Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
common types of malignant tumor in southern China (1). Currently, a combination of
radiotherapy and chemotherapy is the primary clinical treatment for
NPC. However, the average five-year survival rate of patients with
advanced NPC remains low due to tumor metastasis and multi-drug
resistance (2).
A recent study demonstrated that combining
chemotherapy with gene therapy may mediate synergistic actions,
enhance treatment effects and inhibit the development of drug
resistance (3). Furthermore, He
et al (4) developed a drug
and gene co-delivery system, using zinc ions (Zn2+) as
the connecting point. The obtained coordination liposome mediated
the cellular uptake of cisplatin and small interfering (si)RNA, and
enabled efficient endosomal escape in cisplatin-resistant ovarian
cancer cells. Li et al (5)
used poly (carboxybetaine) to conjugate camptothecin, and assembled
cationic liposomes to form a drug and gene dual-carrier, which
demonstrated a synergistic tumor suppression effect in
tumor-bearing mice in vivo. Chang et al (6) assembled drug and gene co-delivering
liposomes from amphiphilic pillar[5]arene capped with ferrocenium,
which exhibited redox sensitivity and effective drug/siRNA
co-delivery.
Previous studies have incorporated hydrophobic
anticancer drugs into the hydrophobic cores of these carriers, and
bound plasmid DNA or siRNA to cationic hydrophilic shells. However,
the self-assembly processes involved in the preparation of micelles
are typically difficult to control, and therefore stable and
uniform complexes are difficult to obtain. Furthermore, micelles
are not stable in blood circulation in vivo, and their
disassembly may result in drug emission (7–9). A
recent study synthesized a star-shaped cyclodextrin (CD)
derivative, consisting of a CD core and poly(L-lysine) dendron
(PLLD) arms (CD-PLLD) (10). The
CD core may interact with hydrophobic drug models, thus avoiding
the complicated micellization process.
For gene delivery, hyperbranched or dendrimer
cationic polymers, including poly (amidoamine)s, have been widely
used and have demonstrated efficiency due to their topologic
structure (11,12). Of these, PLLD and its derivatives
are of interest due to their well-defined architecture, good
biocompatibility, low cytotoxicity, high transfection efficiency
and biodegradability. Previous studies have investigated their
synthesis and potential applications in drug or gene delivery
(13–15). The synthesized CD-PLLD demonstrated
stability and good gene delivery ability in our previous study
(10), and was used to co-deliver
docetaxel (DOC) and a matrix metalloproteinase 9 (MMP-9) siRNA
plasmid for NPC therapy (16). The
present study investigated the distributions of MMP-9 and DOC in
vivo, and the potential of using the CD-PLLD/DOC/MMP-9 complex
for NPC treatment. In addition, it was demonstrated in vitro
that CD-PLLD may co-deliver DOC and MMP-9 effectively into HNE-1
NPC cells, and that these cells underwent apoptosis, therefore
suggesting its potential application in drug and gene
co-delivery.
Materials and methods
Materials
CD-PLLD/DOC/MMP-9 complexes were synthesized
according to our previous study (the loading amount of DOC was
13.20 µg/mg) (16). DOC was
purchased from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany)
and used without further purification. The most efficient
interference MMP-9 siRNA plasmid vector expressing enhanced green
fluorescent protein (EGFP), selected following screening (16), was purchased from Invitrogen;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The optimum
siRNA sequences targeting MMP-9 were as follows: Forward,
5′-TGCTGAAACCGAGTTGGAACCACGACGTTTTGGCCACTGACTGACGTCGTGGTCAACTCGGTTT-3′
and reverse,
5′-CCTGAAACCGAGTTGACCACGACGTCAGTCAGTGGCCAAAACGTCGTGGTTCCAACTCGGTTTC-3′.
Tert-butyl methyl ether and acetonitrile were of analytical grade
and used without further purification. HNE-1 human NPC cells were
provided by Southern Medical University (Guangzhou, China). A total
of 50 female specific pathogen-free BALB/c nude mice (age, 4–5
weeks; weight, 17–20 g) maintained at a specific pathogen free
facility with a constant humidity (60%) and temperature (28°C) at
12/12 h light/dark cycle with free access to food and water were
obtained from the Center for Laboratory Animal Sciences, Southern
Medical University. Ethical approval was obtained from The
Institutional Administration Panel for Laboratory Animal Care of
Southern Medical University. The university guidelines for care and
use of laboratory animals were strictly followed.
In vivo metabolism assay in nude
mice
Nude mice implanted with HNE-1 tumors were used as
the animal model for in vivo analyses. HNE-1 cells (1×107
cells in 200 µl PBS) were injected subcutaneously into the right
axillary flank of female BALB/c nude mice. Once the tumors had
grown to a maximum of 1 cm in diameter, the mice were sacrificed by
cervical dislocation, and the HNE-1 tumors were removed and
sectioned into 2×2×2 mm small pieces in aseptic conditions. The
tumors were subsequently transplanted into the right axillary
flanks of five mice. Following this, all mice were anesthetized by
an intraperitoneal injection of 0.1 ml chloral hydrate (Shanghai
Aladdin Bio-Chem Technology Co., Ltd., China), and injected with
CD-PLLD containing 20 µg/g DOC and 6 µg/g MMP-9 via the tail vein.
