Introduction
Mesenchymal stem cells (MSCs) are a promising cell
source for regenerative medicine therapies, primarily due to their
multipotency and immunosuppressive functions (1). MSCs are also used for cell therapy in
myocardial repair (2) and to
reduce scar size following acute myocardial infarction (3). However, the therapeutic use of MSCs
has been limited by many factors, including the difficulty of
obtaining sufficient numbers of cells. Current methods include the
in vitro expansion of MSCs in plastic adherent culture prior
to in vivo transplantation (4), but the benefits of this cell transfer
method are modest and short-lived. This may be partially attributed
to poor survival and retention of the transplanted cells (5), which limits successful cell therapy
for cardiac repair. Previous studies have identified multiple
factors that are responsible for the poor survival of MSCs
following transplantation into the myocardium, including ischemia,
inflammatory response, hypoxia, and oxidative stress (6,7).
Vunjak-Novakovic and Scadden (8) have classified the cellular and
acellular components of the stem cell niche. The plastic adherent
condition is considered a suitable niche for amplification of MSCs
in vitro, promoting cells survival. To determine the effect
of culture conditions on apoptosis of human MSCs (hMSCs), hMSCs
were cultured in vitro either in ultra-low-adherence culture
plates (to mimic nonadherent conditions) or in standard tissue
culture plates (to mimic adherent conditions), as previously
described (9). Apoptosis was then
analyzed by flow cytometry. In addition, mRNA and protein
expression levels of caspase-3, −7, −8, and −9 were analyzed by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis respectively. The present study
demonstrated that removal from adherent culture conditions resulted
in hMSC apoptosis through activation of the caspase pathway.
Materials and methods
Isolation and expansion of hMSCs
Bone marrow was aspirated from the posterior iliac
crest of three healthy adult volunteers. All procedures were
performed with the approval of the ethics committee of SunYat-sen
Memorial Hospital, Sun Yat-sen University (approval number no.
2014-57; Guangzhou, China) and following informed written consent
by the volunteers. Nucleated cells were isolated with a density
gradient (Lymphoprep, Stemcell Technologies, Inc., Vancouver,
Canada) and resuspended in MSC culture medium (MesenCult
Proliferation kit; Stemcell Technologies, Inc.), according to the
manufacturer's instructions. Nucleated cells (1.2×107)
were plated in 20 ml culture medium in T75 tissue culture flasks
(Corning, Inc., Corning, NY, USA) and incubated at 37°C with 5%
CO2 and 20% O2. Following 24 h, nonadherent
cells were discarded and adherent cells were thoroughly washed
twice with PBS. The culture medium was changed every three days and
following 10 days in culture, cells were 80% confluent. The cells
were then incubated with 0.05% trypsin-EDTA (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 5 min at 37°C, and replated
at 2,500 cells/cm2 in T75 tissue culture flasks.
Following 5 days incubation, the cultured cells were 80% confluent
and were suspended by incubation in 0.05% trypsin-EDTA for 5 min at
37°C and rinsed with 5–7 ml culture medium, followed by collection
in a 50-ml centrifuge tube. Cells were subsequently centrifuged at
300 × g (Sorvall™ ST 16R; Thermo Fisher Scientific, Inc.)
for 5 min at room temperature. Pellets were washed with culture
medium and centrifuged at 300 × g for 5 min at room
temperature again, following which the cells were resuspended in
10% dimethyl sulfoxide and 90% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) at 2×105 cells per
freezing vial, and frozen in liquid nitrogen (passage-1 cells). To
expand a culture, a frozen vial of MSCs was thawed, plated in a T75
tissue culture flask, and incubated for 5 days at 37°C with 5%
CO2 and 20% O2 (passage-2 cells).
Fluorescence-Activated Cell Sorting
(FACS) analysis of MSC surface markers
Passage-1 cells were plated in T75 tissue culture
flasks after being thawed from liquid nitrogen, and incubated at
37°C with 5% CO2 and 20% O2. When the
cultured cells reached 90% confluency (passage-2 cells), they were
harvested, centrifuged at 300 × g for 5 min, and resuspended
in PBS. Cells were then stained with mouse anti-human CD11b (cat.
no. 557321; 1:100), CD14 (cat. no. 557154; 1:100), CD34 (cat. no.
550619; 1:100), CD45 (cat. no. 555482; 1:100), CD73 (cat. no.
561014; 1:100), CD90 (cat. no. 555596; 1:100) and CD105 (cat. no.
561443; 1:100) antibodies (BD Biosciences, Franklin Lakes, NJ, USA)
and analyzed by flow cytometry using a BD FACSCalibur (BD
Biosciences). An isotype control immunoglobulin (cat no. PE-R3-34;
BD Biosciences) was used at the indicated concentration. BD
FACSComp software (version no. 5.1) was used for the data analysis
(BD Biosciences).
Colony-Forming Unit-Fibroblast (CFU-F)
assay
Passage-2 cells were plated into 6-well plates at
100 cells/well in complete Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS,
10 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES;
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, 100
µg/ml streptomycin and incubated at 37°C with 5% CO2 and
20% O2 for 12 days. The colonies were stained with
Giemsa (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). A
cluster of >50 cells was counted as one colony, and images were
acquired using a Leica Application Suite software (version no. 4.0;
Leica Microsystems GmbH, Wetzlar, Germany).
In vitro differentiation assay
The assay was performed with passage-2 cultured
hMSCs according to a previously described protocol (10), for evaluation of their multipotent
differentiation ability. The cells were split into three separate
differentiation-specific media to induce osteoblast, adipocyte, or
chondrocyte differentiation. The media were changed every 3–5 days
for 3–4 weeks. For adipocyte differentiation, the cultures were
incubated in DMEM containing 10% FBS, 10 µM HEPES, 100 U/ml
penicillin, 100 µg/ml streptomycin, 5 µg/ml insulin, 20 µM
indomethacin, 0.5 µM isobutylmethylxanthine (Sigma-Aldrich; Merck
Millipore), and 1×10−7 M dexamethasone (Sigma-Aldrich;
Merck Millipore). Following 28 days' incubation, the cells were
fixed with 4% formalin and stained with 0.5% oil red O. For
osteoblast differentiation, the cells were cultured in DMEM
containing 10% FBS, 10 mM HEPES, 100 U/ml penicillin, 100 U/ml
streptomycin, 50 µg/ml ascorbic acid (Sigma-Aldrich; Merck
Millipore), 1×10−7 M dexamethasone and 10 mM glycerol
phosphate. Following 21 days' incubation, the cells were fixed with
4% formalin and stained with 1% alizarin red S (pH 4.1).
