effect of
mechanical force on cell injury.
Cell proliferation ability following mechanical
loading was detected using a microplate reader at a wavelength of
450 nm, which indicates the number of living cells. The present
study demonstrated that cell viability decreased as mechanical
force increased. As cell viability is an important indicator of the
cellular state, this indicated that the mechanical force was
damaging to HPLFs. In addition, cytoskeletal alterations were
detected by immunofluorescence imaging, using phalloidin staining.
The cytoskeleton is important for maintaining cell morphology, and
consists of microtubules, microfilaments and intermediate
filaments. Microfilaments are spiral structures composed of actin
subunits, which include F- and G-actin, in dynamic equilibrium.
Actin provides structural support to the cells and reacts to
mechanical alterations in the surrounding environment. Lozupone
et al (15) and Vico et
al (16) revealed that cyclic
environmental alterations may affect the structure and function of
the cytoskeleton, including cytoskeletal reorganization,
cytoskeletal fracture, cell dysfunction and cell death. F-actin is
an important component of the cytoskeleton, and its integrity is a
key factor in determining cell function (17). The present study demonstrated that
the cell cytoskeleton depolymerized and rearranged with increasing
mechanical force, indicating that mechanical stress may lead to
F-actin damage in HPLFs.
Transmission electron microscopy was used to observe
the intracellular structure and mitochondrial alterations in
fibroblasts. These data demonstrated that mechanical loading may
damage mitochondrial morphology. Particularly at 5,333 µ, the
internal structure of HPLFs was destroyed and apoptotic bodies were
observed. Mitochondria are the metabolic centers of eukaryotic
cells, providing basic energy to numerous types of cellular
activities. They are a key factor in determining cell survival and
death, and serve an important role in the transduction and
expansion of death signals (18).
In addition, mitochondria are the primary production site of active
oxygen, and exhibit the clearest alterations and damage when cells
are suffering from oxidative stress injury. Furthermore,
mitochondria serve a vital role in the metabolism of free radicals,
which are a further indication of cell oxidative stress. The
present study revealed that an increase in mechanical force was
associated with greater damage to the mitochondria of fibroblasts,
consistent with a previous study, which demonstrated that
mechanical loading may lead to elevated levels of intracellular
oxidative stress (13). Therefore,
it may be hypothesized that increased mechanical force alters
mitochondrial morphology and structure by increasing the level of
oxidative stress, thus affecting the function of cells. In future
studies, it may be useful to further investigate the oxidative
stress model, to understand whether oxidative stress may lead to
structural alterations in mitochondria.
The level of cell senescence was detected using the
β-galactosidase staining kit. It was revealed that the percentage
of cell senescence increased as mechanical force increased. The
underlying mechanisms of cellular senescence primarily include the
external oxidative stress theory and the intrinsic gene regulation
theory. When the body is in a state of oxidative stress, the high
concentration of ROS may mediate cellular senescence by regulating
associated pathways. Potential other pathways involved in oxidative
stress-induced cellular senescence include the DNA-damage-response,
nuclear factor-κB, mitogen-activated protein kinase and microRNA
pathways (19,20). Previous studies have indicated that
alterations in mitochondrial structure and function are closely
associated with cellular senescence (21). Furthermore, mitochondrial
respiratory function, free radical scavenging ability and
mitochondrial DNA mutations may mediate cell senescence (22). Therefore, it was hypothesized that
the increase in cellular senescence, induced by increasing
mechanical force, may be a result of damage to mitochondrial
structures in the cell.
In conclusion, the present study investigated the
effect of mechanical force on HPLFs at the cellular level. It was
demonstrated that, with an increase of mechanical stress loading
within a specific range, HPLFs began to exhibit indicators of
damage, including decreased cell proliferation, increased
cytoskeletal and mitochondrial injury, and increased cell
senescence. These data provide a theoretical basis for further
investigation into the underlying mechanisms of POP.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81270684) and the
Foundation of Collaborative and Innovation Projects of Wuhan
University School of Medicine (grant no. 523-266078).
References
|
1
|
O'Boyle AL, O'Boyle JD, Calhoun B and
Davis GD: Pelvic organ support in pregnancy and postpartum. Int
Urogynecol J Pelvic Floor Dysfunct. 16:69–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gyhagen M, Bullarbo M, Nielsen TF and
Milsom I: Prevalence and risk factors for pelvic organ prolapse 20
years after childbirth: A national cohort study in singleton
primiparae after vaginal or caesarean delivery. BJOG. 120:152–160.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rodrigues AM, De Oliveira LM, Kde F
Martins, Del Roy CA, Sartori MG, Girão MJ and Castro Rde A: Risk
factors for genital prolapse in a Brazilian population. Rev Bras
Ginecol Obstet. 31:17–21. 2009.(In Portuguese). PubMed/NCBI
|
|
4
|
Jones KA and Moalli PA: Pathophysiology of
pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 16:79–89.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gutierrez C, Corbera JA, Morales I,
Morales M and Navarro R: Uterine prolapse in 2 dromedary camels.
