Introduction
The uterus is a female reproductive organ composed
of a well-differentiated endometrium, a thick muscular myometrium,
and an outer serosal layer (1).
Marked progress has been made in understanding the physiology and
pathophysiology of the endometrium, resulting in the development of
important interventions for menstrual function, conception and
contraception. Physiological functions of the endometrium are
differentially regulated by steroid hormones and peptides depending
on the estrous cycle (2). The
mammalian estrous cycle, including follicular and luteal phases, is
regulated by a positive and negative feedback system involving
reproductive hormones that are manufactured and released from
organs including the hypothalamus, pituitary and ovaries (3). In the follicular phase (also called
proliferative phase), estradiol (E2) leads to lining of the uterus
for development and proliferation of endometrium. Following uterus
maturation, ovarian follicles secrete an increasing quantity of E2
to initiate development of a new layer of proliferative endometrium
in the uterus in addition to provoking crypts in the cervix to
produce rich cervical mucus (4).
Following ovulation, E2 production is reduced while the corpus
luteum begins to form and produce progesterone (P4), which serves
an important role in implantation of blastocysts and support of
early pregnancy in the endometrium. High concentrations of P4 have
a negative effect on follicular growth by preventing secretion of
follicle-stimulating hormone (FSH) and luteinizing hormone (LH)
(5). This phase is called the
secretory phase and corresponds to the luteal phase of the estrous
cycle. If female mammals are not pregnant at the luteal phase,
prostaglandin F2α (PGF2α) is released from
the endometrium, and the corpora lutea rapidly regresses and is
degraded. In addition, P4 levels rapidly decline while FSH and LH
gradually increases to stimulate ovarian follicle growth, which is
known as the follicular phase. As ovarian follicles grow, E2
concentrations reach high levels and stimulate ovulation again. The
estrous cycle in mammalian females repeats until pregnancy
(6).
The uterus frequently contracts throughout the
entire estrous cycle. Stable uterine contractile activity is an
essential factor for maintenance of pregnancy in addition to
appropriate timing of childbirth. By contrast, inappropriate
uterine contraction leads to premature birth during pregnancy,
which is currently recognized as one of the leading causes of
perinatal death in the developed world. Premature birth in humans,
defined as labor prior to 37 weeks gestation, occurs in 7–10% of
all births, however, amounts for >85% of all complications and
mortality associated with child birth (7). Preterm labor likely represents a
syndrome rather than a specific diagnosis, due to the fact that the
causes are varied. It may reflect a breakdown in the mechanisms
responsible for maintaining uterine quiescence (8). In mice and rats, P4 promotes
myometrial relaxation and sustains pregnancy, whereas E2 increases
uterine contractility during parturition (9,10).
P4 appears to induce relaxation of the myometrium by repressing
expression of genes that encode factors collectively referred to as
contraction-associated proteins (CAPs), which promote labor. Some
important CAPs include oxytocin receptor (OXTR), prostaglandin (PG)
metabolizing enzyme hydroxyprostaglandin dehydrogenase 15-(NAD)
(HPGD), and gap junction α-1 protein (GJA1) (11). A previous study described a novel
pathway in which P4 coordinately represses expression of two
critical CAP genes, specifically GJA1 (encodes a key gap-junction
protein that synchronizes contractile activity) and OXTR
(determines responsiveness of myometrial cells to OXT, a potent
stimulator of contraction) (12).
In contrast, E2 is known to upregulate expression of CAPs in the
uterus and thereby, stimulates uterine contraction. Thus, correct
balance between P4 and E2 is essential for uterine function and
successful pregnancy. Although regulation of uterine contraction by
steroid hormones during pregnancy and parturition has been studied,
few reports have focused on this issue in non-pregnant animals.
Furthermore, few studies have examined the porcine reproductive
system according to the estrous cycle. It is important to
understand the porcine reproductive system due to the fact that it
is directly connected to the birth ratio of pigs, which is key in
meat production processes.
In the present study, the expression levels of
receptors corresponding with reproductive hormones and CAPs were
examined according to the estrous cycle in the porcine uterus in
order to improve the understanding of the physiological and
endocrine circumstances of the porcine reproductive system.
Materials and methods
Reagents and chemicals
Rabbit anti-β-actin (no. 4967) was purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Rabbit
anti-estrogen receptor 1 (ESR1; sc-542), ESR2 (sc-8974), rabbit
anti-progesterone receptor (PGR; sc-538), rabbit anti-luteinizing
hormone/choriogonadotropin receptor (LHCGR; sc-25828) goat
anti-OXTR (sc-8102) and rabbit anti-HPGD (sc-98907) antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Rabbit anti-GJA1 (ab11370) was purchased from Abcam
(Cambridge, MA, USA). Mouse anti-OXT (MAB5296) was purchased from
Merck Millipore (Darmstadt, Germany). Horseradish peroxidase
(HRP)-conjugated anti-rabbit (sc-2313), anti-mouse (sc-2005) and
anti-goat IgG (sc-2020) secondary antibodies were purchased from
Santa Cruz Biotechnology, Inc.
Tissue preparations
Peripubertal crossbred gilts (n=20, Eoband-dong,
Gimhae-si, Gyeongsangnam-do, South Korea) of 5.5–6.5 months of age
were slaughtered at a local abattoir, and uterus tissues were
collected according to the follicular (n=8) and luteal (n=12)
phases. The tissues were washed with phosphate-buffered saline
(PBS) and fixed in 10% formalin until analysis or stored at −70°C
for further experiments. The animal experiments were approved by
the Pusan National University Institutional Animal Care and Use
Committee.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. The concentration of
total RNA was measured by a spectrophotometer (Biospec-nano;
Shimadzu, Kyoto, Japan). First-strand complementary DNA (cDNA) was
prepared from total RNA (3 µg) by RT using M-MLV reverse
transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.) and
random primers (9-mers; Takara Bio, Inc., Otsu, Japan). RT-qPCR was
performed with cDNA template (2 µl) and 2X Power SYBR Green (6 µl;
Toyobo Co., Ltd., Osaka, Japan) containing specific primers. Primer
sequences for β-actin, S100 calcium-binding protein G (S100G),
ESR1, ESR2, PGR, LHCGR, OXT, OXTR, HPGD and GJA1 are presented in
Table I. RT-qPCR was conducted for
40 cycles using the following parameters: Denaturation at 95°C for
15 sec, followed by annealing and extension at 70°C for 60 sec.