Chondrocyte differentiation was induced with OriCell Human
Mesenchymal Stem Cell Chondrogenic Differentiation Medium (Cyagen
Biosciences, Guangzhou, China), according to the manufacturer's
instructions. The chondrogenic pellets were harvested following 18
days in culture, fixed with 4% formalin, and embedded in paraffin
for Alcian blue staining. Adipocyte and chondrocyte differentiation
assays were carried out similarly for both adherent and nonadherent
cultured cells at 72 h to evaluate their differentiation
potential.
Expansion of MSCs and nonadherent in
vitro culture conditions
Passage-2 cultures were plated in 20 ml complete
DMEM containing 10% FBS, 10 mM HEPES, 100 U/ml penicillin, and 100
µg/ml streptomycin in T75 tissue culture flasks. The cells were
incubated at 37°C with 20% O2 and 5% CO2. The
medium was changed every 5–7 days. When MSCs reached 90%
confluency, they were suspended by incubation in 0.05% trypsin-EDTA
for 5 min at 37°C and then reseeded at 2,500 cells/cm2
in a T75 tissue culture flask (passage-3 cells). To test the effect
of adherent vs. nonadherent culture conditions in MSCs, passage 3
cells were seeded at 2,500 cells/cm2 in adherent culture
plates (BD Biosciences) or in ultra-low-adherence tissue culture
plates (Corning, Inc.) respectively, and allowed to grow for 24 or
72 h prior to experimental assays.
Flow cytometry assay for
apoptosis
Adherent-cultured cells and nonadherent-cultured
cells grown for 24 or 72 h were collected in 1.5 ml Eppendorf (EP)
tubes and centrifuged at 300 × g for 5 min at room
temperature. Pellets were washed twice with PBS, followed by
resuspension in 400 µl binding buffer (as per the kit instructions;
eBioscience, Inc., San Diego, CA, USA), and divided equally into
two new tubes. One sample was used as the negative control, while 5
µl Annexin V-FITC (eBioscience, Inc.) was added to the other sample
and incubated at room temperature for 15 min. Propidium iodide (10
µl) was added to both samples, and they were analyzed by flow
cytometry on a FACSCalibur using CellQuest software (version no.
3.3; BD Biosciences).
RT-qPCR
Adherent-cultured cells and nonadherent-cultured
cells grown for 24 or 72 h were collected in 50-ml centrifuge tubes
and centrifuged at 300 × g for 5 min at room temperature.
Pellets were resuspended with Dulbecco's PBS and transferred into
1.5 ml EP tubes. Total RNA was extracted using the High Pure RNA
Isolation kit (Roche Diagnostics, Ltd., Basel, Switzerland),
according to the manufacturer's instructions. cDNA was synthesized
from 1 µg RNA using SuperScript III and Oligo primers (Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. Primers were as follows: caspase-3, forward 5-CTG GAC
TGT GGC ATT GAG AC-3′ and reverse 5′-ACAAAGCGACTGGATGAACC-3′;
caspase-7, forward 5′-CAAGATCCCAGTGGAAGCTGAC-3′ and reverse
5′-GGCTTGCACAAACCAGGAG-3′; caspase-8, forward
5′-GAAGGTGCTACCATCGTGAGAGTAA-3′ and reverse
5′-CCTGGAGTCTCTGGAATAACATCAA-3′; caspase-9, forward
5′-CGCAAACCAGAGGTTCTCAGAC-3′ and reverse
5′-AGGATGTAAGCCTGCCAGCAC-3′; and GAPDH, forward
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse 5′-TGGTGAAGACGCCAGTGGA-3′.
qPCR analysis was performed using the LightCycler 480 platform and
the LightCycler 480 SYBR-Green I Master kit (Roche Diagnostics,
Ltd.). Each reaction mixture contained 10 ml SYBR Green I master
mix, 6 µl RNase-free H2O, 1 µl 10 mM forward primer, 1
µl 10 mM reverse primer, and 2 µl cDNA (undiluted) in a final
reaction volume of 20 ml. Gene expression was normalized to the
internal reference gene β-actin. The 2−∆∆Cq method
(11) was used to determine
relative fold changes using the LightCycler480 software (Roche
Diagnostics, Ltd.). The experiment was performed three independent
times in triplicate.
Western blot analysis
Adherent-cultured cells and nonadherent-cultured
cells grown for 24 or 72 h were lysed on ice for 5 min using Cell
Lysis Buffer (Cell Signaling Technology, Inc., Danvers, MA, USA)
containing 1 mM phenylmethanesulfonyl fluoride (Beyotime Institute
of Biotechnology, Haimen, China) and protease inhibitors (Roche
Diagnostics, Ltd.), then sonicated briefly and centrifuged for 10
min at 14,000 × g in a cold microfuge, according to the
manufacturer' protocol. Protein concentrations were determined
using the bicinchoninic acid method (Beyotime Institute of
Biotechnology). Total protein samples (30 µg per lane) were
separated by 8% SDS-PAGE and transferred to polyvinylidene fluoride
membranes (Beyotime Institute of Biotechnology). Following blocking
with 5% dried skim milk in TBS/0.1% Tween-20 (TBST) for 1 h at room
temperature, the membranes were incubated overnight at 4β with
primary antibodies (1:1,000 dilution) against caspase-3 (cat. no.
9665; Cell Signaling Technology, Inc.), caspase-7 (cat. no. 12,827;
Cell Signaling Technology, Inc.), caspase-8 (cat. no. AC056;
Beyotime Institute of Biotechnology), caspase-9 (cat. no. 9508;
Cell Signaling Technology, Inc.) and β-actin (cat. no. AA128;
Beyotime Institute of Biotechnology) in primary antibody dilution
buffer. Following 3 washes with TBST, membranes were incubated with
horseradish peroxidase (HRP)-linked anti-mouse immunoglobulin (Ig)
G antibody (cat. no. 7076; 1:2,000 dilution; Cell Signaling
Technology, Inc.) or HRP-linked anti-rabbit IgG antibody (cat. no.
