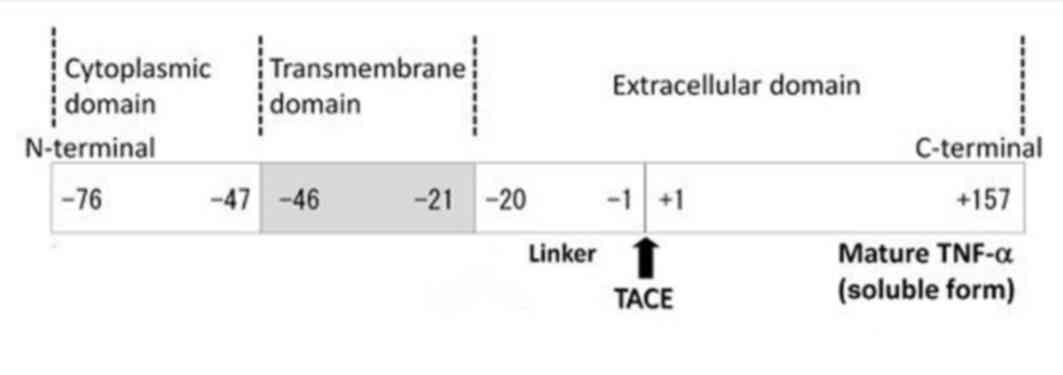
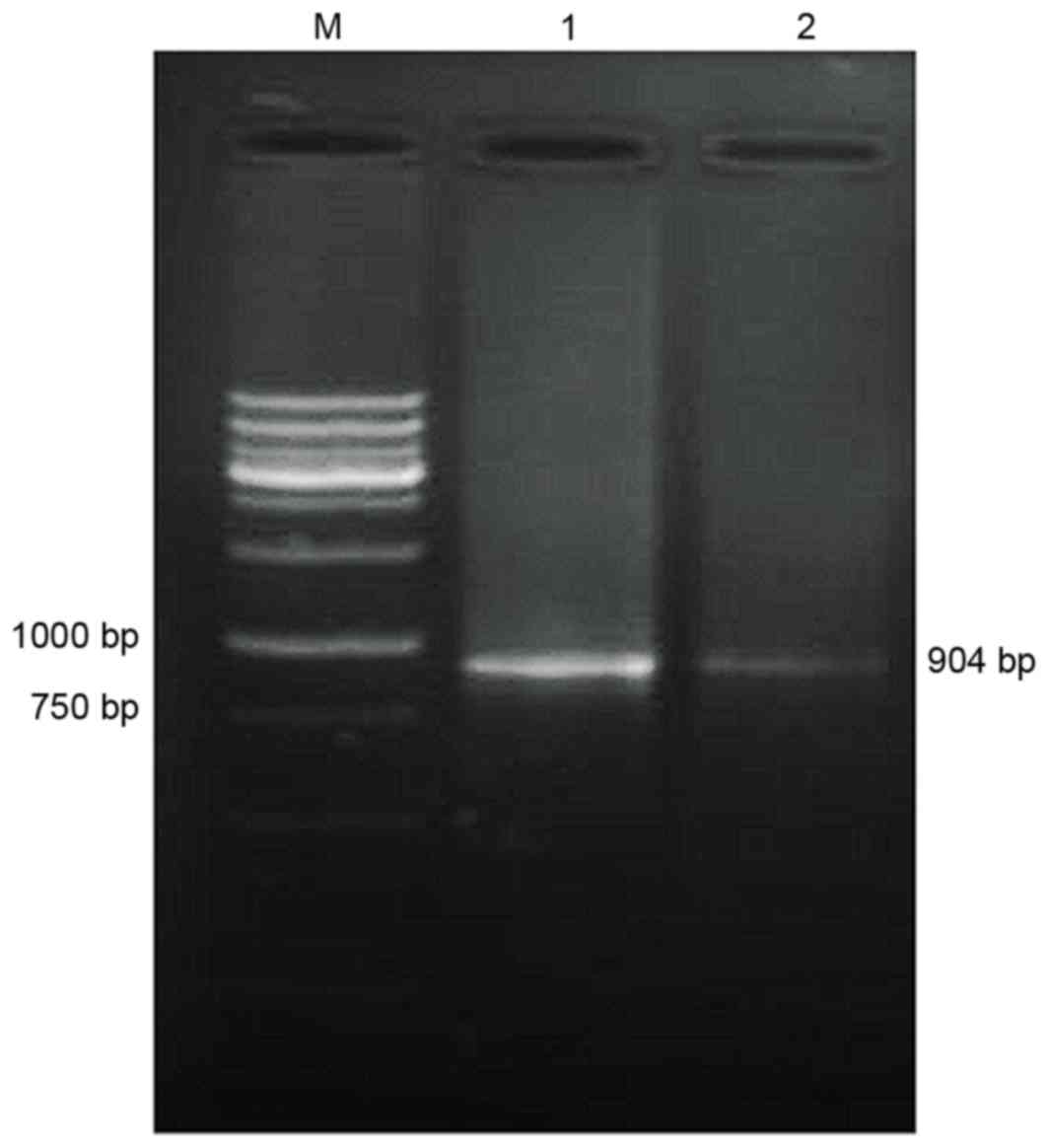