Fluorescence intensity was measured at the end of the extension
phase of each cycle. The threshold value for the fluorescence
intensity of each sample was set manually. The reaction cycle at
which PCR products exceeded this fluorescence intensity threshold
during exponential phase of PCR amplification was considered to be
the quantification cycle (Cq). Expression of the target gene was
quantified relative to that of β-actin, a ubiquitous housekeeping
gene, based on comparison of Cqs at constant fluorescence
intensity.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene name | Primer | Sequence (5′-3′) | Fragment size
(bp) |
|---|
| β-actin | Forward |
TCCCTGGAGAAGAGCTACGA | 149 |
|
| Reverse |
CGCACTTCATGATCGAGTTG |
|
| S100G | Forward |
TCCTGCAGAACTGAAGAGCA | 172 |
|
| Reverse |
TCCATCTCCATTCTTGTCCA |
|
| ESR1 | Forward |
AGCACCCTGAAGTCTCTGGA | 160 |
|
| Reverse |
TGTGCCTGAAGTGAGACAGG |
|
| ESR2 | Forward |
GTGATCACACAACCCGAGTG | 214 |
|
| Reverse |
ATGAAGCCCGGAATTTTCTT |
|
| PGR | Forward |
GATTCAGAAGCCAGCCAGAG | 185 |
|
| Reverse |
CGTCGTTTCTTCCAGCACA |
|
| LHCGR | Forward |
TCCGAAAGCTTCCAGATGTT | 238 |
|
| Reverse |
TGCATCTTCTTCAGGTGTGC |
|
| OXT | Forward |
CGCCTGCTACATCCAGAACT | 193 |
|
| Reverse |
CGGCAGGTAGTTCTCCTCCT |
|
| OXTR | Forward |
TGCTACGGCCTTATCAGCTT | 158 |
|
| Reverse |
GCCTTGGAGATGAGCTTGAC |
|
| HPGD | Forward |
AAGGCGGCATCATTATCAAC | 161 |
|
| Reverse |
GCAAATGGCATTCAGTCTCA |
|
| GJA1 | Forward |
CACCAGGTGGACTGTTTCCT | 151 |
|
| Reverse |
TCTTTCCCTTCACACGATCC |
|
Western blotting analysis
Protein samples were extracted with PRO-PREP
solution (iNtRON Biotechnology, Seoul, South Korea) following the
manufacturer's protocol. A total of 30 µg cytosolic proteins were
separated by 8–12% SDS-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to nitrocellulose membranes (Daeillab
Service Co., Ltd., Seoul, South Korea). Membranes were then blocked
for 2 h with 5% skimmed milk (Difco; BD Biosciences, Sparks, MD,
USA) in PBS with 0.05% Tween-20 (PBS-T). Following blocking,
membranes were incubated with ESR1 (1:500), ESR2 (1:500), PGR
(1:500), LHCGR (1:500), OXTR (1:500), HPGD (1:500) and GJA1
(1:1,000) overnight, followed by HRP-conjugated anti-rabbit and
anti-goat secondary antibodies (1:2,000) in 5% skimmed milk with
PBS-T for 1 h. Luminol reagent (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to visualize antibody binding. Each
blot was then stripped by incubation with 2% SDS and 100 mM
mercaptoethanol in 62.5 mM Tris-HCl (pH 6.8) for 30 min at 50–60°C.
Membranes were subsequently probed with antibody against β-actin
(1:2,000) as an internal control. Blots were scanned using Gel Doc
1000, version 1.5 (Bio-Rad Laboratories, Inc.), and band
intensities were normalized to β-actin levels.
Dot blot analysis
Total proteins prepared from porcine uterus tissues
were transferred to a nitrocellulose membrane using a SlotBlot kit
(Pharmacia Biotech, Inc., Piscataway Township, NJ, USA). The
membrane was incubated with the antibody specific for OXT (1:5,000)
for 1 h, followed by the HRP-conjugated anti-mouse secondary
antibody (1:2,000) in 5% skimmed milk with TBS-T for 1 h. Membranes
were subsequently probed with the antibody against β-actin
(1:2,000) as an internal control. Blots were then visualized by
Luminol reagent and analyzed by Gel Doc 1000 (Bio-Rad Laboratories,
Inc.). Band intensities were normalized to β-actin levels.
Histological analysis
Uterus tissues were fixed with 10% formalin,
embedded in paraffin wax, routinely processed, then sectioned into
4-µm-thick slices. Tissue sections were deparaffinized and
rehydrated through graded alcohol using standard procedures and
then stained with hematoxylin & eosin (H&E; Sigma-Aldrich;
Merck Millipore). Images were captured at a magnification of ×40
using a model BX50F-3 optical microscope (Olympus Corporation,
Tokyo, Japan) and examined for histological analysis.