7074; 1:2,000 dilution; Cell Signaling Technology, Inc.) for 1 h at
room temperature. Protein expression signals were detected with
BeyoECL Plus enhanced chemiluminescence reagent (Beyotime Institute
of Biotechnology) using the Intelligent imaging system (Syngene,
Frederick, MD, USA) and ImageJ software (version no. 1.44)
(12), according to the
manufacturer's instructions.
Statistical analysis
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla,
CA, USA) was used to perform Student's t-test analysis in
order to identify significant differences between culture
conditions. P<0.05 was considered to indicate a statistically
significant difference.
Results
CFU-F and in vitro differentiation
assays
Growth and multipotency are key features of MSCs. To
confirm the functional properties of the hMSCs isolated in the
present study, their clonogenic and multipotent lineage potential
were evaluated by CFU-F and in vitro differentiation assays
respectively. CFUs were detected within the cell population, and
cells within individual colonies exhibited fibroblast-like
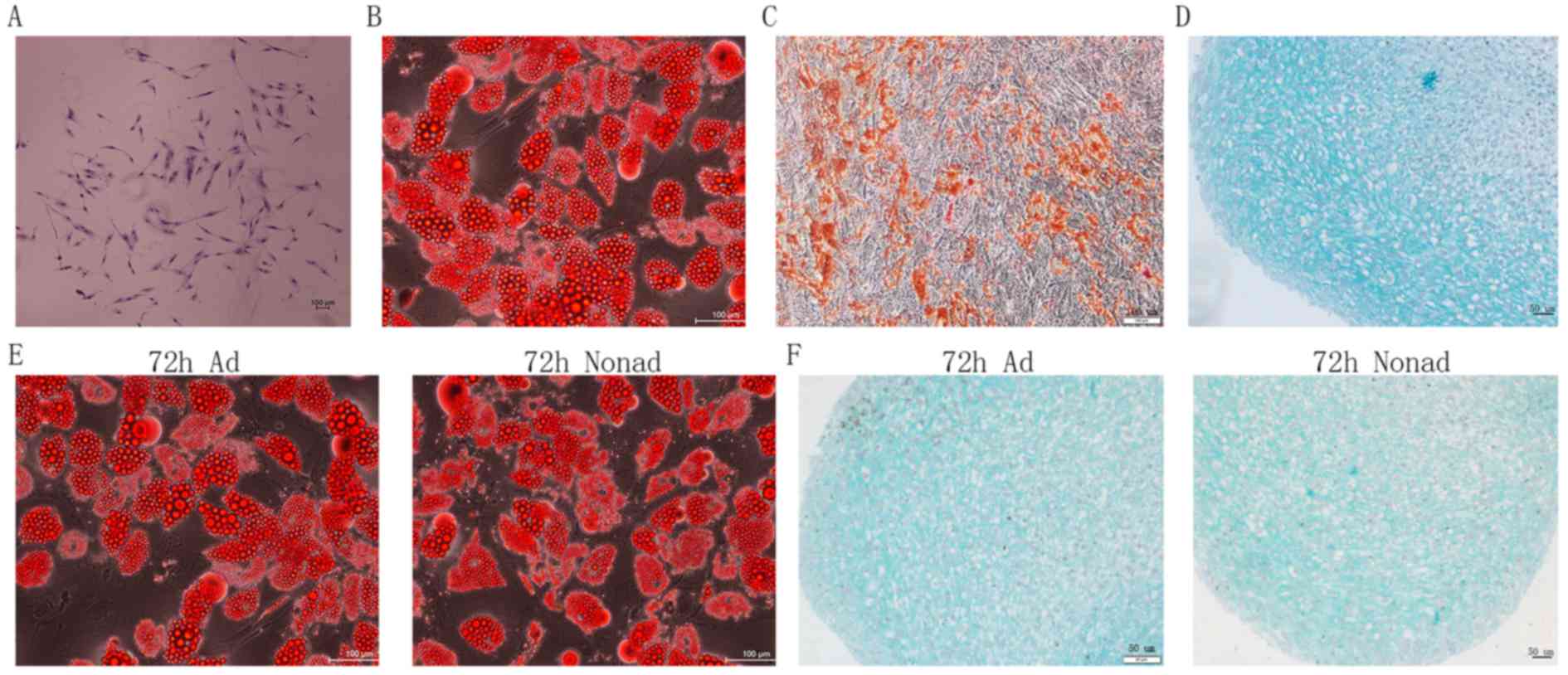
morphology (Fig. 1A), indicating
good self-renewing properties of the isolated cell populations. The
differentiation assays of passage-2 hMSCs demonstrated that the
cells were able to generate adipocytes (Fig. 1B), osteoblasts (Fig. 1C), and chondrocytes (Fig. 1D) in vitro. These findings
suggest that the cells exhibit the multipotent differentiation
potential characteristic of MSCs. In addition, adherent and
nonadherent-cultured cells following 72 h culture also demonstrated
successful adipocyte (Fig. 1E) and
chondrocyte (Fig. 1F)
differentiation.
