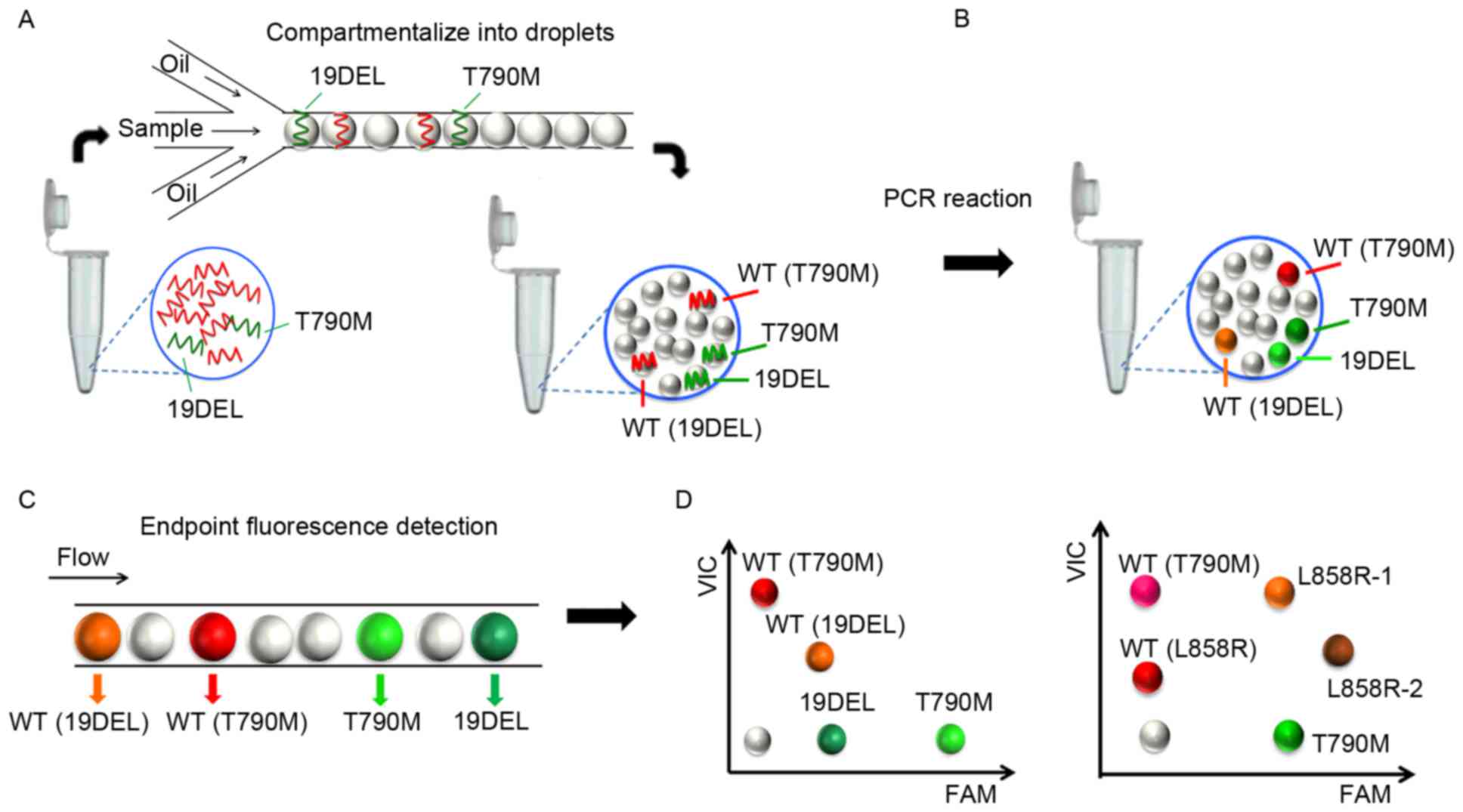
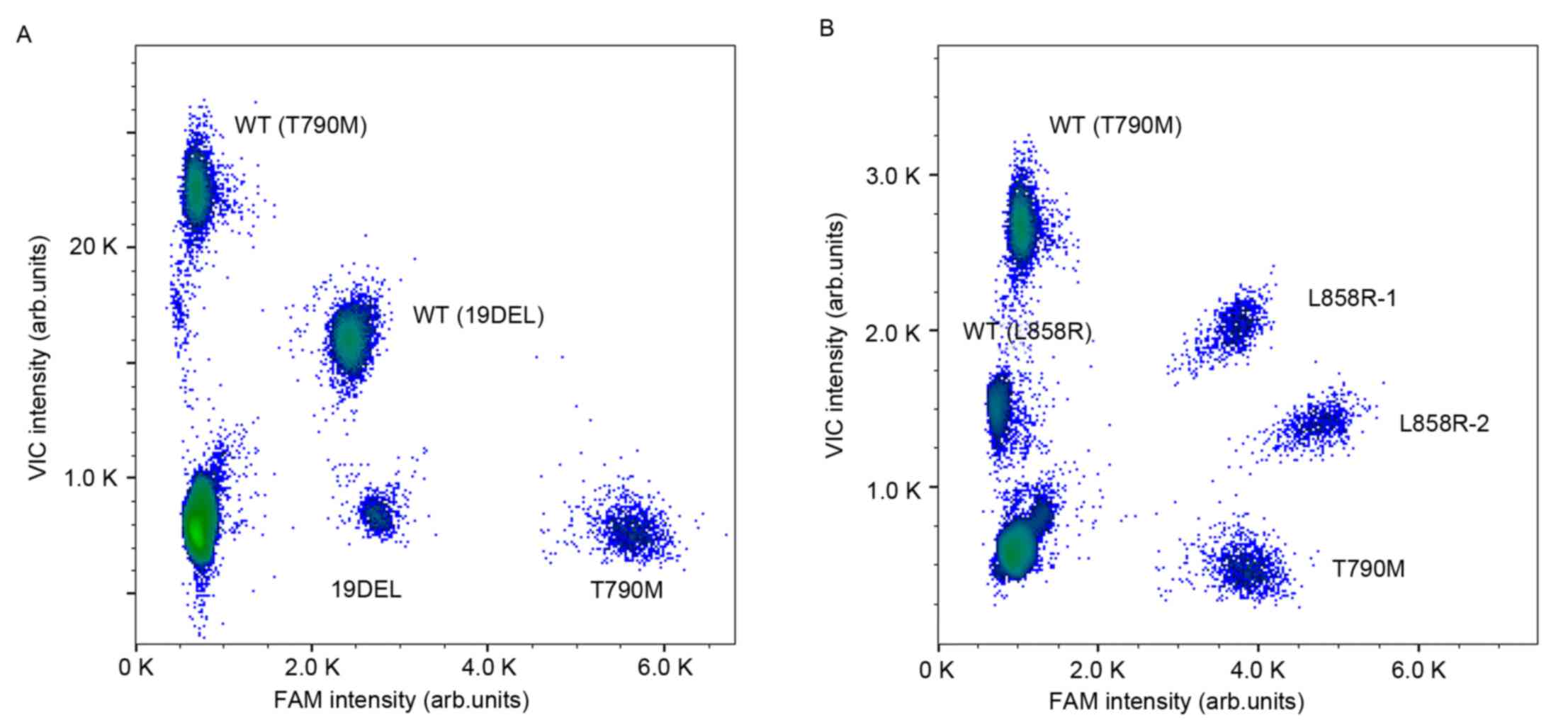
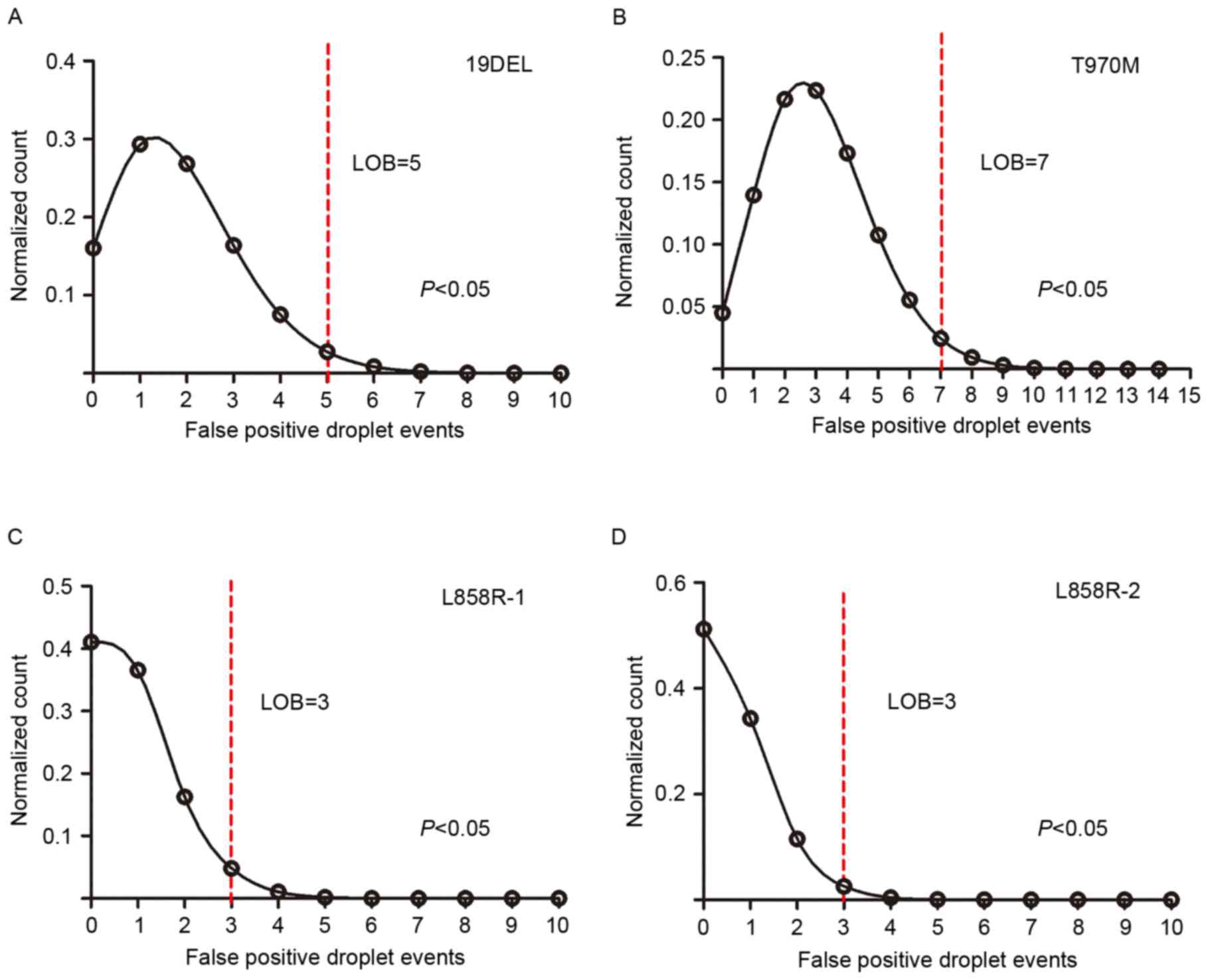
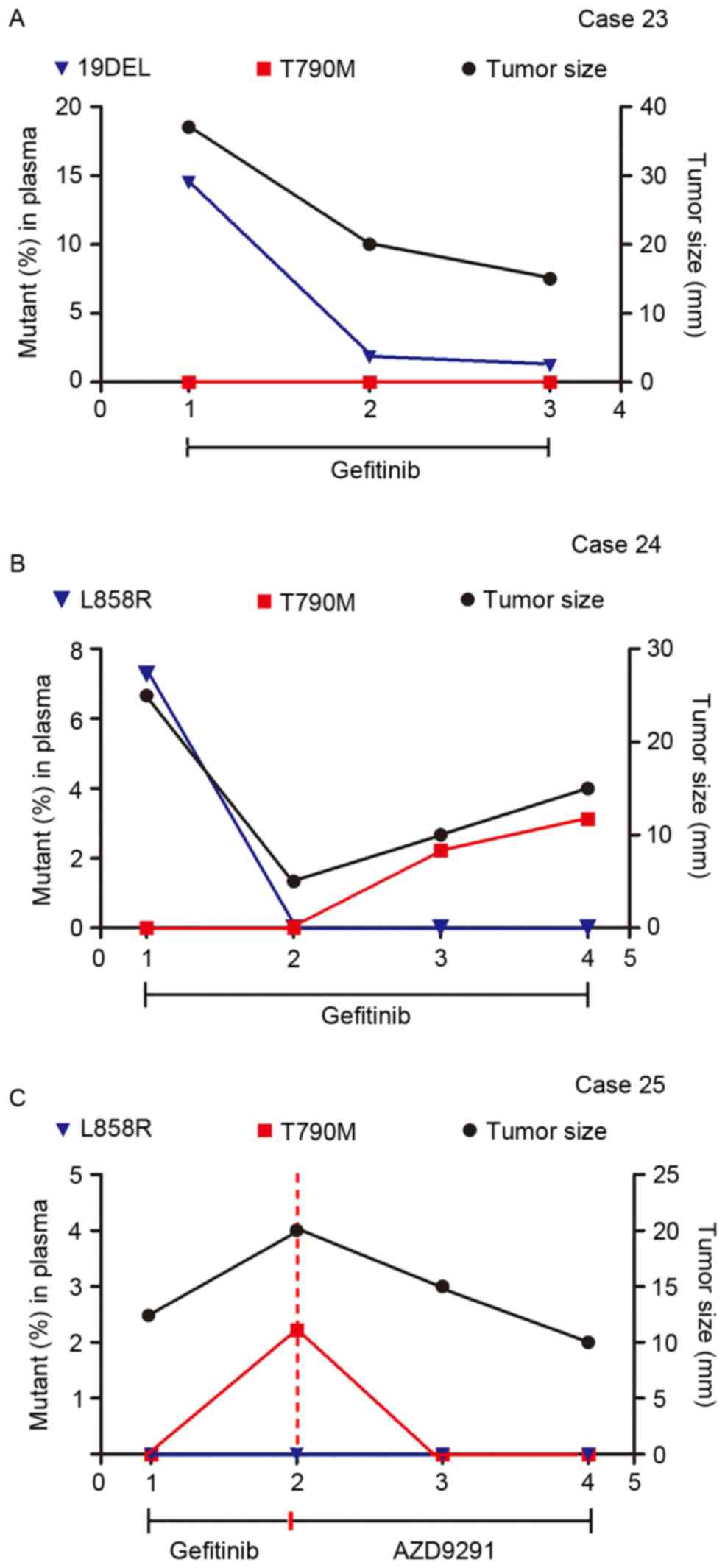
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han JY, Park K, Kim SW, Lee DH, Kim HY,
Kim HT, Ahn MJ, Yun T, Ahn JS, Suh C, et al: First-SIGNAL:
First-line single-agent iressa versus gemcitabine and cisplatin
trial in never-smokers with adenocarcinoma of the lung. J Clin
Oncol. 30:1122–1128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitsudomi T and Yatabe Y: Mutations of the
epidermal growth factor receptor gene and related genes as
determinants of epidermal growth factor receptor tyrosine kinase