Statistical analysis
Results are presented as the mean ± standard
deviation. Data were analyzed using Sigma Plot, version 10.0
(Systat Software, Inc., San Jose, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Preparation of porcine uterus
tissue
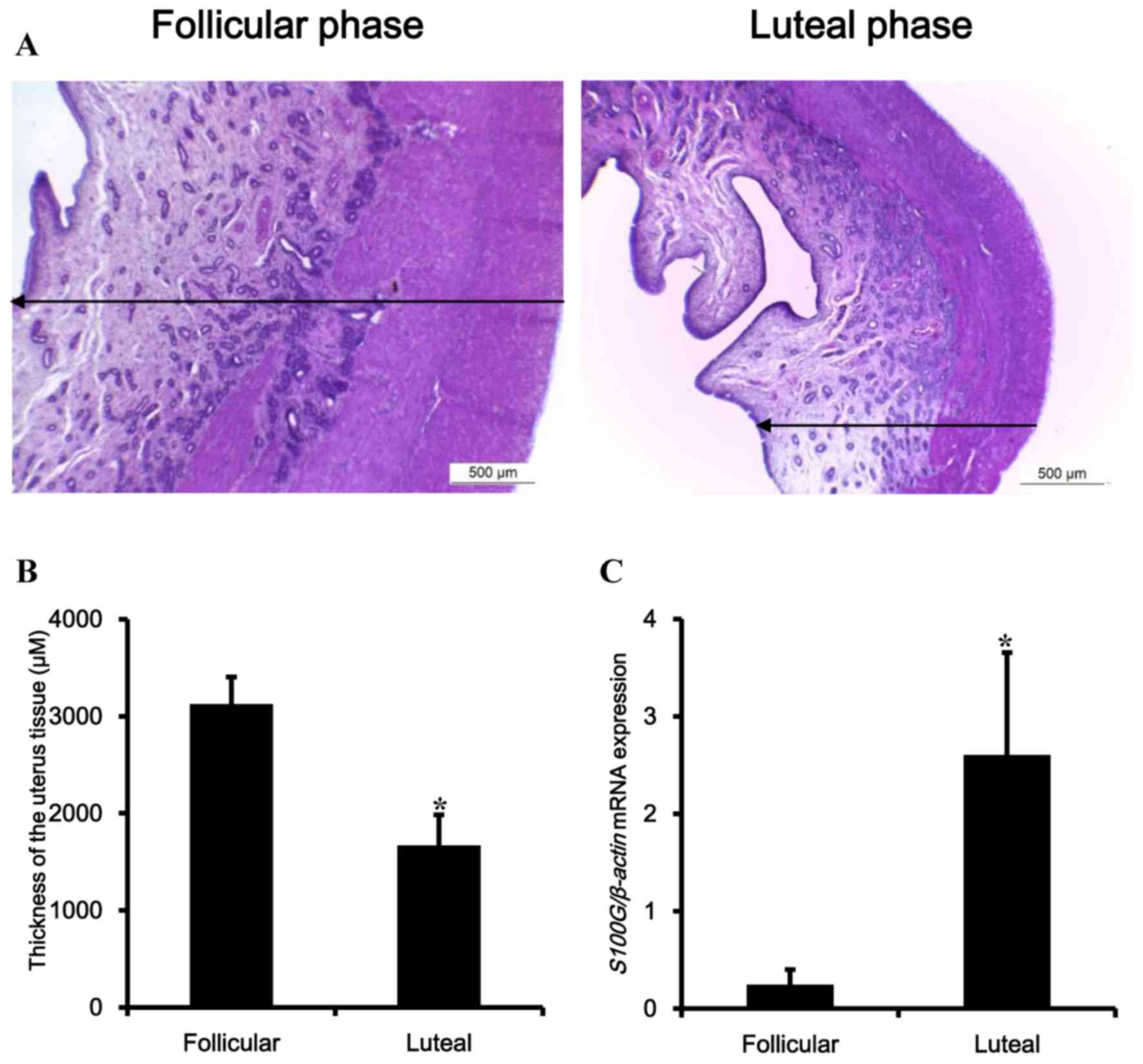
In the first experiment, to distinguish the porcine
uterus according to follicular and luteal phases, porcine uteruses
were examined by histological analysis and luteal phase marker gene
(Fig. 1). The tissues were fixed
and stained with H&E (Fig.
1A). Based on histological analysis by typical characteristics
of estrous uterine endometrium, by observing a developed
endometrial layer that was relatively thicker than that of the
luteal phase, uteruses were divided into the follicular and luteal
phases (Fig. 1B). Additionally,
the transcriptional level of S100G, a biomarker of the luteal phase
of porcine uterus, was observed (13). The transcriptional levels were
increased up to 10-fold in the luteal phase as predicted (Fig. 1C).
