Introduction
Osteosarcoma is the most common type of primary
malignant bone cancer in children and adolescents, accounting for
~35% of all bone cancers (1). One
of the most prominent characteristics of osteosarcoma is local
invasiveness and distant metastatic ability, which influences the
therapy options and prognosis of the disease. With the use of
neoadjuvant chemotherapy, the 5-year survival rate for
non-metastasized cases has improved from 60 to 70%. However, the
5-year survival rate of patients with metastasis remains <30%
(2). Thus, novel therapeutic
targets and strategies are required to improve the outcome in
patients with osteosarcoma.
Hypoxia, caused by a limited blood supply from
aberrant neovascularization and a rapidly growing tumor mass, is a
common characteristic of solid tumors (3). Hypoxic conditions are hypothesized to
facilitate tumor progression by activating signaling pathways
involved in cell proliferation, angiogenesis, apoptosis, invasion
and metastasis (4). The
transcription factor hypoxia-inducible factor-1α (HIF-1α) is a key
element in the cellular response to hypoxia and is widely expressed
in a variety of solid tumors, including osteosarcoma (5). Previous studies have demonstrated
that HIF-1α expression levels are significantly associated with
vascular endothelial growth factor and cyclooxygenase-2 expression
levels in osteosarcoma tissues (6). Furthermore, overexpression of HIF-1α
is predictive of poor prognosis in osteosarcoma patients (7). Thus, HIF-1α-targeted therapy may
offer a novel strategy for the treatment of osteosarcoma.
The Notch signaling pathway is an evolutionarily
conserved signaling pathway. The aberrant expression and activation
of Notch signaling has been demonstrated in various types of
malignancies and has been associated with cell proliferation,
survival, apoptosis and differentiation (8). To date, four Notch receptors have
been identified in mammals, Notch1-4. The binding of a ligand
(jagged 1, jagged 2, delta-like 1 or delta-like 4) to cell-surface
Notch1-4 results in the cleavage and nuclear translocation of the
Notch intracellular domain (NICD), leading to the expression of
downstream targeting genes (9). A
previous study indicated that Notch1 is a downstream signaling
component of HIF-1α under hypoxic conditions (10). However, whether Notch1 inhibition
may reverse the effects induced by hypoxia remains to be
clarified.
Curcumin is a biologically active ingredient
abundantly present in the ground rhizomes of Curcuma longa,
which is widely distributed in Southeast Asia (11). The wide range of bioactive
properties of curcumin have been known for a number of years, and
include anti-oxidative (12),
anti-inflammatory (13),
anticoagulative (14) and
anti-atherosclerotic (15)
properties. The antitumor effect of curcumin is of increasing
interest (16–19). It has been reported that curcumin
may exert antitumor effects in normoxic and hypoxic conditions
(4). As hypoxia is a key
characteristic of the tumor microenvironment in osteosarcoma, the
identification of molecules that contribute to the antitumor
effects of curcumin may provide potential therapeutic targets. The
present study investigated the effects of curcumin on the
biological behaviors of osteosarcoma cells in a hypoxic
microenvironment and the potential underlying molecular
mechanisms.
Materials and methods
Cell culture and reagents
The MG-63 human osteosarcoma cell line was purchased
from the American Type Culture Collection (Manassas, VA, USA) and
was authenticated as to genotype and phenotype by the supplier.
Cells were cultured in α-minimum essential medium (MEM) (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% heat-inactivated fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), 100 U/ml ampicillin
(HyClone; GE Healthcare Life Sciences) and 100 µg/ml streptomycin
(HyClone; GE Healthcare Life Sciences) at 37°C in a humidified
atmosphere of 5% CO2 (normoxic conditions). To establish
hypoxic conditions, cells were incubated in a humidified atmosphere
consisting of 3% O2. Curcumin (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was dissolved in dimethyl sulfoxide
(DMSO; Sigma-Aldrich; Merck Millipore). The cells in the control
group were treated with DMSO only.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) was purchased from Sigma-Aldrich; Merck Millipore.
Cell viability assay
MG-63 cells were seeded into 96-well plates at a
density of 5×103 cells per well and treated with various
concentrations (0, 5 or 10 µM) of curcumin. The cells were cultured
under normoxic or hypoxic conditions. At the indicated time points
(12, 24, 36 or 48 h), cell viability was assessed by the MTT assay
according to the manufacturer's protocol, and the absorbance was
measured at a wavelength of 490 nm using a multiwell microplate
reader (Bio-Tek Instruments, Inc., Winooski, VT, USA).
Matrigel invasion assays
Transwell assays were performed using 8-µm Transwell
chambers (EMD Millipore, Billerica, MA, USA) according to a
protocol described previously (4).
Briefly, the membrane in the upper chamber was coated with Matrigel
(25 µg per filter) 12 h prior to use. MG-63 cells were pretreated
with curcumin for 24 h and suspended in α-MEM containing 0.1% FBS.
