Introduction
Experimental autoimmune neuritis (EAN) is a cluster
of differentiation (CD) 4+ T cell-mediated inflammatory
demyelinating disease of the peripheral nervous system (PNS)
(1). EAN serves as a useful animal
model for Guillain-Barré syndrome (GBS), which is a CD4+
T cell-mediated disease of the PNS in humans (2).
Triggering receptor expressed on myeloid cells-1
(TREM-1) is a 30-kDa glycoprotein associated with DNAX-activation
protein of 12 kDa (DAP12), which is an immunoreceptor
tyrosine-based activation motif-containing adaptor protein
(3). TREM-1 is selectively
expressed on blood neutrophils and on a subset of monocytes
(4,5) and may amplify the inflammatory
response (5–7). TREM-1 induces the production of
chemokines and cytokines via DAP12 (3). TREM-1 additionally regulates
signaling pathways induced by known classes of pattern-recognition
receptors, including Toll-like receptors and neuronal apoptosis
inhibitory proteins (4). Previous
studies have demonstrated that TREM-1 is associated with numerous
types of autoimmune diseases, including inflammatory bowel diseases
(8), acute pancreatitis (9), neoplastic pleural effusions (10) and rheumatoid arthritis (11). TREM-1 influences chronic heart
rejection by regulating the infiltration and differentiation of
CD4+ lymphocytes (12).
In addition, TREM-1 has a tumor promoting effect (13).
Although TREM-1 has been widely studied, its role in
EAN/GBS remains to be investigated. To determine whether TREM-1 is
involved in the pathogenesis of EAN, the present study treated the
animal model of EAN with linear plasmid 17 (LP 17), an analogue
synthetic peptide derived from the extracellular moiety of TREM-1
(14). The symptoms of rats and
the mRNA expression levels of tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and TREM-1 in sciatic nerves and peripheral
blood mononuclear cells (PBMCs) were subsequently assessed. The
results of the present study suggested that TREM-1 may serve a role
in the pathogenesis of EAN.
Materials and methods
Animal models
Animal procedures were approved by Xiangya Hospital
Ethics Committee, Xiangya Hospital (Changsha, China). The
Guidelines for Ethical Conduct in the Care and Use of Vertebrate
Animals in Research and Training by the American Physiological
Society Council were followed (15).
Specific pathogen free male Lewis rats (age, 6–8
weeks; weight, 160–180 g; n=64) were purchased from Vital River
Laboratories Co., Ltd. (Beijing, China) and were housed in groups
of two or three per cage under controlled conditions: Light-dark
cycle, 12-h; background noise, 40±10 dB; relative humidity, 50–60%;
temperature, 20±3°C; and food and water ad libitum. Rats
were acclimatized for one week, following which they were randomly
assigned to four groups: Normal saline (NS), complete Freund's
adjuvant (CFA), EAN and LP 17.
Myelin P2 peptides 53–78
(THR-GLU-SER-PRO-PHE-LYS-ASN-THR-GLU-ILE-SER-PHE-LYS-LEU-GLY-GLN-GLU-PHE-GLU-GLU-THR-THR-ALA-ASP-ASN-ARG)
were synthesized by solid-phase stepwise elongation using a Tecan
peptide synthesizer (GL Biochem Ltd., Shanghai, China).
The EAN animal model was created by injecting the
two hind-foot pads with 200 µl antigen emulsion containing 100 µg
P2 53–78 emulsified in 100 µl saline and 100 µl Freund's incomplete
adjuvant (FIA; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
containing 5 mg/ml heat-inactivated Mycobacterium tuberculosis
H37RA (Beijing Institute of Biological Products, Co., Ltd.,
Beijing, China) (16). The FIA +
Mycobacterium tuberculosis H37RA mixture was referred to as
CFA.
Rats in the NS group were injected with 200 µl
sterile saline, and rats in the CFA group with 200 µl CFA. The NS
and CFA groups served as controls. Rats in the LP 17 group were
injected with 200 µl antigen emulsion and a single dose (4 mg/kg,
dissolved in 500 µl NS and injected intraperitoneally) of LP 17 (GL
Biochem Ltd., Shanghai, China) administered immediately following
the footpad injection.
Body weight and clinical scores were assessed
immediately prior to immunization and every day following
immunization until 33 days post-immunization (PI). The timeline of
the experiment was numbered as days PI. Severity of paresis was
graded as follows: 0=normal; 1=flaccid tail; 2=moderate
paraparesis; 3=severe paraparesis; 4=tetraparesis (16).
