Introduction
Angiogenesis is an essential process in fracture
healing, and defective blood supply at the fracture site is a
primary consideration in cases of non-union. Several factors are
involved in angiogenesis during the osseous repair cascade in a
temporospatial manner, however, vascular endothelial growth factor
(VEGF) is of primary importance (1). During facture healing, VEGF is
released or secreted from different cells, of which osteoblasts
have been extensively investigated although the mechanism is
complicated. Zhu et al (2)
revealed that activating transcription factor 4 is a novel
regulator of the VEGF secretion axis in osteoblasts, whereas Rui
et al reported that the intracellular scaffold G
protein-coupled receptor kinase interacting factor-1 also mediates
the release of VEGF from osteoblasts (3). However, previous studies have
investigated the role of osteoblasts in the expression and
secretion of VEGF (4–6), the mechanism remains to be fully
elucidated.
Brain-derived neurotrophic factor (BDNF) is a small
basic protein, which is a member of the neurotrophin family of
growth factors. BDNF is important in neural development and
functions through the activation of its specific receptor,
tropomyosin-related kinase B (TrkB) (7,8). In
addition, several reports have demonstrated the critical effects of
BDNF and TrkB in non-neural cells or tissues, including endothelial
cells (ECs), chondrosarcoma cells, intervertebral disc degeneration
and cardiac contraction (9–12).
With respect to osteoblasts, the function of BDNF/TrkB signaling is
diverse. For example, Pinski et al (13) found that TrkB is associated with
cell apoptosis, whereas another study characterized the rs6265
polymorphism in BDNF, which regulates its differentiation and
osteoblastic activity (14). In
addition, BDNF/TrkB is involved in fracture healing. A previous
study demonstrated that BDNF is localized at high levels in
osteoblast-like cells through use of a mouse fracture healing model
(15). In human fracture healing,
another study indicated that BDNF and TrkB are expressed in
osteoblast-like cells, and are involved in vessel formation and the
osteogenic process (16). Although
rodent and human histological examinations have revealed that
BDNF/TrkB is involved during fracture healing, the underlying
mechanism remains to be elucidated.
Several reports have shown that BDNF can promote
VEGF-mediated angiogenesis. Silencing of the BDNF gene in multiple
myeloma inhibits osteolytic bone destruction, and reduces
angiogenesis and tumor burden (17). ECs expressing high levels of BDNF
can facilitate tumor angiogenesis and growth (18). However, in different cells, the
downstream signaling molecules of the BDNF axis involved in
regulating the secretion of VEGF vary considerably. In
chondrosarcoma cells, BDNF increases the expression of VEGF and
angiogenesis through the TrkB/phospholipase C (PLC) signaling
pathway, whereas in human umbilical vein ECs, BDNF promotes
angiogenic tube formation through the generation of oxidative
stress (9,11). At present, the role of BDNF in the
expression of VEGF in osteoblasts remains to be fully elucidated.
In the present study, it was demonstrated that BDNF promoted the
expression of VEGF in osteoblasts via activation of the
TrkB/extracellular signal-regulated kinase (ERK)1/2 signaling
pathway. These results indicated that BDNF is important in fracture
healing via VEGF-mediated angiogenesis; thus, it may be a novel
therapeutic target for the treatment of fracture non-union.
Materials and methods
Reagents
Anti-rat phosphorylated (p)-ERK1/2, ERK1/2 and
anti-phosphotyrosine 4G10 antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Anti-rat TrkB
antibody, PD98059 and K252a were purchased from Abcam (Cambridge,
UK). All secondary antibodies used in the present study were
purchased from Beyotime Institute of Biotechnology (Shanghai,
China). BDNF was purchased from Peprotech, Inc. (Rocky Hill, NJ,
USA).
Isolation, culture and stimulation of
rat osteoblasts
A total of 16 Sprague Dawley (SD) rats (age, 1–2
days; male/female, 1:1) were obtained from the Department of Animal
Science, Nanjing Medical University (Nanjing, China). Neonatal rats
were sacrificed by being submerged in 75% ethanol for 20 min. The
neonatal rats were housing with the temperature 18–26°C and the
humidity was 40–70% in dark and quiet conditions and fed by their
maternal rats. The present study was approved by the Ethics
Committee of The Affiliated Drum Tower Hospital of Nanjing
University Medical School (Nanjing, China). Primary neonatal
calvarial osteoblasts were isolated from the rats according to a
previously described method with minor modification (3). Briefly, under aseptic conditions, the
calvaria of the 1–2-day-old SD rats were dissociated and digested
in 0.05% trypsin and 0.1% collagenase (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) for 10 min at 37.8°C in an incubator with
5% CO2. Following termination of digestion, the cell
suspensions were passed through a 70 µm mesh Falcon nylon filter.