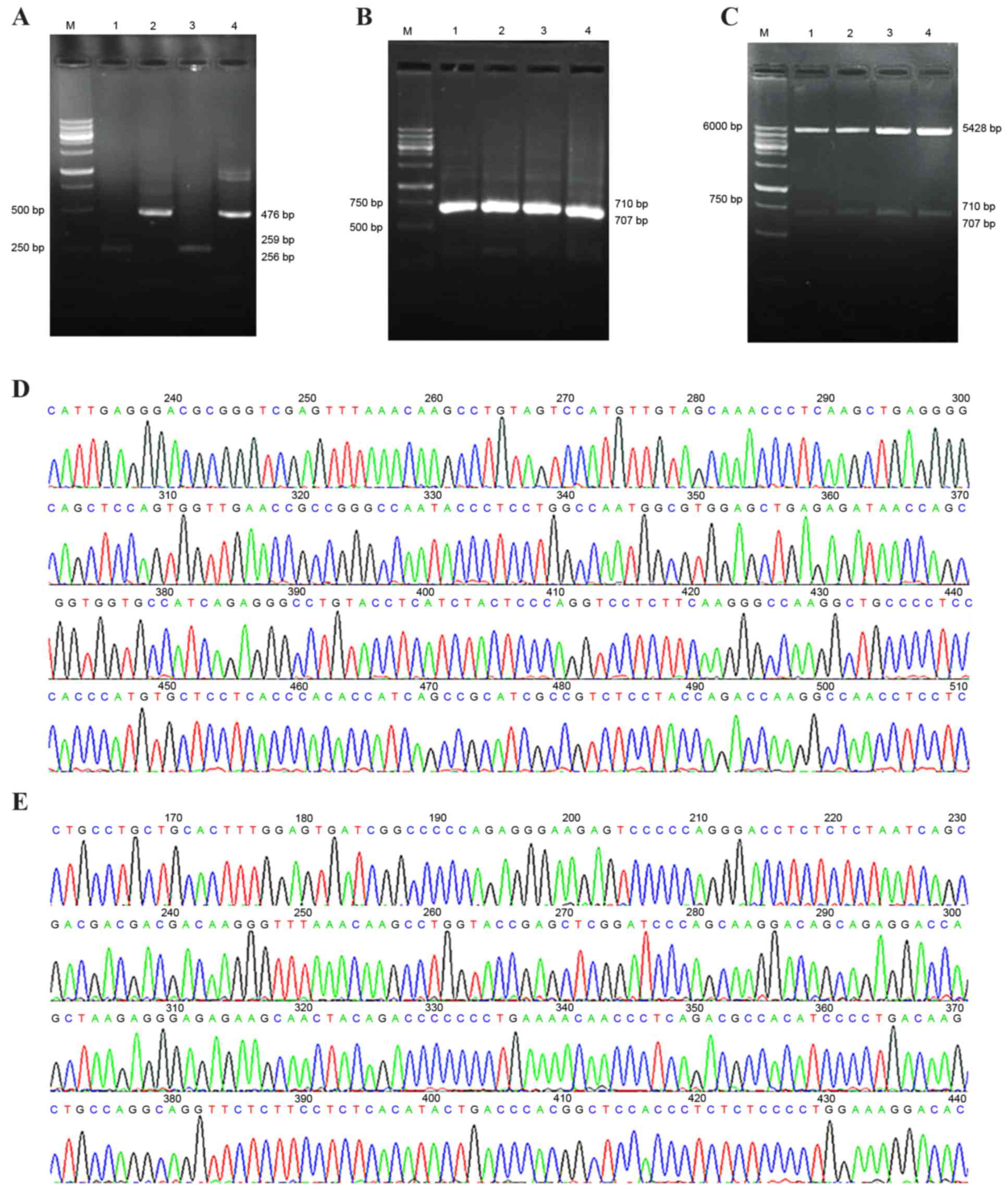
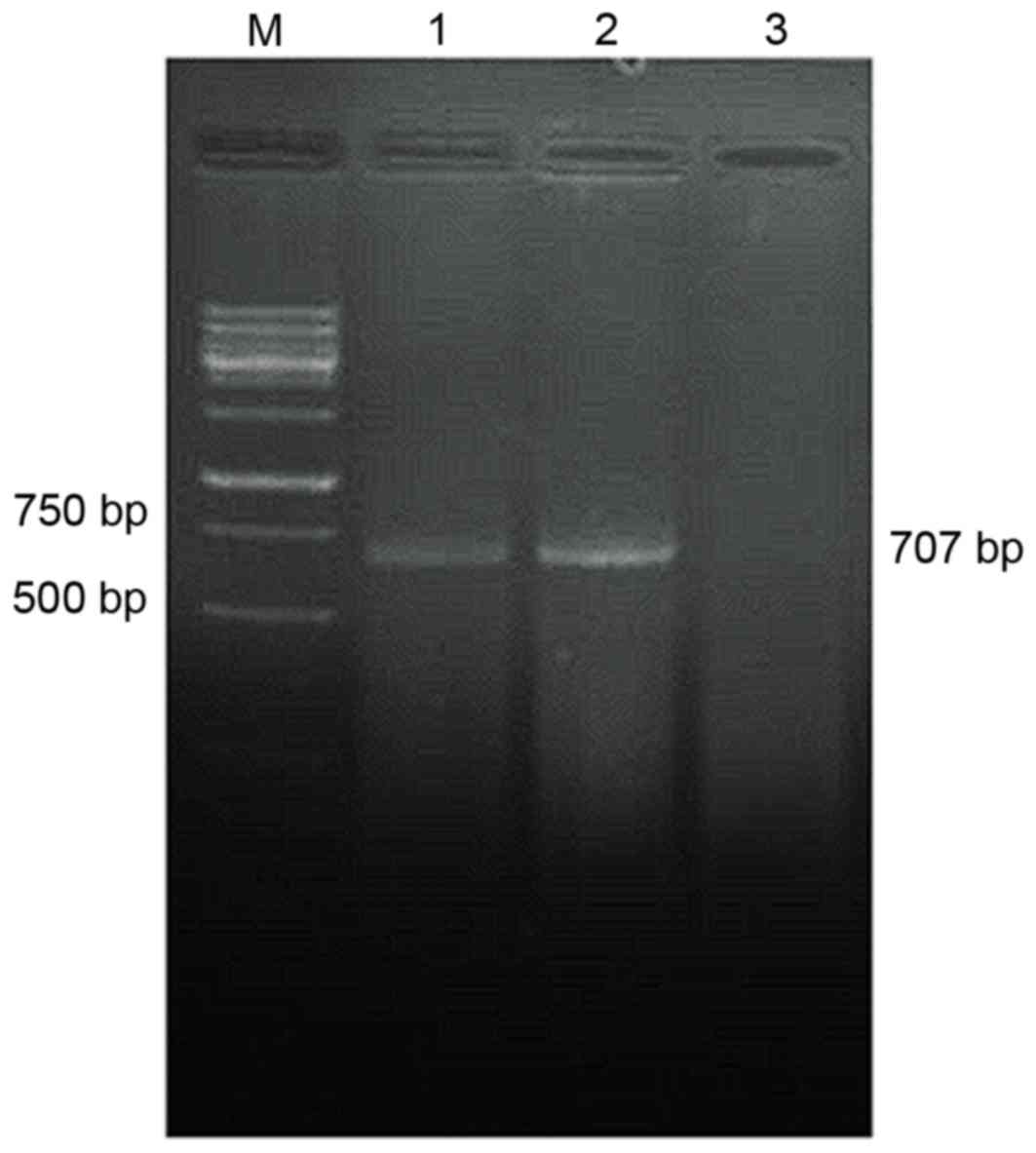
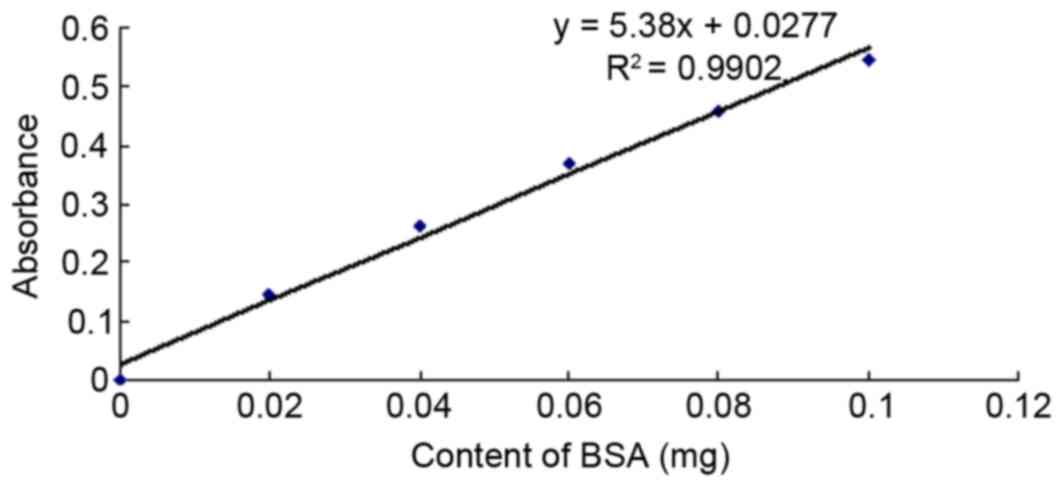