Sample collection
Each group contained four subgroups (n=4/subgroup)
that were sacrificed at different days PI: 7 (phase 1: Onset of
disease), 16 (phase 2: Disease peak), 24 (phase 3: Early recovery
phase) and 33 (phase 4: Late recovery phase). Prior to sacrifice,
rats anesthetized with an intraperitoneal injection of 36 mg/kg 3%
chloral hydrate (Xiangya Hospital, Changsha, China) to draw blood
and to carefully separate the bilateral sciatic nerves (stored at
−80°C for mRNA analysis and collected for histopathological
assessment, respectively), and then the anesthetized rats were
sacrificed in a 0.015 l CO2 gas chamber, where the rats
received a 100% CO2 air flow for 10 min. Mortality was
confirmed with no heartbeat/breath by at least two technicians
(Xiangya Hospital, Changsha, China).
Blood samples (~5 ml) were obtained from the jugular
vein and collected in EDTA-coated tubes. PBMCs were isolated by
gradient centrifugation using LymphoPrep™ solution (Beijing Dingguo
Changsheng Biotechnology Co., Ltd., Beijing, China), and the whole
blood was centrifuged at 1,200 × g for 15 min at 4°C. The
buffy coat was mixed with RPMI-1640 (Sigma-Aldrich; Merck
Millipore). The buffy coat/RPMI mix was layered on top of
endotoxin-free LymphoPrep and centrifuged at 1,200 × g for
25 min at 4°C. PBMCs separated out into a distinct layer that was
removed, washed twice with RPMI-1640 and centrifuged at 600 ×
g for 10 min at 4°C, then 400 × g for 10 min at 4°C.
Following three washes with PBS, PBMCs were collected for RNA
extraction.
Histopathological assessment of
EAN
Sciatic nerves were isolated and fixed overnight in
4% paraformaldehyde at 4°C. Each sciatic nerve was cut into
segments ~5-mm long, which were embedded in paraffin blocks,
sectioned serially (4 µm), and mounted on polylysine-treated slides
(Beijing Dingguo Changsheng Biotechnology Co., Ltd.). Hematoxylin
and eosin (H&E) staining was performed to observe inflammatory
cell infiltration in the nerves (17,18).
This served to validate the EAN animal model.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from PBMCs and sciatic nerves
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). RNA purity and concentration
were confirmed by spectrophotometry with a wavelength of 450 nm
using the NanoDrop ND-1000 Spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.) cDNA was synthesized
using a Reverse-Transcription kit (Toyobo Co., Ltd., Osaka, Japan)
and an ABI StepOnePlus™ PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.).
Primer sequences were as follows: Forward,
5′-TTCAACGGCACAGTCAAG-3′ and reverse, 5′-CCAGCATCACCCCATTT-3′ for
TREM-1; forward, 5′-CCTGGTCACCAAATCAGCATTA-3′ and reverse,
5′-GAAGCTGTCTTCAGGCCAACAT-3′ for TNF-α; forward,
5′-ATGAGAGCATCCAGCTTCAAATC-3′ and reverse,
5′-CACACTAGCAGGTCGTCATCATC-3′ for IL-1β; and forward,
5′-TTCAACGGCACAGTCAAG-3′ and reverse, 5′-CCAGCATCACCCCATTT-3′ for
GAPDH. The primers were synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China). cDNAs were amplified with SYBR Green using the
Platinum SYBR-Green qPCR SuperMix UDG (Invitrogen; Thermo Fisher
Scientific, Inc.). A RevertAid First Strand cDNA synthesis kit
(Thermo Fisher Scientific, Inc.) and nuclease-free water (Thermo
Fisher Scientific, Inc.) were also used during the process of
qPCR.
Cycling conditions were identical for all primer
pairs: An initial denaturation step at 95°C for 1 min, followed by
40 cycles at 95°C for 15 sec and 65°C for 1 min. The results were
automatically analyzed using the ABI StepOnePlus PCR system and the
2−ΔΔCq method was used to analyze mRNA expression (ΔCq
represents the difference in quantification cycle value between the
target gene and the internal control; ΔΔCq represents the
difference in ΔCq between groups) (19). Three independent RT-qPCR reactions
were performed.
Statistical analysis
Data are presented as the mean ± standard deviation.