The filtered medium was centrifuged at 250 × g for 10 min at
20°C and resuspended in Dulbecco's modified Eagle's Medium (DMEM;
Thermo Fisher Scientific, Inc.). The cells were grown in DMEM
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), 100 IU/ml penicillin and 100 IU/ml streptomycin,
and incubated at 37°C in a 5% CO2 atmosphere. Confluent
cells at a density of 5.0×104/ml were passaged in medium
with 0.25% trypsin and EDTA every 3 days and allowed to grow to
confluence. At the third generation, BDNF was added to the medium
only following serum starvation for 30 min at concentrations of 0,
5, 25, 50, 100 and 200 ng/ml and durations of 0, 3, 6, 2, 24 and 48
h for VEGF detection and 0, 0.5, 1, 2, 5 and 10 min for kinase
assay. K252a (200 nM) or PD98059 (25 µM) was added to the medium 30
min prior to the stimulation with BDNF. An equal quantity of
dimethyl sulfoxide was used for incubation of the cells as a
solvent control.
Immunoblotting and
immunoprecipitation
The cells were lysed and proteins were extracted
using RIPA buffer. The protein concentration was quantified using
the Bradford method (19).
Proteins (10 µg) were separated by 10% SDS-polyacrylamide gel, and
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were incubated overnight at 4°C
with the following primary antibodies: ERK1/2 (cat. no. 4695;
1:1,000), p-ERK1/2 (cat. no. 4377; 1:1,000), 4G10 (cat. no. 9411;
1:500); TrkB (cat. no. ab5372; 1:2,000). This was followed by the
addition of a horseradish peroxidase-linked secondary antibodies
(cat. nos. A0208, A0216; 1:1,000) at 37°C for 2 h, and
electrochemiluminescence visualization of the bands (Beyotime
Institute of Biotechnology). Quantification of the bands was
performed using Quantity One densitometric analysis software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). For
immunoprecipitation, the cell lysate was centrifuged at 10,000 ×
g for 10 min at 4°C. A proportion of the lysate (0.5 mg) was
then incubated with 2 µg primary antibody overnight at 4°C on a
rocker platform. Protein A/G-agarose (Santa Cruz Biotechnology
Inc., Dallas, TX, USA) was added and mixed for 2 h at 4°C. The
precipitate was isolated following centrifugation of the mixture at
1,000 × g for 30 sec at 4°C and discarding the supernatant.
The following steps performed were similar to those described above
for western blot analysis.
Immunofluoresence
Coverslips were washed with phosphate-buffered
saline (PBS) three times and then fixed in −20°C cold methanol for
20 min. Following washing with PBS three times, the cells at a
density of 1.0×104/ml were permeated with 0.3% Triton
X-100 for 10 min, followed by blocking with PBS at 37°C containing
10% FBS for 1 h. The coverslips were incubated at 4°C overnight
with TrkB antibody, which was followed by incubation in fluorescein
isothiocyanate (1:100) at 37°C for 1 h. The cells were visualized
using a confocal microscope.
Detection of VEGF content
The osteoblasts were cultured in 24-well plates. To
investigate the downstream signaling pathways, BDNF treatment was
performed; cells at a density of 5.0×105/ml were
pretreated with inhibitors (K252a, 200 nM at 37°C; PD98059, 25 μM
at 37°C) for 30 min prior to the addition of 50 ng/ml BDNF for 12
h. The medium was extracted and examined immediately. A VEGF
(colorimetric ELISA) assay kit (ExCell Biological Products Co.,
Ltd., Shanghai, China) was used to determine the VEGF content in
the culture medium according to the manufacturer's protocol.
Statistical analysis
The data are shown as the mean ± standard error of
the mean. All experiments were repeated three times independently.