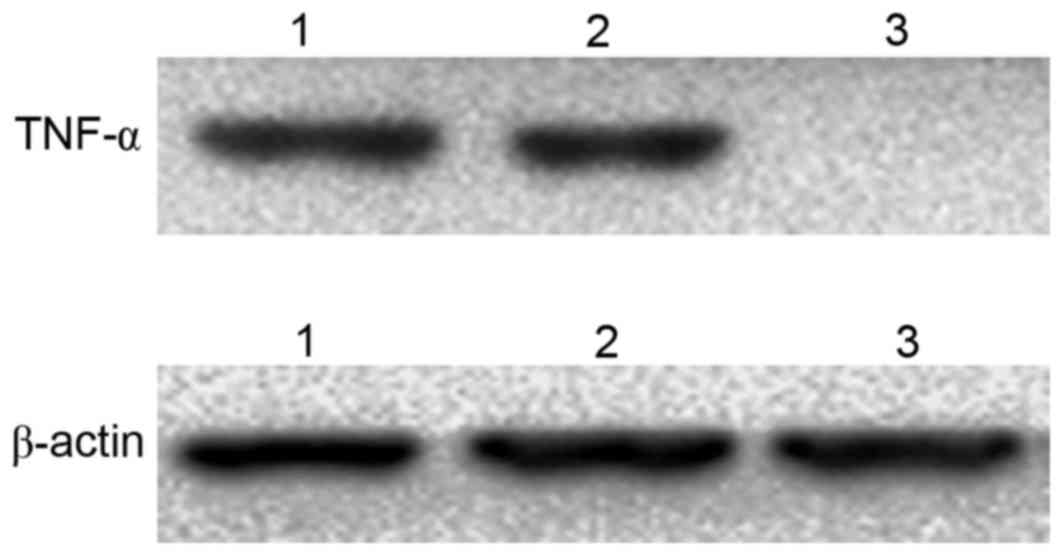
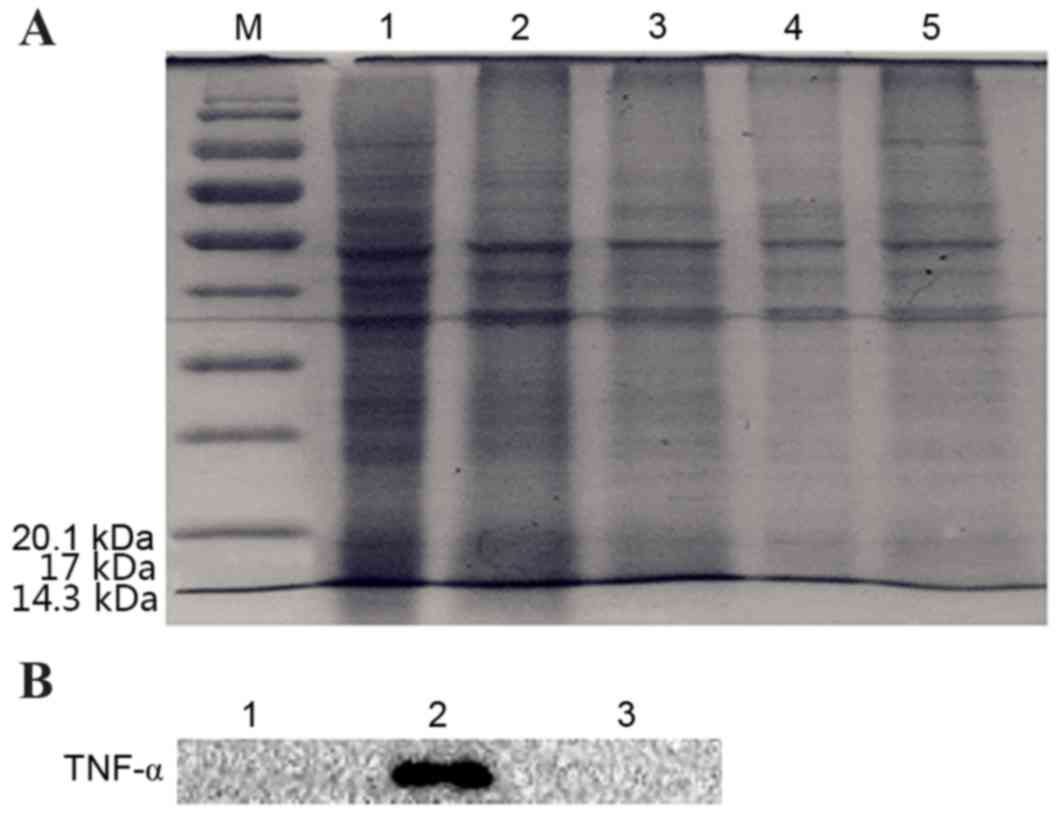