Analyses were performed using SPSS software version 18.0 (SPSS,
Inc., Chicago, IL, USA). Differences between groups were evaluated
by one-way analysis of variance with Bonferroni post-test analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical and histopathological
alterations
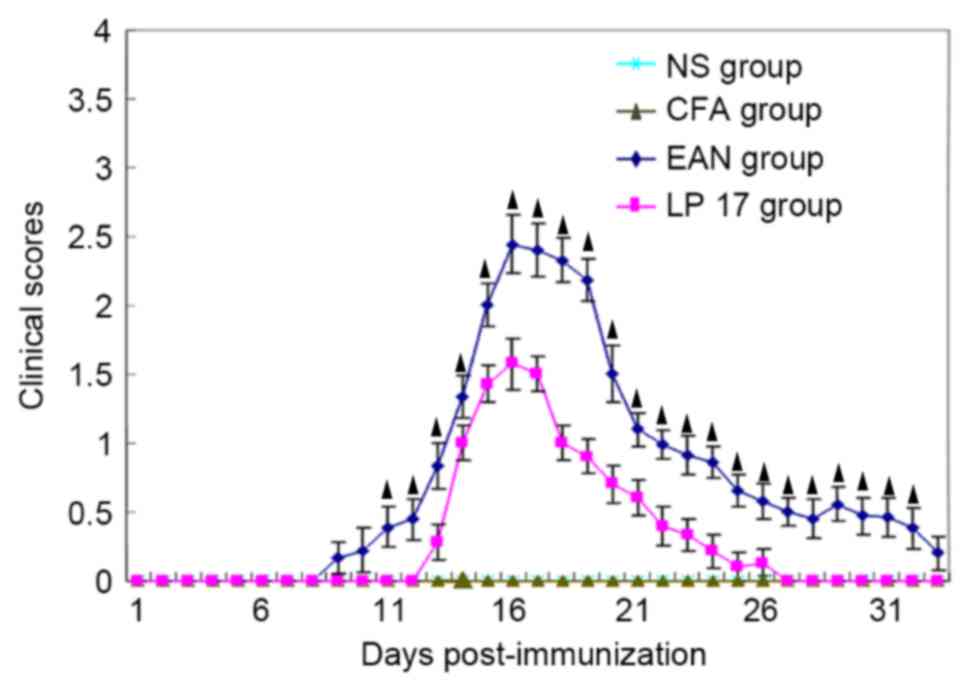
Rats in the EAN group (n=16) appeared healthy prior
to immunization and began to exhibit fatigue and tail weakness 9 to
11 days PI. They had the greatest clinical score (2.44±0.21; n=12)
on day 16 and then gradually recovered, with complete recovery by
day 33 (n=4). Severity of disease in the LP 17 group was reduced
compared with the EAN group (P<0.05; Fig. 1); however, the clinical score of
the LP 17 group also peaked on day 16 (1.57±0.19; n=12; Fig. 1).
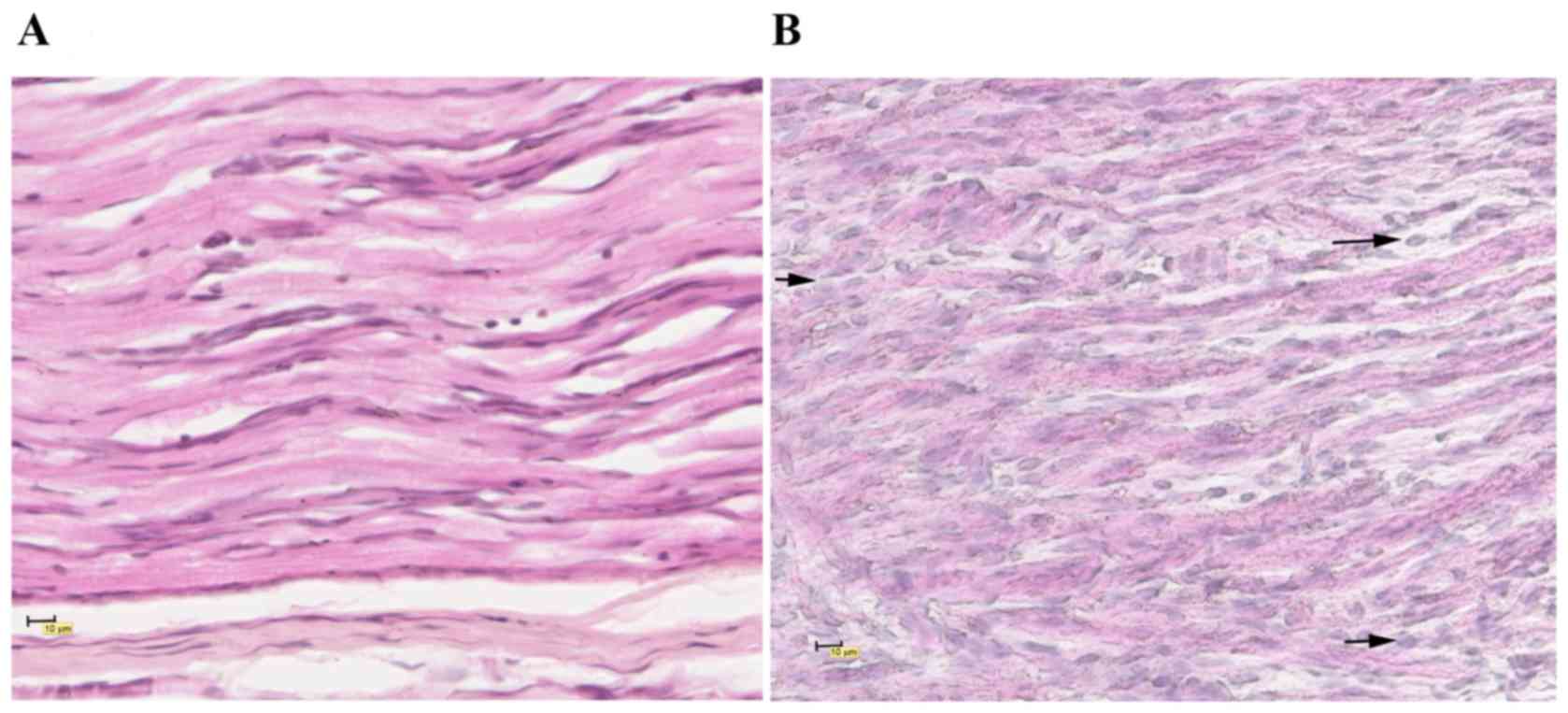
The infiltration of inflammatory cells in the LP 17
group (Fig. 2A) was markedly
reduced compared with the EAN group (Fig. 2B), and fewer histopathological
alterations were observed.