Transcriptional and translational
expression levels of ESR1, ESR2, PGR, and LHCGR in the porcine
uterus according to estrous cycle
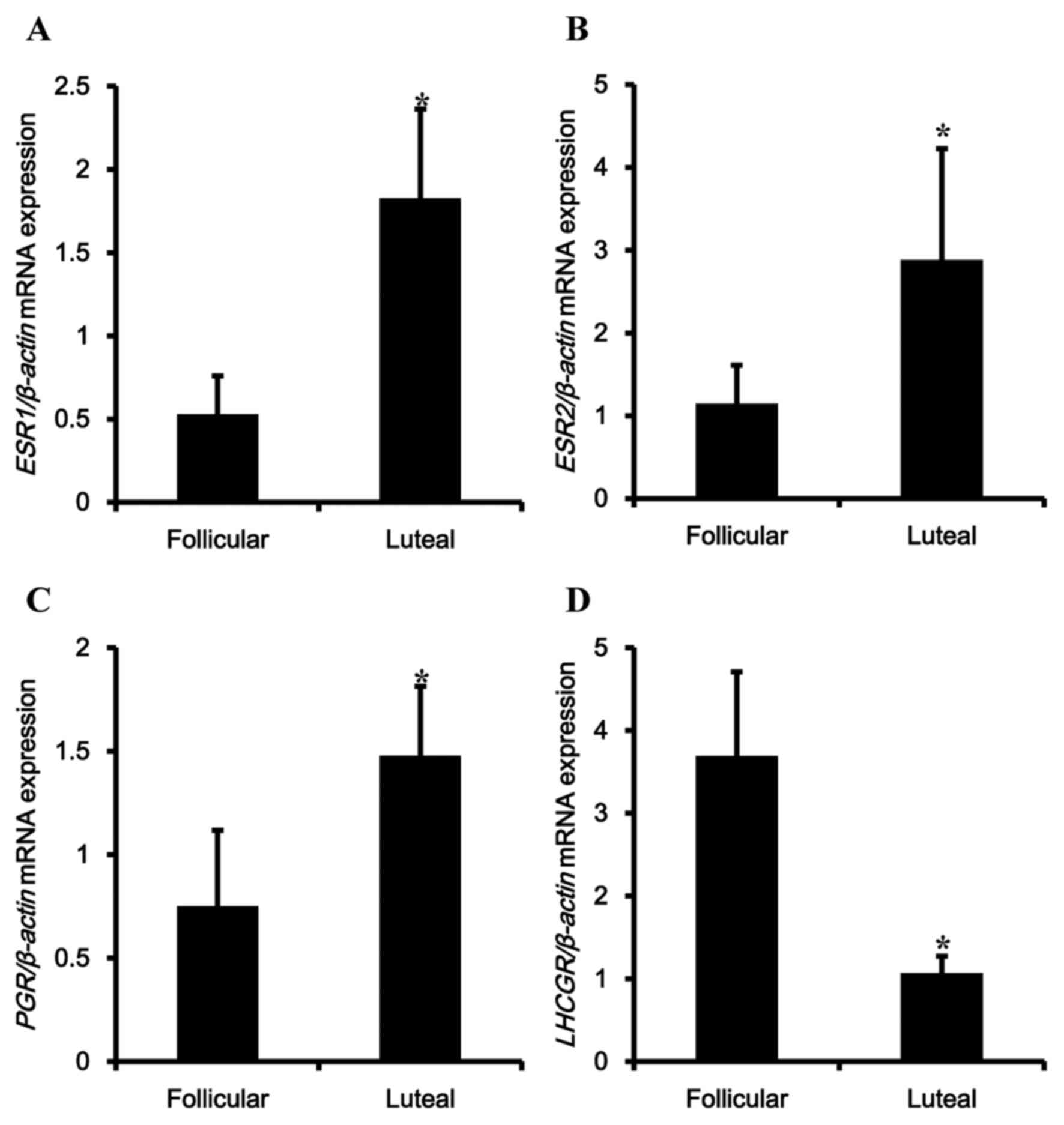
To confirm regulation of contraction-associated
factors according to the estrous cycle, mRNA and protein levels of
reproductive hormonal receptors were examined. The expression
levels of ESR1, ESR2, PGR and LHCGR mRNA were analyzed in the
porcine uterus. Expression of ESR1, ESR2, PGR and LHCGR mRNA were
observed in the uterus, and transcriptional levels of ESR1, ESR2
and PGR were significantly upregulated during the luteal phase
compared with the follicular phase (Fig. 2A-C). In contrast, expression of
LHCGR was significantly reduced in the luteal phase (Fig. 2D).
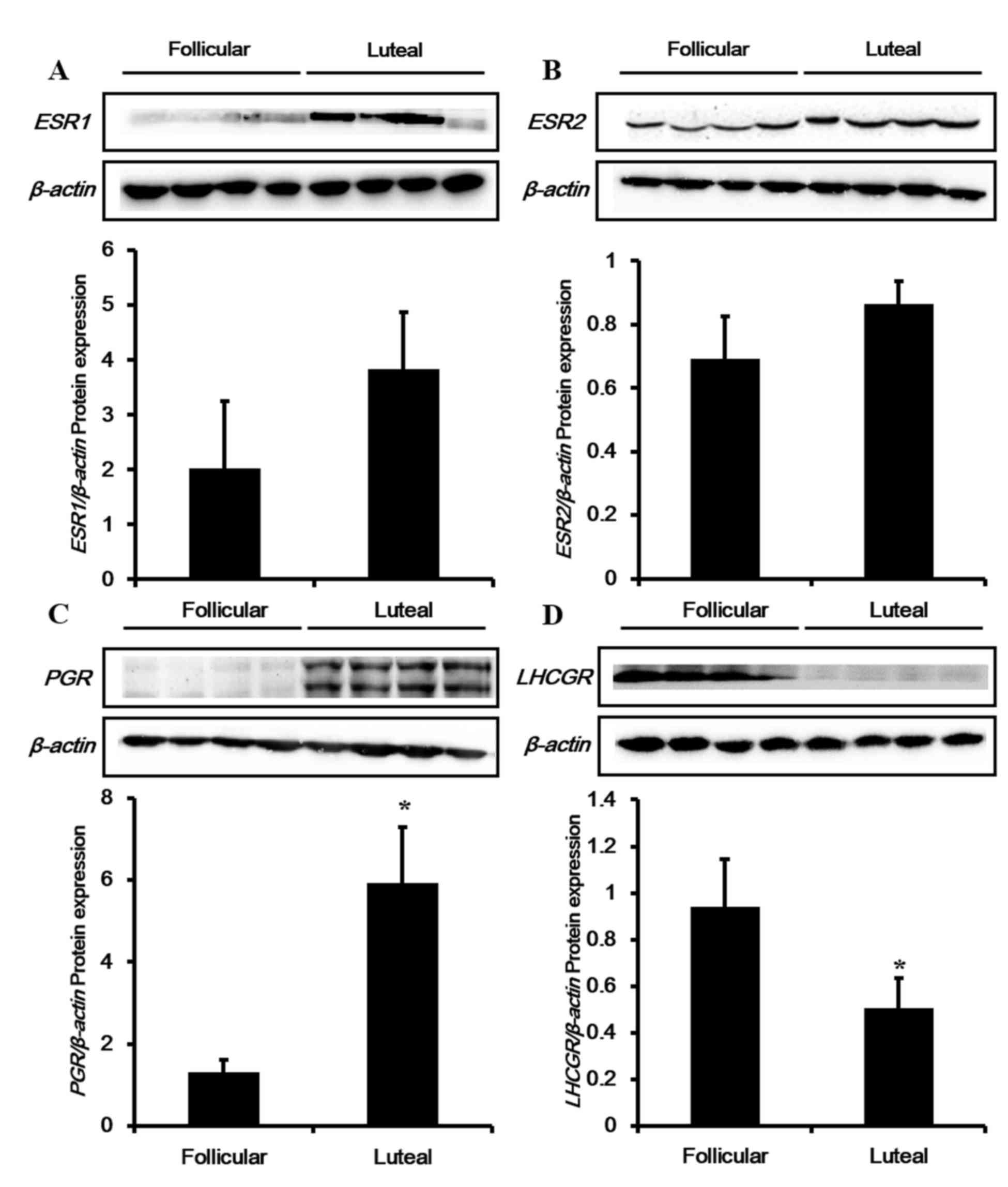
To confirm expression of these genes at the
translational level in the porcine uterus according to the estrous
cycle, western blotting analysis was conducted for ESR1, ESR2, PGR
and LHCGR proteins (Fig. 3).