inhibitors sensitivity in lung cancer. Cancer Sci. 98:1817–1824.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jänne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su KY, Chen HY, Li KC, Kuo ML, Yang JC,
Chan WK, Ho BC, Chang GC, Shih JY, Yu SL and Yang PC: Pretreatment
epidermal growth factor receptor (EGFR) T790M mutation predicts
shorter EGFR tyrosine kinase inhibitor response duration in
patients with non-small-cell lung cancer. J Clin Oncol. 30:433–440.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hata A, Katakami N, Yoshioka H, Takeshita
J, Tanaka K, Nanjo S, Fujita S, Kaji R, Imai Y, Monden K, et al:
Rebiopsy of non-small cell lung cancer patients with acquired
resistance to epidermal growth factor receptor-tyrosine kinase
inhibitor: Comparison between T790M mutation-positive and
mutation-negative populations. Cancer. 119:4325–4332. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z, Chen R, Wang S, Zhong J, Wu M,
Zhao J, Duan J, Zhuo M, An T, Wang Y, et al: Quantification and
dynamic monitoring of EGFR T790M in plasma cell-free DNA by digital
PCR for prognosis of EGFR-TKI treatment in advanced NSCLC. PLoS
One. 9:e1107802014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reck M, Popat S, Reinmuth N, de Ruysscher
D, Kerr KM and Peters S: ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer (NSCLC): ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 25
Suppl 3:iii27–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leighl NB, Rekhtman N, Biermann WA, Huang
J, Mino-Kenudson M, Ramalingam SS, West H, Whitlock S and
Somerfield MR: Molecular testing for selection of patients with
lung cancer for epidermal growth factor receptor and anaplastic
lymphoma kinase tyrosine kinase inhibitors: American society of
clinical oncology endorsement of the College of American
Pathologists/International Association for the study of lung
cancer/association for molecular pathology guideline. J Clin Oncol.