Cells (5×104) were added to the upper chamber of the Transwell
plates, and the lower compartments were filled with α-MEM
containing 10% FBS. Assays were performed at 37°C in normoxic or
hypoxic (3% O2) conditions. Following incubation for 48
h, the non-invasive cells were removed from the upper surface of
the membrane using a cotton-tipped swab. Invading cells on the
bottom surface of the filter were fixed with methanol and stained
with 0.1% crystal violet. The invading cells were counted in ten
representative fields (magnification, ×200) under a light
microscope (Nikon Corporation, Tokyo, Japan).
Notch1 cDNA transfection
The Notch1 expression plasmid was constructed by
cloning the NICD-1 fragment into a pcDNA3.1 vector using the
following primers: Forward, 5′-CACCATGGTGCTGCTGTCCCGCAAGCGCC-3′ and
reverse, 5′-TGCTTTAAATGCCACAGGAATGTGGG-3′. For overexpression of
Notch1, MG-63 cells (0.5×106/well) were seeded into a 6-well plate
for 24 h and subsequently transfected with empty vector or Notch1
expression plasmid (5 µg cDNA) using Lipofectamine®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were
incubated at 37°C for 24 h and the culture medium was replaced with
fresh medium. After a further incubation for 48 h, cell extracts
were prepared and the expression levels of Notch1 were examined by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blotting.
RT-qPCR
Total RNA was prepared from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. cDNA was
subsequently synthesized from 5 µg RNA using the Takara Reverse
Transcription Reagent (Takara Bio, Inc., Otsu, Japan). Relative
expression levels of the Notch1 gene transcript were determined by
qPCR using the SYBR®-Green Master mix (Takara
Biotechnology Co., Ltd., Dalian, China) in the iQ5 Multicolor
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The primer sequences were as follows: Forward,
5′-GAGGCGTGGCAGACTATGC-3′ and reverse, 5′-CTTGTACTCCGTCAGCGTGA-3′
for Notch1; forward, 5′-CATCACTATCGGCAATGAGC-3′ and reverse,
5′-GACAGCACTGTGTTGGCATA-3′ for β-actin. The following amplification
conditions were used: Predenaturation at 94°C for 4 min, followed
by 40 cycles of denaturation at 94°C for 30 sec, annealing at 60°C
