Introduction
Visceral pain refers to a type of pain, which occurs
following noxious stimulation surrounding internal organs.
Accompanied by ambiguous location and internal organ cramps, it
causes severe pain and causes muscle pain in referred areas, which
affects the quality of life of patients and substantially increases
medical treatment burden. At present, how visceral pain occurs and
the pathway of visceral noxious information transmission remain to
be fully elucidated due to limitations in experimental techniques
(1–4).
Substance P (SP), first identified in 1931, is a
type of neurokinin. It is well documented that SP is an excitatory
neurotransmitter released by the first stage of peripheral
nociceptive afferent fibers and is involved in pain transmission.
As its specific receptor, neurokinin 1 receptor (NK1R) has been
demonstrated to be important in the development of pain and
hyperalgesia. However, how NK1R functions when visceral pain occurs
remains to be elucidated (5,6). The
cerebrospinal fluid (CSF)-contacting nucleus (CSF-CN), the neurons
of which contact the internal CSF, was first identified and named
at the Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou
Medical College (Xuzhou, China). Using cholera toxin B subunit
conjugated to peroxidase (CB-HRP), CSF-CN was successfully labeled
and distributed in the ventral periaqueductal central gray matter
of the brainstem (7,8). The CSF-CN is unique owing to its
specific structure. The cell bodies of these neurons are in the
brain parenchyma, whereas their projections are in contact with the
CSF (9,10). Previous studies have demonstrated
that there are subtle changes in the chemical composition of CSF
when pain occurs (11,12). Considering the specific structure
of the CSF-CN, the present study hypothesized that the CSF and the
CSF-CN have certain indivisible connections. Evidence from previous
studies has supported this hypothesis, and suggested that the
CSF-CN is involved in the transduction and regulation of pain
signals (13,14). However, the role of CSF-CN remains
to be fully elucidated. The present study aimed to investigate the
possible functions of NK1R within the CSF-CN under conditions of
visceral pain in order to provide a feasible solution for the
clinical treatment of visceral pain.
Materials and methods
Animals
All experiments were performed according to the
regulations of The Committee for the Ethical Use of Laboratory
Animal of Xuzhou Medical College. Male Sprague-Dawley rats provided
by the Experimental Animal Center of Xuzhou Medical College
(license number, SYXK 2002–0038) were 8–9 weeks old and weighed
250–300 g. The rats were randomly assigned into three groups, each
containing six animals. The rats in the visceral pain group were
subjected to lower colon instillation of 5% formalin; in the sham
group, rats received instillation of saline; in the RP67580 group,
a specific NK1R antagonist was injected into the lateral ventricle
(LV) of rats 24 h prior to visceral pain induction. All animals
were placed in separate rooms, in which conditions and light were
controlled (23±1°C; 12/12 h dark/light cycle with light on at 8:00
a.m.), and food and water were available ad libitum.
Formalin instillation
In the animal experiments, visceral pain was induced
by rectal infusion with dilute formalin. In order to allow the rats
to regain consciousness as soon as possible, a small quantity of
halothane (induction at 3%, then 1.5% in a mixture of 2:3 nitrous
oxide and 1:3 oxygen) was used. While under anesthesia, the animal
was suspended by its tail (<5 min). Subsequently, a polyethylene
tube, which was wrapped in surgical tape at ~30 mm from the edge,
was inserted through the anus. A 5% formalin solution or saline
(100 µl) was injected through the tube slowly to prevent leakage
(15).
Visceral pain behavior score
assessment
All animals were placed in separate observation
boxes for adaptation 30 min prior to the behavioral assessments. On
regaining consciousness, the rats were observed for 3 h to record
pain-associated behaviors. The visceral pain-associated behaviors
included licking of the abdomen (A), back stretching (B),
contraction of flanks (C), and contraction of whole body (D). Based
on the duration of the contraction, the contraction of the whole
body was divided into three levels of <30 sec (W1), 30–60 sec
(W2) and >1 min (W3). Using the following formula, the visceral
pain score (PS) was calculated: PS=L+2B+3C+4W1+5W2+6W3 (16).
Nociceptive assessment
The animals were placed in grid-bottomed cages for
30 min for adaption prior to the evaluation of allodynia.
