Introduction
Traumatic fracture is one of the most common
injuries in daily life. In clinical cases, numerous methods are
used to decrease injury, and the majority of simple fractures heal
with minimal intervention; however, in certain complex cases,
including fractures in diabetics and splintered fractures, there
may be impaired fracture healing and bone defects (1,2).
Although advances have been made, certain patients with independent
factors (fracture pattern, location, displacement, severity of soft
tissue injury, degree of bone loss, quality of surgical treatment
and presence or absence of infection) (3) require prolonged reconstructive
procedures and still experience nonunion (2).
The hypoxia-inducible factor-1α (HIF) pathway is the
central pathway for sensing and responding to alterations in the
local oxygen tension in a wide variety of organisms (4). Activation of the HIF-1α pathway is a
critical mediator of the neoangiogenesis required for skeletal
regeneration, suggesting the application of HIF activators as
therapies for improving bone healing (4). Increasing data suggests that hypoxia
may be considered a powerful stimulus for bone cells by influencing
angiogenesis via the vascular endothelial growth factor (VEGF),
cellular metabolism via the glucose transporter and recruiting
mesenchymal stem cells (MSCs) to areas of matrix damage (5–7). A
more thorough understanding of hypoxia in bone healing may identify
novel underlying cellular and molecular mechanisms that may offer
potential protective effects. In our previous study,
CoCl2 was demonstrated to enhance fracture healing by
inducing bone and cartilage formation and increasing tissue
vascularization; furthermore, CoCl2 may activate the
HIF-1α pathway (8). However,
CoCl2 has certain toxic effects on specific
hematological factors (9).
Therefore, the aim of the present study is to identify a non-toxic
alternative.
Bone ischemia-reperfusion occurs during various
clinical procedures, including orthopedic arthroplasty, plastic
gnathoplasty, spinal surgery and amputation. Fracture of bone
reduces or disrupts the tissue blood supply; during fracture
healing, neovascularization and vascular growth take place. This is
hypothesized to constitute reperfusion of an ischemic region and
the production of oxygen free radicals (10). Simulation of this
hypoxia-reoxygenation physiological process may further elucidate
understanding of the underlying mechanism.
The protective effect of ischemia/hypoxia
pre-conditioning (IPC) has been demonstrated by numerous
experimental and clinical studies. Studies have revealed that IPC
may enhance the expression of HIF-1 and VEGF (11,12).
However, the use of this technique is limited, as treatment must
occur prior to ischemia. A modification of this technique involves
brief coronary artery occlusions and reperfusions performed at
myocardial reperfusion, ischemic post-conditioning (13). In 2003, Zhao et al (14) first demonstrated reproducible
experimental results following this procedure. However, remote
conditioning was first described by Przyklenk et al
(15) in 1993. Remote ischemic
post-conditioning (RIPC) (16,17)
was developed as a protective strategy similar to local
post-conditioning. HIF-1α and VEGF expression are increased in the
ischemic muscle of animals following hind-limb ischemia (18).
The present study hypothesized that RIPC results in
accelerated fracture healing. To determine whether systemic
application of RIPC enhances rates of fracture healing in a
pre-clinical model, the effects of RIPC on callus formation,
fracture healing, callus mechanical strength and integrity and gene
expression in vivo were investigated.
Materials and methods
Animal care
All animal experiments were approved by the Animal
Care and Use Committee of Xuanwu Hospital, Capital Medical
University (Beijing, China), and were conducted according to
guidelines produced by the National Institutes of Health (Bethesda,
MD, USA). A total of 64 adult male Sprague-Dawley (SD) rats (age, 6
weeks; weight, 185–220 g) were purchased from the Laboratory Animal
Center of Capital Medical University. The animals were housed in
groups of 4 in the same animal care facility throughout the
duration of the study and were maintained at a temperature of
23–25°C and a humidity of 50–60%, under a 12-h light/dark cycle,
and with unlimited access to food and water. All efforts were made
to minimize any suffering and to reduce the total number of animals
used.
Animal experiments
Animals were acclimatized to the conditions for 1
week prior to experiments. Surgery was performed with a fracture
device, as described previously (19). Prior to the procedure, the animals
were anesthetized by an intraperitoneal injection of 1% sodium
pentobarbital (4 mg/kg body weight). Fractures were created at the
tuberositas tibiae using a blunt guillotine driven by a drop
weight; a wire driver was used to insert a steel K-wire into the
medullary canal. Radiographs were performed immediately to confirm
the extent of fractures. At 7, 14, 28 and 42 days following
fracture (n=8/group at each time point), radiographs were obtained,
and the rats were sacrificed by cervical dislocation. The K-wire
was removed, the tibiae were dissected and the fracture zone was
prepared for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR), western blotting, immunohistochemistry (IHC),
micro-computed tomography (micro-CT) and biomechanical testing.
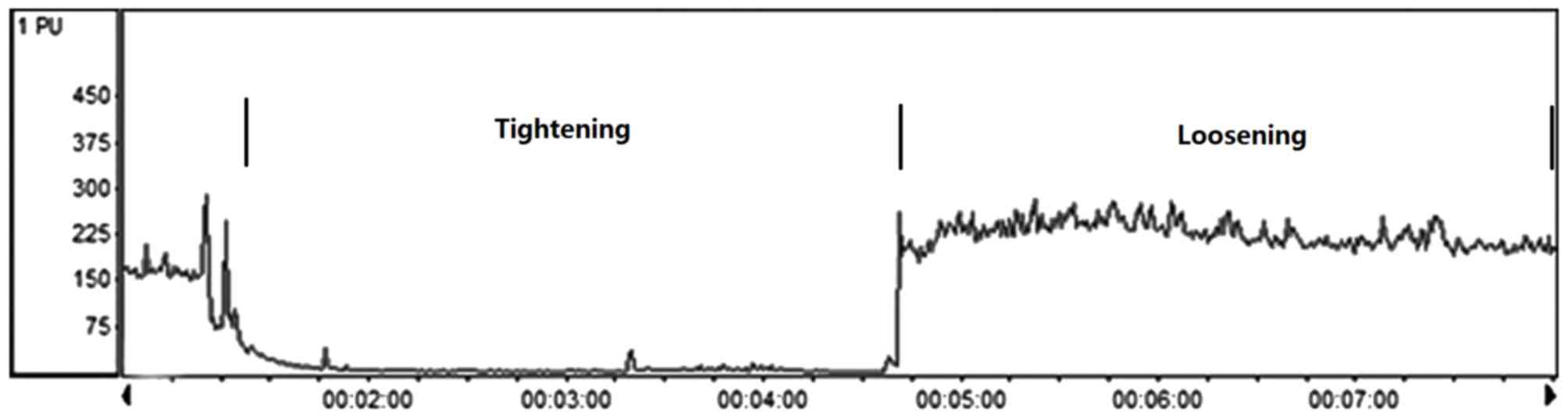
RIPC
A total of 64 male SD rats were assigned to two
groups: Control (n=32) and RIPC (n=32). RIPC was initiated
immediately following the K-wire operation by occluding blood flow
to the other hind limb. Hind limb occlusion was performed by
tightening a tourniquet (18-mm) around the upper thigh and
releasing in for 3 cycles, with each occlusion or release phase
lasting 10 min, this was performed once a day over 7 days (20). This method has been previously
demonstrated to completely occlude the blood, as assessed using the
vascular assessments system (Periflux System 5000; Perimed AB,
Järfälla, Sweden; Fig. 1). The
control group received the same dosage of sodium pentobarbital.