Mice were sacrificed by cervical dislocation 5, 30 or 60 min later
and organs were harvested for high-performance liquid
chromatography (HPLC), or 48 h later for EGFP detection.
Standard curves of DOC concentration
for in vivo distribution
Standard curves of DOC concentration in each tissue
(liver, kidney, lung, heart, spleen, brain and HNE-1 tumor) were
obtained by HPLC analysis. Briefly, 12.16 mg DOC standard samples
were transferred into a 5 ml calibrated flask. The DOC solution was
subsequently diluted with mobile phase solvent consisting of
methanol and purity water (70/30, v/v) to achieve the following
gradient DOC concentrations: 1,216, 608, 304, 152 and 76 µg/ml.
Tissue samples harvested from nude mice bearing HNE-1 tumors were
rinsed with ice-cold saline, dried on filter paper, weighed and
homogenized with saline (g: 2 ml ratio of tissue weight:saline
dosage). Following this, 160 µl tissue sample and 40 µl prepared
DOC standards sample solution with gradient concentration were
mixed, and 1 ml tert-butyl methyl ether was added into each sample
and mixed for 5 min by vortex for extraction. The total organic
layer was separated by centrifugation at 11,180 × g for 10 min at
4°C, transferred to a clean tube and evaporated to dryness at 40°C
under a stream of nitrogen. The drug residue was finally
reconstituted in 0.4 ml acetonitrile followed by centrifugation at
1,1180 × g for 5 min at 4°C prior to analysis, of which 10 µl
supernatant fluid was injected into the HPLC system. The HPLC
analysis of DOC was achieved on a C18 column (Waters
Corporation, Milford, MA, USA) with a mobile phase consisting of
methanol and purity water (70/30, v/v) at a flow rate of 1.0
ml/min. The effluents were monitored at 227 nm and quantized by
comparing the peak areas with the standard curve.
DOC distribution in vivo
Following DOC administration, nude mice bearing
HNE-1 tumors were sacrificed after 5, 30 or 60 min, and the tumors
and aforementioned tissues were rapidly dissected. Samples were
rinsed with ice-cold saline, dried on filter paper and homogenized
with saline at a 1:2 ratio of tumor weight: Saline dosage.
Following this, 0.2 ml of tissue sample and 1.2 ml tert-butyl
methyl ether were mixed for 5 min by vortex to extract, and the
total organic layer was separated by centrifugation at 11,180 × g
for 10 min at 4°C, transferred to a clean tube, and evaporated to
dryness at 40°C under a stream of nitrogen. The drug residue was
subsequently reconstituted in 0.4 ml acetonitrile and centrifuged
at 11,180 × g for 5 min at 4°C prior to analysis. The DOC
concentrations were determined by HPLC analysis, which was
performed using the aforementioned method.
EGFP expression in vivo
Nude mice were sacrificed by cervical dislocation
after 48 h via tail vein injection of nanocomposites. HNE-1 tumor
and the aforementioned tissues were immediately dissected, and EGFP
expression levels in frozen sections of tumor tissue were observed
via fluorescence microscope.
In vivo tumor inhibition
Nude mice implanted with HNE-1 tumors were used as
the animal model to assess the efficacy of CD-PLLD/DOC/MMP-9
against tumor cells in vivo. The mice were randomly divided
into four groups (n=5). Subsequently, the mice were intravenously
injected via the tail vein with 250 µl saline (as control),
CD-PLLD/DOC, CD-PLLD/MMP-9 or CD-PLLD/DOC/MMP-9, using DOC doses of
10 µg/g and MMP-9 doses of 3 µg/g according to the loading amount
of DOC (13.20 µg/mg) and CD-PLLD/MMP-9 at the N/P ratios of 20
(16), on days 1, 6, 11 and 16,
respectively. After three weeks, all mice were sacrificed by
cervical dislocation. The tumor volume was calculated by the
following formula: widest diameter × longest diameter2/2. The
tumors were dissected and fixed in 10% neutral buffered formalin,
embedded in paraffin and sectioned at a thickness of 5 µm. The
proliferating cell nuclear antigen (PCNA) was used to evaluate
proliferation ability of the cells in the HNE-1 tumors. The PCNA
expression in sections was assayed via immunohistochemistry with
the Elivision two-step detection kit (Kit-0015; Fuzhou Maixin
Biotech. Co., Ltd., China). These sections were deparaffinized and
pretreated by boiling the slides in citrate buffer (pH 6.0) for 10
min. The sections were then immersed in 3% hydrogen peroxide for 10
min at room temperature to block endogenous tissue peroxidase
activity. Following washing with phosphate buffered saline (PBS),
sections were incubated with mouse PCNA monoclonal antibody
(catalog no. TA800876; 1:50; Origene Technologies, Beijing, China)
at 4°C overnight, and then sections were incubated with 50 µl
amplifier polymer (reagent A), and 50 µl horse radish
peroxidase-conjugated anti-mouse/rabbit IgG (reagent B, Kit-0015;
Fuzhou Maixin Biotech. Co., Ltd.) for 30 min at room temperature.