Student's t-test or one-way analysis of variance were used to
compare the differences among the groups using SPSS version 16.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
BDNF promotes the expression and
release of VEGF from osteoblasts
ELISA and immunoblotting were utilized to detect the
expression and secretion of VEGF. To evaluate the time-dependent
effect, 50 ng/ml of BDNF was used to stimulate osteoblasts for 0,
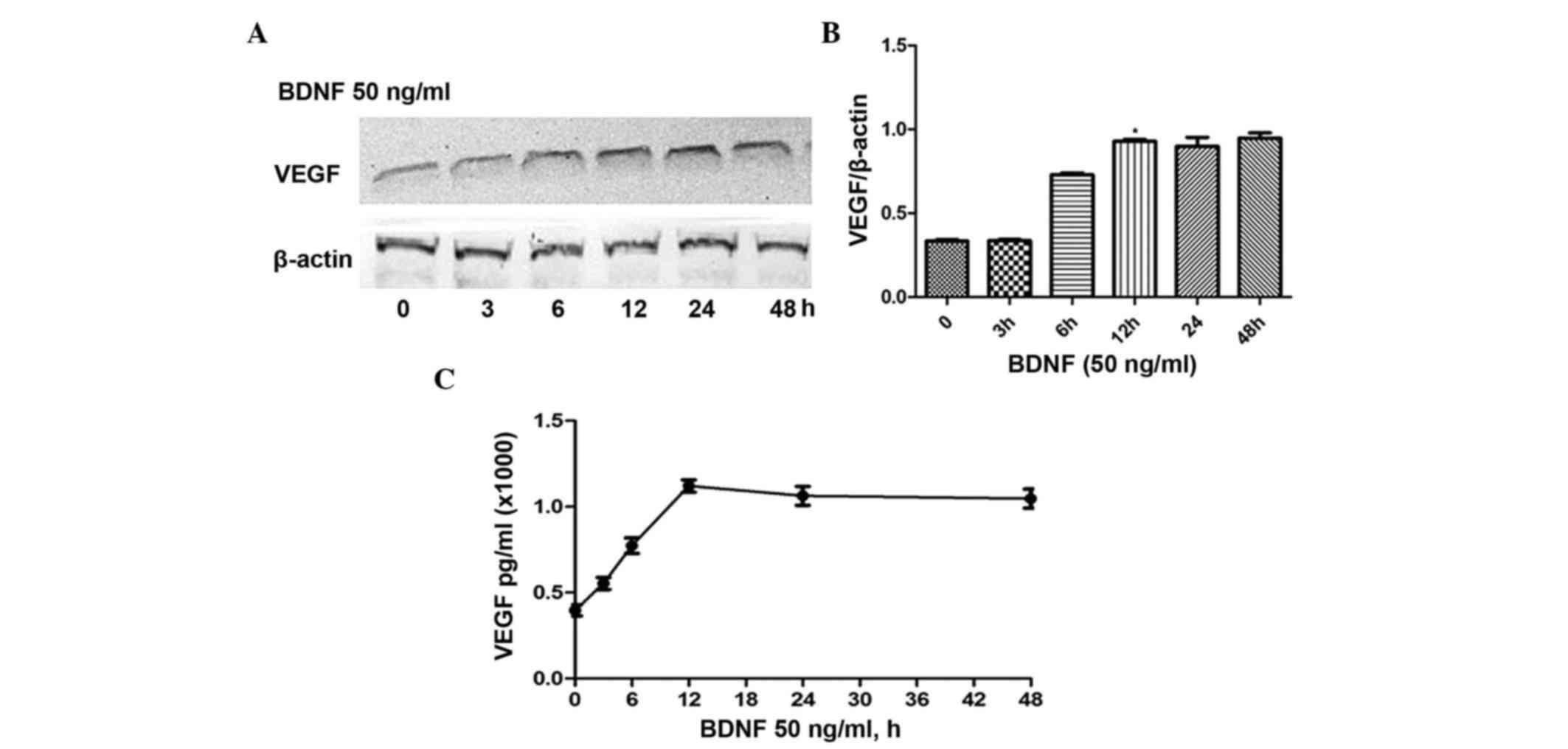
3, 6, 12, 24 and 48 h. As shown in Fig. 1A, the expression of VEGF gradually
increased and peaked at 12 h. No significant differences were found
in expression levels at 12, 24 or 48 h (Fig. 1B). Similar to protein expression,
the ELISA results revealed comparable concentrations of VEGF
released in the medium (Fig. 1C).
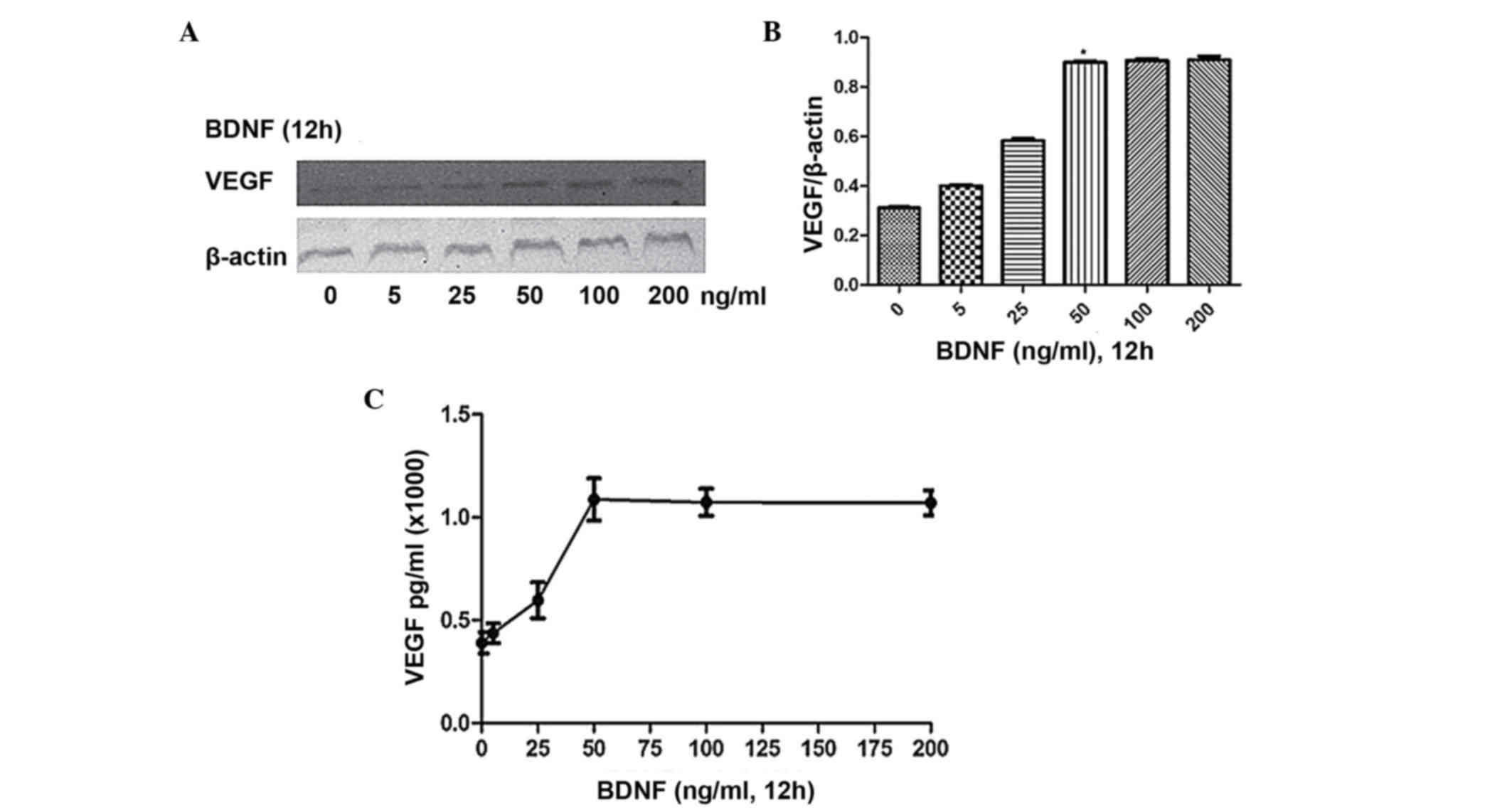
To investigate the dose-dependent effect, the cells were stimulated
for 12 h with BDNF at concentrations of 0, 25, 50, 100 and 200
ng/ml, respectively. As shown in Fig.
2A-C, maximal expression and secretion of VEGF were observed at
a BDNF concentration of 50 ng/ml. Therefore, for subsequent
experiments, a BDNF concentration of 100 ng/ml was used for a
duration of 12 h.
Detection of TrkB in osteoblasts
Immunoblotting and immunofluoresence were performed
to detect the expression and distribution of TrkB in osteoblasts.
The results revealed that TrkB was expressed at a high level in the
cells, predominantly located in the cytoplasm and cytomembrane
(data not shown).
In BDNF-stimulated osteoblasts, the
activities of TrkB and ERK1/2 are increased
To confirm the association between protein kinases
in signaling pathways involved in the BDNF-stimulated release of
VEGF, the present study detected the phosphorylation levels of
ERK1/2 and TrkB separately. BDNF (50 ng/ml) was used to stimulate
the osteoblasts for 0, 0.5, 1, 2, 5 and 10 min, respectively. The
results of the statistical analysis revealed that ERK1/2 (Fig 3A and B) and TrkB (Fig. 3C and D) were rapidly activated
following BDNF stimulation, and peaked at 1 min.