for 30 sec and extension at 72°C for 30 sec. The relative
expression levels of Notch1 mRNA transcripts to GAPDH were
determined using the 2-ΔΔCq method as previously
described (20).
Western blot analysis
Total proteins were prepared from cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Guangzhou, China) and protein concentration was
determined using the Bicinchoninic Acid Protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Equal quantities (25 µg) of protein were subjected to 10%
SDS-PAGE and transferred to 0.22 µm polyvinylidene difluoride
membranes. The membranes were blocked using 5% non-fat milk powder
at room temperature for 1 h and incubated with primary anti-Notch1
(1:800; rabbit; cat. no. 3439; Cell Signaling Technology, Inc.,
Danvers, MA, USA) or anti-β-actin (1:1,000; rabbit; cat. no. 4970;
Cell Signaling Technology, Inc.) or anti-HIF-1α (1:500; rabbit;
cat. no. BS3514; Bioworld Technology, Inc., St. Louis Park, MN,
USA) antibodies overnight at 4°C. Following washing, the membranes
were incubated with goat anti-rabbit horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:5,000; cat. no. ab97051;
Abcam, Cambridge, MA, USA) at room temperature for 2 h. The bands
were visualized using an Enhanced Chemiluminescence Detection
system (GE Healthcare Life Sciences, Chalfont, UK). Quantity
One® software (version 4.6.2; Bio-Rad Laboratories,
Inc.) was used to analyze the densitometry of each band; β-actin
served as an internal loading control.
Statistical analysis
Experiments were repeated three times with each
sample analyzed in triplicate. The results are expressed as the
mean ± standard deviation. Differences between groups were
evaluated using a one-way analysis of variance followed by
Dunnett's post hoc test. Statistical analysis was performed in SPSS
software version 15.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Curcumin inhibits hypoxia-induced
proliferation of osteosarcoma cells
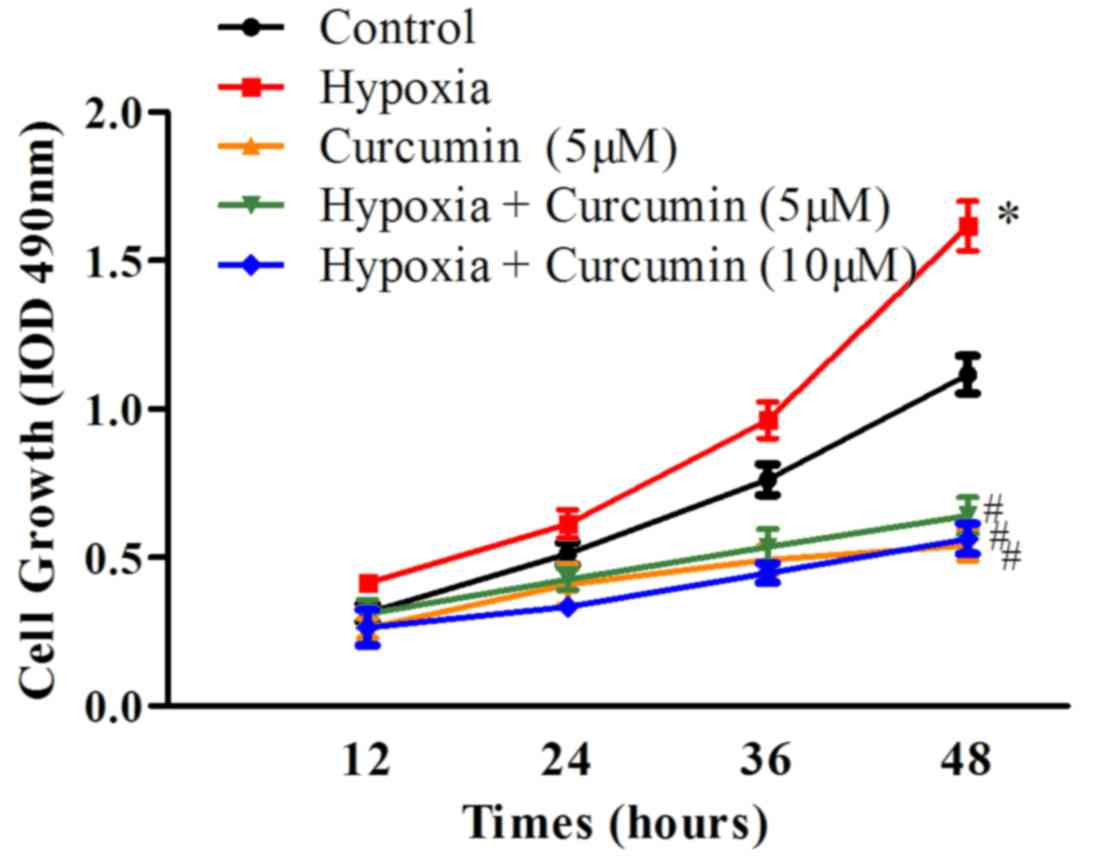
Firstly, the effects of curcumin on the viability of
cancer cells under hypoxic conditions were examined. MG-63
osteosarcoma cells were treated with 5 or 10 µM curcumin under
normoxic or hypoxic conditions, for 12, 24, 36 or 48 h, and cell