Flow cytometry analysis of MSC surface
markers
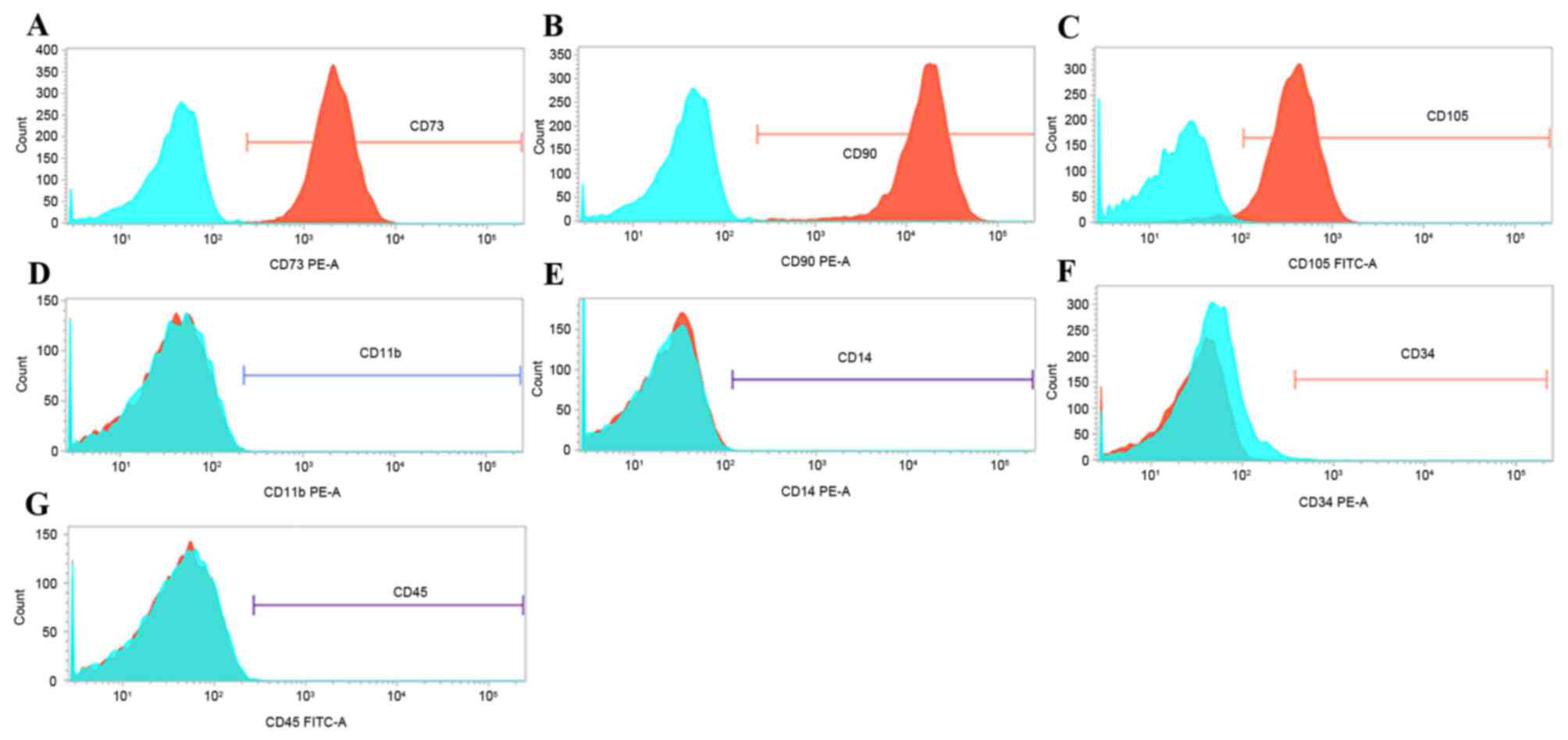
The expression of surface markers, including CD11b,
CD14, CD34, CD45, CD73, CD90 and CD105, has been previously
reported as a means to confirm the correct cell identity for MSCs
(13). Therefore, expression of
these surface markers was analyzed by flow cytometry in passage-2
cells. Greater than 99% of passage-2 cells expressed CD73, CD90,
and CD105 (Fig. 2A-C). By
contrast, less than 1% of cells expressed CD11b, CD14, CD34 and
CD45 (Fig. 2D-G, respectively).
These findings suggest that the cells isolated in the present study
are enriched in phenotypically defined hMSCs.
Morphology of adherent-cultured and
nonadherent-cultured hMSCs
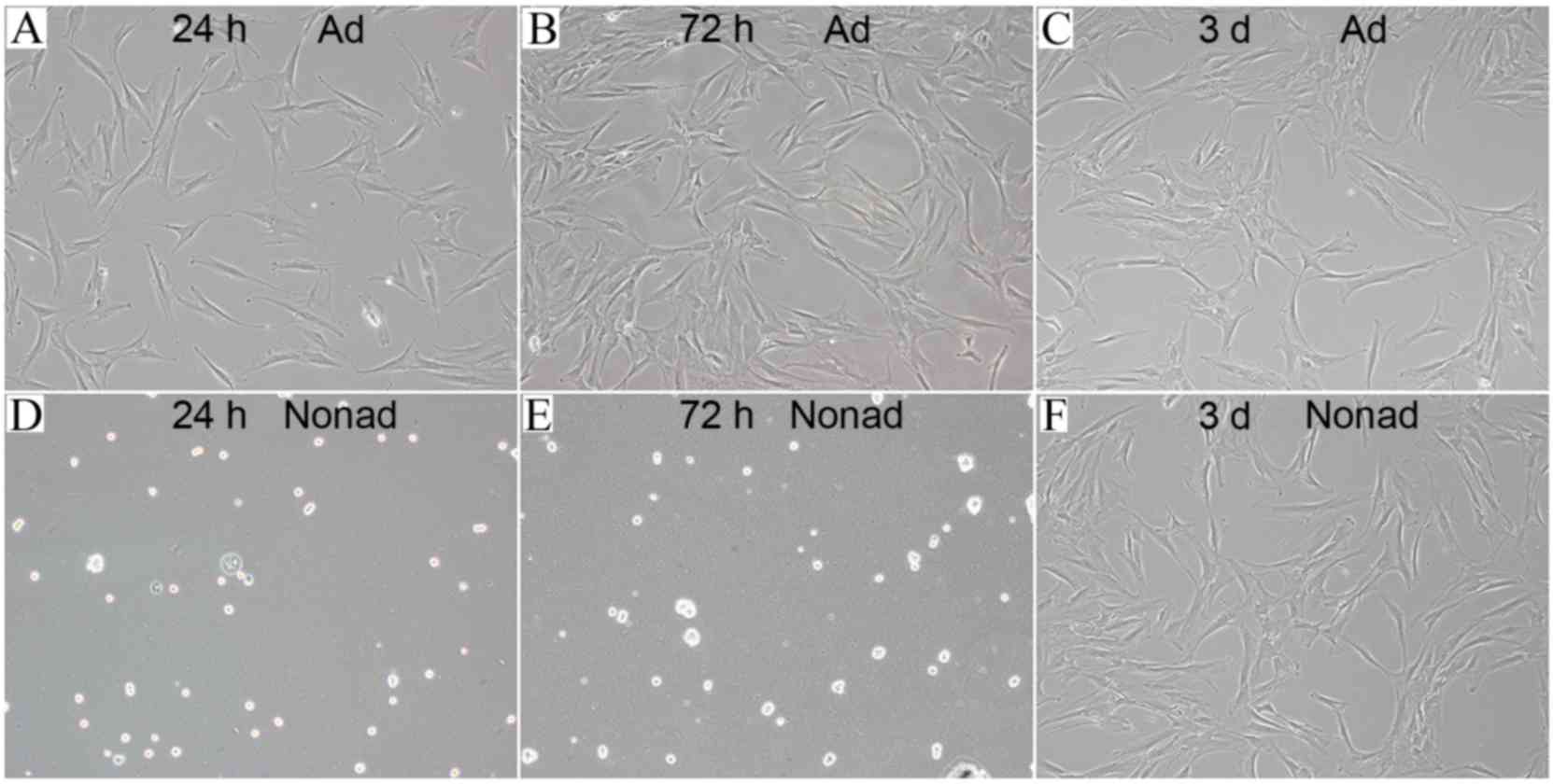
hMSCs seeded in tissue culture plates (at 2,500
cells/well) were attached to the bottom of the plates and exhibited
flat morphology following 24 (Fig.