Subsequently, to determine the hyperalgesia of the referred area,
von Frey hairs (VFHs; Semmes-Weinstein Monofilaments, North Coast
Medical, Inc., San Jose, CA, USA) were used. On stimulation of the
abdomen with increasing force (0.16, 0.4, 0.6, 1, 1.4, 2, 4, 6, 8,
10 and 15 g), the rats showed withdrawal responses, which included
sharp abdominal retraction, licking or scratching the site of
application of the hair and jumping with all four paws off the
floor (17). Each VFH test lasted
for 6 sec, or until a withdrawal response was observed. Once a
withdrawal reaction occurred, the next descending force of VFH was
applied to retest the area where the former VFH was placed until no
response was detected. The minimum force was recorded as the
abdomen withdrawal threshold in grams. If the result of allodynia
was ≤2 g VFH, it was considered innocuous to the rats.
Drugs administration
The animals were anesthetized with sodium
pentobarbital (40 mg/kg, i.p.). When immobilized in stereotaxic
instrument, the rat was injected with 3 µl of 30% CB-HRP
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) into the LV
according to stereotaxic coordinates (Brega, −1.2±0.4 mm; depth,
3.2±0.4 mm; right to median sagittal plane, 1.4±0.2 mm) 48 h prior
to the behavioral assessments. The NK1R antagonist, RP67580 (Tocris
Biosciences, Bristol, United Kingdom) was dissolved in dimethyl
sulfoxide, and 100 pmol was injected into the LV of rats in the
RP67580 group 24 h prior to the behavioral assessments.
Immunohistochemistry and imaging
Following the intracerbroventricular injections, the
rats were administered with the formalin instillation and visceral
pain behavioral assessments were performed. Subsequently, the rats
were deeply anesthetized by injection of pentobarbital sodium (50
mg/kg, i.p.) and then successively perfused with 150 ml of
phosphate buffered saline (PBS; 0.01 M; pH 7.4) and 4%
paraformaldehyde. Following isolation and overnight fixation, the
brainstem was immersed in 30% sucrose solution for 2 days. On a
cryostat microtome (Leica CM1900; Leica Microsystems GmbH, Wetzlar,
Germany), all tissues were successively cut into 35 µm slices in
the transverse plane. The sections were then incubated in donkey
serum (1:100 dilution; cat. no. 566460; Merck KGaA) for 1 h at
37°C, following which the sections were treated with goat anti-CB
(1:400 dilution; cat. no. 227040; Merck KGaA) and anti-rabbit NK1R
(1:100 dilution; cat. no. BA3678; Wuhan Boster Biological
Technology Ltd., Wuhan, China). Following reaction with goat
anti-IgG coupled to Alexa 546 (1:400 dilution; cat no. A11056;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and rabbit
anti-IgG coupled to Alexa 488 (1:400 dilution; cat. no. A21206;
Thermo Fisher Scientific, Inc.), the slices were thoroughly rinsed
three times with 0.01 mol/ml PBS at the end of each step. The
tissue sections were transferred onto slides and allowed to air
dry, prior to sealing with glycerol. Finally, ultrathin sections
(75×25×1 mm) were prepared and examined under a laser-scanning
confocal microscope (TCS SP2; Leica Microsystems). Using Image-Pro
Plus (version 6.0; Media Cybernetics, Inc., Rockville, MD, USA),
images were captured, cropped and adjusted.
Western blot analysis
Following the behavior assessments, the rats were
sacrificed and the CSF-CN region of rat brain was removed and
stored at −80°C. The tissues were homogenized by an electric
homogenizer for 20 sec at room temperature. Following
centrifugation at 13,523 × g for 15 min at 4°C, supernatant
collection and denaturation, the lysates were electrophoresed on 8%
SDS-polyacrylamide gel. According to the analysis, the semi-dry
western blot transfer method was used, and the polyvinylidene
difluoride membranes were blocked with 5% skim milk for 1 h at room
temperature. The membranes were then incubated with anti-rabbit
NK1R (1:500 dilution; cat. no. BA3678; Wuhan Boster Biological
Technology Ltd.) and anti-rabbit GAPDH (1:5,000 dilution; cat. no.