Can Vet J. 42:803–804. 2001.PubMed/NCBI
|
|
6
|
Kim EJ, Chung N, Park SH, Lee KH, Kim SW,
Kim JY, Bai SW and Jeon MJ: Involvement of oxidative stress and
mitochondrial apoptosis in the pathogenesis of pelvic organ
prolapse. J Urol. 189:588–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li BS, Hong L, Min J, Wu DB, Hu M and Guo
WJ: The expression of glutathione peroxidase-1 and the anabolism of
collagen regulation pathway transforming growth
factor-beta1-connective tissue growth factor in women with uterine
prolapse and the clinic significance. Clin Exp Obstet Gynecol.
40:586–590. 2013.PubMed/NCBI
|
|
8
|
Hong S, Li H, Wu D, Li B, Liu C, Guo W,
Min J, Hu M, Zhao Y and Yang Q: Oxidative damage to human
parametrial ligament fibroblasts induced by mechanical stress. Mol
Med Rep. 12:5342–5348. 2015.PubMed/NCBI
|
|
9
|
Lu M, Gong X, Lu Y, Guo J, Wang C and Pan
Y: Molecular cloning and functional characterization of a
cell-permeable superoxide dismutase targeted to lung adenocarcinoma
cells. Inhibition cell proliferation through the Akt/p27kip1
pathway. J Biol Chem. 281:13620–13627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matthews BD, Overby DR, Alenghat FJ,
Karavitis J, Numaguchi Y, Allen PG and Ingber DE: Mechanical
properties of individual focal adhesions probed with a magnetic
microneedle. Biochem Biophys Res Commun. 313:758–764. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kapeta S, Chondrogianni N and Gonos ES:
Nuclear erythroid factor 2-mediated proteasome activation delays
senescence in human fibroblasts. J Biol Chem. 285:8171–8184. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bao R, Liu X and Zhou J: Primary culture
and identification of human fibroblasts. J XinX Med Coll.
5:0152011.(In Chinese).
|
|
13
|
Ding WJ, Fang G, Hong Li, Hong SS, Hu M,
Min J, Wu DB, Yang Q, Zhang XH and Zhao Y: Effects of oxidative
damage in human parametrial ligament fibroblasts induced by
mechanical stress. Chinese Journal of Clinicians. 23:10775–10779.
2013.(In Chinese).
|
|
14
|
Chen Y and Yuan M: Progress in research on
pathogenesis of pelvic organ prolapsed. Chinese Journal of Woman
and Child Health Research. 19:507–509. 2008.(In Chinese).
|
|
15
|
Lozupone E, Favia A and Grimaldi A: Effect
of intermittent mechanical force on bone tissue in vitro:
Preliminary results. J Bone Miner Res. 7 Suppl 2:S407–S409. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vico L, Lafage-Proust MH and Alexandre C:
Effects of gravitational changes on the bone system in vitro and in
vivo. Bone. 22 Suppl 5:95S–100S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stricker J, Falzone T and Gardel ML:
Mechanics of the F-actin cytoskeleton. J Biomech. 43:9–14. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sims NR and Muyderman H: Mitochondria,
oxidative metabolism and cell death in stroke. Biochim Biophys
Acta. 1802:80–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rai P, Onder TT, Young JJ, McFaline JL,
Pang B, Dedon PC and Weinberg RA: Continuous elimination of
oxidized nucleotides is necessary to prevent rapid onset of
cellular senescence. Proc Natl Acad Sci USA. 106:169–174. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ito K, Hirao A, Arai F, Takubo K, Matsuoka
S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y and Suda T:
Reactive oxygen species act through p38 MAPK to limit the lifespan
of hematopoietic stem cells. Nat Med. 12:446–451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Booth FW: Perspectives on molecular and
cellular exercise physiology. J Appl Physiol (1985). 65:1461–1471.
1988.PubMed/NCBI
|
|
22
|
Lustbader JW, Cirilli M, Lin C, Xu HW,
Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, et al:
ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's
disease. Science. 304:448–452. 2004. View Article : Google Scholar : PubMed/NCBI
|