3A) and 72 h (Fig. 3B) of
culture. However, hMSCs seeded in the ultra-low-adherence culture
plates appeared suspended and scattered throughout the medium, and
exhibited a rounded morphology, following 24 (Fig. 3D) and 72 h (Fig. 3E) of culture. In order to test
whether this morphology change was a transient or permanent
feature, adherent and nonadherent cells were harvested at 72 h and
then reseeded in tissue culture plates. Following incubation for 3
days in adherent culture conditions, hMSCs reseeded from the
nonadherent cultures exhibited a similar flat morphology (Fig. 3F) to the ones reseeded from the
adherent cultures (Fig. 3C),
suggesting that nonadherent cells were capable of adherent growth
and fibroblast-like morphology.
Effect of nonadherent culture
conditions on apoptosis in hMSCs
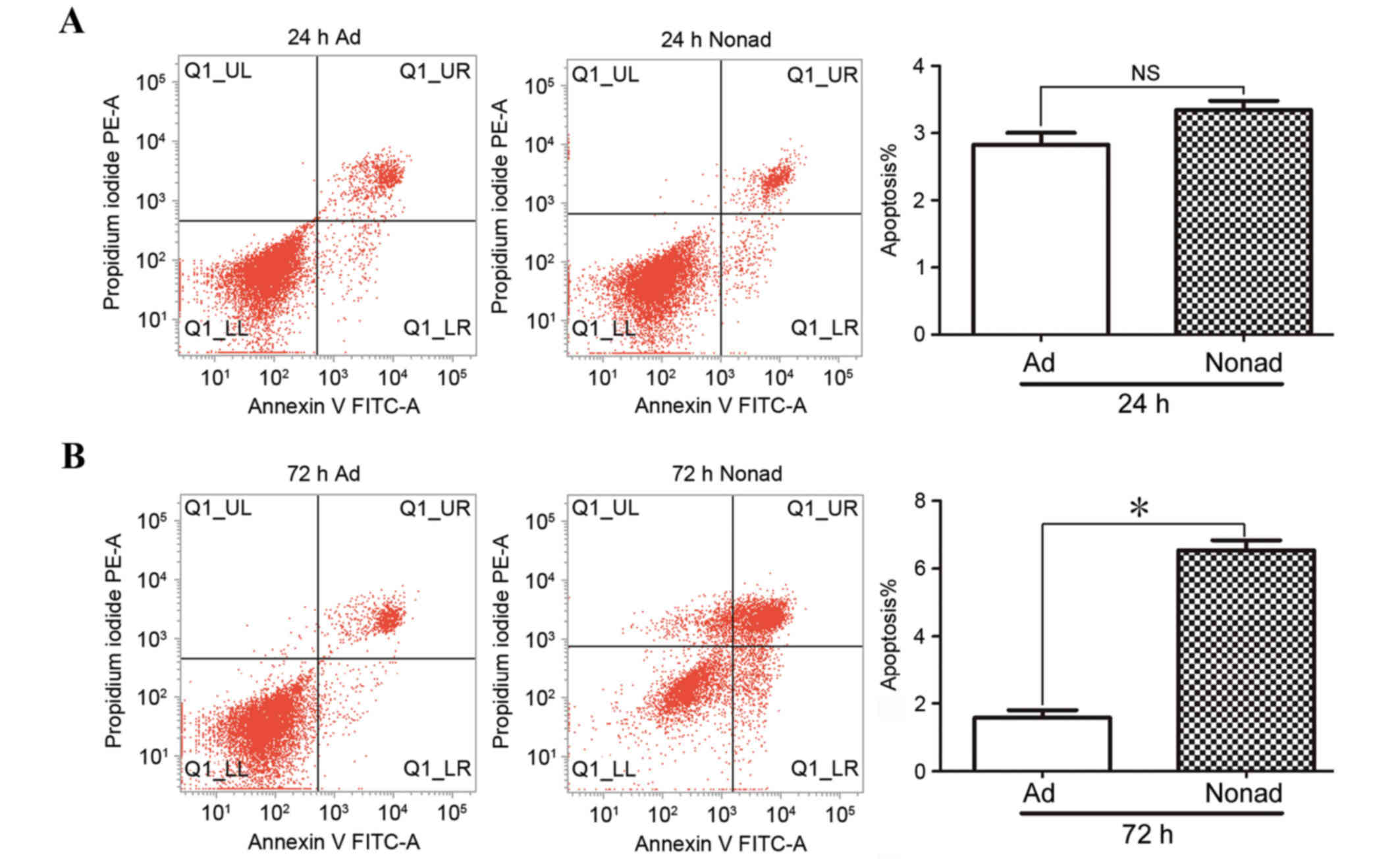
Apoptosis of passage-3 hMSCs was analyzed by flow
cytometry in three independent repeats. At 24 h, the % of apoptotic
cells in nonadherent-cultured cells was not significantly different
compared with the control adherent-cultured cells (P>0.05;
Fig. 4A). At 72 h, the % of
apoptotic cells was significantly higher in the
nonadherent-cultured cells compared with the adherent-cultured
cells (P<0.05; Fig. 4B),
indicating that nonadherent culture conditions result in increased
apoptosis in hMSCs.