sc-25778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C
overnight. The membranes were washed with TBS-Tween 3 times and
incubated with secondary anti-rabbit IgG antibody (1:1,000
dilution; cat. no. A0545; Sigma-Aldrich; Merck KGaA) for 2 h at
room temperature. The densities of the bands were analyzed using
ImageJ software (version 1.47; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis and image
analysis
Quantitative immunohistochemistry was used to assess
the expression of NK1R in the CSF-CN. Image analysis software
(Image-Pro Plus Version 6.0; Media Cybernetics, Inc.) was used for
digital image analysis. On examination of every tissue section,
details were recorded on the number, area and density of
immunoreactive cells. Dual labeling of neurons with CB-HRP/NK1R
enabled counting via a yellow fluorescence reaction, with areas
between 40 and 1,000 µm2. Data are expressed as the mean
± standard deviation and were imported into Microsoft Excel 2003
(Microsoft Corporation, Redmond, WA, USA). Using SPSS v.13.0 (SPSS,
Inc., Chicago, IL, USA), all data were analyzed using one-way
analysis of variance or Student's t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Behavior in formalin-induced visceral
pain
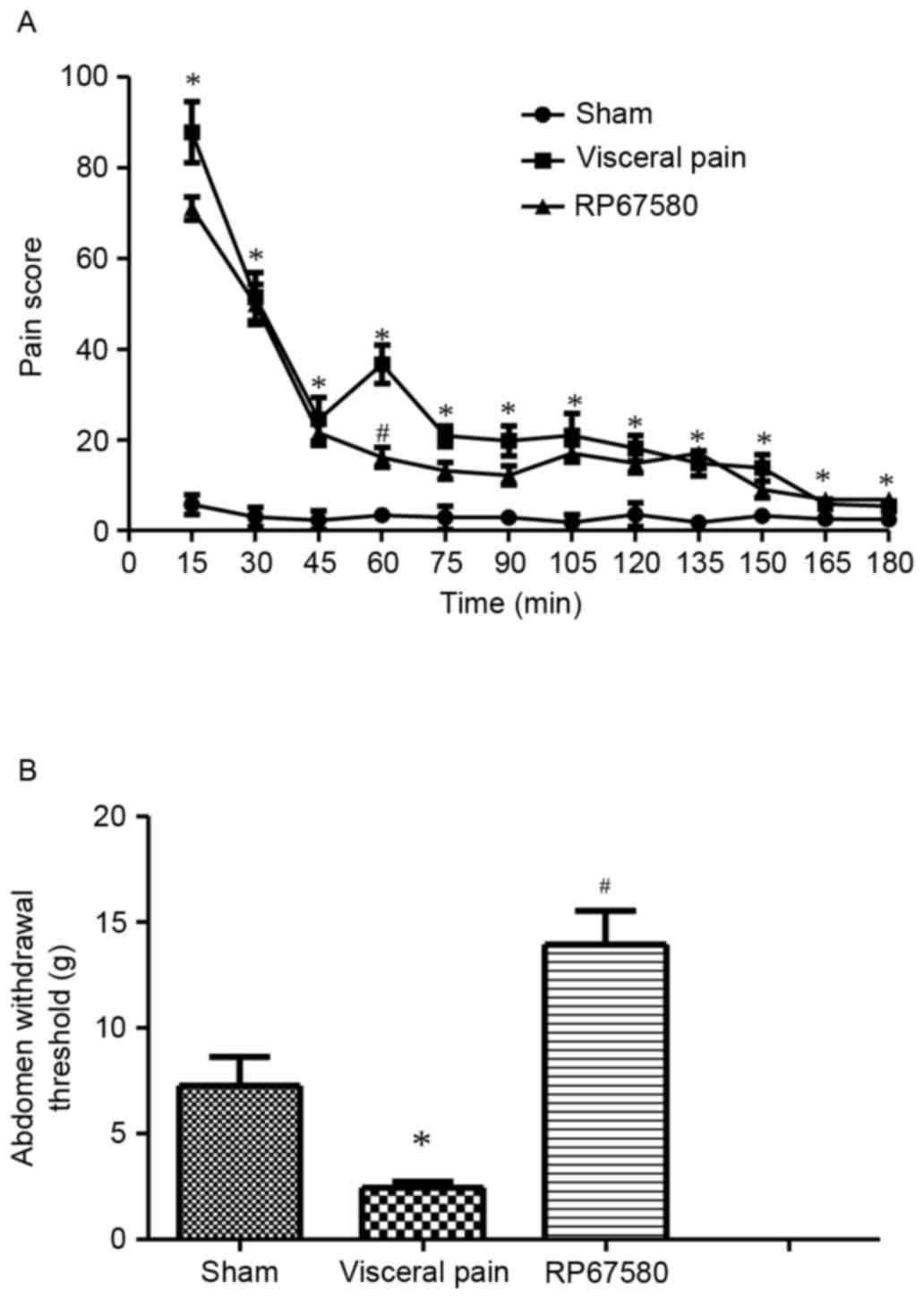
Prior to surgery, the PS of visceral pain behavior
at baseline was determined in untreated rats, which was 0.8±0.7.