Micro-CT analysis
The tibia was scanned using an Inveon Micro-CT
(Siemens AG, Munich, Germany). The scan protocol was as follows: 15
µm specimen, 500 µa, 80 kv. A 3D reconstruction was performed using
Mimics software version 16.0 (Materialise NV, Leuven, Belgium). The
3D measurement area extended 2.5 mm proximally and distally from
the osteotomy line (21). The
thresholds were defined by averaging the values determined visually
by the analyses of the software (n=3 per group). Callus volumes and
gray values were quantified. Micro-CT scans at multiple time points
post-fracture (14, 28 and 42 days) were assessed blindly by three
independent investigators (22).
RNA extraction and RT-qPCR
Total RNA was extracted from fresh bone tissues of
each group using TRIzol® reagent according to the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The RNA quantity and quality were
determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). Total RNA (500 ng) was
reverse transcribed using the ReverTra Ace® (Toyobo Co.,
Ltd., Osaka, Japan) according to the manufacturer's instructions.
qPCR was performed to measure the mRNA expression levels relative
to β-actin (ACTB), using an iCycle iQ Real-Time PCR Detection
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
SYBR-Green® Master mix (Toyobo Co., Ltd.). The primers
used were as follows: Forward, 5′-CCCCTACTATGTCGCTTTCTTGG-3′ and
reverse, 5′-GGTTTCTGCTGCCTTGTATGG-3′ for HIF-1α; forward,
5′-CGACAAGGCAGACTATTCAACG-3′ and reverse,
5′-GGCACGATTTAAGAGGGGAAT-3′ for VEGF; forward,
5′-CCCACGAATGCACTATCCAG-3′ and reverse, 5′-GGCTTCCATCAGCGTCAACA-3′
for Runx2; forward, 5′-GGACGGTGAACGGGAGAAC-3′ and reverse,
5′-CCCTCAGAACAGGGTGCGTAG-3′ for ALP; forward,
5′-CGGACCACATTGGCTTCCAG-3′ and reverse, 5′-GCTGTGCCGTCCATACTTTCG-3′
for OCN; and forward, 5′-CCGTAAAGACCTCTATGCCAACA-3′ and reverse,
5′-CGGACTCATCGTACTCCTGCT-3′ for ACTB; forward,
5′-GGCAAGTTCAACGGCACAG-3′ and reverse, 5′-CGCCAGTAGACTCCACGACA-3′.
The thermocycling conditions were as follows: An initial
predenaturation step at 95°C for 2 min, followed by 40 cycles of
denaturation at 95°C for 10 sec and annealing at 60°C for 30 sec.
The expression values were normalized against GAPDH (23) using the 2−∆∆Cq method
(24). All experiments were
performed in triplicate and were repeated at least three times.
IHC analysis
Sections were prepared and processed using standard
techniques following a previously described method (25). Briefly, tissues generated from
callus were cut into 3 µm and mounted on slides. Following
deparaffinization and hydration, antigen retrieval was performed
using 0.05% trypsin, and sections were incubated with 3%
H2O2 in methanol for 10 min to inhibit
endogenous peroxide. Subsequently, slides were incubated with
primary antibodies in blocking solution (10% goat serum; Thermo
Fisher Scientific, Inc.) in a humidified chamber at 4°C overnight.
The following primary antibodies were used: Mouse anti-Runx2
(1:200; cat. no. ab76956; Abcam, Cambridge, UK), rabbit anti-ALP
(1:300; cat. no. ab95462; Abcam) and mouse anti-OCN (1:200; cat.
no. ab13420; Abcam). Following this, sections were incubated with
the biotinylated goat anti-mouse (cat. no. ab6788; dilution 1:250;
Abcam) and goat anti-rabbit IgG (cat. no. ab6720, dilution 1:100;
Abcam) for 10 min at 37°C. Following washing with PBS, the sections
were incubated with streptavidin-peroxidase conjugate (Rockland
Immunochemicals Inc. Limerick, PA, USA) for 10 min. Color was
developed using diaminobenzidine tetrahydrochloride solution
(Ventana Medical Systems, Inc., Tucson, AZ, USA). Sections were
counterstained with hematoxylin, dehydrated and mounted. Negative
controls included replacement of the primary antibody with PBS of
the same concentration.
Scoring was performed by two independent observers
who had no knowledge of the rat outcome or corresponding
pathological parameters. The percentage of stained cells and
staining intensity were scored. Staining intensity was scored as
follows: 0, no staining; 1, weak intensity; 2, moderate intensity;
and 3, high intensity. The number of positive cells was evaluated
as follows: 0 (negative), <10% positive cells; 1 (weak), <30%
positive cells; 2 (moderate), <50% positive cells; and 3
(strong), >70% positive cells.
Biomechanical testing
Hydrated tibias were tested in torsion using
previously published methods (26). The rat tibias from each group were
assessed to failure by three-point bending using a material testing
system (ELF 3400; Enduratec Systems Group; Bose Corporation,
Framingham, MA, USA). Biomechanical parameters including breaking
force (maximum load), stiffness (average slope of linear portion of
the curve before yielding) and work-to-fracture (bend strain at
maximum and bend strength at maximum) were calculated from the
force displacement data.
Western blot analysis
Western blot analysis was used to assess HIF-1α,
VEGF, ALP, Runx2 and OCN protein expression levels. Protein was
isolated from the rat callus region at 7, 14, 28 and 48 days
following fracture. Radioimmunoprecipitation assay buffer
containing protease inhibitors (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) was used to prepare tissue lysates with 1% SDS.
