|
1
|
Jankovic J: Parkinson's disease: Clinical
features and diagnosis. J Neurol Neurosurg Psychiatry. 79:368–376.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kowal SL, Dall TM, Chakrabarti R, Storm MV
and Jain A: The current and projected economic burden of
Parkinson's disease in the United States. Mov Disord. 28:311–318.
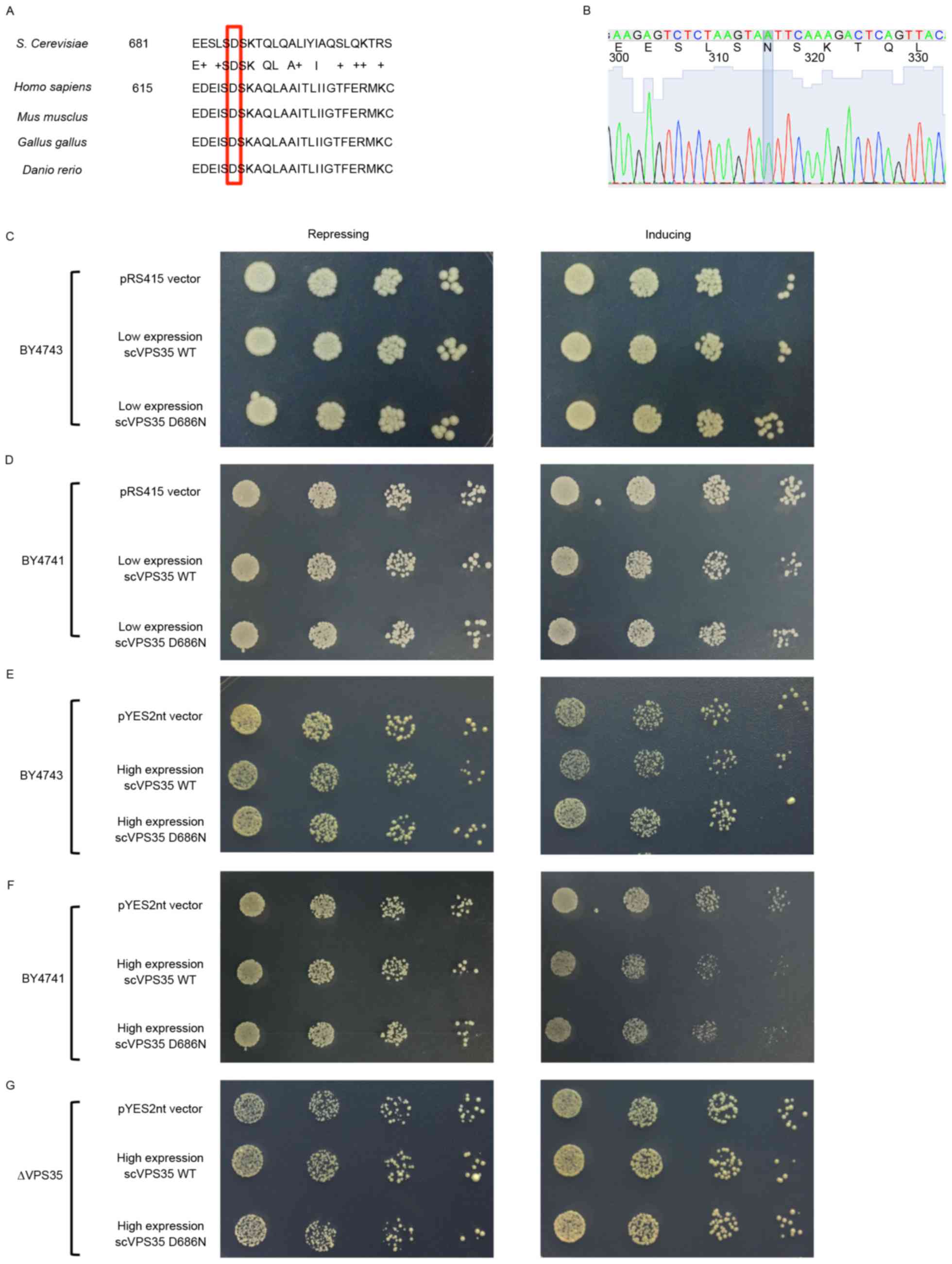
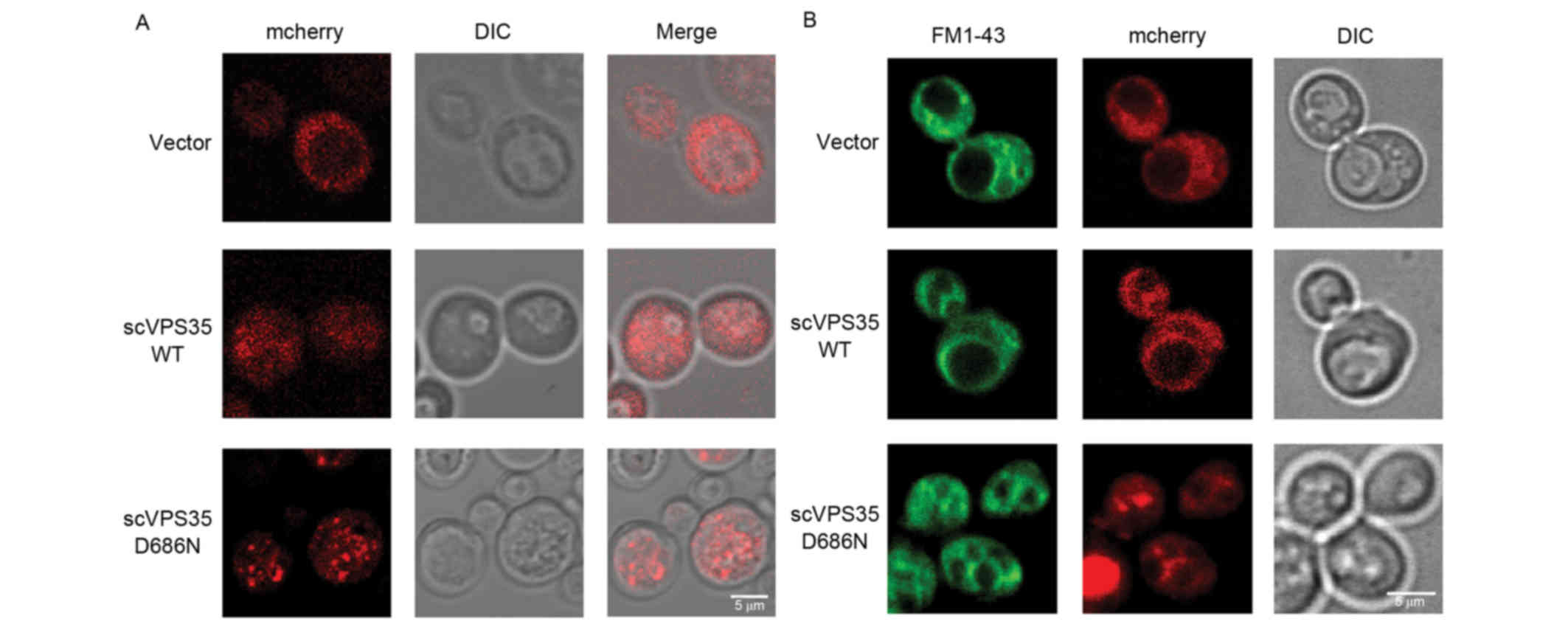
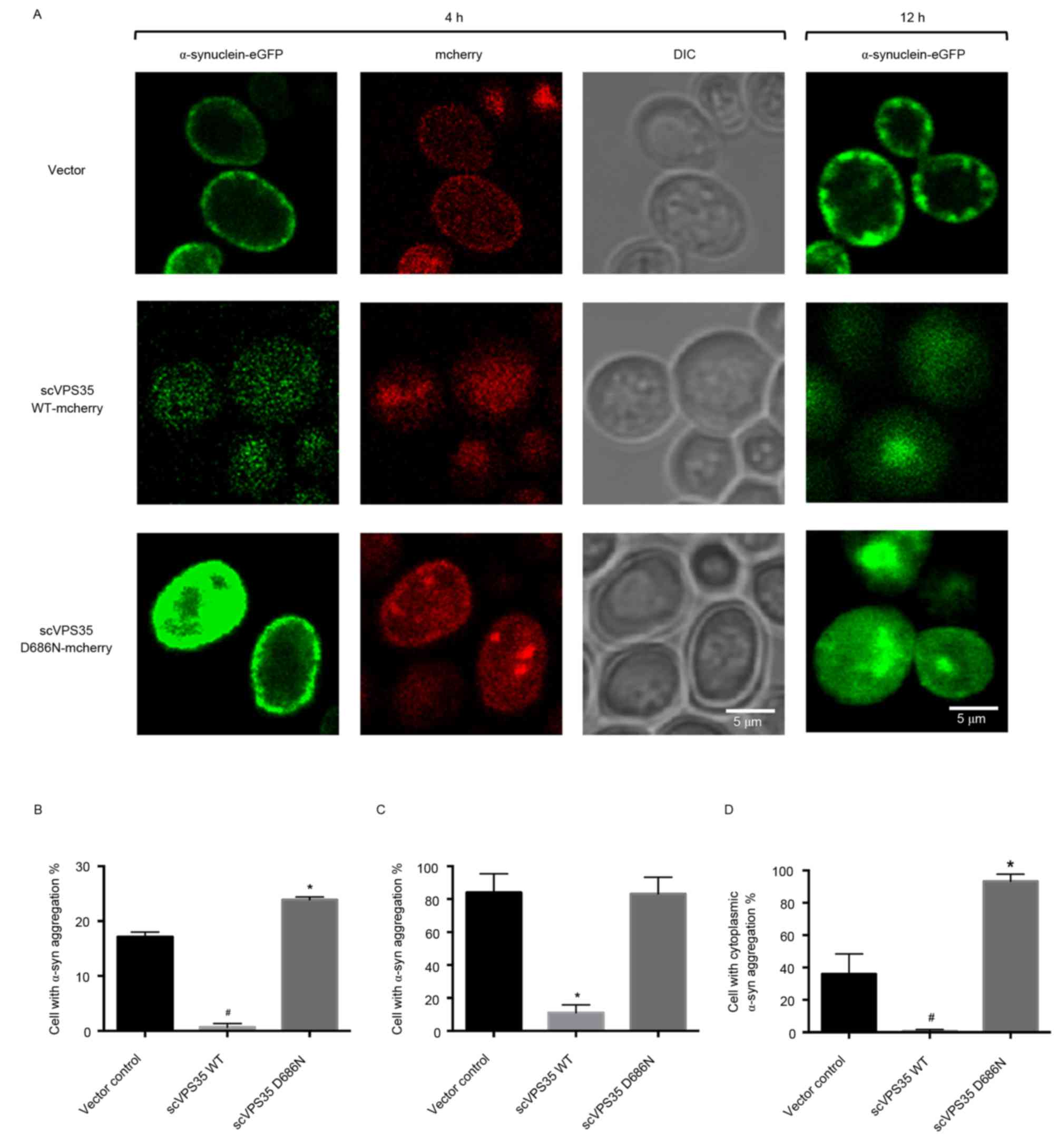
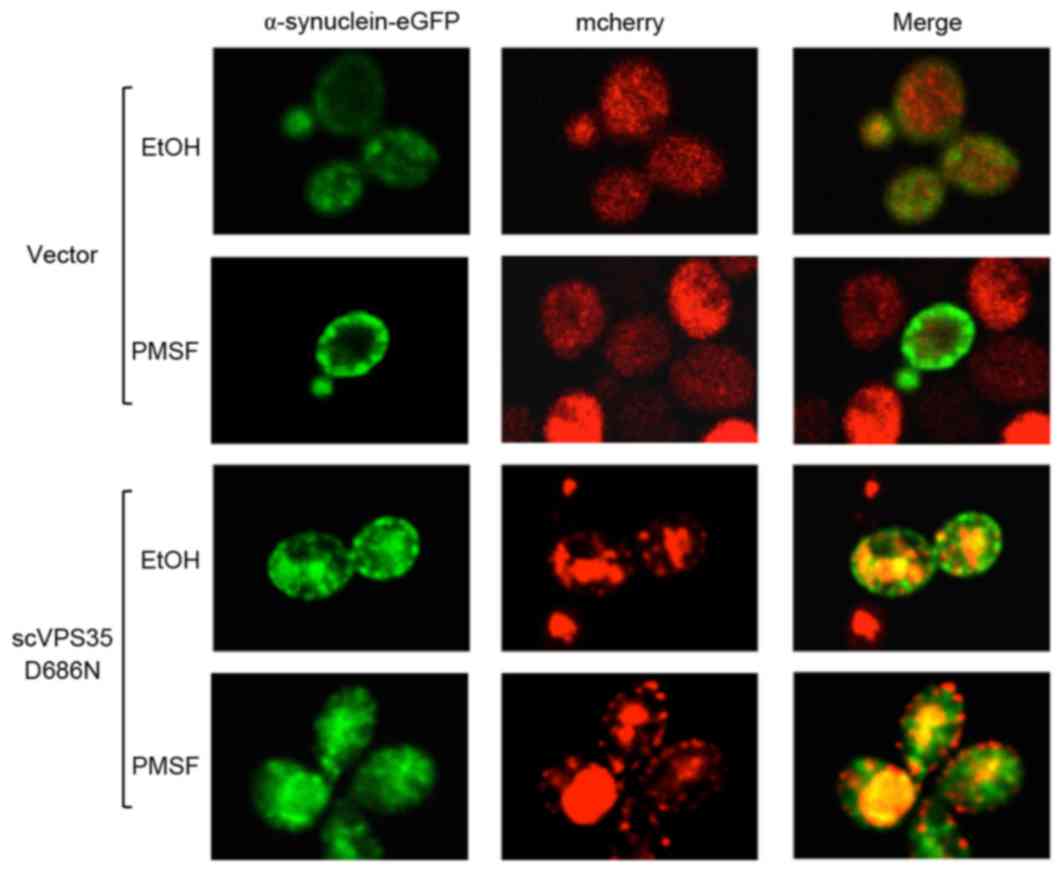
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
LeWitt PA and Fahn S: Levodopa therapy for
Parkinson disease: A look backward and forward. Neurology. 86:(14