Following formalin instillation, a series of discrete behavioral
episodes were observed, including licking of the upper abdomen,
stretching of the whole body and arching of the back against the
floor. Compared with the PS of rats in the sham group, the PS of
rats in the visceral pain group presented with a typical biphasic
time course. The first peak occurred at ~15 min post-instillation
(PS=87.8±5.4), which was 15 times higher, compared with that in the
saline instillation group (P<0.05). The second PS peak occurred
at 60 min post-instillation (PS=36.7±3.3), which was 12 times
higher, compared with that of the sham group (P<0.05). The PS of
the RP67580 group was significantly decreased at 60 min
post-formalin instillation, compared with that of the visceral pain
group (P<0.05; Fig. 1A).
Mechanical allodynia in rats following
formalin instillation
In the present study, the von Frey assessment was
used to measure the abdomen withdrawal threshold. The values of the
abdomen withdrawal threshold in rats of the visceral pain group
were significantly lower 60 min post-formalin instillation,
compared with that of the sham group (P<0.05). The values were
significantly upregulated in rats pretreated with RP67580
(P<0.05; Fig. 1B).
Immunohistochemical analysis of NK1R
in the CSF-CN of rats following formalin instillation
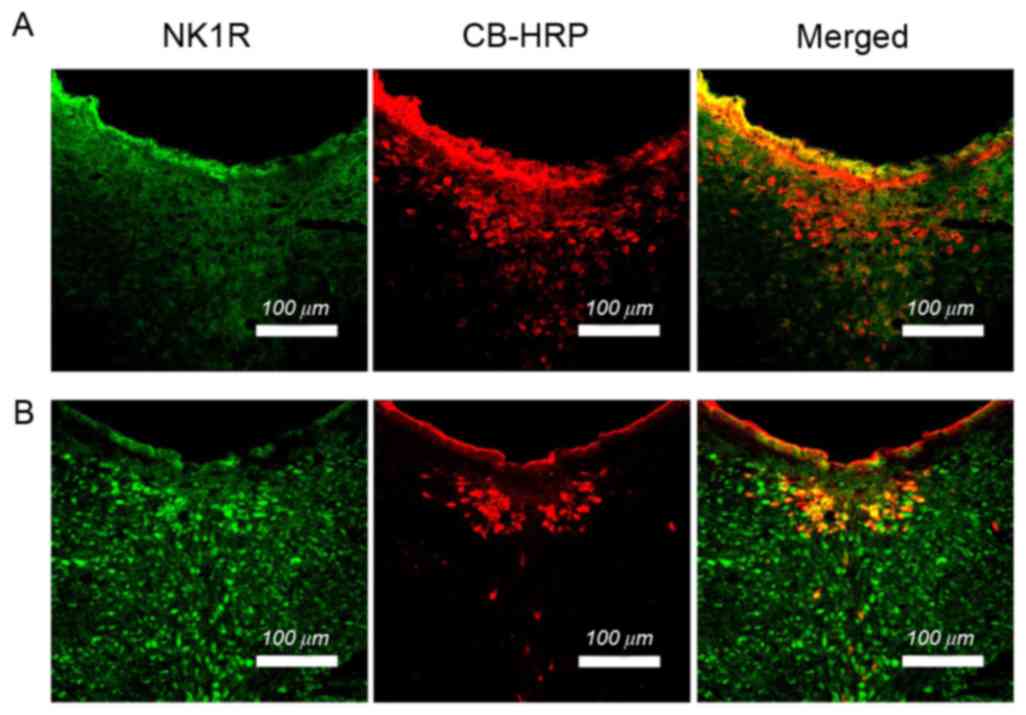
The CSF-CN was first identified by injecting CB-HRP,
as a fluorescent tracer, into the LV of the rats, which was used as
a reliable method to label CSF-CN. As shown in the images in
Fig. 2, the neurons labeled by
CB-HRP were predominantly located around the midline of the
aqueduct midbrain and ventral periaqueductal gray matter of the
brainstem. In order to determine whether the CSF-CN expressed NK1R,
a double-labeled immunofluorescence technique was used. The results
indicated that NK1R-immunoreactive neurons (green) were distributed
in the CSF-CN. The total CSF-CN sample of the visceral pain group
did not differ from that of the sham group (P>0.05). The number
of simple labeled NK1R-containing neurons counted in the visceral
pain group was significantly higher, compared with that in the sham
group (P<0.05). Compared with the sham group, then number of
dual-labeled CB-HRP/NK1R neurons counted in the visceral group was
significantly upregulated (P<0.05; Fig. 2; Table
I).