The total proteins (50 µg) were resolved on a 10% SDS-PAGE gels and
transferred onto polyvinylidene difluoride membranes
(Sigma-Aldrich; Merck Millipore). The membranes were blocked in 5%
skim milk in TBS containing 0.1% Tween-20 (TBST) for 1 h and
incubated overnight at 4°C with the following primary antibodies:
Mouse anti-HIF-1α (cat. no. ab463; Abcam), rabbit anti-VEGF (cat.
no. ab46154; Abcam), rabbit anti-ALP (cat. no. ab95462; Abcam),
mouse anti-Runx2 (cat. no. ab76956; Abcam), mouse anti-OCN (cat.
no. ab13420; Abcam) and actin (cat. no. sc-47778; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). All antibodies were diluted
1:1,000 in Tris-buffered saline. The blots were washed in TBST and
then incubated for 1 h with anti-goat horseradish
peroxidase-conjugated immunoglobulin G (IgG) (1:2,000; cat. nos.
ZB-2305 and ZB-2301; OriGene Technologies, Inc., Beijing, China) at
37°C. Bands and band intensity were detected using Super ECL Plus
kit (cat. no. P1010; Applygen Technologies, Inc., Beijing, China).
β-actin served to verify equal loading.
Statistical analysis
Data are expressed as the mean ± standard deviation,
and statistical analyses were performed with SPSS software, version
19.0 (IBM SPSS, Armonk, NY, USA). For comparisons between the two
groups, Student's t-test was performed. In all cases, P<0.05 was
considered to indicate a statistically significant difference.