viability was assessed using an MTT assay. As presented in Fig. 1, cell growth was accelerated in
hypoxic conditions compared with control normoxic conditions
(P=0.032). Curcumin treatment (5 µM) inhibited cell growth in
normoxic conditions (P=0.028). Furthermore, hypoxia-induced cell
proliferation was prevented by curcumin treatment at 5 and 10 µM
concentrations (P=0.016). These results indicated that curcumin may
inhibit the growth of osteosarcoma cells in normoxic and hypoxic
conditions.
Curcumin inhibits hypoxia-induced
invasion of osteosarcoma cells
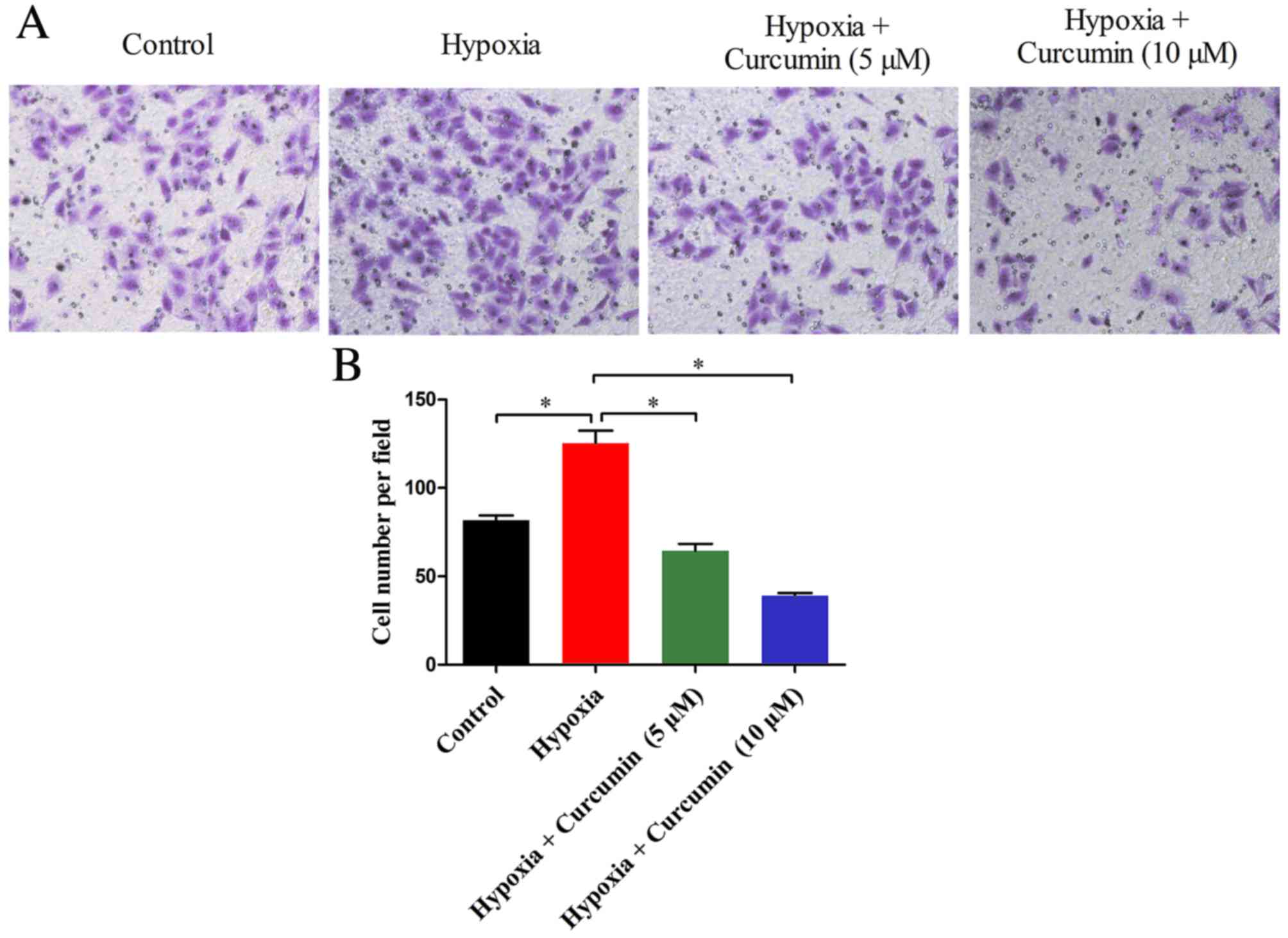
Cancer cells exposed to hypoxic conditions
demonstrate enhanced invasiveness and metastasis (21,22).
To investigate the effect of curcumin on the invasiveness of the
MG-63 osteosarcoma cell line, a Transwell assay was performed. As
presented in Fig. 2, the number of
invaded cells cultured in hypoxic conditions was significantly
greater compared with control normoxic conditions (P=0.005).
However, hypoxia-mediated cell invasiveness was markedly inhibited
by curcumin treatment, which significantly reduced the number of
invaded cells in a dose-dependent manner. These findings suggested
that curcumin may inhibit the invasiveness of cancer cells under
hypoxic conditions.
Curcumin inhibits hypoxia-induced
Notch1 upregulation
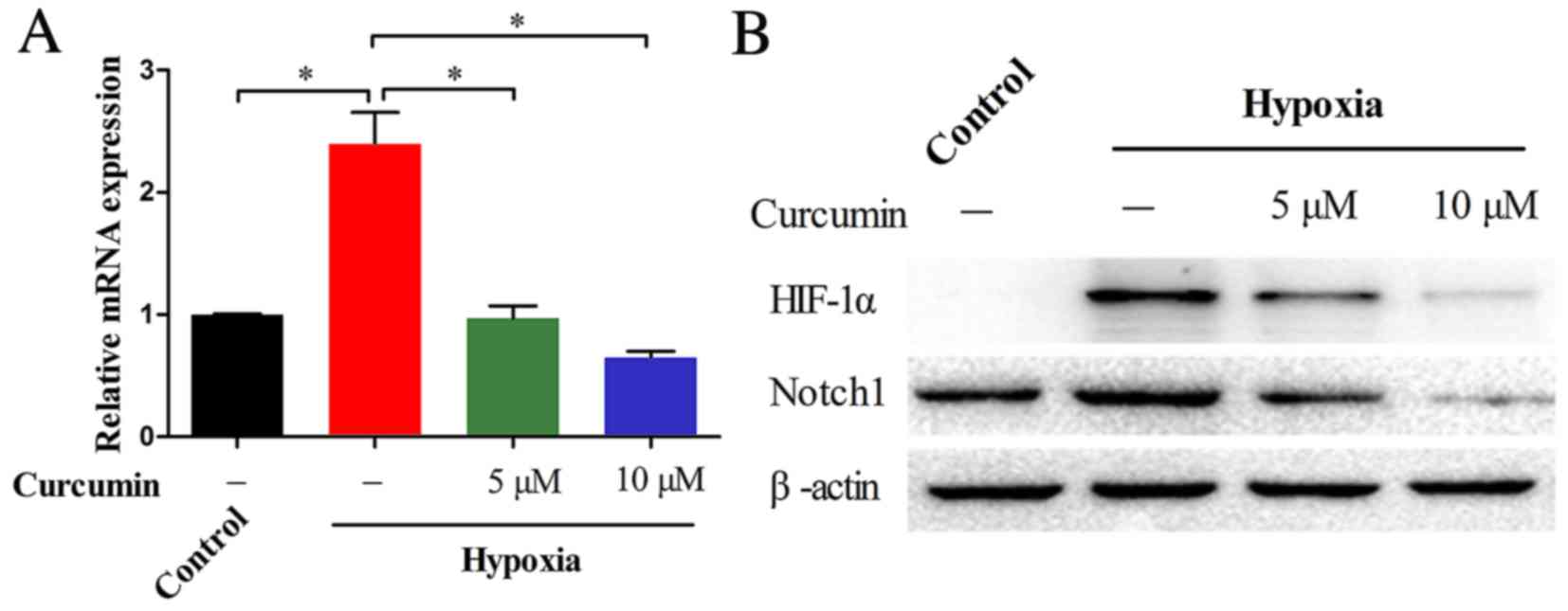
Previous studies indicated that Notch1 is required
for the hypoxia-induced proliferation and invasion of cancer cells
(23). The present study
investigated whether curcumin has an effect on hypoxia-induced
Notch1 upregulation in MG-63 cells. As presented in Fig. 3A, RT-qPCR revealed that the
relative mRNA expression levels of Notch1 in cells cultured in
hypoxic conditions were markedly greater compared with those from
normoxic conditions (P=0.007). However, the hypoxia-induced Notch1
mRNA expression levels were markedly reduced by 5 (P=0.007) and 10
µM (P=0.003) curcumin. These observations were further supported at
the protein level by western blotting (Fig. 3B). Treatment with curcumin markedly
diminished the hypoxia-induced upregulation of the HIF-1α and
Notch1 proteins. Together, these data indicated that curcumin may
inhibit hypoxia-induced Notch1 upregulation in a dose-dependent
manner.