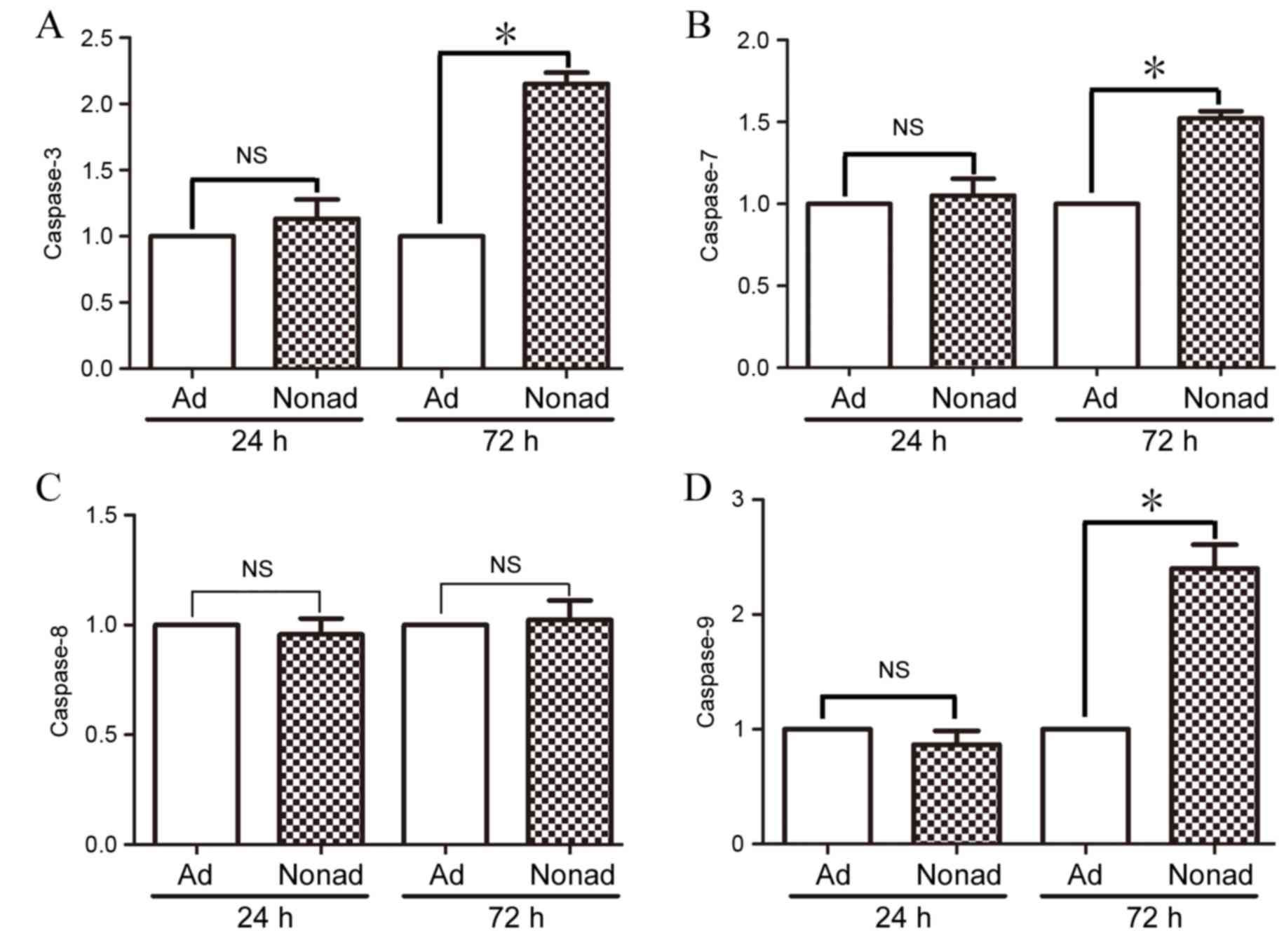
Effect of nonadherent culture
conditions on caspase mRNA expression in hMSCs
RT-qPCR was used to compare levels of caspase-3, −7,
−8, and-9 mRNA expression in adherent and nonadherent-cultured
cells at 24 and 72 h. At 24 h, mRNA expression levels of all the
caspases examined were not significantly different in
nonadherent-cultured cells compared with the adherent-cultured
cells (P>0.05; Fig. 5A-D,
respectively). At 72 h however, mRNA expression levels of
caspase-3, −7, and −9 were significantly increased in
nonadherent-cultured cells compared with the control
adherent-cultured cells (P<0.05; Fig. 5A, B and D, respectively).
Expression of caspase-8 did not differ significantly between the
two groups at either time point (P>0.05; Fig. 5C).