 | Table I.Expression of CB-HRP, NK1R and
CB-HRP/NK1R in the sham group and visceral pain group. |
Table I.
Expression of CB-HRP, NK1R and
CB-HRP/NK1R in the sham group and visceral pain group.
| Group | CB-HRP | NK1R | CB-HRP/NK1R |
|---|
| Sham | 398±16 | 114±7 | 70±3 |
| Visceral pain | 407±12 | 604±12a | 353±7a |
Western blot analysis of NK1R in the
CSF-CN of rats experiencing visceral pain
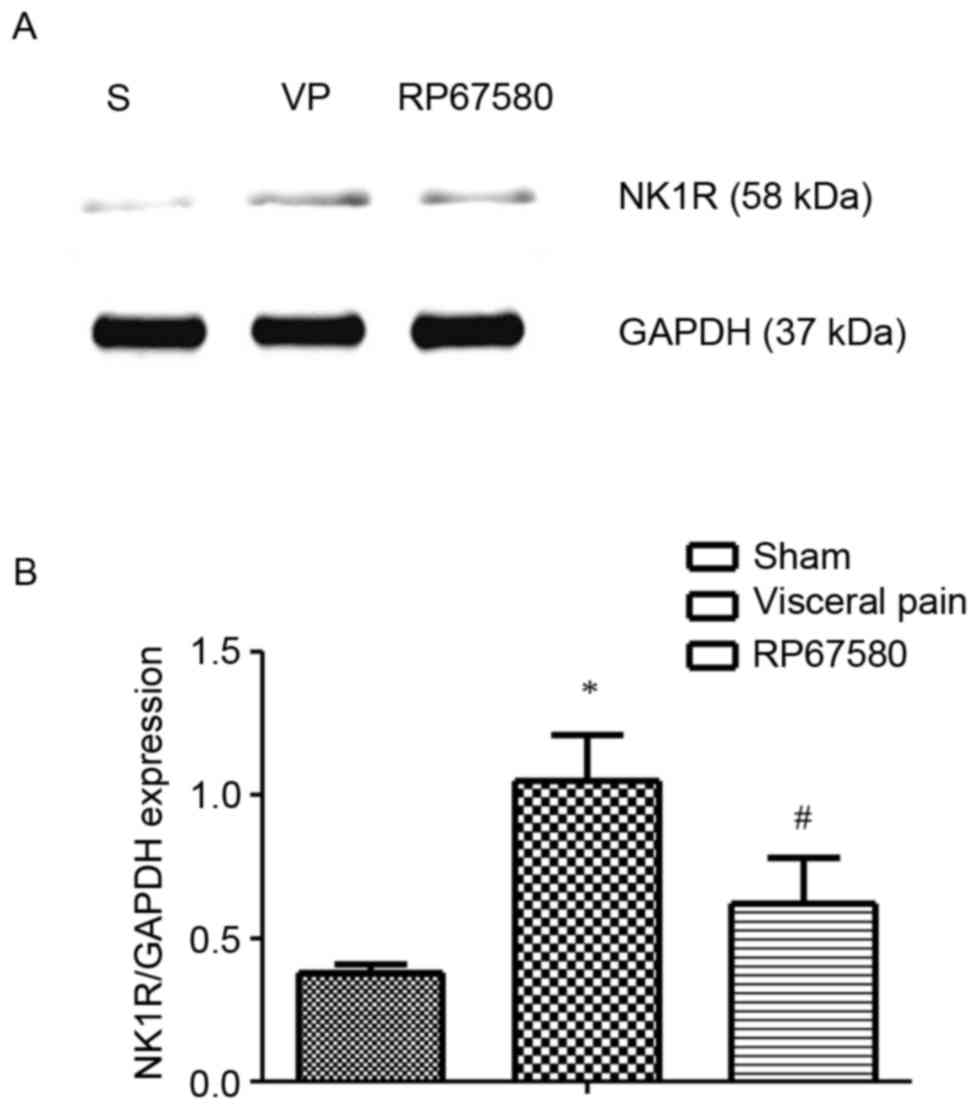
Based on the above-mentioned findings, the present
study investigated the role of NK1R in visceral pain. Western blot
analysis was used to examine the protein expression levels of NK1R,
the result of which indicated that NK1R was upregulated 60 min
following formalin instillation, compared with that of the sham
group (P<0.05).