Overexpression of Notch1 reduces
curcumin-induced cell growth inhibition under hypoxia
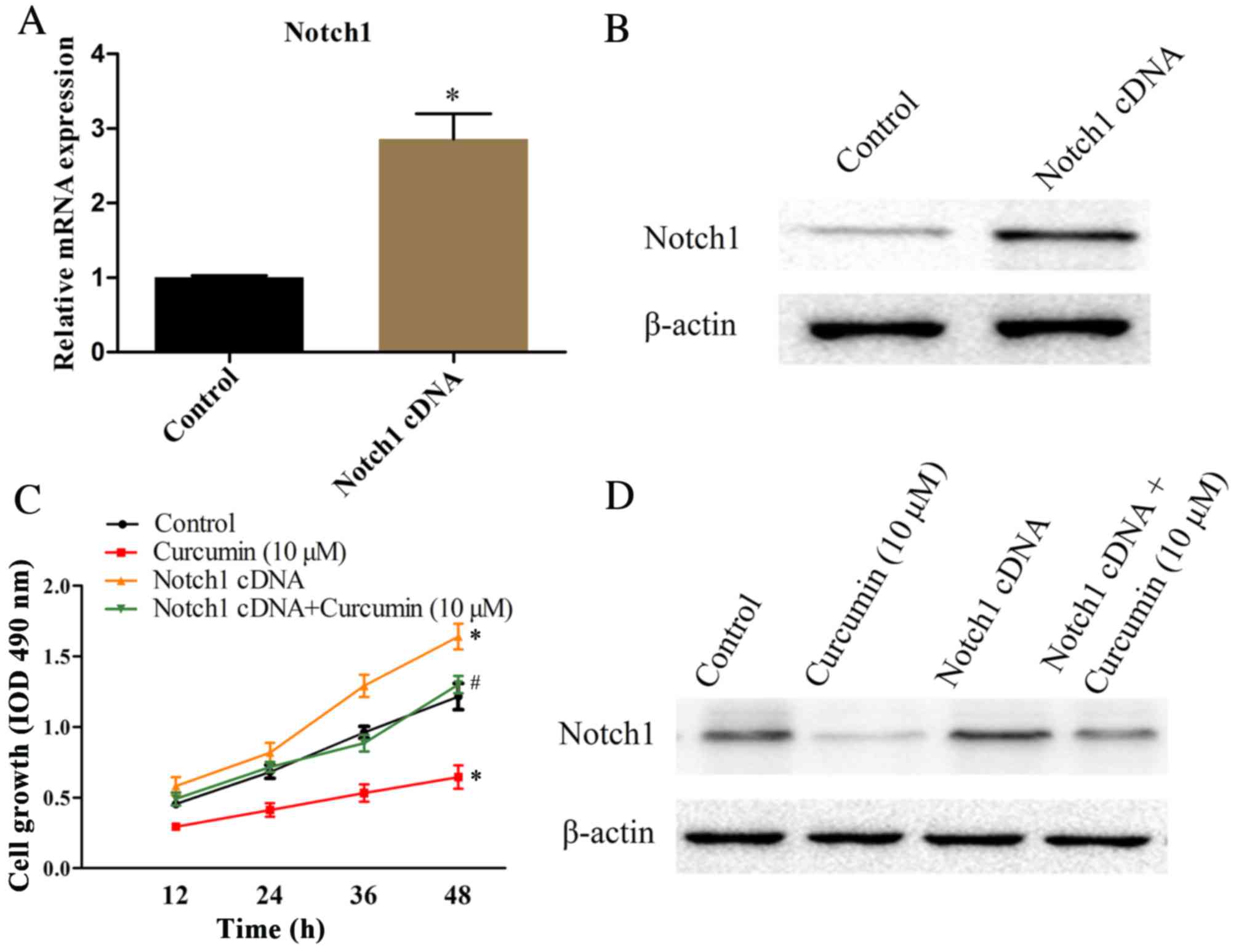
To determine the underlying mechanisms of Notch1 in
curcumin-inhibited cell growth under hypoxic conditions, MG-63
cells were transfected with the Notch1 cDNA. The mRNA and protein
expression levels of Notch1 in MG-63 cells were detected by RT-qPCR
and western blotting, respectively. As presented in Fig. 4A, RT-qPCR revealed that Notch1 mRNA
expression levels were approximately 3-fold greater in Notch1
cDNA-transfected cells compared with cells transfected with an
empty vector (P=0.0007). These results were supported by western
blotting data (Fig. 4B). The
effects of Notch1 overexpression on curcumin-mediated cell growth
inhibition were subsequently assessed under hypoxia in MG-63 cells.