Consistent with the mRNA results, protein expression levels of
ESR1, ESR2 and PGR were enhanced during the luteal phase compared
with the follicular phase, although the results for ESR1 and ESR2
were not significant (Fig. 3A-C).
Similar to the mRNA results, the protein levels of LHCGR (Fig. 3D) were significantly reduced during
the luteal phase in the porcine uterus.
Transcriptional and translational
expression of CAPs in porcine uterus according to the estrous
cycle
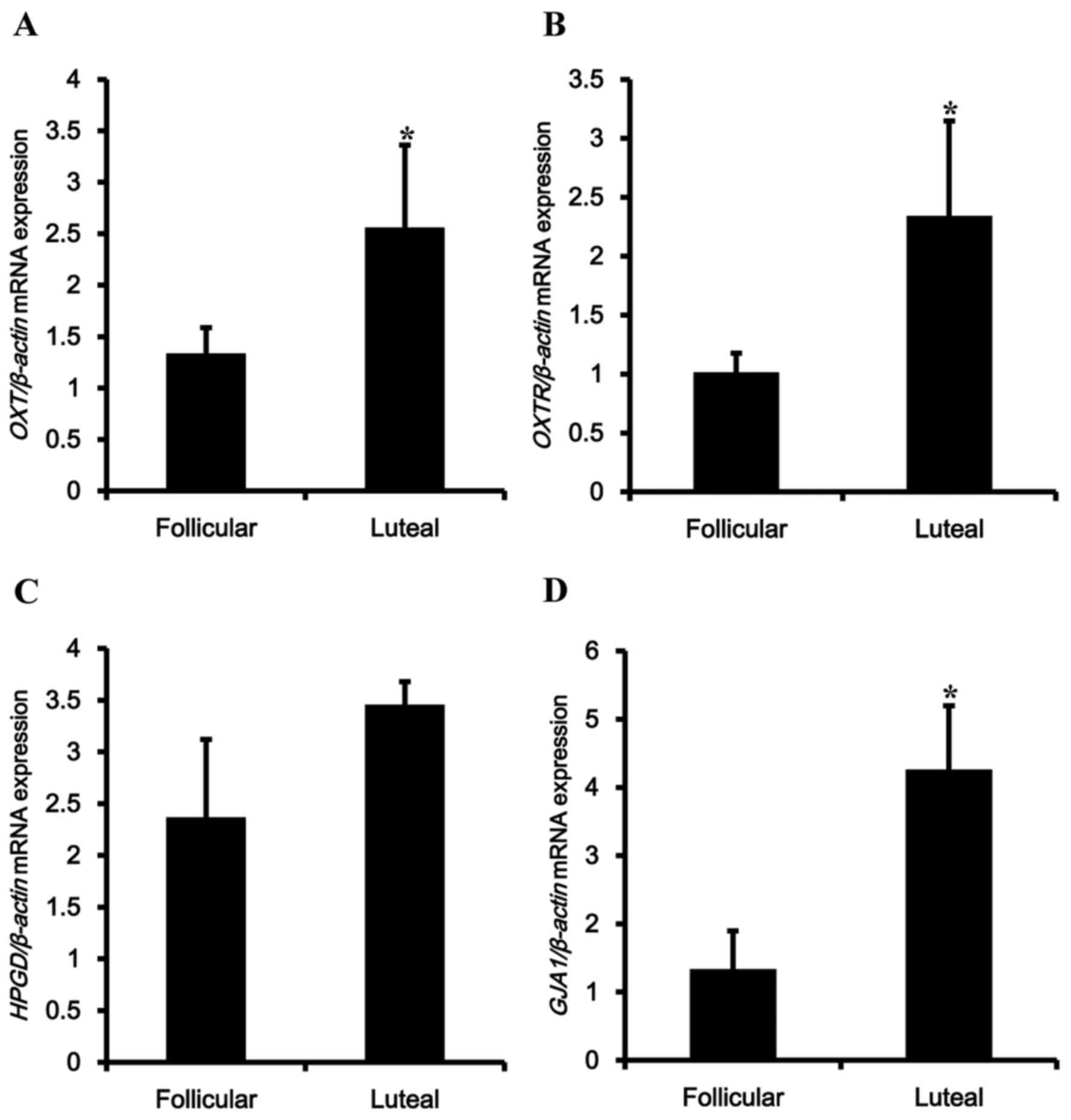
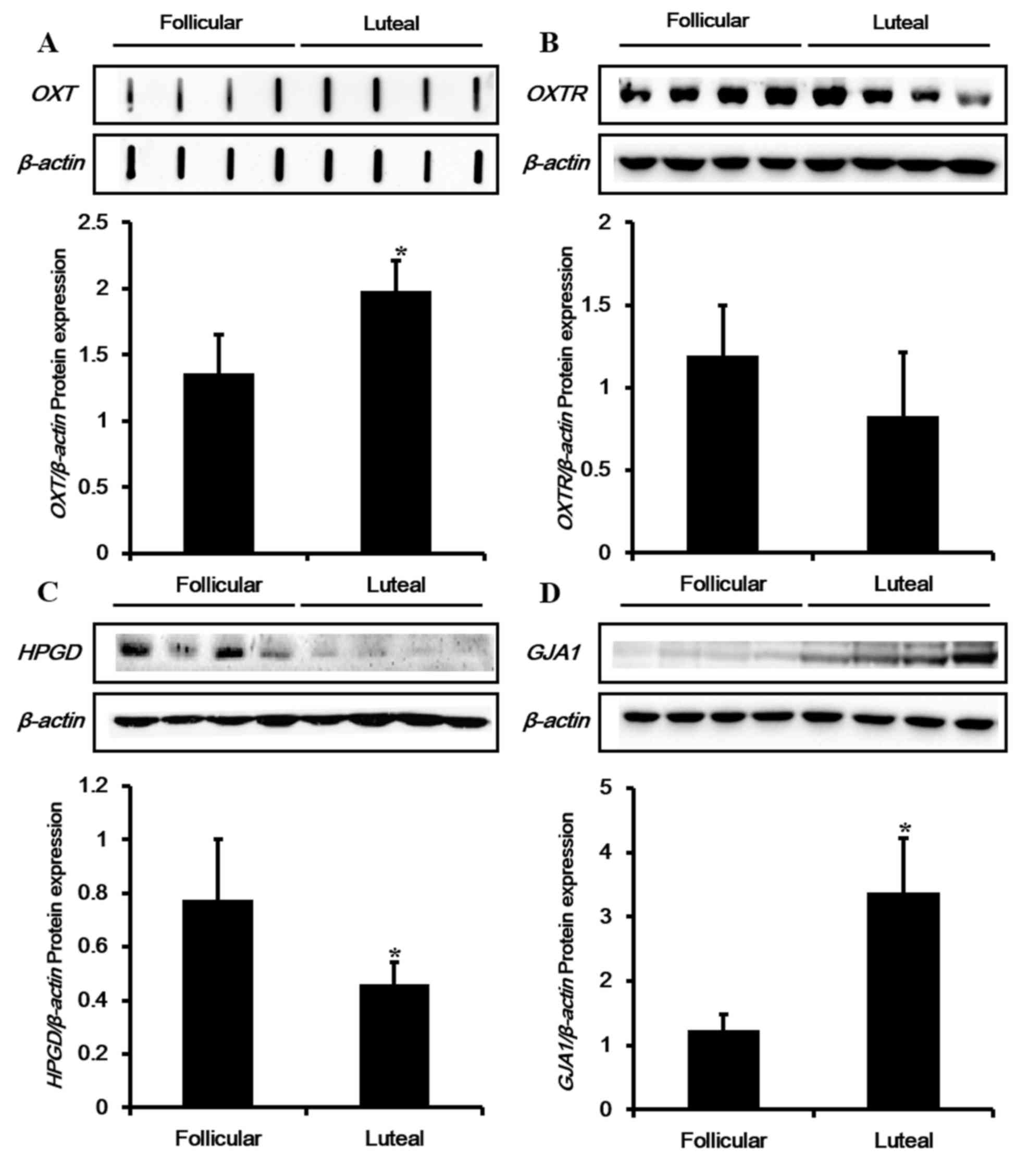
To examine regulation of CAPs depending on the
estrous cycle, the mRNA expression levels of OXT-associated genes,
including OXT and OXTR, were examined (Fig. 4A and B). Transcriptional levels of
OXT and OXTR were upregulated in the luteal phase. The PGF
metabolic enzyme HPGD exhibited no significant differences in mRNA
expression depending on the estrous cycle (Fig. 4C). Among the tested genes, mRNA
expression of GJA1 was upregulated by 4-fold in the luteal phase
compared with the follicular phase (Fig. 4D).
Subsequently, protein expression of CAPs, including
OXTR, HPGD and GJA1 were examined during the follicular and luteal
phases of the porcine uterus by western blot analyses. In addition,
a dot-blot assay was conducted in order to detect the signal of
OXT, due to its low molecular weight (Fig. 5). Protein expression patterns of
OXT and GJA1 were similar to their mRNA levels, which were
significantly upregulated during the luteal phase compared with the
follicular phase (Fig. 5A and D).
However, protein expression levels of OXTR and HPGD were different
from the transcription results (Fig.
5B and C). Although mRNA levels of OXTR and HPGD were augmented
in the luteal phase, protein levels were observed to decline in the
luteal phase.
Discussion
Study of the porcine reproductive system is
important, due to the fact that pigs breed only twice a year, and
pork consumption is increasing. The porcine estrous cycle spans a
period of 18–24 days and consists of an follicular phase of 5–7
days and a luteal phase of 13–15 days (3). Following ovulation, the developing
corpora lutea produces an increasing amount of P4, reaching peak
concentrations 8–9 days after ovulation (14). Subsequently, luteolysis occurs
around 15 days after ovulation if pigs are not pregnant in the
luteal phase.