32:3673–3679. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Comprehensive Cancer Network:
NCCN guidelines for patients. Version 1. 2015, Non-Small Cell Lung
Cancer (S/OL); November 6–2015
|
|
15
|
Taly V, Pekin D, Benhaim L, Kotsopoulos
SK, Le Corre D, Li X, Atochin I, Link DR, Griffiths AD, Pallier K,
et al: Multiplex picodroplet digital PCR to detect KRAS mutations
in circulating DNA from the plasma of colorectal cancer patients.
Clin Chem. 59:1722–1731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yung TK, Chan KC, Mok TS, Tong J, To KF
and Lo YM: Single-molecule detection of epidermal growth factor
receptor mutations in plasma by microfluidics digital PCR in
non-small cell lung cancer patients. Clin Cancer Res. 15:2076–2084.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watanabe M, Kawaguchi T, Isa S, Ando M,
Tamiya A, Kubo A, Saka H, Takeo S, Adachi H, Tagawa T, et al:
Ultra-sensitive detection of the pretreatment EGFR T790M mutation
in non-small cell lung cancer patients with an EGFR-activating
mutation using droplet digital PCR. Clin Cancer Res. 21:3552–3560.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thermo Scientific. User Guide: Phusion
site-directed mutagenesis kit. January 4–2015
|
|
19
|
Nishino M, Jackman DM, Hatabu H, Jänne PA,
Johnson BE and Van den Abbeele AD: Imaging of lung cancer in the
era of molecular medicine. Acad Radiol. 18:424–436. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu G, Ye X, Dong Z, Lu YC, Sun Y, Liu Y,
McCormack R, Gu Y and Liu X: Highly sensitive droplet digital PCR
method for detection of EGFR-activating mutations in plasma
cell-Free DNA from patients with advanced non-small cell lung
cancer. J Mol Diagn. 17:265–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heitzer E, Ulz P and Geigl JB: Circulating
tumor DNA as a liquid biopsy for cancer. Clin Chem. 61:112–123.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Diaz LA Jr and Bardelli A: Liquid
biopsies: Genotyping circulating tumor DNA. J Clin Oncol.
32:579–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bai H, Mao L, Wang HS, Zhao J, Yang L, An
TT, Wang X, Duan CJ, Wu NM, Guo ZQ, et al: Epidermal growth factor
receptor mutations in plasma DNA samples predict tumor response in
Chinese patients with stages IIIB to IV non-small-cell lung cancer.