Results
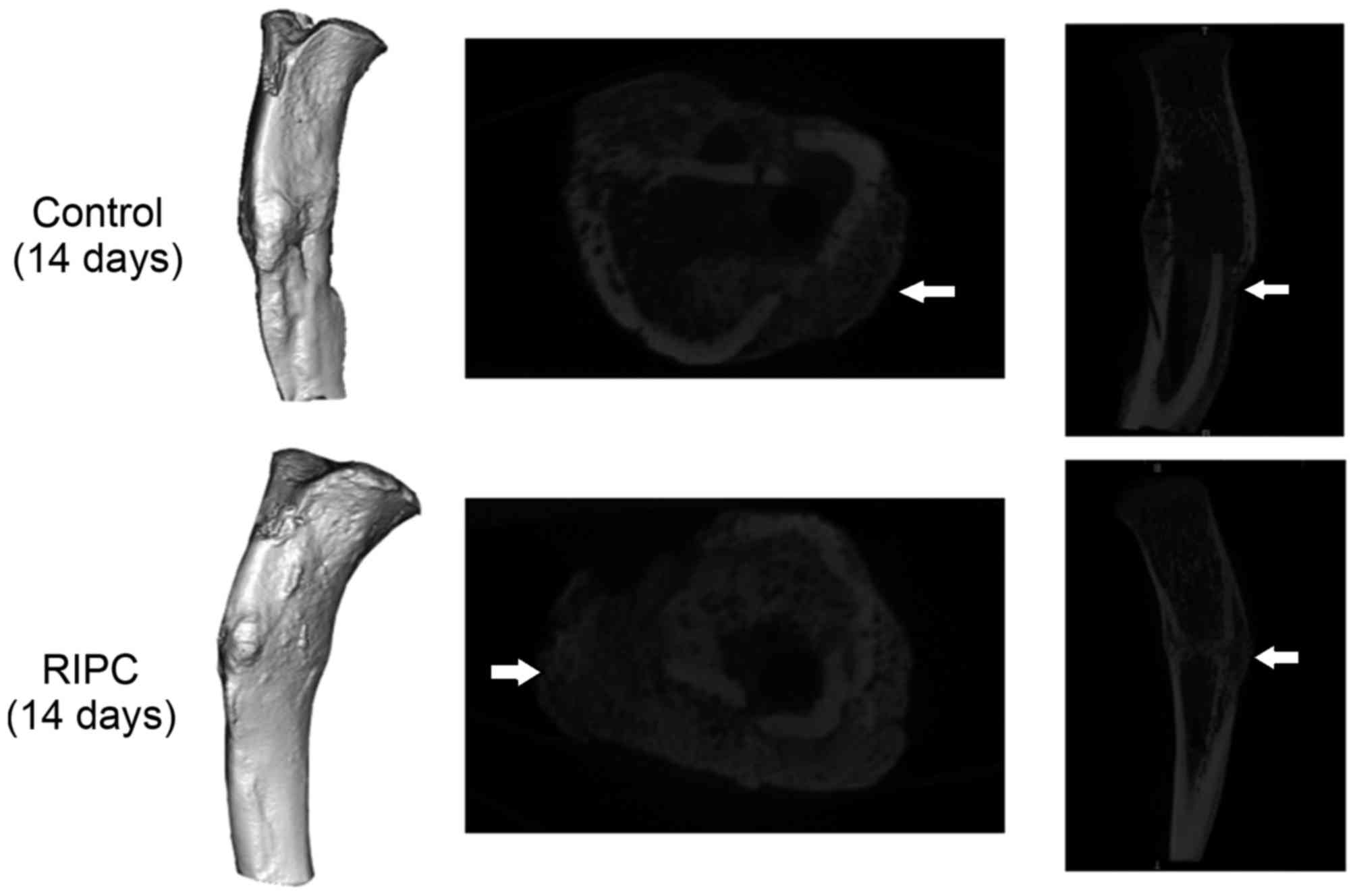
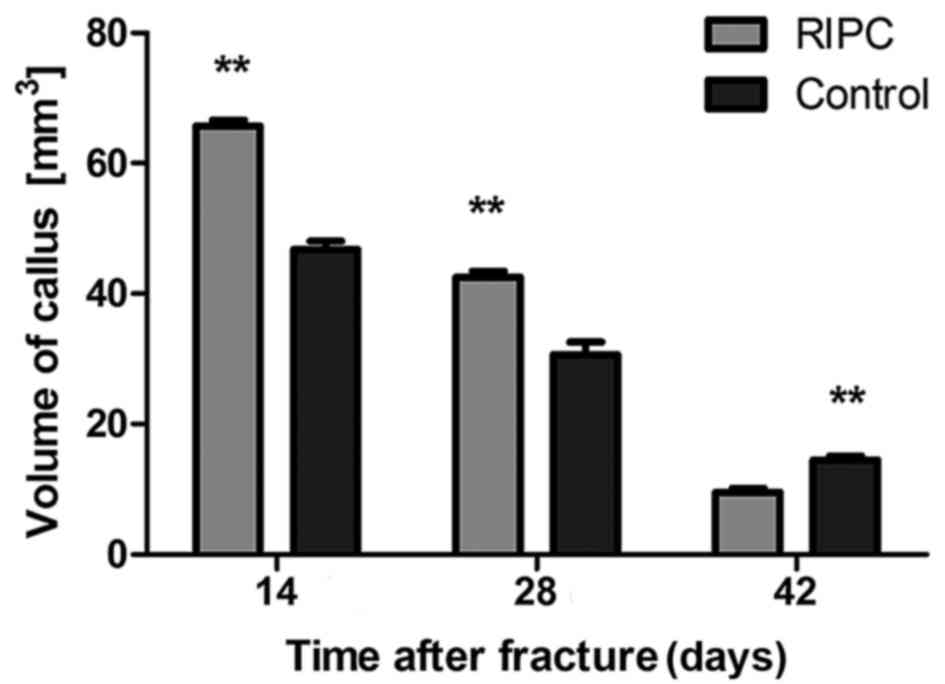
Micro CT scans of the interfragmentary
zone
After 14 and 28 days of fracture, a significant
increase in the callus volume of specimens was detected in the
group that underwent RIPC treatment compared with the control
animals (P<0.01; Figs. 2 and
3). Following 42 days, the
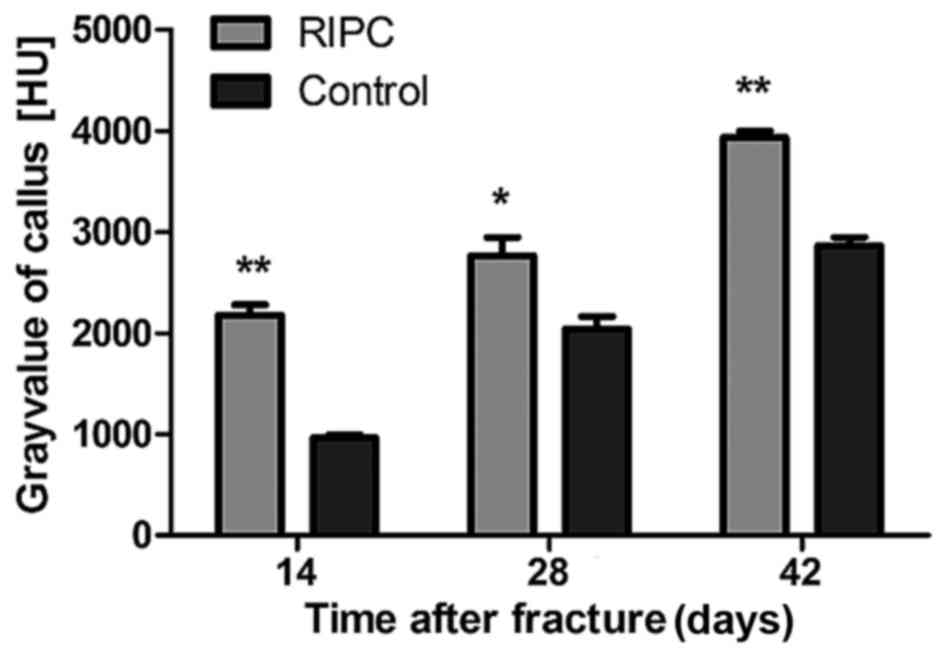
opposite trend was observed (P<0.01). To quantify the formation
of the callus, the gray value generated by micro CT scans within
the defined area was analyzed. After 14 (P<0.01), 28 (P<0.05)
and 42 (P<0.01) days, a significant increase of the gray value
was detected in the RIPC compared with the control group (Fig. 4).
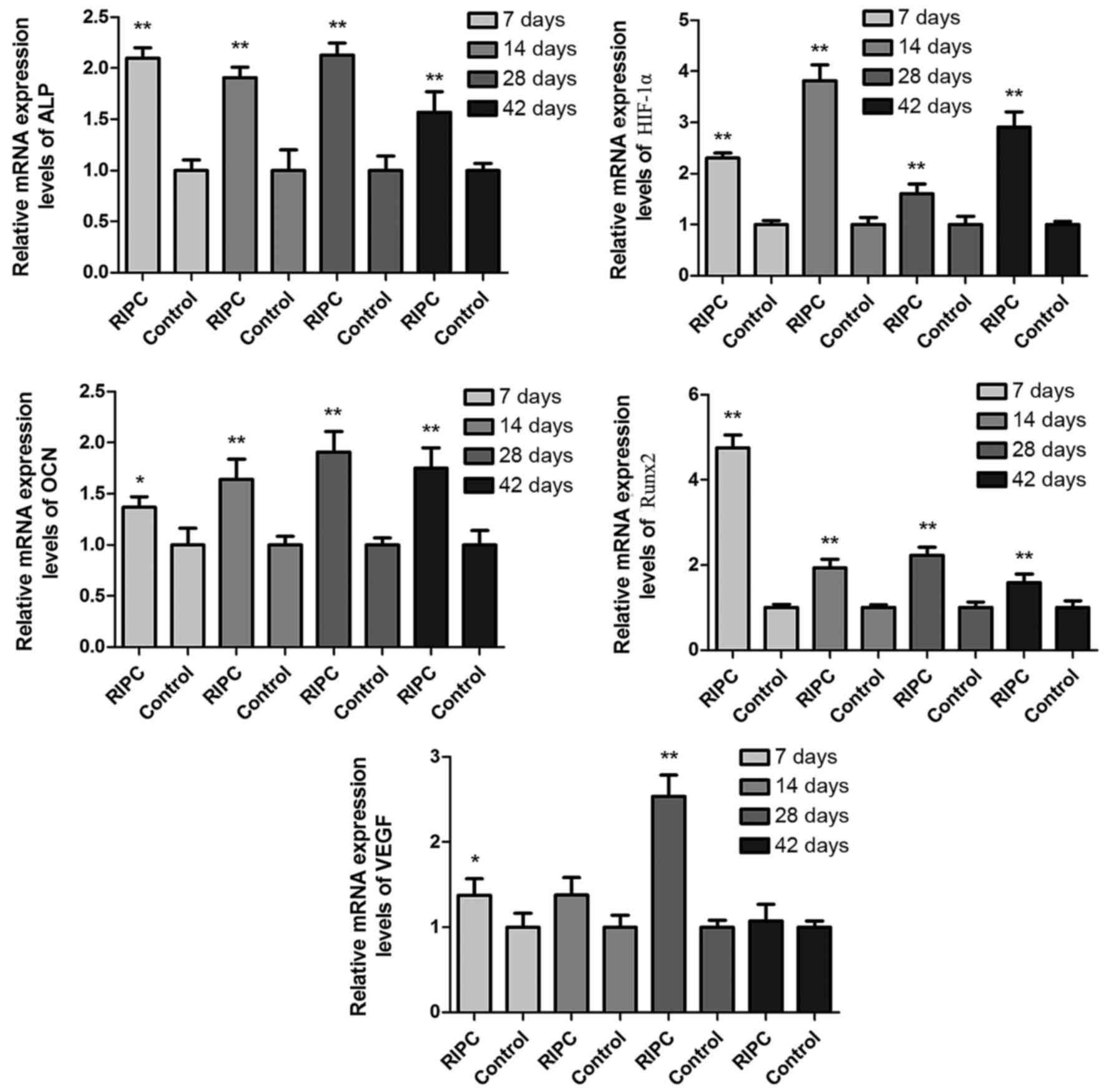
Protein and mRNA expression levels of
angiogenic mediators and osteoblast markers
Protein (Fig. 5)
and mRNA (Fig. 6) expression
levels of HIF-1α were increased in RIPC compared with control
groups at all time points (P<0.01). To further evaluate if
HIF-1α had a direct functional role in this process, the expression
of downstream genes of HIF-1α were investigated. The results of the
present study demonstrated that RIPC increased VEGF, Runx2, ALP and
OCN protein (Fig. 5) and mRNA
(Fig. 6) expression. These results
suggested that the effect of RIPC on fracture healing is partially
mediated via the HIF-1α signaling pathway.