Notch1 cDNA-transfected and empty vector-transfected control cells
were cultured in the absence or presence of 10 µM curcumin under
normoxia, for 12, 24, 36 or 48 h, and cell viability was assessed
by an MTT assay. As presented in Fig.
4C, the promoted cell growth in hypoxia was prevented by
curcumin treatment at a concentration of 10 µM (P=0.001). Cells
transfected with Notch1 cDNA demonstrated increased cell growth
compared with empty vector-transfected control cells under normoxia
(P=0.007). Notably, curcumin-inhibited cell growth in hypoxia was
reversed by Notch1 overexpression (P=0.019). The Notch1 protein
expression levels in each group were detected by western blotting
(Fig. 4D). Collectively, these
data indicated that Notch1 may be involved in curcumin-inhibited
cell growth in hypoxia.
Discussion
Although osteosarcoma is a relatively rare type of
malignant cancer, the high incidence in children and adolescents
make it a major public health issue worldwide. In the past decade,
despite progress in the understanding of osteosarcoma and the
advent of multi-agent chemotherapy, there has been no significant
improvement in prognosis for patients with osteosarcoma.
Developments in molecular biology have provided insight into the
molecular pathogenesis of osteosarcoma (24,25).
Previous studies (5,26) have demonstrated that hypoxia is a
common phenomenon within solid tumors, including osteosarcoma.
Hypoxia-mediated upregulation of HIF-1α may act on multiple
downstream genes associated with tumor progression (27). HIF-1α overexpression is a frequent
event in osteosarcoma and has been identified as an independent
prognostic biomarker in osteosarcoma (28), indicating that hypoxia is a feature
of osteosarcoma. Therefore, it may be more relevant to examine the
response of osteosarcoma to chemotherapeutics under hypoxic
conditions.
Evidence from experimental and clinical studies have
indicated a critical role for hypoxia in the malignant progression
of solid tumors (27). Hypoxia has
become a central issue in tumor physiology and cancer treatment due
to its multiple roles in cancer cell genomic instability (29), angiogenesis (30), invasiveness (31), metastasis (32), resistance to cell death (33), metabolism reprogramming (34), chemoresistance (35) and radioresistance (36). A hypoxic microenvironment has been
confirmed by histological studies of osteosarcoma specimens
(37). It was reported that
elevated HIF-1α protein expression levels were associated with
shortened disease-free survival and treatment resistance in
osteosarcoma patients (6). In
addition, a recent study demonstrated that osteosarcoma cells
exhibited enhanced proliferation and invasiveness when exposed to
hypoxic conditions (38).
Consistent with this, the present study observed that the
proliferation and invasiveness of MG-63 cells were markedly
promoted by hypoxic conditions.
A previous study indicated that curcumin is a potent
anticancer agent, which acts by targeting multiple cell signaling
pathways involved in cancer progression (16). To date, various anti-cancer effects
of curcumin have been revealed, including its ability to suppress
proliferation, induce apoptosis and inhibit the invasion and
metastasis of cancer cells (39).
However, previous studies were primarily focused on the anticancer
effects of curcumin under normoxic conditions, rather than focusing
on its anticancer effects under hypoxic conditions. Duan et
al (40) reported that
curcumin may inhibit hypoxia-induced proliferation and
epithelial-mesenchymal transition of hepatic carcinoma cells. This
study used the chemical CoCl2 to establish hypoxic
conditions, which is a poor reflection of real hypoxic conditions,
although CoCl2 may induce HIF-1α accumulation in cancer
cells. In the present study, a culture condition containing 3%
O2 was used to examine the effects of curcumin on
osteosarcoma cell proliferation and invasion. These results
demonstrated that curcumin may inhibit hypoxia-induced
proliferation and invasion of osteosarcoma cells. This suggested
that curcumin may serve pivotal roles in tumor suppression under
normoxic and hypoxic conditions.
Notch signaling is a highly conserved cell signaling
system involved in normal organ development. Recent studies
(41,42) have suggested that the Notch1
signaling pathway serves a critical role in osteosarcoma
pathogenesis, development, invasion and metastasis, which indicates
that Notch1 is a potential therapeutic target in osteosarcoma. In
the present study, in addition to enhanced proliferation and
invasiveness, the Notch1 expression levels in MG-63 cells were
markedly increased by hypoxia treatment. Furthermore, curcumin,
which has been revealed to inhibit hypoxia-induced HIF-1α
expression, may suppress the expression levels of Notch1. These
results indicated that Notch1 may be a downstream gene of HIF-1α in
osteosarcoma cells and suggested that curcumin may serve as a
potential anticancer agent for the treatment of osteosarcoma.