In the present study, the histological patterns of
the uterus were examined in order to determine the follicular and
luteal phases in the porcine uterus. In addition, examination of
S100G expression confirmed the correct classification of porcine
uterine tissues according to the estrous cycle. S100G is a
cytosolic calcium-binding protein that is expressed predominantly
in the duodenum, placenta and uterus. Although few studies have
investigated the estrous cycle in the porcine uterus, regulation of
the S100G gene during the porcine estrous cycle has been
investigated in a previous study, indicating that the S100G gene is
a useful marker gene for the luteal phase of the porcine uterus
(13).
Understanding the physiological characteristics of
the uterus depending on concentrations of endogenous hormones and
the estrous cycle is critically important due to the fact it
contributes to enhanced livestock production, and physiological
studies of this nature are currently lacking. A previous study
observed that plasma levels of E2 were highly regulated during the
follicular phase in pigs (15). In
another study, P4 concentration in the luteal phase increased
gradually during formation of the corpus luteum throughout the
porcine estrous cycle (16).
In the present study, ESR1, ESR2, PGR and LHCGR were
all expressed in the porcine uterus. Transcriptional and
translational levels of ESR1, ESR2, and PGR were elevated during
luteal phase compared to follicular phase, although alteration of
ESR1 and ESR2 protein expression was not significant. Both mRNA and
protein expression levels of LHCGR were significantly reduced in
the luteal phase. These results indicate that signaling of E2, P4
and LH can be dynamically regulated via expression of their
cognitive receptors. Furthermore, it was previously reported that
E2 maintains a high concentration during the follicular phase,
whereas LH is highly produced in the luteal phase (17). Thereby, it is possible that
regulation of ESR1, regulation of ESR1, ESR2 and LHCGR is under the
negative feedback influence of their agonists, E2 and LH.
To the best of our current knowledge, only previous
study has investigated the regulation of steroid receptor during
the estrous cycle. Sukjumlong et al (18), examined the mRNA expression levels
of ESR1, ESR2 and PGR in the uterine endometrium and myometrium of
cyclic sows. It was identified that the level of ESR1 mRNA in the
endometrium exhibited no significant differences depending on the
estrous cycle, although ESR1 mRNA expression was greater in the
follicular phase compared with the luteal phase in the myometrium.
In the case of ESR2, mRNA expression was low in all stages with no
significant difference depending on the estrous cycle. The mRNA
level of PGR in follicular phase was higher than in the luteal
phase in the sow uterus (18).
However, these experiments were limited to only mRNA measurements,
these results differ from those of the current study due to the
distinct environmental conditions for pigs used.
In the uterus, regulation of myometrial
contractility is regulated by expression of genes encoding CAPs,
which is under the control of E2 and P4. Since expression of ESRs
and PGR was differentially regulated during the estrous cycle, it
was hypothesized that CAPs as indicators of uterine contractile
activity may also be regulated during the estrous cycle. In order
to confirm this hypothesis, mRNA and protein expression levels of
CAPs were evaluated in the non-pregnant stage of the porcine uterus
according to the estrous cycle. OXT and OXTR mRNA expression levels
were observed to be significantly increased during the luteal phase
compared with the follicular phase, although protein levels of OXTR
were marginally reduced. At present, there is insufficient evidence
available regarding regulation of OXT and OXTR in the uterus. In
experimental animals, E2 was demonstrated to increase OXTR in the
uterus of non-pregnant and pregnant rats (19–21).
HPGD, which oxidizes and inactivates PG at the
15-hydroxyl group, was expressed in the porcine uterus, and its
mRNA levels exhibited no significant differences depending on the
estrous cycle. However, protein expression of HPGD significantly
increased during the follicular phase compared with the luteal
phase. It was reported that E2 increases activity of HPGD in the
uteruses of rats and humans (22,23).
In the present study, GJA1, a transmembrane and gap junction
protein that serves an important role in contraction of muscle
tissues, was identified to be expressed in the porcine uterus, and
its mRNA level was significantly increased during the luteal phase
compared with the follicular phase. Consistent with the mRNA
results, protein expression of GJA1 was enhanced during the luteal
phase. Although different tissue was tested, protein expression of
GJA1 in the bovine corpus luteum was highest in the early luteal
phase and decreased as the estrous cycle progressed (24). In summary, expression levels of
specific receptors for E2, P4 and LH were observed together with
CAPs in the porcine uterus during the estrous cycle. The results
indicated that these proteins are dynamically regulated during the
estrous cycle and may serve critical roles in the physiological
function and contraction of the uterus.
Acknowledgements
The current study was supported by the National
Research Foundation of Korea grant funded by the Korea government
(MEST; grant no. 2014R1A1A2057387).
References
|
1
|
An BS, Ahn HJ, Kang HS, Jung EM, Yang H,
Hong EJ and Jeung EB: Effects of estrogen and estrogenic compounds,
4-tert-octylphenol, and bisphenol A on the uterine contraction and
contraction-associated proteins in rats. Mol Cell Endocrinol.
375:27–34. 2013. View Article : Google Scholar
|
|
2
|
Dawe ST, Husband AJ and Langford CM:
Effects of induction of parturition in ewes with dexamethasone or
oestrogen on concentrations of immunoglobulins in colostrum, and
absorption of immunoglobulins by lambs. Aust J Biol Sci.
35:223–230. 1982. View Article : Google Scholar
|
|
3
|
Soede N, Langendijk P and Kemp B:
Reproductive cycles in pigs. Anim Reprod Sci. 124:251–258. 2011.
View Article : Google Scholar
|
|
4
|
Bullivant SB, Sellergren SA, Stern K,
Spencer NA, Jacob S, Mennella JA and McClintock MK: Women's sexual
experience during the menstrual cycle: Identification of the sexual
phase by noninvasive measurement of luteinizing hormone. J Sex Res.