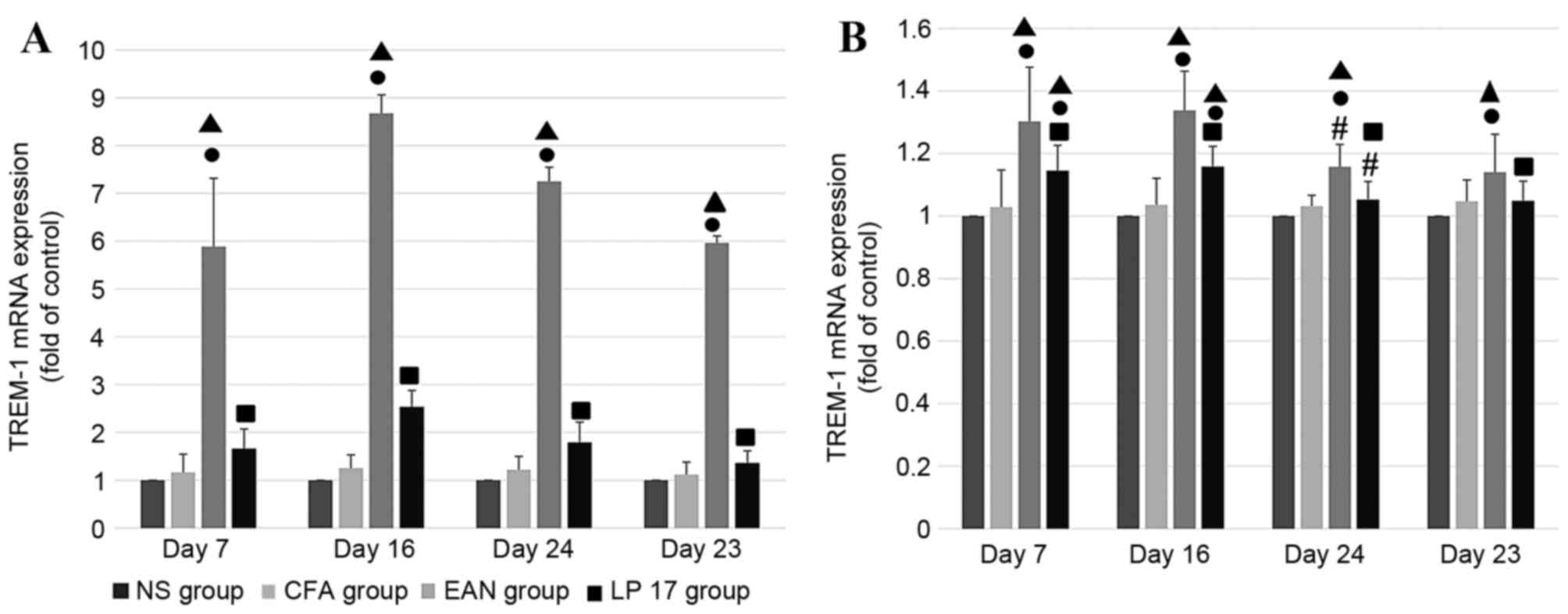
TREM-1 mRNA expression levels
In sciatic nerves (Fig.
3A) and PBMCs (Fig. 3B), mRNA
expression levels of TREM-1 were increased across all phases in the
EAN group compared with the CFA and NS groups (P<0.05). The
expression levels of TREM-1 mRNA were markedly increased in sciatic
nerves compared with PBMCs. In the PBMCs, mRNA expression levels of
TREM-1 in the EAN and LP 17 groups differed significantly between
the disease peak phase and early recovery phase (P<0.05).