In conclusion, the current study demonstrated that
the MG-63 osteosarcoma cell line exhibited increased rates of
proliferation and enhanced invasiveness upon exposure to hypoxic
conditions. However, the effects induced by hypoxia in MG-63 cells
were prevented by curcumin treatment. Further investigation
revealed that curcumin may inhibit the Notch1 upregulation induced
by hypoxia. Overexpression of Notch1 by Notch1 cDNA transfection
ameliorated curcumin-inhibited MG-63 cell growth under hypoxic
conditions. Taken together, the results of the present study
revealed that curcumin may suppress the growth of osteosarcoma
cells in hypoxia via inhibition of Notch1 signaling.
Acknowledgements
The authors thank Medjaden Bioscience Limited (Hong
Kong, China) for assisting in the preparation of the
manuscript.
Glossary
Abbreviations
Abbreviations:
|
HIF-1α
|
hypoxia inducible factor-1α
|
|
NICD
|
Notch intracellular domain
|
References
|
1
|
Bacci G, Longhi A, Bertoni F, Briccoli A,
Versari M, Pignotti E and Picci P: Bone metastases in osteosarcoma
patients treated with neoadjuvant or adjuvant chemotherapy: The
Rizzoli experience in 52 patients. Acta Orthop. 77:938–943. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruan K, Song G and Ouyang G: Role of
hypoxia in the hallmarks of human cancer. J Cell Biochem.
107:1053–1062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan C, Zhang L, Cheng X, Lin XF, Lu RR,
Bao JD and Yu HX: Curcumin inhibits hypoxia-induced migration in K1
papillary thyroid cancer cells. Exp Biol Med (Maywood).
240:925–935. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizobuchi H, Garcia-Castellano JM, Philip
S, Healey JH and Gorlick R: Hypoxia markers in human osteosarcoma:
An exploratory study. Clin Orthop Relat Res. 466:2052–2059. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y, Wang CM, Shi YQ and Yang Y:
Expression of hypoxia-inducible factor 1alpha in osteosarcoma and
its value in predicting chemosensitivity. Zhonghua Zhong Liu Za
Zhi. 34:899–904. 2012.(In Chinese). PubMed/NCBI
|
|
7
|
Yang QC, Zeng BF, Dong Y, Shi ZM, Jiang ZM
and Huang J: Overexpression of hypoxia-inducible factor-1alpha in
human osteosarcoma: Correlation with clinicopathological parameters
and survival outcome. Jpn J Clin Oncol. 37:127–134. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Hussaini H, Subramanyam D, Reedijk M
and Sridhar SS: Notch signaling pathway as a therapeutic target in
breast cancer. Mol Cancer Ther. 10:9–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong Y, Jesse AM, Kohn A, Gunnell LM,
Honjo T, Zuscik MJ, O'Keefe RJ and Hilton MJ: RBPjkappa-dependent
Notch signaling regulates mesenchymal progenitor cell proliferation
and differentiation during skeletal development. Development.
137:1461–1471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiang L, Wu T, Zhang HW, Lu N, Hu R, Wang
YJ, Zhao L, Chen FH, Wang XT, You QD and Guo QL: HIF-1a is critical
for hypoxia-mediated maintenance of glioblastoma stem cells by
activating Notch signaling pathway. Cell Death Differ. 19:284–294.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh S and Khar A: Biological effects of
curcumin and its role in cancer chemoprevention and therapy.
Anticancer Agents Med Chem. 6:259–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Panchal HD, Vranizan K, Lee CY, Ho J, Ngai
J and Timiras PS: Early anti-oxidative and anti-proliferative
curcumin effects on neuroglioma cells suggest therapeutic targets.
Neurochem Res. 33:1701–1710. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurup VP and Barrios CS: Immunomodulatory
effects of curcumin in allergy. Mol Nutr Food Res. 52:1031–1039.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim DC, Ku SK and Bae JS: Anticoagulant
activities of curcumin and its derivative. BMB Rep. 45:221–226.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dou X, Fan C, Wo L, Yan J, Qian Y and Wo
X: Curcumin up-regulates LDL receptor expression via the sterol
regulatory element pathway in HepG2 cells. Planta Med.
74:1374–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shishodia S, Chaturvedi MM and Aggarwal
BB: Role of curcumin in cancer therapy. Curr Probl Cancer.
31:243–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang YJ, Huang CY, Hung CS, Chen WY and
Wei PL: GRP78 mediates the therapeutic efficacy of curcumin on
colon cancer. Tumour Biol. 36:633–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye M and Zhang J and Zhang J, Miao Q, Yao
L and Zhang J: Curcumin promotes apoptosis by activating the
p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer.
Cancer Lett. 357:196–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin AR, Haque A, Rahman MA, Chen ZG,
Khuri FR and Shin DM: Curcumin induces apoptosis of upper
aerodigestive tract cancer cells by targeting multiple pathways.
PLoS One. 10:e01242182015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C (T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao X, Gao S, Ren H, Sun W, Zhang H, Sun
J, Yang S and Hao J: Hypoxia-inducible factor-1 promotes pancreatic
ductal adenocarcinoma invasion and metastasis by activating
transcription of the actin-bundling protein fascin. Cancer Res.