Following LV injection of RP67580, the rats
experienced significant relief from pain on formalin instillation,
which suggested that NK1R in the CSF-CN may be associated with the
alleviation of visceral pain. Western blot analysis was performed
to observe the expression of NK1R 24 h following the injection of
RP67580. Compared with the rats in the visceral pain group, there
was a significant downregulation in the expression of NK1 in the
rats pretreated with RP67580 (P<0.05; Fig. 3).
Discussion
In previous studies, acute colitis has been used as
a model to simulate visceral pain of pelvis organs, including the
lower colon, rectum and bladder (16,17).
Through the rectal infusion of formalin, inflammatory pain can be
successfully simulated in regions of regions of the pelvic organs
(18). In the present study, this
method was used to induce visceral pain. The results showed that
the visceral pain behavior scores of rats in the visceral pain
group were significantly increased at each time point, compared
with those of rats in the sham group, which suggested establishment
of the visceral pain model had been successful. The present study
demonstrated that the intestinal perfusion of formalin mediated a
biphasic reaction of visceral pain, which was similar to the
reaction of formalin-induced somatic pain (19). The specific mechanism underlying
this biphasic pain remains to be fully elucidated and requires
further investigation.
Our previous investigations demonstrated that the
CSF-CN participated in the transmission of information within the
brain, with chemical messages transferred in a cell-to-cell manner
or in the CSF (20). It has been
found that the neurons of the CSF-CN have a specific cell
structure, with cell bodies located within the brain parenchyma and
projections extending into the CSF through ependymal barriers
(7). In addition, signal
transmission by the CSF-CN may affect the composition of the CSF,
which may lead to neuromodulation or neuroendocrinal regulation
(21).
NK1R is a G protein-coupled receptor, which can
specifically combine with SP. It has been well documented that SP
is involved in the pain regulation process (6). Several studies have suggested that
CSF-CN is involved in the transmission of pain signals by the
expression of SP, transient receptor potential cation channel,
subfamily C, member 6 and extracellular-signal-regulated kinase 5
(10,22,23).
In the present study, using a double-labeled immunofluorescent
technique, it was observed that NK1R was expressed in the CSF-CN
when visceral pain occurred, and that there were alterations in the
PS and abdomen withdrawal threshold in rats exposed to formalin
instillation, compared with those of naive rats. To assess the
involvement of NK1R of the CSF-CN in the behavioral nociceptive
symptoms due to visceral inflammation and to semi-quantitatively
analyze variations in the expression of NK1R in visceral pain,
western blot analysis was used. The results showed that, at 60 min
post-formalin instillation, when the second phase of visceral pain
occurred, the protein expression level of NK1R within the CSF-CN
was upregulated. In addition, RP67580, a specific NK1R antagonist,
significantly alleviated visceral pain in rats and reduced the
expression of NK1R, suggesting that the upregulation of NK1R may be
crucial in the process of establishing inflammation-induced
visceral pain. Based on these findings, it was hypothesized that
NK1R was involved within the CSF-CN in visceral pain.