Compared with the control groups, mRNA expression levels of TREM-1
in PBMCs were increased in the LP 17 group at the onset of disease
(day 7) and disease peak (day 16) phases (P<0.05).
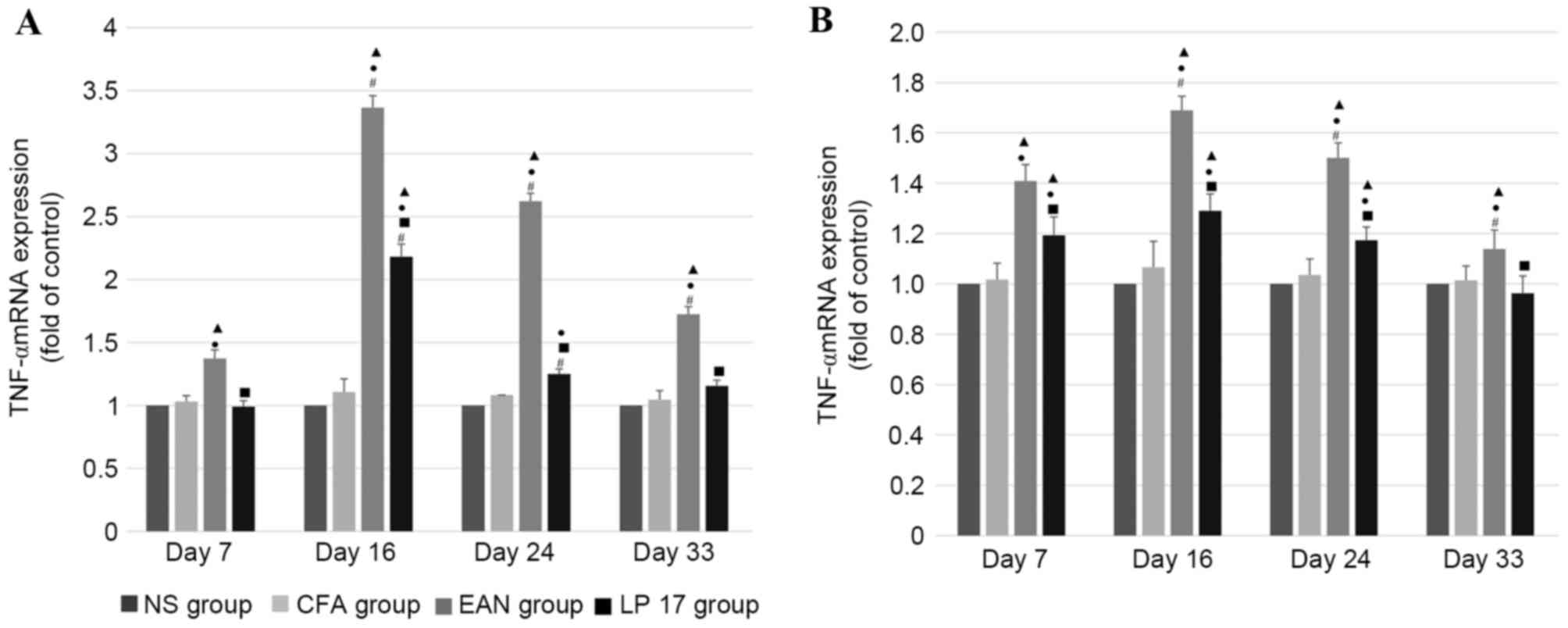
TNF-α mRNA expression levels
In sciatic nerves (Fig.
4A) and PBMCs (Fig. 4B), mRNA
expression levels of TNF-α were increased in the EAN group compared
with the control and LP 17 groups across all phases (P<0.05),
and there was a significant difference between each phase within
the EAN group (P<0.05).
In sciatic nerves, the mRNA expression levels of
TNF-α were significantly different across the first three phases
within the LP 17 group (P<0.01). mRNA expression levels of TNF-α
in the sciatic nerves of the LP 17 group were increased compared
with the NS and CFA groups at disease peak (P<0.05) and were
increased compared with the NS group in the early recovery phase
(P<0.05). TNF-α mRNA levels were markedly increased in the EAN
group compared with the LP 17 group (P<0.01).
In PBMCs, TNF-α mRNA expression levels were
increased in the LP 17 group compared with the control groups in
the first three phases (P<0.05), and there were significant
differences between the EAN and LP 17 groups across all phases
(P<0.05).