Suppl 1). S3–S12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trinh J and Farrer M: Advances in the
genetics of Parkinson disease. Nat Rev Neurol. 9:445–454. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Warner TT and Schapira AH: Genetic and
environmental factors in the cause of Parkinson's disease. Ann
Neurol. 53:(Suppl 3). S16–S25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mouradian MM: Recent advances in the
genetics and pathogenesis of Parkinson disease. Neurology.
58:179–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gasser T: Update on the genetics of
Parkinson's disease. Mov Disord. 22:(Suppl 17). S343–S350. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polymeropoulos MH, Lavedan C, Leroy E, Ide
SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et
al: Mutation in the alpha-synuclein gene identified in families
with Parkinson's disease. Science. 276:2045–2047. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baba M, Nakajo S, Tu PH, Tomita T, Nakaya
K, Lee VM, Trojanowski JQ and Iwatsubo T: Aggregation of
alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and
dementia with Lewy bodies. Am J Pathol. 152:879–884.
1998.PubMed/NCBI
|
|
10
|
Tofaris GK: Lysosome-dependent pathways as
a unifying theme in Parkinson's disease. Mov Disord. 27:1364–1369.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee HJ, Khoshaghideh F, Patel S and Lee
SJ: Clearance of alpha-synuclein oligomeric intermediates via the
lysosomal degradation pathway. J Neurosci. 24:1888–1896. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chu Y, Dodiya H, Aebischer P, Olanow CW
and Kordower JH: Alterations in lysosomal and proteasomal markers
in Parkinson's disease: Relationship to alpha-synuclein inclusions.
Neurobiol Dis. 35:385–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vilarino-Guell C, Wider C, Ross OA,
Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA,
Wilhoite GJ, Bacon JA, et al: VPS35 mutations in Parkinson disease.