Following washing with PBS, the sections were stained with DAB
(3,3-dimethylbenzidine; Fuzhou Maixin Biotech. Co., Ltd.) and
counterstained with hematoxylin (Fuzhou Maixin Biotech. Co., Ltd.),
differentiated using hydrochloric acid in ethanol, blued by washing
with water and sealed with conventional resin. The PCNA expression
was examined with tan or brown staining in cell nucleus and no
specific color in cytoplasm by light microscopy. The PCNA positive
rates as positive cell index (PI) were calculated in five visual
fields (x400) selected randomly in each section.
Statistical analysis
The peak area, DOC concentration and tumor size in
each group were compared by two-way analysis of variance (ANOVA)
and the PCNA expression of tumors in each group were compared by
one-way ANOVA, followed by Tukey's post hoc test. Data was analyzed
using SPSS software version 11.5 (SPSS, Inc., Chicago, IL, USA).
All data are presented as the mean ± standard deviation. P<0.05
was considered to indicate a statically significant difference.
Results
In vivo metabolism assay
A linear association was determined between DOC
concentration (X) and peak area (Y) in each tissue. The standard
curve of DOC and regression equations were as follows: HNE-1 tumor,
Y=2474.3 X + 799.96; liver, Y=2693.0 X-846.12; kidney, Y=2655.5 X +