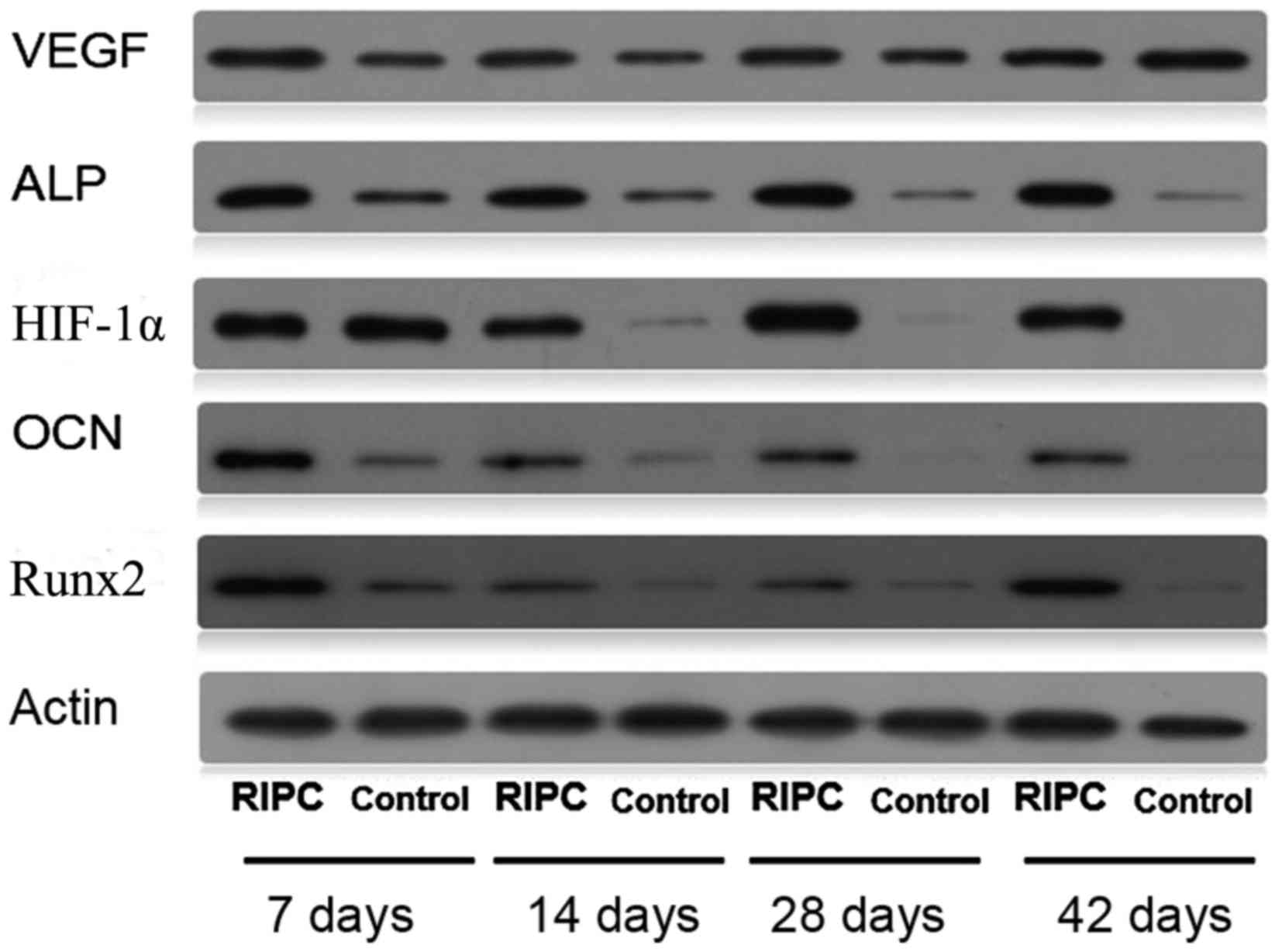 | Figure 5.Western blot analysis of bone lysates
using HIF-1α, VEGF, Runx2, ALP, OCN and β-actin antibodies. RIPC,
remote ischemic post-conditioning; HIF-1α, hypoxia-inducible
factor-1α; VEGF, vascular endothelial growth factor; Runx2,
Runt-related transcription factor 2; ALP, alkaline phosphatase;
OCN, osteocalcin. |
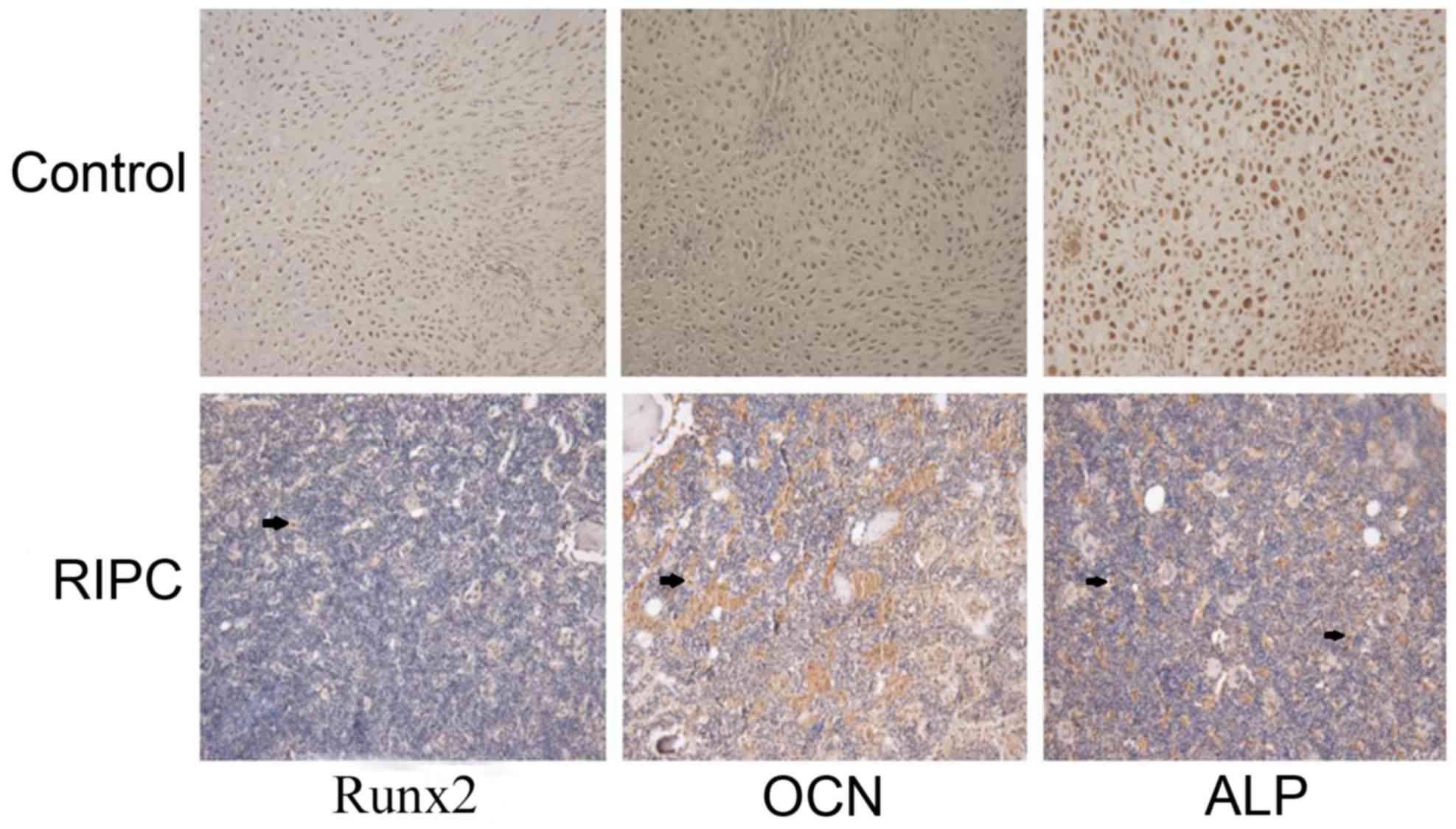
IHC analysis
Runx2 is considered to be an osteoblast-specific
transcriptional factor and is involved in chondrocyte maturation
and osteoblast differentiation (27,28).
Osteoblast-specific gene expression, including ALP and OCN, are
important markers (29). Based on
the above data, the expression of Runx2, ALP and OCN was analyzed
by IHC in bone tissues 42 days after the fracture. Increased
expression of Runx2, ALP and OCN was observed in the RIPC compared
with the control group (Fig. 7).
These data further suggested that RIPC induces fracture healing
through activation of HIF-1α and its target genes.
Biomechanical assessment
To further assess the functional features of
fracture healing, the influence of RIPC on the mechanical
properties of the tibias of rats was investigated. The dimensions
of all testing specimens were performed according to the guidelines
of the American Society for Testing and Materials for the uniaxial
strength testing (26). The RIPC
group was significantly stronger than the control group (P<0.05;
Table I). The force required to
break the bone and structural stiffness of the fractured tibia was
49.1 and 47.9% greater, respectively, compared with the control
group at 42 days (P<0.01).
 | Table I.Biomechanical testing evaluated by
three-point bending. |
Table I.
Biomechanical testing evaluated by
three-point bending.
|
| Control group
(day) | RIPC group
(day) |
|---|
|
|
|
|
|---|
| Parameter | 28 | 42 | 28 | 42 |
|---|
| Maximum force
(N) | 72.4±2.9 | 121.6±7.1 |
82.9±5.4a |
181.3±12.7b |
| Stiffness
(N/mm) | 201.1±12.1 | 310.5±21.3 |
242.1±12.1a |
459.2±19.7b |
| Work to fracture
(N-mm) | 19.9±1.1 | 35.2±3.2 | 21.9±2.5 |
51.2±4.2a |
Discussion
Normal fracture healing is a complex process
involving cellular recruitment, specific gene expression and
synthesis of compounds that regenerate native tissue to restore the
mechanical integrity and function of damaged bone (30). There are numerous aspects of
fracture healing, including biological, nutritional, physical and
genetic factors. A previous study demonstrated that hypoxia may
promote fracture healing and that the osteoblast-specific