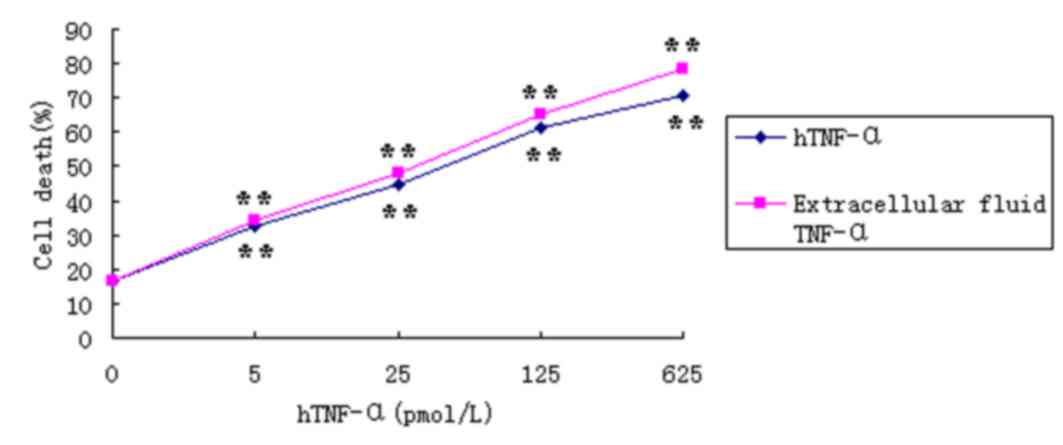
|
1
|
Itai T, Tanaka M and Nagata S: Processing
of tumor necrosis factor by the membrane-bound TNF-alpha-converting
enzyme, but not its truncated soluble form. Eur J Biochem.