1238.8; lung, Y=2581.3 X-172.32; heart, Y=2608.7 X-701.39; spleen,
Y=2470.4 X + 266.92; and brain, Y=2755.0 X + 1459.2. Following
this, tissue DOC concentrations were calculated 5, 30 and 60 min
after intravenous injection with CD-PLLD/DOC/MMP-9 complexes. HPLC
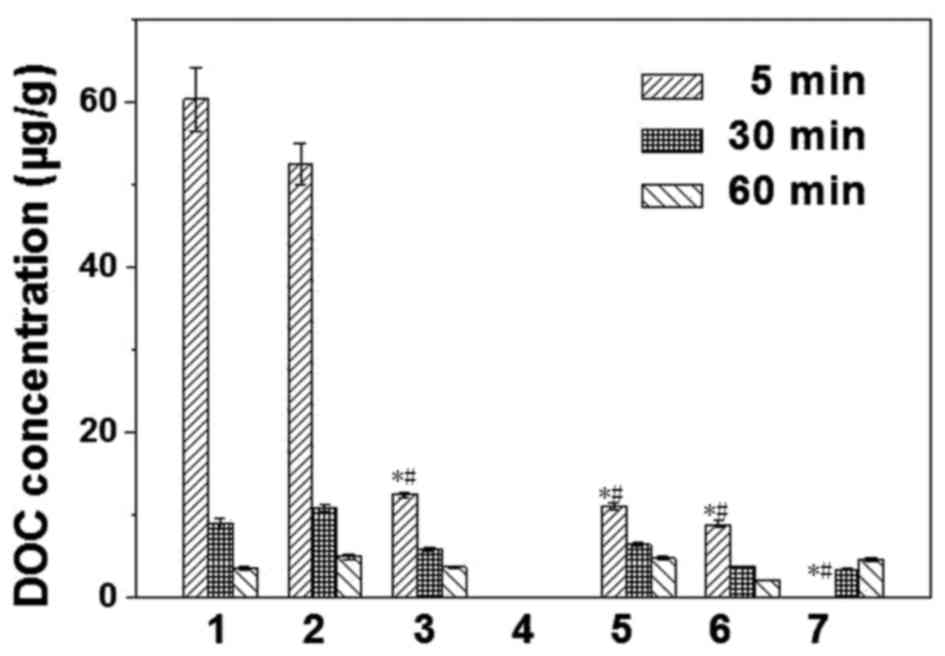
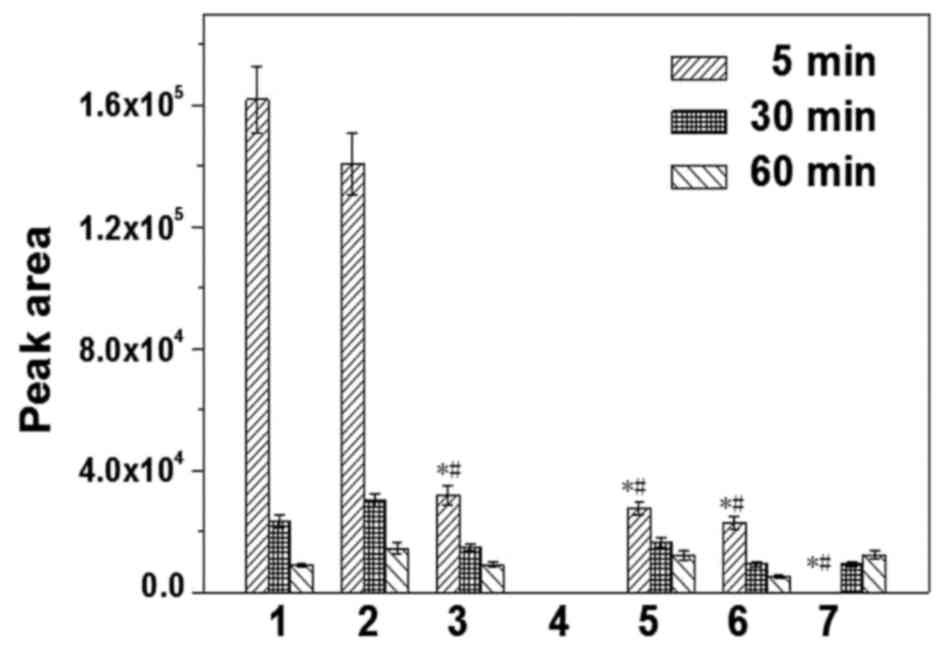
analysis and DOC concentration in tissues are presented in Figs. 1 and 2, respectively. The results demonstrated
that DOC was absorbed rapidly and distributed widely in the
majority of tissues, and that DOC concentration was the greatest in
the liver and kidney, followed by the heart, spleen and lung
(P<0.05). After 60 min, DOC concentration markedly decreased,
and there were no significant differences between organs
(P>0.05). No DOC was detected in the brains of the nude mice.
DOC concentrations in HNE-1 tumors increased with prolonged blood
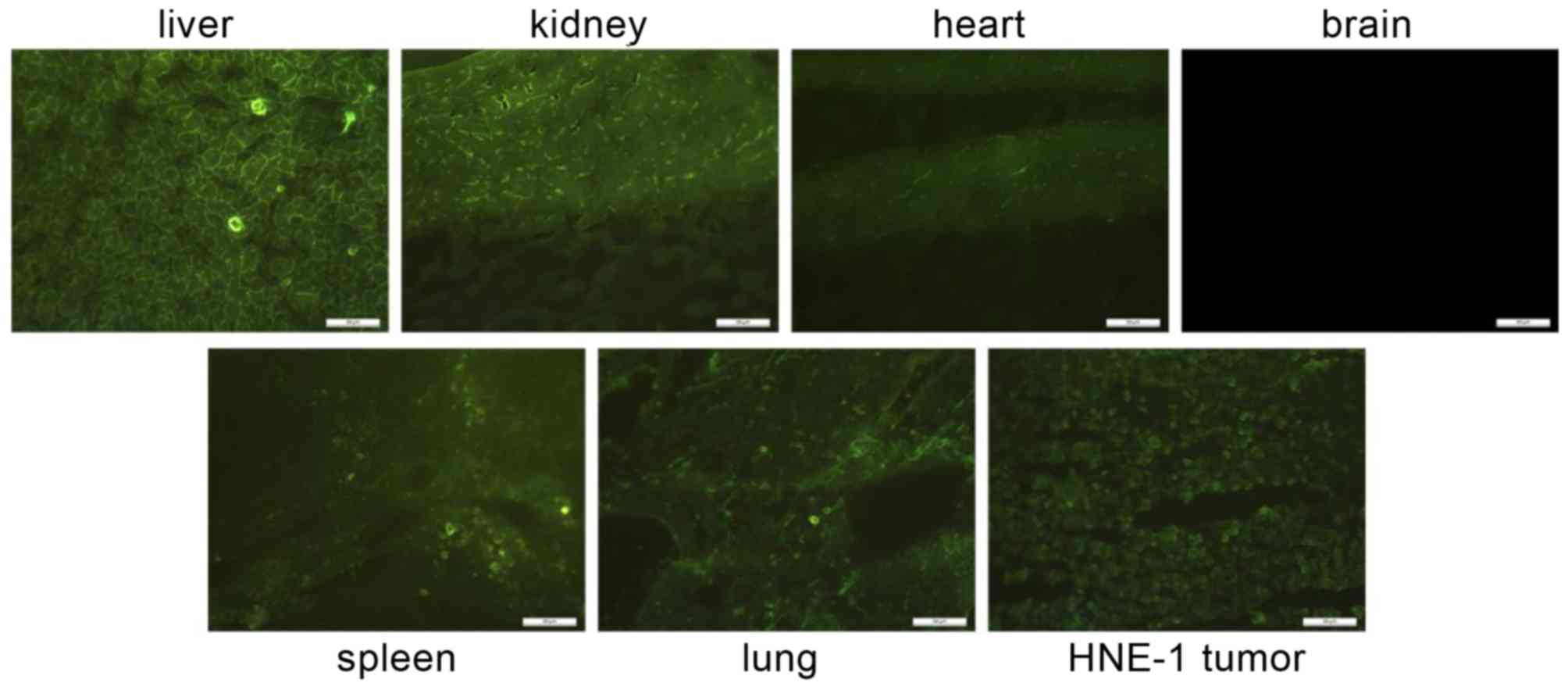
circulation. Following intravenous injection with CD-PLLD/DOC/MMP-9
complexes, EGFP expression after 48 h in frozen tissue sections is
presented in Fig. 3. Similar to
DOC distribution, EGFP expression was detectable in all assayed
organs except the brain, and the liver and kidney frozen sections
exhibited greater GFP expression. EGFP expression was observed in
HNE-1 tumors, consistent with the DOC concentration analysis.