transcriptional factor Runx2 and the osteoblast-specific genes ALP
and OCN are increased (8). These
findings provide a potential strategy to induce bone tissue
regeneration.
Remote ischemic conditioning (RIC) has been
demonstrated to induce intramyocardial cardioprotection across
different coronary artery areas (15). However, it has since been indicated
that the heart may be protected by an RIC stimulus applied to an
organ remote from the heart, including the kidney, small intestine,
brain, liver, pancreas and lung (31). The non-invasive hind limb ischemia
model was reported by Oxman et al (32), and was demonstrated to reduce
reperfusion arrhythmias in a rat heart following a sustained
ischemic insult. Numerous studies have since confirmed the use of
the lower limb for RIC due to its ease of access (11,33–35).
This method has been revealed to be effective and reproducible in
reducing injury of other organs in animals and humans (36).
Growing evidence indicated that HIF-1α may be
involved in the early phase of IPC, within min of the IPC stimulus
(37). In the present study,
upregulated expression of HIF-1α was detected in bone tissue.
HIF-1α interacts with the core DNA sequence of 5′-[AG]CGTG-3′ at
the hypoxia response element target gene promoters to upregulate
numerous hypoxia-sensitive genes, including VEGF (38). The present study revealed that RIPC
has positive effects on the expression of Runx2, which is
considered to be the primary controlling transcription factor in
osteoblast differentiation (39);
ALP is another essential factor. Runx2 and ALP are required for
osteogenesisin vivo (40,41).
OCN is an important marker of mature osteoblasts. The protein
serves a role in the differentiation of osteoblast progenitor
cells, with significant upregulation observed in matrix synthesis
and mineralization (42,43). Micro-CT in the present study
demonstrated that the volume of callus in the RIPC group was
significantly larger compared with the control group during the
callus formation stage (at 14 days), and the gray value, which
represents bone mineral density, was additionally increased in the
RIPC group. At 42 days, the gray value of the RIPC group approached
healthy cortical bone (gray-value=4120±112 HU). The biomechanical
properties of the healing construct may be attributed to density
and quantity of tissue; biomechanical analysis revealed that RIPC
significantly increased the maximum load, stiffness and energy
absorption. Taken together, these results suggested that RIPC
enhanced the osteoblast proliferation rate and recovered bone
nodular mineralization.