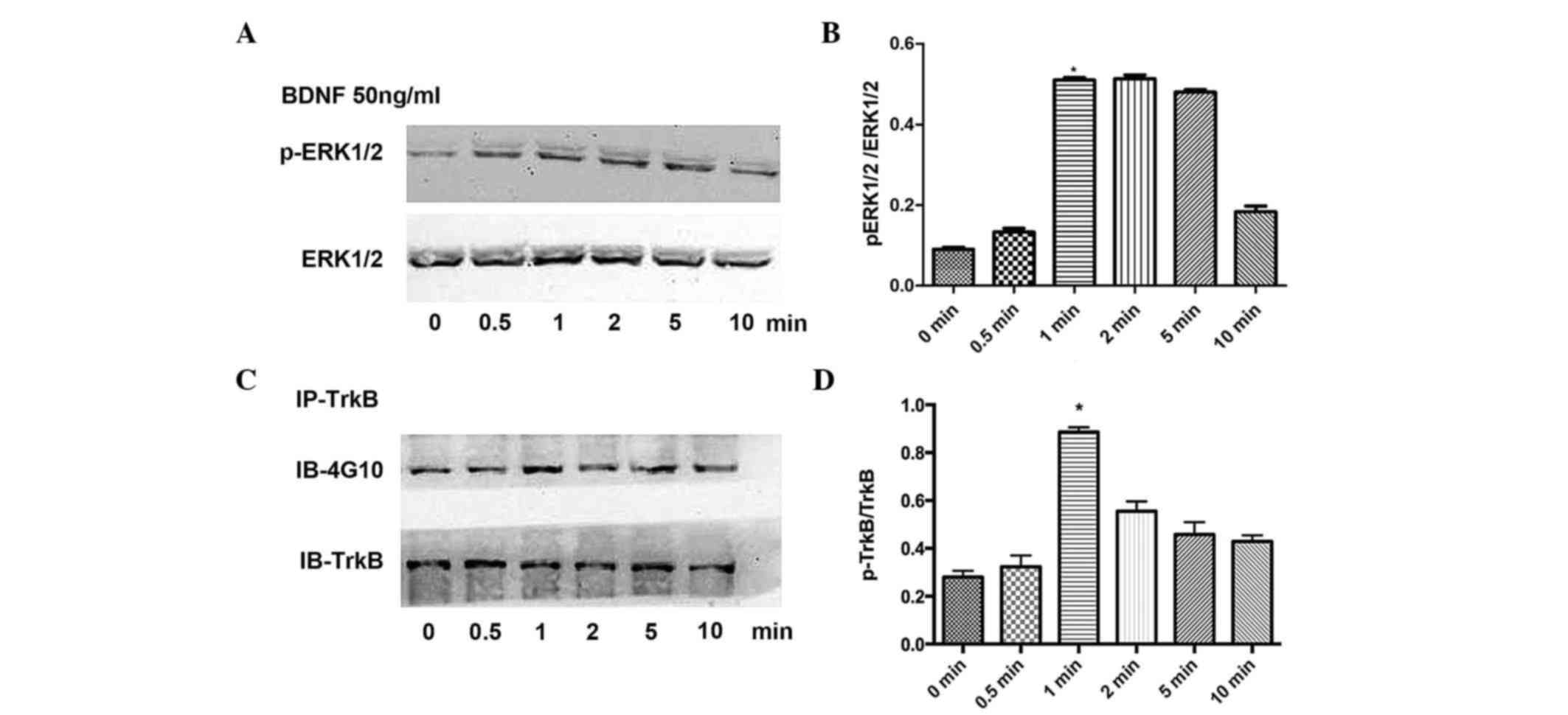 | Figure 3.Effects of BDNF on the phosphorylation
of TrkB and ERK1/2. (A) Effects of BDNF stimulation on the
phosphorylation of ERK1/2 at 0, 0.5, 1, 2, 5 and 10 min with (B)
results of statistical analysis. (C) Effects of BDNF stimulation on
the phosphorylation of TrkB at 0, 0.5, 1, 2, 5 and 10 min with (D)
results of statistical analysis. Data are presented as the mean ±
standard error of the mean. *P<0.05, compared with the control
group. BDNF, brain-derived neurotrophic factor; TrkB,
tropomyosin-related kinase B; ERK, extracellular signal-regulated
kinase; p-, phosphorylated; IP, immunoprecipitation; IB,
immunoblotting. |
TrkB is involved in the regulation of VEGF through
the activation of ERK1/2 in BDNF-stimulated osteoblasts. In the
present study, K252a, a specific inhibitor of TrkB, was used to
pretreat the osteoblasts. The results showed that K252a
significantly inhibited the phosphorylation of ERK1/2 (Fig. 4A and B) and inhibited the
BDNF-induced expression and release of VEGF, (Fig. 4C). To further confirm the role of
ERK1/2, PD98059, the ERK1/2 specific inhibitor, was used for
pretreatment of the cells. The results demonstrated that PD98059
had the same effect on VEGF as K252a, however, no significant
changes were observed in the activity of TrkB (Fig. 4).