 | Figure 1.HPLC analysis of DOC in mouse tissues.
BALB/c nude mice were implanted with HNE-1 human nasopharyngeal
carcinoma cell tumors and intravenously injected with
β-cyclodextrin-poly(L-lysine) dendron/DOC/matrix metalloproteinase
9. Tissues were harvested 5, 30 or 60 min later for HPLC analysis
of DOC concentrations. Data are expressed as the mean ± standard
deviation. 1, liver; 2, kidney; 3, heart; 4, brain; 5, spleen; 6,
lung; 7, HNE-1 tumor. *P<0.05 vs. liver at 5 min;
#P<0.05 vs. kidney at 5 min. HPLC, high-performance
liquid chromatography; DOC, docetaxel. |
In vivo tumor therapy
The antitumor effects of CD-PLLD/DOC/MMP-9 were
assessed in vivo, using nude mice implanted with HNE-1
tumors. Representative tumor images are presented in Fig. 4. Tumor volume was measured every 3
days following implantation, until mice were sacrificed at day 21
(Fig. 5). Tumor volume in the
saline-treated control group increased greatly during this period.
Treatment with CD-PLLD/DOC, CO-PLLD/MMP-9 or CD-PLLD/DOC/MMP-9
inhibited tumor growth; CD-PLLD/DOC/MMP-9 to the greatest extent
(P<0.05). Inhibition of tumor volume and weight were most marked
in the CD-PLLD/DOC/MMP-9-treated group compared with the control
group, with reductions of 61.32 and 60.00%, respectively. Tumor
volume and weight in the CD-PLLD/MMP-9-treated group were reduced
19.94 and 18.75%, respectively, and in the
CD-PLLD/DOC/MMP-9-treated group were reduced 41.40 and 40.00%,
respectively, compared with the control group. Tumor PCNA
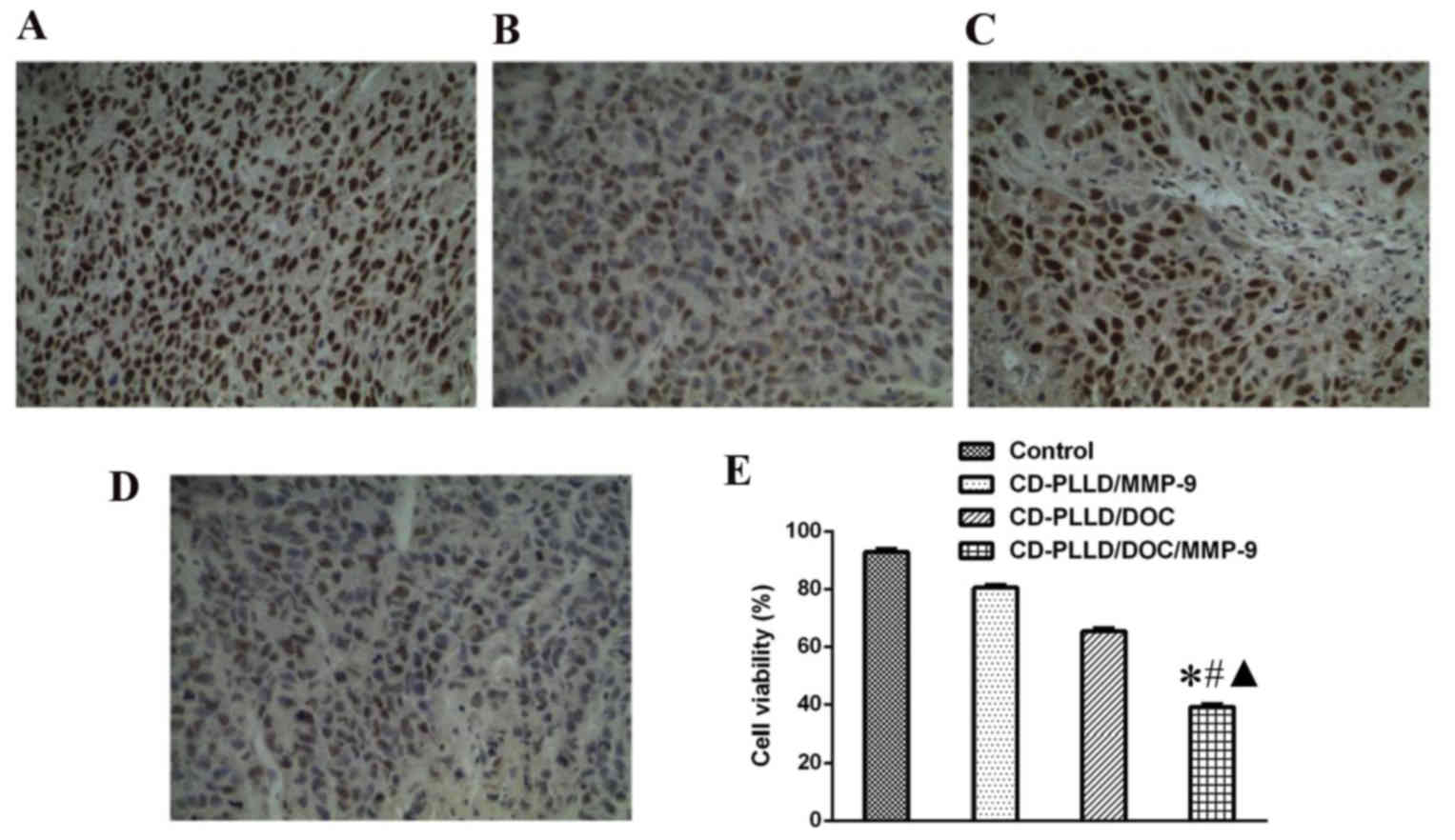
expression in each group is presented in Fig. 6. Compared with the control group,
PCNA expression levels in the CD-PLLD/MMP-9 group were slightly
reduced, with a positive cell index (PI) of 80.62 vs. 92.83% in the
control group; however, no significant differences were observed
(P>0.05). PI in the CD-PLLD/DOC and CD-PLLD/DOC/MMP-9 groups
were markedly reduced compared with the control group (65.36 and
39.52%, respectively), to a greater extent in the CD-PLLD/DOC/MMP-9
group (P<0.05).
Discussion
NPC is one of the most common types of malignant
tumor in southern China. Currently, a combination of radiotherapy
and chemotherapy is the primary clinical treatment for NPC.
However, the average five-year survival rate of advanced NPC
patients remains ~50% due to tumor metastasis and multi-drug
resistance. The co-delivery of drugs and genes has become a primary