41:82–93. 2004. View Article : Google Scholar
|
|
5
|
Rzucidlo S, Weigl R and Tilton J:
Myometrial LH/hCG receptors during the estrous cycle and pregnancy
in pigs. Anim Reprod Sci. 51:249–257. 1998. View Article : Google Scholar
|
|
6
|
Krzymowski T and Stefańczyk-Krzymowska S:
The oestrous cycle and early pregnancy-a new concept of local
endocrine regulation. Vet J. 168:285–296. 2004. View Article : Google Scholar
|
|
7
|
Rush RW, Keirse MJ, Howat P, Baum JD,
Anderson AB and Turnbull AC: Contribution of preterm delivery to
perinatal mortality. Br Med J. 2:965–968. 1976. View Article : Google Scholar :
|
|
8
|
López Bernal A, Rivera J, Europe-Finner
GN, Phaneuf S and Asbóth G: Parturition: Activation of stimulatory
pathwaysor loss of uterine quiescence? Adv Exp Med Biol.
395:435–451. 1994.
|
|
9
|
Wu WX, Ma XH, Coksaygan T, Chakrabarty K,
Collins V, Rose J and Nathanielsz PW: Prostaglandin mediates
premature delivery in pregnant sheep induced by estradiol at 121
days of gestational age. Endocrinology. 145:1444–1452. 2004.
View Article : Google Scholar
|
|
10
|
Csapo AL, Knobil E, Van Der Molen HJ and
Wiest WG: Peripheral plasma progesterone levels during human
pregnancy and labor. Am J Obstet Gynecol. 110:630–632. 1971.
View Article : Google Scholar
|
|
11
|
Mesiano S and Welsh TN: Steroid hormone
control of myometrial contractility and parturition. Seminars in
cell & developmental biology. 18:pp. 321–331. 2007; View Article : Google Scholar
|
|
12
|
Renthal NE, Chen CC, Williams KC, Gerard
RD, Prange-Kiel J and Mendelson CR: miR-200 family and targets,
ZEB1 and ZEB2, modulate uterine quiescence and contractility during
pregnancy and labor. Proc Natl Acad Sci USA. 107:pp. 20828–20833.
2010; View Article : Google Scholar :
|
|
13
|
Yun SM, Choi KC, Kim IH, An BS, Lee GS,
Hong EJ, Oh GT and Jeung EB: Dominant expression of porcine
Calbindin-D9k in the uterus during a luteal phase. Mol Reprod Dev.
67:251–256. 2004. View Article : Google Scholar
|
|
14
|
Knox R: Recruitment and selection of
ovarian follicles for determination of ovulation rate in the pig.
Domest Anim Endocrinol. 29:385–397. 2005. View Article : Google Scholar
|
|
15
|
Sukjumlong S, Kaeoket K, Dalin AM and
Persson E: Immunohistochemical Studies on Oestrogen Receptor Alpha
(ER Alpha) and the Proliferative Marker Ki-67 in the Sow Uterus at
Different Stages of the Oestrous Cycle. Reprod Domest Anim.
38:5–12. 2003. View Article : Google Scholar
|
|
16
|
Ribeiro LA, Bacci ML, Seren E, Tamanini C
and Forni M: Characterization and differential expression of
vascular endothelial growth factor isoforms and receptors in swine
corpus luteum throughout estrous cycle. Mol Reprod Dev. 74:163–171.
2007. View Article : Google Scholar
|
|
17
|
Cassidy A, Bingham S and Setchell KD:
Biological effects of a diet of soy protein rich in isoflavones on
the menstrual cycle of premenopausal women. Am J Clin Nutr.
60:333–340. 1994.
|
|
18
|
Sukjumlong S, Persson E, Dalin AM, Janson
V and Sahlin L: Messenger RNA levels of estrogen receptors alpha
and beta and progesterone receptors in the cyclic and
inseminated/early pregnant sow uterus. Anim Reprod Sci.
112:215–228. 2009. View Article : Google Scholar
|
|
19
|
Murata T, Narita K, Honda K and Higuchi T:
Changes of receptor mRNAs for oxytocin and estrogen during the
estrous cycle in rat uterus. J Vet Med Sci. 65:707–712. 2003.
View Article : Google Scholar
|
|
20
|
Murata T, Narita K, Honda K, Matsukawa S
and Higuchi T: Differential regulation of estrogen receptor alpha
and beta mRNAs in the rat uterus during pregnancy and labor:
Possible involvement of estrogen receptors in oxytocin receptor
regulation. Endocr J. 50:579–587. 2003. View Article : Google Scholar
|
|
21
|
Murata T, Murata E, Liu C, Narita K, Honda
K and Higuchi T: Oxytocin receptor gene expression in rat uterus:
Regulation by ovarian steroids. J Endocrinol. 166:45–52. 2000.
View Article : Google Scholar
|
|
22
|
Matsuo M, Ensor CM and Tai HH:
Characterization of the genomic structure and promoter of the mouse
NAD+-dependent 15-hydroxyprostaglandin dehydrogenase gene. Biochem
Biophys Res Commun. 235:582–586. 1997. View Article : Google Scholar
|
|
23
|
Kelly RW, Linan C, Thong J, Yong EL and
Baird DT: Prostaglandin inactivation is increased in endometrium
after exposure to clomiphene. Prostaglandins Leukot Essent Fatty
Acids. 50:235–238. 1994. View Article : Google Scholar
|
|
24
|
Grazul-Bilska AT, Redmer DA, Johnson ML,
Jablonka-Shariff A, Bilski JJ and Reynolds LP: Gap junctional
protein connexin 43 in bovine corpora lutea throughout the estrous
cycle. Biol Rep. 54:1279–1287. 1996. View Article : Google Scholar
|