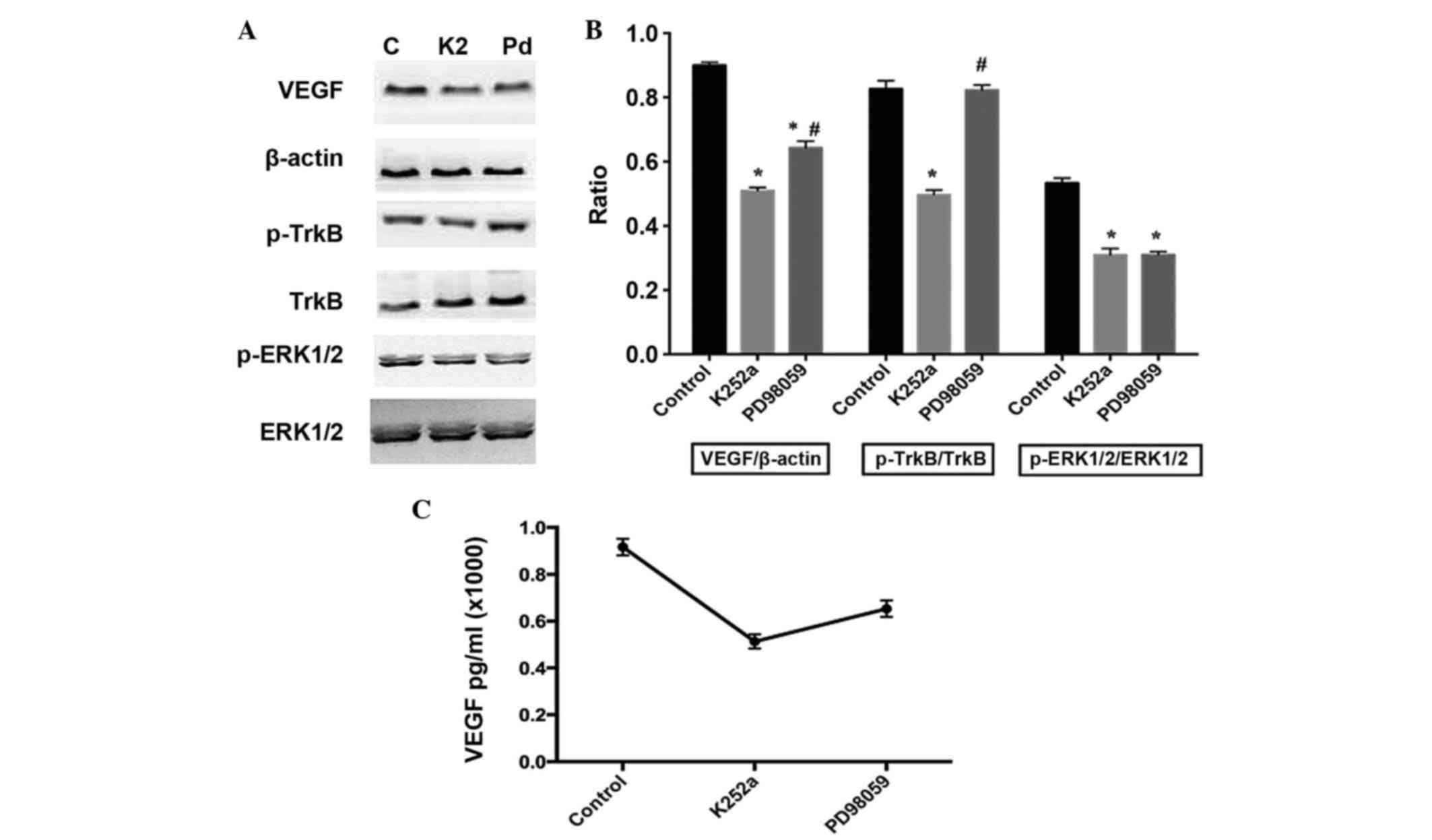 | Figure 4.Effects of K252a and PD98059 on the
phosphorylation of TrkB and ERK1/2, and expression of VEGF in
BDNF-treated osteoblasts. K252a (200 nM) or PD98059 (25 µM) were
respectively added to the culture medium 30 min prior to BDNF
stimulation. The expression of VEGF was detected 12 h following
BDNF stimulation. (A) Expression of VEGF, and activation of TrkB
and ERK1/2 with (B) results of statistical analysis. (C)
Concentrations of VEGF in the media were detected using ELISA. Data
are presented as the mean ± standard error of the mean. *P<0.05,
compared with the control group; #P<0.05, compared
with the K252a-treated group. C, control; K2, K252a; Pd, PD98059;
BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin-related
kinase B; ERK, extracellular signal-regulated kinase; p-,
phosphorylated; VEGF, vascular endothelial growth factor. |
Discussion
The present study investigated the expression and
distribution of TrkB in rat calvarial osteoblasts in vitro,
and confirmed that BDNF regulated the expression and release of
VEGF through activation of the TrkB/ERK1/2 pathway.
Fracture healing is a complex process, which
requires the involvement of several growth factors in a
temporospatial manner. Using a mouse fracture healing model, Asaumi
et al (15) first
demonstrated that BDNF was expressed at high levels and localized
in osteoblast-like cells, and another study found that the mRNA
expression and protein distribution of BDNF and its specific
receptor TrkB were detected in osteoblast-like cells obtained from
fracture gaps in a human healing diaphyseal fracture (16). However, the mechanism of BDNF in
regulating fracture healing remains to be elucidated. The present
study demonstrated that BDNF promoted the expression and secretion
of VEGF from osteoblasts in a time- and dose-dependent manner. VEGF
is one of the most extensively investigated angiogenic growth
factors during fracture repair. Inhibition of the activity of VEGF
by a VEGF antagonist results in impaired healing of femoral
fractures and cortical bone loss in mice (20). In BDNF signaling, several studies
have shown that VEGF is involved through the regulation of
angiogenesis only. For example, Lin et al (9) found that BDNF can promote the
expression of VEGF in human chondrosarcoma cells, and that
knockdown of BDNF significantly reduces the expression of VEGF and
angiogenesis in vivo. In multiple myeloma, the stable
interference of BDNF in multiple myeloma cells significantly
inhibits osteolytic bone destruction, and reduces angiogenesis and
tumor burden (17). However,
compared with multiple myeloma and chondrosarcoma cells, the
effects of BDNF on the promotion of the expression and release of
VEGF from osteoblasts are more evident. Thus, it can be concluded
that BDNF has a positive role in fracture healing by controlling
VEGF-mediated angiogenesis.