strategy for the treatment of cancer and other diseases, due to its
potential to mediate synergistic actions, improve target
selectivity and inhibit the development of drug resistance
(17).
MMP-9, the enzyme with the largest molecular weight
in the MMP family, may accelerate tumor invasion and metastasis due
to its ability to degrade the basement membrane and extracellular
matrix (18). It has previously
been revealed to mediate tumor cell proliferation and inhibit
apoptosis; therefore, MMP-9 inhibition via an siRNA plasmid may be
a potential therapeutic strategy (19).
DOC, an analog of paclitaxel, is a novel anticancer
agent of the taxoid family. It has been demonstrated to mediate
tubulin assembly in microtubules and inhibit their
depolymerization, leading to mitotic arrest in the G2M
phase of the cell cycle (20,21).
DOC may induce apoptosis of tumor cells and inhibit tumor growth,
and has demonstrated antitumor effects in cancer chemotherapy
(22,23). However, DOC is poorly soluble in
organic solvents and water and therefore does not disperse well in
plasma. Taxotere®, the clinical formulation of DOC, is formulated
in polysorbate 80, which has been identified to cause serious
side-effects including hypersensitivity reactions, nephrotoxicity,
cardiotoxicity and neurotoxicity (24), limiting the clinical application of
DOC. Furthermore, single-drug chemotherapy may cause additional
side-effects and tumor drug-resistance. Therefore, nanocarriers
that co-deliver DOC and a target gene may provide a platform to
overcome these limitations.
CD is a cyclic oligosaccharide with seven glucose
units bonded by a α-1, 4-linkage. It increases the water solubility
of certain medicinal agents, via noncovalent inclusion complexation
with a hydrophilic outer surface and a lipophilic central cavity,
which encapsulates hydrophobic drug molecules or parts of these
molecules. To co-deliver a chemotherapy drug and gene, our previous
study prepared a biodegradable star-shaped CD-PLLD via chemical
reactions with a CD surface modified with dendritic poly(L-lysine).
The CD core interacted with the hydrophobic drug and the cationic
arms bound to the target gene. It was demonstrated that such a
conjugate may be used directly for drug and gene delivery without a
complicated micellization process. In vitro assays indicated
that CD-PLLD/DOC/MMP-9 complexes induce synergistic anti-tumor
effects (16).