J Clin Oncol. 27:2653–2659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Douillard JY, Ostoros G, Cobo M, Ciuleanu
T, Cole R, McWalter G, Walker J, Dearden S, Webster A, Milenkova T
and McCormack R: Gefitinib treatment in EGFR mutated caucasian
NSCLC: Circulating-free tumor DNA as a surrogate for determination
of EGFR status. J Thorac Oncol. 9:1345–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HR, Lee SY, Hyun DS, Lee MK, Lee HK,
Choi CM, Yang SH, Kim YC, Lee YC, Kim SY, et al: Detection of EGFR
mutations in circulating free DNA by PNA-mediated PCR clamping. J
Exp Clin Cancer Res. 32:502013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Couraud S, Vaca-Paniagua F, Villar S,
Oliver J, Schuster T, Blanché H, Girard N, Trédaniel J,
Guilleminault L, Gervais R, et al: Noninvasive diagnosis of
actionable mutations by deep sequencing of circulating free DNA in
lung cancer from never-smokers: A proof-of-concept study from
BioCAST/IFCT-1002. Clin Cancer Res. 20:4613–4624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishii H, Azuma K, Sakai K, Kawahara A,
Yamada K, Tokito T, Okamoto I, Nishio K and Hoshino T: Digital PCR
analysis of plasma cell-free DNA for non-invasive detection of drug
resistance mechanisms in EGFR mutant NSCLC: Correlation with paired
tumor samples. Oncotarget. 6:30850–30858. 2015.PubMed/NCBI
|
|
29
|
Seki Y, Fujiwara Y, Kohno T, Takai E,
Sunami K, Goto Y, Horinouchi H, Kanda S, Nokihara H, Watanabe S, et
al: Picoliter-droplet digital polymerase Chain reaction-based
analysis of cell-free plasma DNA to assess EGFR mutations in lung
adenocarcinoma that confer resistance to tyrosine-kinase
inhibitors. Oncologist. 21:156–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JY, Qing X, Xiumin W, Yali B, Chi S,
Bak SH, Lee HY, Sun JM, Lee SH, Ahn JS, et al: Longitudinal
monitoring of EGFR mutations in plasma predicts outcomes of NSCLC
patients treated with EGFR TKIs: Korean lung cancer consortium
(KLCC-12-02). Oncotarget. 7:6984–6993. 2016.PubMed/NCBI
|
|
31
|
Taniguchi K, Okami J, Kodama K,
Higashiyama M and Kato K: Intratumor heterogeneity of epidermal
growth factor receptor mutations in lung cancer and its correlation
to the response to gefitinib. Cancer Sci. 99:929–935. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bai H, Wang Z, Wang Y, Zhuo M, Zhou Q,
Duan J, Yang L, Wu M, An T, Zhao J and Wang J: Detection and
clinical significance of intratumoral EGFR mutational heterogeneity
in Chinese patients with advanced non-small cell lung cancer. PLoS
One. 8:e541702013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen ZY, Zhong WZ, Zhang XC, Su J, Yang
XN, Chen ZH, Yang JJ, Zhou Q, Yan HH, An SJ, et al: EGFR mutation
heterogeneity and the mixed response to EGFR tyrosine kinase
inhibitors of lung adenocarcinomas. Oncologist. 17:978–985. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shimizu K, Yukawa T, Hirami Y, Okita R,
Saisho S, Maeda A, Yasuda K and Nakata M: Heterogeneity of the EGFR
mutation status between the primary tumor and metastatic lymph node
and the sensitivity to EGFR tyrosine kinase inhibitor in non-small
cell lung cancer. Target Oncol. 8:237–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sillence KA, Roberts LA, Hollands HJ,
Thompson HP, Kiernan M, Madgett TE, Welch CR and Avent ND: Fetal
sex and RHD genotyping with digital PCR demonstrates greater
sensitivity than real-time PCR. Clin Chem. 61:1399–1407. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reinert T, Schøler LV, Thomsen R, Tobiasen
H, Vang S, Nordentoft I, Lamy P, Kannerup AS, Mortensen FV,
Stribolt K, et al: Analysis of circulating tumor DNA to monitor
disease burden following colorectal cancer surgery. Gut.
65:625–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cross DA, Ashton SE, Ghiorghiu S, Eberlein
C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ,
et al: AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated
resistance to EGFR inhibitors in lung cancer. Cancer Discov.
4:1046–1061. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang T and Zhou C: Clinical activity of
the mutant-selective EGFR inhibitor AZD9291 in patients with EGFR
inhibitor-resistant non-small cell lung cancer. Transl Lung Cancer
Res. 3:370–372. 2014.PubMed/NCBI
|