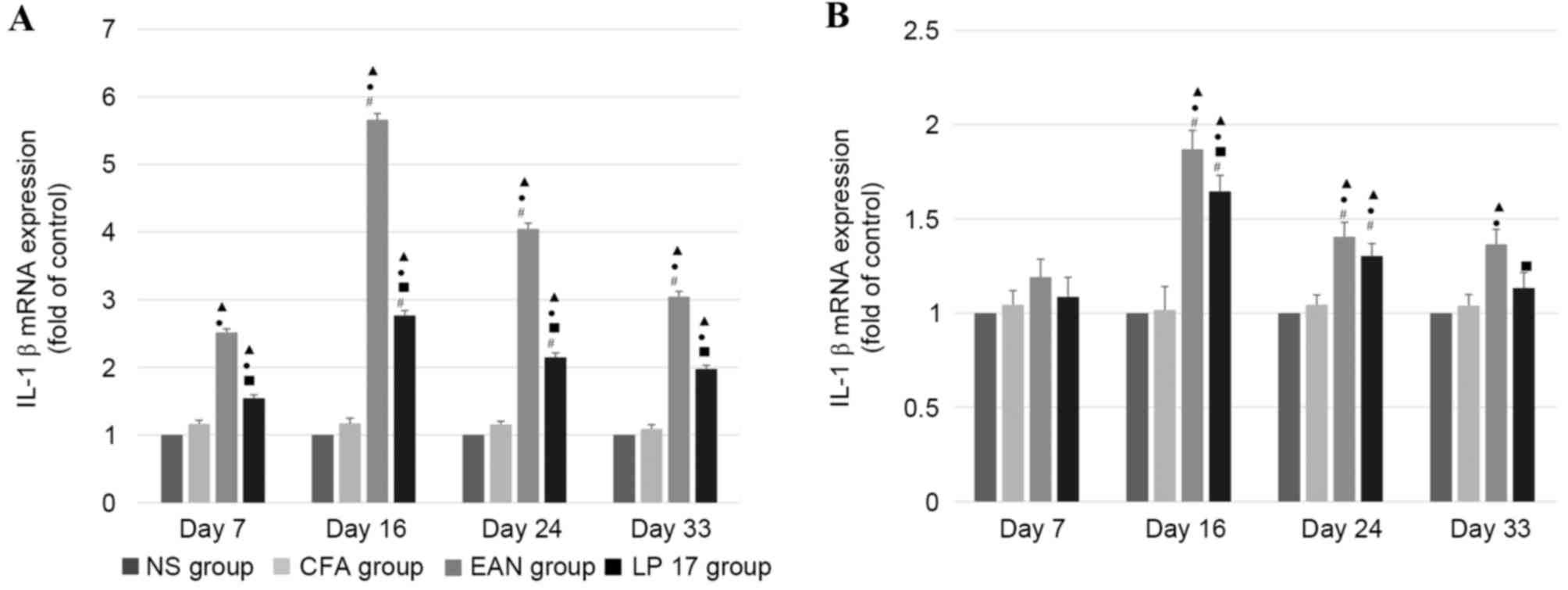
IL-1β mRNA expression levels
In sciatic nerves (Fig.
5A), mRNA expression levels of IL-1β were increased in the EAN
group compared with the control and LP 17 groups across all phases
(P<0.05), and were significantly different between each phase
within the EAN group (P<0.05). IL-1β mRNA expression levels in
the LP 17 group were increased compared with the control groups;
however, were reduced compared with the EAN group across all phases
(P<0.05).
IL-1β mRNA expression levels in PBMCs (Fig. 5B) were increased in the EAN group
compared with the LP 17 group at the disease peak and late recovery
phases (P<0.05). Compared with the control groups, mRNA
expression levels of IL-1β were increased in the EAN and LP 17
groups in the disease peak and early recovery phases
(P<0.05).
In sciatic nerves and PBMCs, IL-1β mRNA expression
levels were significantly different between the first three phases
within the EAN and LP 17 groups (P<0.05).
Discussion
TREM-1 potently amplifies inflammatory responses by
enhancing degranulation and secretion of proinflammatory mediators
(8). Simultaneously, the
activation of TREM-1 increases the expression levels of
costimulatory molecules on the surface of mononuclear leucocytes
(20).
Elevated TREM-1 expression levels may serve as an
early indicator of poor prognosis. Previous studies have suggested
that TREM-1 may represent a valuable marker for inflammatory
severity and be a potential therapeutic target for the treatment of
sepsis (21,22).
In the present study, the mRNA expression levels of
TREM-1 closely paralleled the clinical characteristics of the
disease, and mRNA expression levels of TNF-α, IL-1β and TREM-1 were
greatest in the EAN group. Compared with the EAN group, mRNA
expression levels of TREM-1 were markedly decreased in the LP 17
group, suggesting that LP 17 significantly inhibited the activation
of the TREM-1 signaling pathway. Compared with PBMCs, TREM-1 mRNA
expression levels were increased markedly in the sciatic nerve.
These levels were elevated on day 7 in sciatic nerves and PBMCs,
and peaked on day 16. This indicated that TREM-1 is involved in the
early immune activation process of EAN.