For transmitting signals, BDNF interacts with its
receptors, including high affinity TrkB, low affinity p75
neurotrophin receptor (NTR) and integrin α9β1 (7,21).
In the present study, the expression, distribution and function of
TrkB were investigated. Consistent with prior immunohistological
staining (data not shown), TrkB was expressed at a high level in
osteoblasts, and was located predominantly in the cytoplasm and
cytomembrane, and these characteristics made it possible to
transmit the effects of BDNF (15,16).
In addition to TrkB signaling, the p75 NTR and integrin α9β1
pathways are also involved in VEGF-mediated angiogenesis. A
previous study found that p75 (NTR−/−) mice had reduced
hypoxia-inducible factor-1α stabilization, expression of VEGF and
angiogenesis following retinal hypoxia (22), whereas Walsh et al (23) demonstrated that integrin α9β1
promotes pathological angiogenesis in glioma through direct
interaction with neurotrophic factor. Therefore, further
investigations are required to elucidate the roles of p75 NTR and
integrin α9β1, and the interaction among the three receptors, in
VEGF-mediated angiogenesis during fracture healing.
Following binding of BDNF, three downstream cascades
of TrkB can be activated, including PLCγ, AKT and ERK1/2 (7). PLCγ, a member of the PLC
serine/threonine family, has been shown to mediate the expression
of VEGF and angiogenesis in human chondrosarcoma cells following
exposure to BDNF/TrkB, whereas in BDNF-treated ECs, the activation
of Akt is a key step for angiogenic tube formation (9,11).
In the present study, it was demonstrated that the ERK1/2 inhibitor
antagonized the BDNF-mediated expression and release of VEGF,
suggesting that the activation of ERK1/2 is essential in the
BDNF-induced expression and secretion of VEGF in osteoblasts. In
addition, the results of the present and previous studies indicated
that the above three proteins of the BDNF/TrkB cascade may produce
a feedback effect in TrkB signaling (7,24),
although PD98059 had no effect on the level of TrkB phosphorylation
in the present study. Taken together, the results of the present
study provided evidence that BDNF upregulated the expression and
secretion of VEGF in rat calvarial osteoblasts via the TrkB
receptor and ERK1/2 signaling pathways.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that during fracture
healing, BDNF promoted the expression and secretion of VEGF in
osteoblasts through TrkB and subsequent ERK1/2 activation.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81401795 and 81401793), the
Fundamental Research Funds for the Central Universities (grant no.
20620140712) and the Key Project supported by the Medical Science
and Technology Development Foundation, Nanjing Department of Health
(grant no. YKK14076).
References
|
1
|
Beamer B, Hettrich C and Lane J: Vascular
endothelial growth factor: An essential component of angiogenesis
and fracture healing. HSS J. 6:85–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu K, Jiao H, Li S, Cao H, Galson DL,
Zhao Z, Zhao X, Lai Y, Fan J, Im HJ, et al: ATF4 promotes bone
angiogenesis by increasing VEGF expression and release in the bone
environment. J Bone Miner Res. 28:1870–1884. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rui Z, Li X, Fan J, Ren Y, Yuan Y, Hua Z,
Zhang N and Yin G: GIT1Y321 phosphorylation is required for ERK1/2-
and PDGF-dependent VEGF secretion from osteoblasts to promote
angiogenesis and bone healing. Int J Mol Med. 30:819–825.
2012.PubMed/NCBI
|
|
4
|
Steinbrech DS, Mehrara BJ, Saadeh PB, Chin
G, Dudziak ME, Gerrets RP, Gittes GK and Longaker MT: Hypoxia
regulates VEGF expression and cellular proliferation by osteoblasts
in vitro. Plast Reconstr Surg. 104:738–747. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim IS, Song JK, Zhang YL, Lee TH, Cho TH,
Song YM, Kim DK, Kim SJ and Hwang SJ: Biphasic electric current
stimulates proliferation and induces VEGF production in
osteoblasts. Biochim Biophys Acta. 1763:907–916. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen CY, Su CM, Hsu CJ, Huang CC, Wang SW,
Liu SC, Chen WC, Fuh LJ and Tang CH: CCN1 promotes VEGF production
in osteoblasts and induces endothelial progenitor cells
angiogenesis by inhibiting miR-126 expression in rheumatoid
arthritis. J Bone Miner Res. 32:34–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z, Fan J, Ren Y, Zhou W and Yin G:
The release of glutamate from cortical neurons regulated by BDNF
via the TrkB/Src/PLC-γ1 pathway. J Cell Biochem. 114:144–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Z, Zhou W, Fan J, Ren Y and Yin G:
G-protein-coupled receptor kinase interactor-1 serine 419
accelerates premature synapse formation in cortical neurons by
interacting with Ca(2+)/calmodulin-dependent protein kinase IIβ.