268:2074–2082. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang AM, Creasey AA, Ladner MB, Lin LS,
Strickler J, van Arsdell JN, Yamamoto R and Mark DF: Molecular
cloning of the complementary DNA for human tumor necrosis factor.
Science. 228:149–154. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kriegler M, Perez C, DeFay K, Albert I and
Lu SD: A novel form of TNF/cachectin is a cell surface cytotoxic
transmembrane protein: Ramifications for the complex physiology of
TNF. Cell. 53:45–53. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Black RA, Rauch CT, Kozlosky CJ, Peschon
JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P,
Srinivasan S, et al: A metalloproteinase disintegrin that releases
tumour-necrosis factor-alpha from cells. Nature. 385:729–733. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abu-Amer Y, Erdmann J, Alexopoulou L,
Kollias G, Ross FP and Teitelbaum SL: Tumor necrosis factor
receptors types 1 and 2 differentially regulate osteoclastogenesis.
J Biol Chem. 275:27307–27310. 2000.PubMed/NCBI
|
|
6
|
Nagano K, Alles N, Mian AH, Shimoda A,
Morimoto N, Tamura Y, Shimokawa H, Akiyoshi K, Ohya K and Aoki K:
The tumor necrosis factor type 2 receptor plays a protective role
in tumor necrosis factor-alpha-induced bone resorption lacunae on
mouse calvariae. J Bone Miner Metab. 29:671–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horiuchi T, Mitoma H, Harashima S,
Tsukamoto H and Shimoda T: Transmembrane TNF-alpha: Structure,
function and interaction with anti-TNF agents. Rheumatology
(Oxford). 49:1215–1228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bjornberg F, Lantz M, Olsson I and
Gullberg U: Mechanisms involved in the processing of the p55 and
the p75 tumor necrosis factor (TNF) receptors to soluble receptor
forms. Lymphokine Cytokine Res. 13:203–211. 1994.PubMed/NCBI
|
|
9
|
Jonasson P, Liljeqvist S, Nygren PA and
Ståhl S: Genetic design for facilitated production and recovery of
recombinant proteins in Escherichia coli. Biotechnol Appl Biochem.
35:91–105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blobel G and Dobberstein B: Transfer of
proteins across membranes. I. Presence of proteolytically processed
and unprocessed nascent immunoglobulin light chains on
membrane-bound ribosomes of murine myeloma. J Cell Biol.
67:835–851. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pennica D, Nedwin GE, Hayflick JS, Seeburg
PH, Derynck R, Palladino MA, Kohr WJ, Aggarwal BB and Goeddel DV:
Human tumour necrosis factor: Precursor structure, expression and
homology to lymphotoxin. Nature. 312:724–729. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hehlgans T and Pfeffer K: The intriguing
biology of the tumour necrosis factor/tumour necrosis factor
receptor superfamily: Players, rules and the games. Immunology.
115:1–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin YW, Cheng C, Wang HB, Gao YJ, Shao XY
and Shen AG: Role of P38 in LPS induced TNF-α expression in Schwann
cells. Chin J Biochemistry Mol Biol. 05:382–387. 2007.(In
Chinese).
|
|
14
|
Li YP, Pei YY, Ding J, Shen ZM, Zhang XY,
Gu ZH and Zhou JJ: PEGylated recombinant human tumor necrosis
factor alpha: Preparation and anti-tumor potency. Acta Pharmacol
Sin. 22:549–555. 2001.PubMed/NCBI
|
|
15
|
Mao H: A self-cleavable sortase fusion for
one-step purification of free recombinant proteins. Protein Expr
Purif. 37:253–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SO and Lee YI: High-level expression
and simple purification of recombinant human insulin-like growth
factor I. J Biotechnol. 48:97–105. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh SM and Panda AK: Solubilization and
refolding of bacterial inclusion body proteins. J Biosci Bioeng.