As expected, rats in the LP 17 group experienced
less weight loss and had reduced clinical scores compared with the
EAN group. LP 17 did not completely inhibit EAN; however, the
results of the present study demonstrated that LP 17 attenuates the
symptoms of EAN in rats, indicating that TREM-1 is a potential
target for the treatment of EAN.
Similarly, H&E staining revealed that
infiltration of inflammatory cells in sciatic nerves was less
marked in the LP 17 group on day 16 compared with the EAN group.
Therefore, TREM-1 may serve a role in a series of proinflammatory
processes underlying EAN.
TNF-α may increase the permeability of the blood
nerve barrier (20) and is
involved in the pathogenesis of EAN in rats (21). Expression levels of TNF-α were
greatest in the EAN group on day 7 (onset of disease); this is
consistent with its role in the early pathological phase of EAN
(22). In GBS, TNF-α produced by
infiltrating T cells has a direct myelinotoxic effect (23), which may contribute to the
increased expression levels of TNF-α in the sciatic nerves.
A previous study demonstrated that IL-1β mRNA is
expressed in the lymph nodes and sciatic nerves of rats during the
early onset phase of EAN, which may be associated with the
infiltration of mononuclear macrophages (24). Similarly, the results of the
present study revealed that the mRNA expression levels of IL-1β
were increased in the sciatic nerves of the EAN group across all
phases, and in the LP 17 group were greatest at day 16. A possible
explanation is that IL-1β, mediated by calpain, which is activated
by calcium influx following nerve injury (25), was immediately and continuously
released at the site of injury following injection of the antigen
emulsion. In addition, peripheral inflammation may influence
neurotransmitter metabolism, neuroendocrine function, as well as
growth factor production, which modifies neural circuitry and may
additionally account for the increased expression levels of IL-1β
(25,26).
Although there are therapeutic benefits to the
selective inhibition of TREM-1, a previous study has suggested that
TREM-1 signaling is necessary for successful antimicrobial
responses (24) and that
inappropriate modulation of the TREM-1 signaling pathway may have
profound and potentially detrimental effects on septic
patients.
The present study demonstrates that TREM-1 is a
potential therapeutic target for the treatment of GBS. Further
studies involving expression and silencing of the TREM-1 gene are
required to determine whether the protein itself is expressed, and
which cells produce it.
Glossary
Abbreviations
Abbreviations:
|
EAN
|
experimental autoimmune neuritis
|
|
TREM-1
|
triggering receptor expressed on
myeloid cells-1
|
|
PNS
|
peripheral nervous system
|
|
GBS
|
Guillain-Barré syndrome
|
|
DAP12
|
DNAX-activation protein of 12 kDa
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
NS
|
normal saline
|
|
CFA
|
complete Freund's adjuvant
|
|
FIA
|
Freund's incomplete adjuvant
|
|
PI
|
post-immunization
|
|
H&E
|
hematoxylin and eosin
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Katzav A, Bina H, Aronovich R and Chapman
J: Treatment for experimental autoimmune neuritis with clodronate
(Bonefos). Immunol Res. 56:334–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu H, Li XL, Yue LT, Li H, Zhang M, Wang
S, Wang CC and Duan RS: Therapeutic potential of
atorvastatin-modified dendritic cells in experimental autoimmune
neuritis by decreased Th1/Th17 cytokines and up-regulated T
regulatory cells and NKR-P1(+) cells. J Neuroimmunol. 269:28–37.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Montalvo V, Quigley L, Vistica BP, Boelte
KC, Nugent LF, Takai T, McVicar DW and Gery I: Environmental
factors determine DAP12 deficiency to either enhance or suppress
immunopathogenic processes. Immunology. 140:475–482. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arts RJ, Joosten LA, van der Meer JW and
Netea MG: TREM-1: Intracellular signaling pathways and interaction
with pattern recognition receptors. J Leukoc Biol. 93:209–215.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palazzo SJ, Simpson T and Schnapp LM:
Triggering receptor expressed on myeloid cells type 1 as a
potential therapeutic target in sepsis. Dimens Crit Care Nurs.
31:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bouchon A, Facchetti F, Weigand MA and
Colonna M: TREM-1 amplifies inflammation and is a crucial mediator
of septic shock. Nature. 410:1103–1107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zangi L, Klionsky YZ, Yarimi L,
Bachar-Lustig E, Eidelstein Y, Shezen E, Hagin D, Ito Y, Takai T,
Reich-Zeliger S, et al: Deletion of cognate CD8 T cells by immature
dendritic cells: A novel role for perforin, granzyme A, TREM-1, and
TLR7. Blood. 120:1647–1657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schenk M, Bouchon A, Seibold F and Mueller
C: TREM-1-expressing intestinal macrophages crucially amplify
chronic inflammation in experimental colitis and inflammatory bowel
diseases. J Clin Invest. 117:3097–3106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamei K, Yasuda T, Ueda T, Qiang F,
Takeyama Y and Shiozaki H: Role of triggering receptor expressed on
myeloid cells-1 in experimental severe acute pancreatitis. J
Hepatobiliary Pancreat Sci. 17:305–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu CL, Hsieh WY, Wu CL, Kuo HT and Lu YT:
Triggering receptor expressed on myeloid cells-1 in pleural
effusions: A marker of inflammatory disease. Respir Med.