74:2455–2464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu N, Wang Y, Zhou Y, Pang H, Zhou J,
Qian P, Liu L and Zhang H: Kruppel-like factor 8 involved in
hypoxia promotes the invasion and metastasis of gastric cancer via
epithelial to mesenchymal transition. Oncol Rep. 32:2397–2404.
2014.PubMed/NCBI
|
|
23
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:6392–6397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: A
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He JP, Hao Y, Wang XL, Yang XJ, Shao JF,
Guo FJ and Feng JX: Review of the molecular pathogenesis of
osteosarcoma. Asian Pac J Cancer Prev. 15:5967–5976. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El NA, Clarkson P, Zhang F, Mathers J,
Tognon C and Sorensen PH: Expression and stability of hypoxia
inducible factor 1a in osteosarcoma. Pediatr Blood Cancer.
59:1215–1222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koh MY, Spivak-Kroizman TR and Powis G:
HIF-1alpha and cancer therapy. Recent Results Cancer Res.
180:15–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao H, Wu Y, Chen Y and Liu H: Clinical
significance of hypoxia-inducible factor 1 and VEGF-A in
osteosarcoma. Int J Clin Oncol. 20:1233–1243. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nelson DA, Tan TT, Rabson AB, Anderson D,
Degenhardt K and White E: Hypoxia and defective apoptosis drive
genomic instability and tumorigenesis. Genes Dev. 18:2095–2107.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moeller BJ, Cao Y, Vujaskovic Z, Li CY,
Haroon ZA and Dewhirst MW: The relationship between hypoxia and
angiogenesis. Semin Radiat Oncol. 14:215–221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu Q, He Y, Ji J, Yao Y, Shen W, Luo J,
Zhu W, Cao H, Geng Y, Xu J, et al: Hypoxia-inducible factor 1a
(HIF-1a) and reactive oxygen species (ROS) mediates
radiation-induced invasiveness through the SDF-1a/CXCR4 pathway in
non-small cell lung carcinoma cells. Oncotarget. 6:10893–10907.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu X and Kang Y: Hypoxia and
hypoxia-inducible factors: Master regulators of metastasis. Clin
Cancer Res. 16:5928–5935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim M, Park SY, Pai HS, Kim TH, Billiar TR
and Seol DW: Hypoxia inhibits tumor necrosis factor-related
apoptosis-inducing ligand-induced apoptosis by blocking Bax
translocation. Cancer Res. 64:4078–4081. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bel Aiba RS, Dimova EY, Görlach A and
Kietzmann T: The role of hypoxia inducible factor-1 in cell
metabolism-a possible target in cancer therapy. Expert Opin Ther
Targets. 10:583–599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Selvendiran K, Bratasz A, Kuppusamy ML,
Tazi MF, Rivera BK and Kuppusamy P: Hypoxia induces chemoresistance
in ovarian cancer cells by activation of signal transducer and
activator of transcription 3. Int J Cancer. 125:2198–2204. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zou YM, Hu GY, Zhao XQ, Lu T, Zhu F, Yu SY
and Xiong H: Hypoxia-induced autophagy contributes to
radioresistance via c-Jun-mediated Beclin1 expression in lung
cancer cells. J Huazhong Univ Sci Technolog Med Sci. 34:761–767.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guan G, Zhang Y, Lu Y, Liu L, Shi D, Wen
Y, Yang L, Ma Q, Liu T, Zhu X, et al: The HIF-1a/CXCR4 pathway
supports hypoxia-induced metastasis of human osteosarcoma cells.
Cancer Lett. 357:254–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun Y, Wang H, Liu M, Lin F and Hua J:
Resveratrol abrogates the effects of hypoxia on cell proliferation,
invasion and EMT in osteosarcoma cells through downregulation of
the HIF-1α protein. Mol Med Rep. 11:1975–1981. 2015.PubMed/NCBI
|
|
39
|
Shehzad A, Wahid F and Lee YS: Curcumin in
cancer chemoprevention: Molecular targets, pharmacokinetics,
bioavailability, and clinical trials. Arch Pharm (Weinheim).
343:489–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Duan W, Chang Y, Li R, Xu Q, Lei J, Yin C,
Li T, Wu Y, Ma Q and Li X: Curcumin inhibits hypoxia inducible
factor-1α-induced epithelial-mesenchymal transition in HepG2
hepatocellular carcinoma cells. Mol Med Rep. 10:2505–2510.
2014.PubMed/NCBI
|
|
41
|
Wang L, Jin F, Qin A, Hao Y, Dong Y, Ge S
and Dai K: Targeting Notch1 signaling pathway positively affects
the sensitivity of osteosarcoma to cisplatin by regulating the
expression and/or activity of Caspase family. Mol Cancer.
13:1392014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tao J, Jiang MM, Jiang L, Salvo JS, Zeng
HC, Dawson B, Bertin TK, Rao PH, Chen R, Donehower LA, et al: Notch
activation as a driver of osteogenic sarcoma. Cancer Cell.
26:390–401. 2014. View Article : Google Scholar : PubMed/NCBI
|