Am J Hum Genet. 89:162–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zimprich A, Benet-Pagès A, Struhal W, Graf
E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC,
Lichtner P, et al: A mutation in VPS35, encoding a subunit of the
retromer complex, causes late-onset Parkinson disease. Am J Hum
Genet. 89:168–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Follett J, Norwood SJ, Hamilton NA, Mohan
M, Kovtun O, Tay S, Zhe Y, Wood SA, Mellick GD, Silburn PA, et al:
The Vps35 D620N mutation linked to Parkinson's disease disrupts the
cargo sorting function of retromer. Traffic. 15:230–244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zavodszky E, Seaman MN, Moreau K,
Jimenez-Sanchez M, Breusegem SY, Harbour ME and Rubinsztein DC:
Mutation in VPS35 associated with Parkinson's disease impairs WASH
complex association and inhibits autophagy. Nat Commun. 5:38282014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miura E, Hasegawa T, Konno M, Suzuki M,
Sugeno N, Fujikake N, Geisler S, Tabuchi M, Oshima R, Kikuchi A, et
al: VPS35 dysfunction impairs lysosomal degradation of α-synuclein
and exacerbates neurotoxicity in a Drosophila model of Parkinson's
disease. Neurobiol Dis. 71:1S–13S. 2014. View Article : Google Scholar
|
|
18
|
Tang FL, Liu W, Hu JX, Erion JR, Ye J, Mei
L and Xiong WC: VPS35 deficiency or mutation causes dopaminergic
neuronal loss by impairing mitochondrial fusion and function. Cell
Rep. 12:1631–1643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Wang X, Fujioka H, Hoppel C, Whone
AL, Caldwell MA, Cullen PJ, Liu J and Zhu X: Parkinson's
disease-associated mutant VPS35 causes mitochondrial dysfunction by
recycling DLP1 complexes. Nat Med. 22:54–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wen L, Tang FL, Hong Y, Luo SW, Wang CL,
He W, Shen C, Jung JU, Xiong F, Lee DH, et al: VPS35
haploinsufficiency increases Alzheimer's disease neuropathology. J
Cell Biol. 195:765–779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dhungel N, Eleuteri S, Li LB, Kramer NJ,
Chartron JW, Spencer B, Kosberg K, Fields JA, Stafa K, Adame A, et
al: Parkinson's disease genes VPS35 and EIF4G1 interact genetically
and converge on α-synuclein. Neuron,. 85:76–87. 2015. View Article : Google Scholar
|
|
22
|
Tang FL, Erion JR, Tian Y, Liu W, Yin DM,
Ye J, Tang B, Mei L and Xiong WC: VPS35 in dopamine neurons is
required for Endosome-to-Golgi retrieval of Lamp2a, a receptor of
chaperone-mediated autophagy that is critical for α-synuclein
degradation and prevention of pathogenesis of Parkinson's disease.
J Neurosci. 35:10613–10628. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Outeiro TF and Lindquist S: Yeast cells
provide insight into alpha-synuclein biology and pathobiology.
Science. 302:1772–1775. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Franssens V, Boelen E, Anandhakumar J,
Vanhelmont T, Büttner S and Winderickx J: Yeast unfolds the road
map toward alpha-synuclein-induced cell death. Cell Death Differ.
17:746–753. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edelheit O, Hanukoglu A and Hanukoglu I:
Simple and efficient site-directed mutagenesis using two
single-primer reactions in parallel to generate mutants for protein
structure-function studies. BMC Biotechnol. 9:612009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anandhan A, Rodriguez-Rocha H, Bohovych I,
Griggs AM, Zavala-Flores L, Reyes-Reyes EM, Seravalli J, Stanciu
LA, Lee J, Rochet JC, et al: Overexpression of alpha-synuclein at
non-toxic levels increases dopaminergic cell death induced by
copper exposure via modulation of protein degradation pathways.
Neurobiol Dis. 81:76–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Auluck PK, Caraveo G and Lindquist S:
α-Synuclein: Membrane interactions and toxicity in Parkinson's
disease. Annu Rev Cell Dev Biol. 26:211–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li D, Shi JJ, Mao CJ, Liu S, Wang JD, Chen
J, Wang F, Yang YP, Hu WD, Hu LF and Liu CF: Alteration of dynein
function affects α-synuclein degradation via the
autophagosome-lysosome pathway. Int J Mol Sci. 14:24242–24254.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonifacino JS and Hurley JH: Retromer.
Curr Opin Cell Biol. 20:427–436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsika E, Glauser L, Moser R, Fiser A,
Daniel G, Sheerin UM, Lees A, Troncoso JC, Lewis PA, Bandopadhyay
R, et al: Parkinson's disease-linked mutations in VPS35 induce
dopaminergic neurodegeneration. Hum Mol Genet. 23:4621–4638. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang HS, Toh J, Ho P, Tio M, Zhao Y and
Tan EK: In vivo evidence of pathogenicity of VPS35 mutations in the
Drosophila. Mol Brain. 7:732014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sahay S, Ghosh D, Singh PK and Maji SK:
Alteration of structure and aggregation of a-synuclein by familial
Parkinson's disease associated mutations. Curr Protein Pept Sci.
Mar 14–2016.(Epub ahead of print). PubMed/NCBI
|