Previous clinical and experimental studies have
confirmed the presence of NK1R on postsynaptic dorsal column (PSDC)
neurons in inflammation of the colon. These findings may explain
why DC lesions can relieve visceral pain in patients (16). Studies on NK1R gene-deficient mice
have shown that NK1R can mediate the central nociceptive and
peripheral inflammatory response. It can cause neurogenic
inflammation and regulate the peripheral inflammatory responses to
noxious stimuli (24). Evidence
has suggested that the perception of visceral pain may be completed
by an ascending excitatory pathway in the DC, particularly under
the conditions of peripheral inflammation. There is increasing
evidence that this pathway may contain an amplification loop, which
enhances the responsiveness of spinal cord neurons through a
descending facilitatory pathway, possibly originating in the
rostroventral medulla. PSDC and other projection neurons may be
affected by this amplification loop and lead to potentiation
(25). AsNK1R is expressed in PSDC
neurons under conditions of visceral pain, PSDC neurons may be
involved in the amplification mechanism mediated by the NK1R. NK1R
may regulate visceral pain through the transfer of information
between the brain parenchyma and CSF, or be involved in the DC
pathway via its expression in PSDC neurons. Additionally, the
results of the present study showed that the visceral pain was not
completely relieved following pre-injection of RP67580, which
suggested that there may be other mechanisms for regulating
visceral pain. A substantial number of neurons of the CSF-CN did
not express NK1R, and other neurotransmitters or signaling pathways
may affect the regulation of visceral pain.
In conclusion, the present study provided novel
evidence that NK1R was expressed in the CSF-CN, and that NK1R
within the CSF-CN may be involved in the regulation of visceral
pain. However, the way in which NK1R in the CSF-CN affects the
regulation of visceral pain remains to be elucidated in future
investigations.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no. 81371243)
and the Natural Science Foundation of Jiangsu Province (grant no.
BK2012580).
References
|
1
|
Ness TJ and Gebhart GF: Colorectal
distension as a noxious visceral stimulus: Physiologic and
pharmacologic characterization of pseudaffective reflexes in the
rat. Brain Res. 450:153–169. 1988. View Article : Google Scholar
|
|
2
|
Ness TJ and Gebhart GF: Visceral pain: A
review of experimental studies. Pain. 41:167–234. 1990. View Article : Google Scholar
|
|
3
|
Laird JM, Martinez-Caro L, Garcia-Nicas E
and Cervero F: A new model of visceral pain and referred
hyperalgesia in the mouse. Pain. 92:335–342. 2001. View Article : Google Scholar
|
|
4
|
Greenwood-Van Meerveld B, Gibson MS,
Johnson AC, Venkova K and Sutkowski-Markmann D: NK 1
receptor-mediated mechanisms regulate colonic hypersensitivity in
the guinea pig. Pharmacol Biochem Behav. 74:1005–1013. 2003.
View Article : Google Scholar
|
|
5
|
Allen BJ, Rogers SD, Ghilardi JR, Menning
PM, Kuskowski MA, Basbaum AI, Simone DA and Mantyh PW: Noxious
cutaneous thermal stimuli induce a graded release of endogenous
substance P in the spinal cord: Imaging peptide action in vivo. J
Neurosci. 17:5921–5927. 1997.
|
|
6
|
Steinhoff MS, von Mentzer B, Geppetti P,
Pothoulakis C and Bunnett NW: Tachykinins and their receptors:
Contributions to physiological control and the mechanisms of
disease. Physiol Rev. 94:265–301. 2014. View Article : Google Scholar :
|
|
7
|
Zhang LC, Zeng YM, Ting J, Cao JP and Wang
MS: The distributions and signaling directions of the cerebrospinal
fluid contacting neurons in the parenchyma of a rat brain. Brain
Res. 989:1–8. 2003. View Article : Google Scholar
|
|
8
|
Liu H, Yan WW, Lu XX, Zhang XL, Wei JQ,
Wang XY, Wang T, Wu T, Cao J, Shao CJ, et al: Role of the
cerebrospinal fluid-contacting nucleus in the descending inhibition
of spinal pain transmission. Exp Neurol. 261:475–485. 2014.
View Article : Google Scholar
|
|
9
|
Du J, Yang X, Zhang L and Zeng YM:
Expression of TRPM8 in the distal cerebrospinal fluid-contacting
neurons in the brain mesencephalon of rats. Cerebrospinal Fluid
Res. 6:32009. View Article : Google Scholar :
|
|
10
|
Lu X, Geng X, Zhang L, Zeng Y, Dong H and
Yu H: Substance P expression in the distal cerebrospinal
fluid-contacting neurons and spinal trigeminal nucleus in
formalin-induced the orofacial inflammatory pain in rats. Brain Res
Bull. 78:139–144. 2009. View Article : Google Scholar
|
|
11
|
Miyajima M, Nakajima M, Motoi Y, Moriya M,
Sugano H, Ogino I, Nakamura E, Tada N, Kunichika M and Arai H:
Leucine-rich α2-glycoprotein is a novel biomarker of
neurodegenerative disease in human cerebrospinal fluid and causes
neurodegeneration in mouse cerebral cortex. PLoS One. 8:e744532013.