99:303–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Daly R and Hearn MT: Expression of
heterologous proteins in Pichia pastoris: A useful experimental
tool in protein engineering and production. J Mol Recognit.
18:119–138. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chaudhuri TK, Horii K, Yoda T, Arai M,
Nagata S, Terada TP, Uchiyama H, Ikura T, Tsumoto K, Kataoka H, et
al: Effect of the extra n-terminal methionine residue on the
stability and folding of recombinant alpha-lactalbumin expressed in
Escherichia coli. J Mol Biol. 285:1179–1194. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen H, Shaffer PL, Huang X and Rose PE:
Rapid screening of membrane protein expression in transiently
transfected insect cells. Protein Expr Purif. 88:134–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng J, Ren F, Yu X, Lai BC and Wang Y:
Expression of HPV16E6 protein with insect-baculovirus expression
system. J Northwest Univ (Natural Sci Ed). 05:775–778. 2008.(In
Chinese).
|
|
22
|
Makadiya N, Brownlie R, van den Hurk J,
Berube N, Allan B, Gerdts V and Zakhartchouk A: S1 domain of the
procine epidemic diarrhea virus spike protein as a vaccine antigen.
Virol J. 13:572016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stock J, Sarkari P, Kreibich S, Brefort T,
Feldbrügge M and Schipper K: Applying unconventional secretion of
the endochitinase Cts1 to export heterologous proteins in Ustilago
maydis. J Biotechnol. 161:80–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arnau J, Lauritzen C, Petersen GE and
Pedersen J: Current strategies for the use of affinity tags and tag
removal for the purification of recombinant proteins. Protein Expr
Purif. 48:1–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu J, Qin H, Gao FP and Cross TA: A
systematic assessment of mature MBP in membrane protein production:
Overexpression, membrane targeting and purification. Protein Expr
Purif. 80:34–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klein S, Geiger T, Linchevski I,
Lebendiker M, Itkin A, Assayag K and Levitzki A: Expression and
purification of active PKB kinase from Escherichia coli. Protein
Expr Purif. 41:162–169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leviatan S, Sawada K, Moriyama Y and
Nelson N: Combinatorial method for overexpression of membrane
proteins in Escherichia coli. J Biol Chem. 285:23548–23556. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim DJ, Jang HJ, Pyun YR and Kim YS:
Cloning, expression, and characterization of thermostable DNA
polymerase from Thermoanaerobacter yonseiensis. J Biochem Mol Biol.
35:320–329. 2002.PubMed/NCBI
|
|
29
|
Magliery TJ, Wilson CG, Pan W, Mishler D,
Ghosh I, Hamilton AD and Regan L: Detecting protein-protein
interactions with a green fluorescent protein fragment reassembly
trap: Scope and mechanism. J Am Chem Soc. 127:146–157. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han S, Li H, Jin Z, Huang D, Ren C and Lin
Y: Yeast cell surface display and its application of enzymatic
synthesis in non-aqueous phase. Sheng Wu Gong Cheng Xue Bao.
25:1784–1788. 2009.(In Chinese). PubMed/NCBI
|
|
31
|
Jia D, Yang H, Wan L, Cheng J and Lu X:
Production of bioactive, SUMO-modified, and native-like TNF-α of
the rhesus monkey, Macaca mulatta, in Escherichia coli. Appl
Microbiol Biotechnol. 93:2345–2355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alizadeh AA, Hamzeh-Mivehroud M,
Farajzadeh M, Moosavi-Movahedi AA and Dastmalchi S: A simple and
rapid method for expression and purification of functional TNF-α
using GST fusion system. Curr Pharm Biotechnol. 16:707–715. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Evans DH, Willer DO and Yao XD: DNA
joining method US Patent 7575860 B2. March 7–2001, issued August
18, 2009.
|