Although various studies have demonstrated that
remote conditioning shares similar mechanistic signaling pathways
with local conditioning (44,45),
evidence indicates that the IPC and RIPC signaling pathways are not
identical (46). The following
three potential underlying mechanisms of RIPC have been proposed:
i) Humoral factors released in the pre-conditioned organ are
transported via the blood circulation to protect the target organ
(47); ii) neurogenic transmission
with involvement of muscle afferents and the autonomic nervous
system (47); and iii)
immunomodulation (48). Several
studies have suggested that the underlying protective mechanisms of
RIPC are associated with its ability to attenuate the production of
free radicals, promote the cell survival pathway, modulate the
immune system and inhibit the apoptotic cell signaling pathways
(49–51). However, further research is
required to determine the exact mechanisms underlying RIPC.
In recent years, RIPC has emerged as an effective
strategy for reducing myocardial ischemia/reperfusion injury. The
ability to use transient limb ischemia as a remote conditioning
stimulus has facilitated progression from the bench to the bedside
and has applications in various clinical settings (11). The present study supports the use
of RIPC as a valid strategy to enhance fracture healing. These
experimental observations may be translated into the clinical
setting. In addition, the present study adds information regarding
the mechanisms of RIPC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant. no. 81541135).
References
|
1
|
Marsell R and Einhorn TA: The biology of
fracture healing. Injury. 42:551–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giannoudis PV, Jones E and Einhorn TA:
Fracture healing and bone repair. Injury. 42:549–550. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bishop JA, Palanca AA, Bellino MJ and
Lowenberg DW: Assessment of compromised fracture healing. J Am Acad
Orthop Surg. 20:273–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wan C, Gilbert SR, Wang Y, Cao X, Shen X,
Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC,
et al: Activation of the hypoxia-inducible factor-1alpha pathway
accelerates bone regeneration. Proc Natl Acad Sci USA. 105:686–691.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raheja LF, Genetos DC and Yellowley CE:
Hypoxic osteocytes recruit human MSCs through an OPN/CD44-mediated
pathway. Biochem Biophys Res Commun. 366:1061–1066. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Street J, Bao M, deGuzman L, Bunting S,
Peale FV Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL,
Daugherty A, et al: Vascular endothelial growth factor stimulates
bone repair by promoting angiogenesis and bone turnover. Proc Natl
Acad Sci USA. 99:9656–9661. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loboda A, Jazwa A, Wegiel B, Jozkowicz A
and Dulak J: Heme oxygenase-1-dependent and -independent regulation
of angiogenic genes expression: Effect of cobalt protoporphyrin and
cobalt chloride on VEGF and IL-8 synthesis in human microvascular
endothelial cells. Cell Mol Biol (Noisy-le-grand). 51:347–355.
2005.PubMed/NCBI
|
|
8
|
Huang J, Liu L, Feng M, An S, Zhou M, Li
Z, Qi J and Shen H: Effect of CoCl2 on fracture repair in a rat
model of bone fracture. Mol Med Rep. 12:5951–5956. 2015.PubMed/NCBI
|
|
9
|
Saeedi SS, Karami S, Karami B and
Shokrzadeh M: Toxic effects of cobalt chloride on hematological
factors of common carp (Cyprinus carpio). Biol Trace Elem Res.
132:144–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baik SW, Park BS, Kim YH, Kim YD, Kim CH,
Yoon JY and Yoon JU: Effects of remifentanil preconditioning on
osteoblasts under hypoxia-reoxygenation condition. Int J Med Sci.
12:583–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalakech H, Tamareille S, Pons S,
Godin-Ribuot D, Carmeliet P, Furber A, Martin V, Berdeaux A, Ghaleh
B and Prunier F: Role of hypoxia inducible factor-1α in remote limb
ischemic preconditioning. J Mol Cell Cardiol. 65:98–104. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stefanini MO, Wu FT, Mac Gabhann F and
Popel AS: A compartment model of VEGF distribution in blood,
healthy and diseased tissues. BMC Syst Biol. 2:772008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szijártó A, Czigány Z, Turóczi Z and
Harsányi L: Remote ischemic perconditioning-a simple, low-risk
method to decrease ischemic reperfusion injury: Models, protocols
and mechanistic background. A review. J Surg Res. 178:797–806.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F,
Wang NP, Guyton RA and Vinten-Johansen J: Inhibition of myocardial
injury by ischemic postconditioning during reperfusion: Comparison
with ischemic preconditioning. Am J Physiol Heart Circ Physiol.
285:H579–H588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Przyklenk K, Bauer B, Ovize M, Kloner RA
and Whittaker P: Regional ischemic ‘preconditioning’ protects
remote virgin myocardium from subsequent sustained coronary
occlusion. Circulation. 87:893–899. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren C, Yan Z, Wei D, Gao X, Chen X and
Zhao H: Limb remote ischemic postconditioning protects against
focal ischemia in rats. Brain Res. 1288:88–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiong J, Liao X, Xue FS, Yuan YJ, Wang Q
and Liu JH: Remote ischemia conditioning-an endogenous
cardioprotective strategy from outside the heart. Chin Med J
(Engl). 124:2209–2215. 2011.PubMed/NCBI
|
|
18
|
Leosco D, Rengo G, Iaccarino G, Sanzari E,
Golino L, De Lisa G, Zincarelli C, Fortunato F, Ciccarelli M,
Cimini V, et al: Prior exercise improves age-dependent vascular
endothelial growth factor downregulation and angiogenesis responses
to hind-limb ischemia in old rats. J Gerontol A Biol Sci Med Sci.
62:471–480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petersen W, Wildemann B, Pufe T, Raschke M
and Schmidmaier G: The angiogenic peptide pleiotrophin (PTN/HB-GAM)
is expressed in fracture healing: An immunohistochemical study in
rats. Arch Orthop Trauma Surg. 124:603–607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren C, Wang P, Wang B, Li N, Li W, Zhang
C, Jin K and Ji X: Limb remote ischemic per-conditioning in
combination with post-conditioning reduces brain damage and
promotes neuroglobin expression in the rat brain after ischemic
stroke. Restor Neurol Neurosci. 33:369–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Komrakova M, Weidemann A, Dullin C, Ebert
J, Tezval M, Stuermer KM and Sehmisch S: The impact of strontium
ranelate on metaphyseal bone healing in ovariectomized rats. Calcif
Tissue Int. 97:391–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bouxsein ML, Boyd SK, Christiansen BA,
Guldberg RE, Jepsen KJ and Muller R: Guidelines for assessment of
bone microstructure in rodents using micro-computed tomography. J
Bone Miner Res. 25:1468–1486. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou M, Shen D, Head JE, Chew EY,
Chévez-Barrios P, Green WR and Chan CC: Ocular clusterin expression
in von Hippel-Lindau disease. Mol Vis. 13:2129–2136.