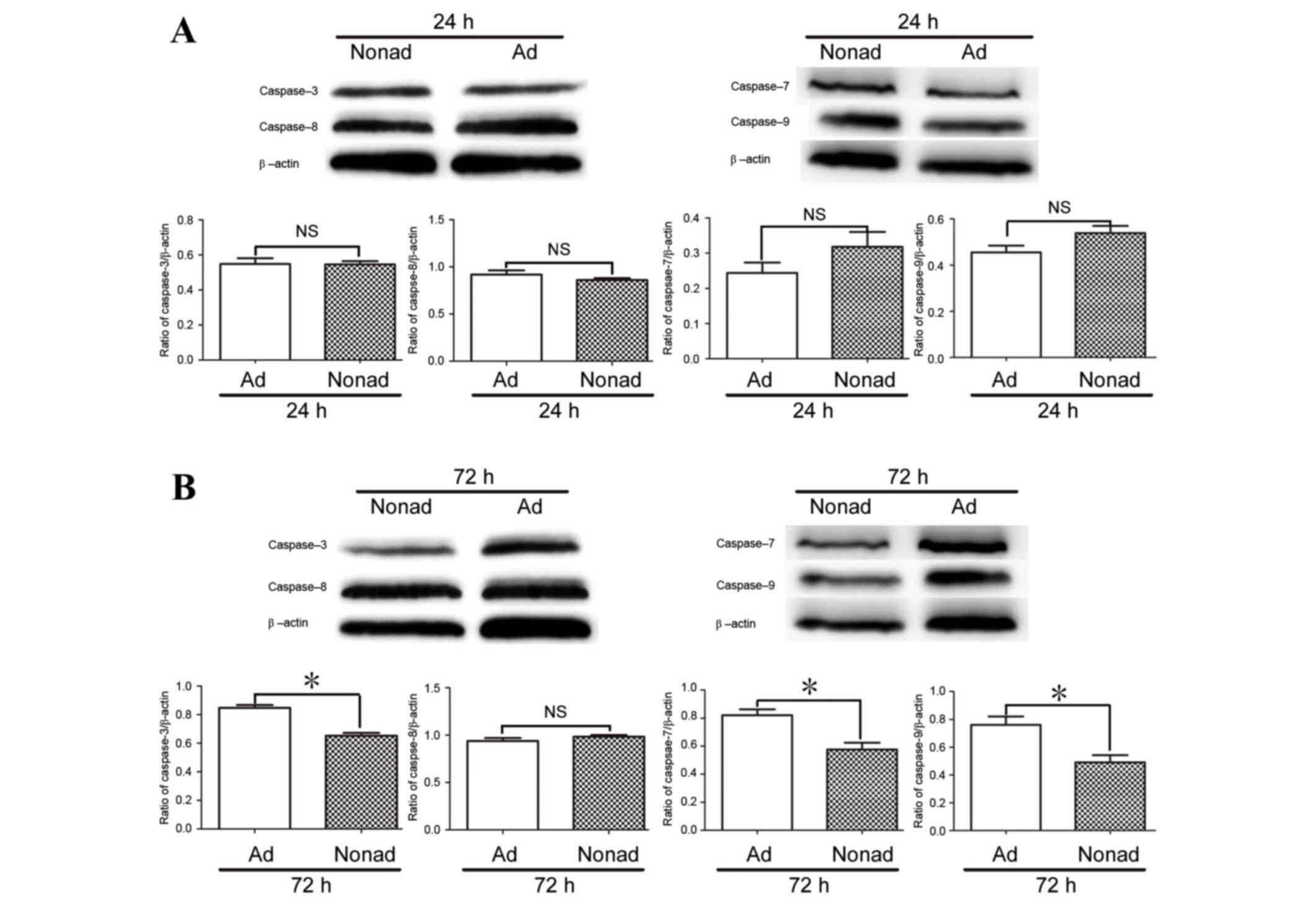
Effect of nonadherent culture
conditions on expression of caspase proteins in hMSCs
Since caspase-3, −7, and −9 mRNA expression at 72 h
differed between adherent and nonadherent-cultured cells, protein
levels were also examined by western blot analysis. The results
demonstrated that at 24 h, the protein expression levels of
caspases 3, 7, 8, and 9 did not differ significantly between
culture conditions (P>0.05; Fig.
6A). At 72 h, levels of caspase-3, caspase-7, and caspase-9
proteins were significantly lower in the nonadherent-cultured cells
compared with the adherent-cultured cells (P<0.05; Fig. 6B), while the level of caspase-8
protein did not differ significantly between the two populations at
24 or 72 h (P>0.05; Fig.
6).
Discussion
Numerous studies have demonstrated the efficiency of
MSCs in stem cell therapy recovery of the infarcted myocardium,
however, a major limitation of this method is that MSCs are
short-lived following transplantation (5,14).
Apoptosis of MSCs following transplantation is an important factor
in this lack of long-term survival. In the present study, a
previously described method was utilized by seeding MSCs in
ultra-low-adherence culture plates to assess the apoptotic
properties of hMSCs removed from adherent culture conditions.
To isolate hMSCs, bone marrow was aspirated from the
posterior iliac crest. The criteria used to define hMSCs were as
follows: i) MSCs must be adherent when maintained under standard
culture conditions, ii) MSCs must express CD105, CD73, and CD90,
and lack expression of CD45, CD34, CD14 and CD11b surface
molecules, and iii) MSCs must be able to differentiate to
osteoblasts, adipocytes and chondroblasts in vitro (13). The isolated cells were confirmed
for surface marker expression by flow cytometric analysis and for
clonogenicity and multipotency by in vitro CFU-F and
differentiation assays. These findings demonstrated that the
isolated cells had the characteristics of hMSCs. In addition,
adherent and nonadherent-cultured cells at 72 h also demonstrated
adipocyte and chondrocyte differentiation potential and plastic
adherence ability.
Apoptosis rates were then compared between
nonadherent and adherent-cultured cells at 24 and 72 h using flow
cytometric analysis. The % of apoptotic cells in
nonadherent-cultured cells increased significantly compared with
adherent-cultured cells at 72 h. These data indicated that
apoptosis increased in hMSCs following removal from adherent
culture conditions. Baksh et al (15,16)
demonstrated that bone marrow-derived nonhematopoietic progenitor
cells proliferate in a cytokine-dependent manner, as individual
cells in stirred suspension cultures. In the present study, neither
adherent or nonadherent cultures contained any exogenously added
cytokines, and both were cultured in static conditions. In
addition, hMSCs were isolated by adherence to plastic, the hMSCs
were a different cell population compared with nonhematopoietic
progenitor cells. This implies that some cytokines may be critical
for the survival of MSCs following removal from adherent culture
conditions.