Brain Res Bull. 95:70–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin CY, Hung SY, Chen HT, Tsou HK, Fong
YC, Wang SW and Tang CH: Brain-derived neurotrophic factor
increases vascular endothelial growth factor expression and
enhances angiogenesis in human chondrosarcoma cells. Biochem
Pharmacol. 91:522–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Binch AL, Cole AA, Breakwell LM, Michael
AL, Chiverton N, Cross AK and Le Maitre CL: Expression and
regulation of neurotrophic and angiogenic factors during human
intervertebral disc degeneration. Arthritis Res Ther. 16:4162014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Usui T, Naruo A, Okada M, Hayabe Y and
Yamawaki H: Brain-derived neurotrophic factor promotes angiogenic
tube formation through generation of oxidative stress in human
vascular endothelial cells. Acta Physiol (Oxf). 211:385–394. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng N, Huke S, Zhu G, Tocchetti CG, Shi
S, Aiba T, Kaludercic N, Hoover DB, Beck SE, Mankowski JL, et al:
Constitutive BDNF/TrkB signaling is required for normal cardiac
contraction and relaxation. Proc Natl Acad Sci USA. 112:1880–1885.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pinski J, Weeraratna A, Uzgare AR, Arnold
JT, Denmeade SR and Isaacs JT: Trk receptor inhibition induces
apoptosis of proliferating but not quiescent human osteoblasts.
Cancer Res. 62:986–989. 2002.PubMed/NCBI
|
|
14
|
Deng FY, Tan LJ, Shen H, Liu YJ, Liu YZ,
Li J, Zhu XZ, Chen XD, Tian Q, Zhao M and Deng HW: SNP rs6265
regulates protein phosphorylation and osteoblast differentiation
and influences BMD in humans. J Bone Miner Res. 28:2498–2507. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asaumi K, Nakanishi T, Asahara H, Inoue H
and Takigawa M: Expression of neurotrophins and their receptors
(TRK) during fracture healing. Bone. 26:625–633. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kilian O, Hartmann S, Dongowski N, Karnati
S, Baumgart-Vogt E, Härtel FV, Noll T, Schnettler R and Lips KS:
BDNF and its TrkB receptor in human fracture healing. Ann Anat.
196:286–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ai LS, Sun CY, Wang YD, Zhang L, Chu ZB,
Qin Y, Gao F, Yan H, Guo T, Chen L, et al: Gene silencing of the
BDNF/TrkB axis in multiple myeloma blocks bone destruction and
tumor burden in vitro and in vivo. Int J Cancer. 133:1074–1084.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lam CT, Yang ZF, Lau CK, Tam KH, Fan ST
and Poon RT: Brain-derived neurotrophic factor promotes
tumorigenesis via induction of neovascularization: Implication in
hepatocellular carcinoma. Clin Cancer Res. 17:3123–3133. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hammond JB and Kruger NJ: The bradford
method for protein quantitation. Methods Mol Biol. 3:25–32.
1988.PubMed/NCBI
|
|
20
|
Street J, Bao M, deGuzman L, Bunting S,
Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL,
Daugherty A, et al: Vascular endothelial growth factor stimulates
bone repair by promoting angiogenesis and bone turnover. Proc Natl
Acad Sci USA. 99:9656–9661. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Staniszewska I, Sariyer IK, Lecht S, Brown
MC, Walsh EM, Tuszynski GP, Safak M, Lazarovici P and Marcinkiewicz
C: Integrin alpha9 beta1 is a receptor for nerve growth factor and
other neurotrophins. J Cell Sci. 121:504–513. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le Moan N, Houslay DM, Christian F,
Houslay MD and Akassoglou K: Oxygen-dependent cleavage of the p75
neurotrophin receptor triggers stabilization of HIF-1α. Mol Cell.
44:476–490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Walsh EM, Kim R, Del Valle L, Weaver M,
Sheffield J, Lazarovici P and Marcinkiewicz C: Importance of
interaction between nerve growth factor and α9β1 integrin in glial
tumor angiogenesis. Neuro Oncol. 14:890–901. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kommaddi RP, Thomas R, Ceni C, Daigneault
K and Barker PA: Trk-dependent ADAM17 activation facilitates
neurotrophin survival signaling. FASEB J. 25:2061–2070. 2011.
View Article : Google Scholar : PubMed/NCBI
|