View Article : Google Scholar :
|
|
12
|
Sainaghi PP, Collimedaglia L, Alciato F,
Molinari R, Sola D, Ranza E, Naldi P, Monaco F, Leone M, Pirisi M
and Avanzi GC: Growth arrest specific gene 6 protein concentration
in cerebrospinal fluid correlates with relapse severity in multiple
sclerosis. Mediators Inflamm. 2013:4064832013. View Article : Google Scholar :
|
|
13
|
Wang MS and Zhang LC: Methodological
comparison on the tracing CSF-CNs with HRP and CB-HRP. Acad Med.
12:286–287. 1992.
|
|
14
|
Lu X, Geng X, Zhang L and Zeng Y: The
methodology for labeling the distal cerebrospinal fluid-contacting
neurons in rats. J Neurosci Methods. 168:98–103. 2008. View Article : Google Scholar
|
|
15
|
Zhang MM, Ji W, Pei LY, Wang W, Chen T,
Wang W, Li H, Zhang T, Wu SX and Li YQ: Acute colitis induces
neurokinin 1 receptor internalization in the rat lumbosacral spinal
cord. PLoS One. 8:e592342013. View Article : Google Scholar :
|
|
16
|
Palecek J, Paleckova V and Willis WD:
Postsynaptic dorsal column neurons express NK1 receptors following
colon inflammation. Neuroscience. 116:565–572. 2003. View Article : Google Scholar
|
|
17
|
Palecek J, Paleckova V and Willis WD: Fos
expression in spinothalamic and postsynaptic dorsal column neurons
following noxious visceral and cutaneous stimuli. Pain.
104:249–257. 2003. View Article : Google Scholar
|
|
18
|
Miampamba M, Chéry-Croze S, Gorry F,
Berger F and Chayvialle JA: Inflammation of the colonic wall
induced by formalin as a model of acute visceral pain. Pain.
57:327–334. 1994. View Article : Google Scholar
|
|
19
|
Bai L, Wang W, Dong YL, Wang W, Huang J,
Wang XY, Wang LY, Li YQ and Wu SX: Attenuation of mouse somatic and
emotional inflammatory pain by hydralazine through scavenging
acrolein and inhibiting neuronal activation. Pain Physician.
15:311–326. 2012.
|
|
20
|
Calle M, Claassen IE, Veening JG, Kozicz
T, Roubos EW and Barendregt HP: Opioid peptides, CRF, and urocortin
in cerebrospinal fluidcontacting neurons in Xenopus laevis. Ann N Y
Acad Sci. 1040:249–252. 2005. View Article : Google Scholar
|
|
21
|
Vigh B, e Silva MJ Manzano, Frank CL,
Vincze C, Czirok SJ, Szabó A, Lukáts A and Szél A: The system of
cerebrospinal fluid-contacting neurons. Its supposed role in the
nonsynaptic signal transmission of the brain. Histol Histopathol.
19:607–628. 2004.
|
|
22
|
Wu TT, Zhao ZJ, Xu C and Zhang LC:
Distribution of TRPC6 in the cerebrospinal fluid-contacting nucleus
of rat brain parenchyma and its expression in morphine dependence
and withdrawal. Neurochem Res. 36:2316–2321. 2011. View Article : Google Scholar
|
|
23
|
Wang CG, Ding YL, Zheng TF, Wei JQ, Liu H,
Chen YF, Wang JY and Zhang LC: Extracellular signal-regulated
kinase 5 in the cerebrospinal fluid-contacting nucleus contributes
to morphine physical dependence in rats. J Mol Neurosci.
50:215–220. 2013. View Article : Google Scholar
|
|
24
|
Laird JM, Olivar T, Roza C, De Felipe C,
Hunt SP and Cervero F: Deficits in visceral pain and hyperalgesia
of mice with a disruption of the tachykinin NK1 receptor gene.
Neuroscience. 98:345–352. 2000. View Article : Google Scholar
|
|
25
|
Palecek J: The role of dorsal columns
pathway in visceral pain. Physiol Res. 53 Suppl 1:S125–S130.
2004.
|