The present study investigated the in vivo
applications for NPC therapy. Using a DOC distribution analysis,
compared with the liquid-liquid anhydrous diethyl ether extraction
method (25), it was demonstrated
that tissue samples extracted by low-toxicity tert-butyl methyl
ether are easily dried by nitrogen, acquiring an increased DOC
extraction rate (26). Due to its
size, the nanomedicine is easily delivered to the tumor cell, and
accumulates in tumor tissues for enhanced permeability and
retention (27,28). Therefore, DOC concentrations in
HNE-1 tumors increased with prolonged blood circulation. DOC
concentrations were increased in organs rich in blood supply,
including the liver and spleen, and decreased over time. GFP
expression in each tissue was consistent with DOC distribution
in vivo. These results indicated that CD-PLLD/DOC/MMP-9
complexes may be stable in the blood circulation. However, the
complexes were easily phagocytized in reticuloendothelial systems
including the liver and spleen; this requires further study.
Furthermore, the complexes did not cross the blood-brain barrier
due to their large molecular size, demonstrating their relative
safety. Additionally, the complexes mediated increased DOC
concentrations compared with prolonged blood circulation and EGFP
expression in HNE-1 tumors.
In the present study, in a tumor-bearing mouse
model, no significant differences were observed following treatment
with CD-PLLD/MMP-9 compared with the control group. This may be due
to removal of CD-PLLD/MMP-9 complexes by reticular endothelial
cells in the liver and spleen, low gene concentrations in tumor, or
because multiple genes are involved in tumor development, and
therefore single-gene therapy is not effective. The antitumor
effects were most marked in the CD-PLLD/DOC/MMP-9-treated group, as
demonstrated by a clear decrease in PCNA expression levels. This
may be due to the nano-characteristics of CD-PLLD, enabling it to
accumulate DOC and MMP-9 in tumor tissue, and penetrate into the
tumor to exert its effects in vivo (29). PCNA is a cofactor of DNA polymerase
δ, which is required for cell proliferation, and is therefore often
used as an index of DNA replication and cell proliferation
(30). The present study
demonstrated that tumor PCNA expression levels in
CD-PLLD/DOC/MMP-9-treated mice were significantly decreased and
exhibited the greatest anti-tumor effects, as demonstrated by
reduced weight and volume.
In conclusion, the present study investigated the
co-delivery of a hydrophobic drug and target gene. In vivo
assays demonstrated that CD-PLLD/DOC/MMP-9 may inhibit HNE-1 tumor
growth and decrease PCNA expression levels. These results indicated
a potential novel strategy for NPC therapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81573000, 81260406,
81272687 and 81372477), the Guangdong Provincial Natural Science
Foundation of China (grant no. 2015A030313864) and the Zhejiang
Provincial Natural Science Foundation of China (grant no.
LZ13H160004).
References
|
1
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW,
Millward MJ, Hong RL, Whang-Peng J, Ma BB, To KF, et al:
Multicenter, phase II study of cetuximab in combination with
carboplatin in patients with recurrent or metastatic nasopharyngeal
carcinoma. J Clin Oncol. 23:3568–3576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dai X and Tan C: Combination of microRNA
therapeutics with small-molecule anticancer drugs: Mechanism of
action and co-delivery nanocarriers. Adv Drug Deliv Rev.
81:184–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He C, Liu D and Lin W: Self-assembled
nanoscale coordination polymers carrying siRNAs and cisplatin for
effective treatment of resistant ovarian cancer. Biomaterials.
36:124–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Liu R, Yang J, Ma G, Zhang Z and
Zhang X: Dual sensitive and temporally controlled camptothecin
prodrug liposomes codelivery of siRNA for high efficiency tumor
therapy. Biomaterials. 35:9731–9745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang Y, Yang K, Wei P, Huang S, Pei Y,
Zhao W and Pei Z: Cationic vesicles based on amphiphilic pillar
[5]arene capped with ferrocenium: A redox-responsive system for
drug/siRNA co-delivery. Angew Chem Int Ed Engl. 53:13126–13130.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma D, Zhang HB, Tu K and Zhang LM: Novel
supramolecular hydrogel/micelle composite for co-delivery of
anticancer drug and growth factor. Soft Matter. 8:3665–3672. 2012.
View Article : Google Scholar
|
|
8
|
Wang AT, Liang DS, Liu YJ and Qi XR: Roles
of ligand and TPGS of micelles in regulating internalization,
penetration and accumulation against sensitive or resistant tumor
and therapy for multidrug resistant tumors. Biomaterials.
53:160–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Q, van Gaal EV, Brundel P, Ippel H,
Hackeng T, Rijcken CJ, Storm G, Hennink WE and Prakash J: A novel
approach for the intravenous delivery of leuprolide using
core-cross-linked polymeric micelles. J Control Release.
205:98–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma D, Zhang HB, Chen YY, Lin JT and Zhang
LM: New cyclodextrin derivative containing poly(L-lysine) dendrons
for gene and drug co-delivery. J Colloid Interface Sci.