101:903–909. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuai J, Gregory B, Hill A, Pittman DD,
Feldman JL, Brown T, Carito B, O'Toole M, Ramsey R, Adolfsson O, et
al: TREM-1 expression is increased in the synovium of rheumatoid
arthritis patients and induces the expression of pro-inflammatory
cytokines. Rheumatol. 48:1352–1358. 2009. View Article : Google Scholar
|
|
12
|
Schiechl G, Brunner SM, Kesselring R,
Martin M, Ruemmele P, Mack M, Hirt SW, Schlitt HJ, Geissler EK and
Fichtner-Feigl S: Inhibition of innate co-receptor TREM-1 signaling
reduces CD4(+) T cell activation and prolongs cardiac allograft
survival. Am J Transplant. 13:1168–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liao R, Sun TW, Yi Y, Wu H, Li YW, Wang
JX, Zhou J, Shi YH, Cheng YF, Qiu SJ and Fan J: Expression of
TREM-1 in hepatic stellate cells and prognostic value in hepatitis
B-related hepatocellular carcinoma. Cancer Sci. 103:984–992. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gibot S, Alauzet C, Massin F, Sennoune N,
Faure GC, Béné MC, Lozniewski A, Bollaert PE and Lévy B: Modulation
of the triggering receptor expressed on myeloid cells-1 pathway
during pneumonia in rats. J Infect Dis. 194:975–983. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guiding principles for the care and use of
vertebrate animals in research and training. Am Physiol Soc Counc.
2014.
|
|
16
|
Ramkalawan H, Wang YZ, Hurbungs A, Yang
YF, Tian FF, Zhou WB, Li J, Yang H, Xiao B and Zhang W:
Pioglitazone, PPARγ agonist, attenuates experimental autoimmune
neuritis. Inflammation. 35:1338–1347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou J, Chai F, Lu G, Hang G, Chen C, Chen
X and Shi J: TREM-1 inhibition attenuates inflammation and tumor
within the colon. Int Immunopharmacol. 17:155–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun M, Zhu M, Chen K, Nie X, Deng Q,
Hazlett LD, Wu Y, Li M, Wu M and Huang X: TREM-2 promotes host
resistance against Pseudomonas aeruginosa infection by suppressing
corneal inflammation via a PI3K/Akt signaling pathway. Invest
Ophthalmol Vis Sci. 54:3451–3462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aronovich R, Katzav A and Chapman J: The
strategies used for treatment of experimental autoimmune neuritis
(EAN): A beneficial effect of glatiramer acetate administered
intraperitoneally. Clin Rev Allergy Immunol. 42:181–188. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Horst SA, Linnér A, Beineke A, Lehne S,
Höltje C, Hecht A, Norrby-Teglund A, Medina E and Goldmann O:
Prognostic value and therapeutic potential of TREM-1 in
Streptococcus pyogenes- induced sepsis. J Innate Immun. 5:581–590.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, Liu S, Wu S, Zhu Q, Ou G, Liu C,
Wang Y, Liao Y and Sun Z: Blocking TREM-1 signaling prolongs
survival of mice with Pseudomonas aeruginosa induced sepsis. Cell
Immunol. 272:251–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abbott NJ, Rönnbäck L and Hansson E:
Astrocyte-endothelial interactions at the blood-brain barrier. Nat
Rev Neurosci. 7:41–53. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang HL, Hassan MY, Zheng XY, Azimullah
S, Quezada HC, Amir N, Elwasila M, Mix E, Adem A and Zhu J:
Attenuated EAN in TNF-alpha deficient mice is associated with an
altered balance of M1/M2 macrophages. PLoS One. 7:e381572012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Langert KA, VonZee CL and Stubbs EB Jr:
Cdc42 GTPases facilitate TNF-alpha-mediated secretion of CCL2 from
peripheral nerve microvascular endoneurial endothelial cells. J
Peripher Nerv Syst. 18:199–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Malemud CJ and Miller AH: Pro-inflammatory
cytokine-induced SAPK/MAPK and JAK/STAT in rheumatoid arthritis and
the new anti-depression drugs. Expert Opin Ther Targets.
12:171–183. 2008. View Article : Google Scholar : PubMed/NCBI
|