2007.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gerstenfeld LC, Wronski TJ, Hollinger JO
and Einhorn TA: Application of histomorphometric methods to the
study of bone repair. J Bone Miner Res. 20:1715–1722. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gutierrez GE, Edwards JR, Garrett IR,
Nyman JS, McCluskey B, Rossini G, Flores A, Neidre DB and Mundy GR:
Transdermal lovastatin enhances fracture repair in rats. J Bone
Miner Res. 23:1722–1730. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inada M, Yasui T, Nomura S, Miyake S,
Deguchi K, Himeno M, Sato M, Yamagiwa H, Kimura T, Yasui N, et al:
Maturational disturbance of chondrocytes in Cbfa1-deficient mice.
Dev Dyn. 214:279–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lian JB, Javed A, Zaidi SK, Lengner C,
Montecino M, van Wijnen AJ, Stein JL and Stein GS: Regulatory
controls for osteoblast growth and differentiation: Role of
Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 14:1–41. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mimori K, Komaki M, Iwasaki K and Ishikawa
I: Extracellular signal-regulated kinase 1/2 is involved in
ascorbic acid-induced osteoblastic differentiation in periodontal
ligament cells. J Periodontol. 78:328–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Komatsu DE and Warden SJ: The control of
fracture healing and its therapeutic targeting: Improving upon
nature. J Cell Biochem. 109:302–311. 2010.PubMed/NCBI
|
|
31
|
Lim SY and Hausenloy DJ: Remote ischemic
conditioning: From bench to bedside. Front Physiol. 3:272012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oxman T, Arad M, Klein R, Avazov N and
Rabinowitz B: Limb ischemia preconditions the heart against
reperfusion tachyarrhythmia. Am J Physiol. 273:H1707–H1712.
1997.PubMed/NCBI
|
|
33
|
Jeanneteau J, Hibert P, Martinez MC,
Tual-Chalot S, Tamareille S, Furber A, Andriantsitohaina R and
Prunier F: Microparticle release in remote ischemic conditioning
mechanism. Am J Physiol Heart Circ Physiol. 303:H871–H877. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Karakoyun R, Koksoy C, Yilmaz TU, Altun H,
Banli O, Albayrak A, Alper M and Sener Z: The angiogenic effects of
ischemic conditioning in experimental critical limb ischemia. Eur J
Vasc Endovasc Surg. 47:172–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Liu X, Yan F, Min L, Ji X and Luo
Y: Protective effects of remote ischemic preconditioning in rat
hindlimb on ischemia- reperfusion injury. Neural Regen Res.
7:583–587. 2012.PubMed/NCBI
|
|
36
|
Tapuria N, Kumar Y, Habib MM, Amara Abu M,
Seifalian AM and Davidson BR: Remote ischemic preconditioning: A
novel protective method from ischemia reperfusion injury-a review.
J Surg Res. 150:304–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi W and Vinten-Johansen J: Endogenous
cardioprotection by ischaemic postconditioning and remote
conditioning. Cardiovasc Res. 94:206–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Formenti F, Constantin-Teodosiu D,
Emmanuel Y, Cheeseman J, Dorrington KL, Edwards LM, Humphreys SM,
Lappin TR, McMullin MF, McNamara CJ, et al: Regulation of human
metabolism by hypoxia-inducible factor. Proc Natl Acad Sci USA.
107:12722–12727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Komori T: Regulation of osteoblast
differentiation by transcription factors. J Cell Biochem.
99:1233–1239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Banerjee C, Javed A, Choi JY, Green J,
Rosen V, van Wijnen AJ, Stein JL, Lian JB and Stein GS:
Differential regulation of the two principal Runx2/Cbfa1 n-terminal
isoforms in response to bone morphogenetic protein-2 during
development of the osteoblast phenotype. Endocrinology.
142:4026–4039. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao J, Fu S, Zeng Z, Li F, Niu Q, Jing D
and Feng X: Cyclic stretch promotes osteogenesis-related gene
expression in osteoblast-like cells through a cofilin-associated
mechanism. Mol Med Rep. 14:218–224. 2016.PubMed/NCBI
|
|
42
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Murshed M, Harmey D, Millán JL, McKee MD
and Karsenty G: Unique coexpression in osteoblasts of broadly
expressed genes accounts for the spatial restriction of ECM
mineralization to bone. Genes Dev. 19:1093–1104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hausenloy DJ and Yellon DM: Remote
ischaemic preconditioning: Underlying mechanisms and clinical
application. Cardiovasc Res. 79:377–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Selzner N, Boehnert M and Selzner M:
Preconditioning, postconditioning, and remote conditioning in solid
organ transplantation: Basic mechanisms and translational
applications. Transplant Rev (Orlando). 26:115–124. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Grall S, Prunier-Mirebeau D, Tamareille S,
Mateus V, Lamon D, Furber A and Prunier F: Endoplasmic reticulum
stress pathway involvement in local and remote myocardial ischemic
conditioning. Shock. 39:433–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kanoria S, Jalan R, Seifalian AM, Williams
R and Davidson BR: Protocols and mechanisms for remote ischemic
preconditioning: A novel method for reducing ischemia reperfusion
injury. Transplantation. 84:445–458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao H: The protective effects of ischemic
postconditioning against stroke: From rapid to delayed and remote
postconditioning. Open Drug Discov J. 5:138–147. 2011.PubMed/NCBI
|
|
49
|
Hoda MN, Fagan SC, Khan MB, Vaibhav K,
Chaudhary A, Wang P, Dhandapani KM, Waller JL and Hess DC: A 2×2
factorial design for the combination therapy of minocycline and
remote ischemic perconditioning: Efficacy in a preclinical trial in
murine thromboembolic stroke model. Exp Transl Stroke Med.
6:102014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang JY, Shen J, Gao Q, Ye ZG, Yang SY,
Liang HW, Bruce IC, Luo BY and Xia Q: Ischemic postconditioning
protects against global cerebral ischemia/reperfusion-induced
injury in rats. Stroke. 39:983–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xing B, Chen H, Zhang M, Zhao D, Jiang R,
Liu X and Zhang S: Ischemic postconditioning inhibits apoptosis
after focal cerebral ischemia/reperfusion injury in the rat.
Stroke. 39:2362–2369. 2008. View Article : Google Scholar : PubMed/NCBI
|