405:305–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li M, Zhou X, Zeng X, Wang C, Xu J, Ma D
and Xue W: Folate-targeting redox hyperbranched poly(amido amine)s
delivering MMP-9 siRNA for cancer therapy. J Mater Chem B.
4:547–556. 2016. View Article : Google Scholar
|
|
12
|
Luo K, Li C, Li L, She W, Wang G and Gu Z:
Arginine functionalized peptide dendrimers as potential gene
delivery vehicles. Biomaterials. 33:4917–4927. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma D, Lin QM, Zhang LM, Liang YY and Xue
W: A star-shaped porphyrin-arginine functionalized poly(L-lysine)
copolymer for photo-enhanced drug and gene co-delivery.
Biomaterials. 35:4357–4367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pang JD, Zhuang BX, Mai K, Chen RF, Wang J
and Zhang LM: Click modification of helical amylose by
poly(l-lysine) dendrons for non-viral gene delivery. Mater Sci Eng
C Mater Biol Appl. 49:485–492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma D, Liu ZH, Zheng QQ, Zhou XY, Zhang Y,
Shi YF, Lin JT and Xue W: Star-shaped polymer consisting of a
porphyrin core and poly(L-lysine) dendron arms: Synthesis, drug
delivery, and in vitro chemo/photodynamic therapy. Macromol Rapid
Commun. 34:548–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu T, Xue W, Ke B, Xie MQ and Ma D:
Star-shaped cyclodextrin-poly(L-lysine) derivative co-delivering
docetaxel and MMP-9 siRNA plasmid in cancer therapy. Biomaterials.
35:3865–3872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Creixell M and Peppas NA: Co-delivery of
siRNA and therapeutic agents using nanocarriers to overcome cancer
resistance. Nano Today. 7:367–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: An imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rao JS, Bhoopathi P, Chetty C, Gujrati M
and Lakka SS: MMP-9 short interfering RNA induced senescence
resulting in inhibition of medulloblastoma growth via p16(INK4a)
and mitogen-activated protein kinase pathway. Cancer Res.
67:4956–4964. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ajani JA: Chemotherapy for advanced
gastric or gastroesophageal cancer: Defining the contributions of
docetaxel. Expert Opin Pharmacother. 7:1627–1631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bouvier E, Thirot S, Schmidt F and
Monneret C: First enzymatically activated Taxotere prodrugs
designed for ADEPT and PMT. Bioorg Med Chem. 12:969–977. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sánchez-Moreno P, Boulaiz H,
Ortega-Vinuesa JL, Peula-García JM and Aránega A: Novel drug
delivery system based on docetaxel-loaded nanocapsules as a
therapeutic strategy against breast cancer cells. Int J Mol Sci.
13:4906–4919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cortes J, Rodriguez J, Aramendia JM,
Salgado E, Gurpide A, Garcia-Foncillas J, Aristu JJ, Claver A,
Bosch A, Lopez-Picazo JM, et al: Front-line paclitaxel/cisplatin
based chemotherapy in brain metastases from non-small-cell lung
cancer. Oncology. 64:28–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng SS and Chien S: Chemotherapeutic
engineering: Application and further development of chemical
engineering principles for chemotherapy of cancer and other
diseases. Chem Eng Sci. 58:4087–4114. 2003. View Article : Google Scholar
|
|
25
|
Zhao Y, Zhai D, Chen X, Yu Q, He H, Sun Y,
Gao Z, Wang L, Wang H, Han D and Ji H: Determination of nimodipine
in human plasma by HPLC-ESI-MS and its application to a
bioequivalence study. J Chromatogr Sci. 48:81–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ju P, Liu Z, Jiang Y, Zhao S, Zhang L,
Zhang Y, Gu L, Tang X, Bi K and Chen X: Determination of a novel
anticancer c-Met inhibitor LS-177 in rat plasma and tissues with a
validated UPLC-MS/MS method: Application to pharmacokinetics and
tissue distribution study. Biomed Chromatogr. 29:1103–1111. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sledge GW Jr and Miller KD: Exploiting the
hallmarks of cancer: The future conquest of breast cancer. Eur J
Cancer. 39:1668–1675. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teicher BA: Molecular targets and cancer
therapeutics: Discovery, development and clinical validation. Drug
Resist Updat. 3:67–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brannon-Peppas L and Blanchette JO:
Corrigendum to ‘Nanoparticle and targeted systems for cancer
therapy’ Advanced Drug Delivery Reviews 56(2004)1649–1659. Adv Drug
Deliv Rev. 61:3642009. View Article : Google Scholar
|
|
30
|
Kato K, Kawashiri S, Yoshizawa K, Kitahara
H, Okamune A, Sugiura S, Noguchi N and Yamamoto E: Expression form
of p53 and PCNA at the invasive front in oral squamous cell
carcinoma: Correlation with clinicopathological features and
prognosis. J Oral Pathol Med. 40:693–698. 2011. View Article : Google Scholar : PubMed/NCBI
|