Mammalian cell apoptosis is primarily activated via
two distinct pathways, the intrinsic and extrinsic apoptotic
pathways (17). Caspase-8 and −3
are involved in the extrinsic apoptotic pathway (18), while caspase-3, −7 and −9
participate in the intrinsic apoptotic pathway (19). In the present study, the protein
levels of caspase-3, −7, and −9 were significantly reduced
following 72 h of nonadherent culture, whereas caspase-8 was
unchanged. These data indicate that the intrinsic apoptotic pathway
may be involved in the apoptosis of nonadherent hMSCs. Caspase-9 is
activated by cleavage, then acts on downstream targets, including
caspase-3 and −7 (19,20). The levels of mRNA expression for
caspase-3, −7, and −9 genes were increased at 72 h. By contrast,
due to protein cleavage, the protein expression levels of
caspase-3, −7, and −9 were decreased in nonadherent-cultured cells
compared with control adherent-cultured cells. Taken together,
these data suggest that caspase-3, −7 and −9 proteins may be
involved in regulating apoptosis in hMSCs following removal from
plastic-adherent culture conditions, through the intrinsic
apoptotic pathway.
Previous studies indicated that ischemia,
inflammatory response, hypoxia and oxidative stress are the major
factors impairing MSC survival following transplantation (6,7,21).
The present study demonstrated another possible reason for the low
survival of MSCs. In conclusion, loss of adherence in vitro
increases apoptosis in hMSCs, through activation of caspase-3, −7
and −9. The results of the present study may provide novel insights
into factors affecting MSC survival following transplantation.
Acknowledgements
We thank Hong Qian of the Center for Hematology and
Regenerative Medicine of Karolinska Institute (Stockholm, Sweden)
for valuable advice in this study. This study was supported by
grants from the International Program of Project 985, Sun Yat-sen
University and the ‘One Hundred Talented Scholars’ of Sun Yat-sen
University (grant no. F002009011) and Zhongshan Postdoctoral
Sustentation Fund (grant no. E2015681).
References
|
1
|
Satija NK, Singh VK, Verma YK, Gupta P,
Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP and Gurudutta
GU: Mesenchymal stem cell-based therapy: A new paradigm in
regenerative medicine. J Cell Mol Med. 13:4385–4402. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Novotny NM, Ray R, Markel TA, Crisostomo
PR, Wang M, Wang Y and Meldrum DR: Stem cell therapy in myocardial
repair and remodeling. J Am Coll Surg. 207:423–434. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deuse T, Peter C, Fedak PW, Doyle T,
Reichenspurner H, Zimmermann WH, Eschenhagen T, Stein W, Wu JC,
Robbins RC and Schrepfer S: Hepatocyte growth factor or vascular
endothelial growth factor gene transfer maximizes mesenchymal stem
cell-based myocardial salvage after acute myocardial infarction.
Circulation. 120(11 Suppl): S247–S254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rabani V, Shahsavani M, Gharavi M, Piryaei
A, Azhdari Z and Baharvand H: Mesenchymal stem cell infusion
therapy in a carbon tetrachloride-induced liver fibrosis model
affects matrix metalloproteinase expression. Cell Biol Int.
34:601–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burlacu A: Tracking the mesenchymal stem
cell fate after transplantation into the infarcted myocardium. Curr
Stem Cell Res Ther. 8:284–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei H, Li Z, Hu S, Chen X and Cong X:
Apoptosis of mesenchymal stem cells induced by hydrogen peroxide
concerns both endoplasmic reticulum stress and mitochondrial death
pathway through regulation of caspases, p38 and JNK. J Cell
Biochem. 111:967–978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Potier E, Ferreira E, Meunier A, Sedel L,
Logeart-Avramoglou D and Petite H: Prolonged hypoxia concomitant
with serum deprivation induces massive human mesenchymal stem cell
death. Tissue Eng. 13:1325–1331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vunjak-Novakovic G and Scadden DT:
Biomimetic platforms for human stem cell research. Cell Stem Cell.
8:252–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng B, Deng W, Xiao P, Zeng K, Zhang S,
Zhang H, Deng DY and Yang Y: Nonadherent culture method
downregulates stem cell antigen-1 expression in mouse bone marrow
mesenchymal stem cells. Exp Ther Med. 10:31–36. 2015.PubMed/NCBI
|
|
10
|
Qian H, Le Blanc K and Sigvardsson M:
Primary mesenchymal stem and progenitor cells from bone marrow lack
expression of CD44 protein. J Biol Chem. 287:25795–25807. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH image to imageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sheikh AY, Huber BC, Narsinh KH, Spin JM,
van der Bogt K, de Almeida PE, Ransohoff KJ, Kraft DL, Fajardo G,
Ardigo D, et al: In vivo functional and transcriptional profiling
of bone marrow stem cells after transplantation into ischemic
myocardium. Arterioscler Thromb Vasc Biol. 32:92–102. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baksh D, Davies JE and Zandstra PW: Adult
human bone marrow-derived mesenchymal progenitor cells are capable
of adhesion-independent survival and expansion. Exp Hematol.
31:723–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baksh D, Zandstra PW and Davies JE: A
non-contact suspension culture approach to the culture of
osteogenic cells derived from a CD49elow subpopulation of human
bone marrow-derived cells. Biotechnol Bioeng. 98:1195–1208. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parrish AB, Freel CD and Kornbluth S:
Cellular mechanisms controlling caspase activation and function.
Cold Spring Harb Perspect Biol. 5:pii: a0086722013. View Article : Google Scholar
|
|
18
|
Berghe T Vanden, Van Loo G, Saelens X, Van
Gurp M, Brouckaert G, Kalai M, Declercq W and Vandenabeele P:
Differential signaling to apoptotic and necrotic cell death by
Fas-associated death domain protein FADD. J Biol Chem.
279:7925–7933. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rowinsky EK: Targeted induction of
apoptosis in cancer management: The emerging role of tumor necrosis
factor-related apoptosis-inducing ligand receptor activating
agents. J Clin Oncol. 23:9394–9407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Philchenkov A, Zavelevich M, Kroczak TJ
and Los M: Caspases and cancer: Mechanisms of inactivation and new
treatment modalities. Exp Oncol. 26:82–97. 2004.PubMed/NCBI
|
|
21
|
Deschepper M, Oudina K, David B, Myrtil V,
Collet C, Bensidhoum M, Logeart-Avramoglou D and Petite H: Survival
and function of mesenchymal stem cells (MSCs) depend on glucose to
overcome exposure to long-term, severe and continuous hypoxia. J
Cell Mol Med. 15:1505–1514. 2011. View Article : Google Scholar : PubMed/NCBI
|