Introduction
Acute lung injury (ALI) is a common respiratory in
which the symptoms include an increase in pulmonary microvascular
permeability, and diffuse pulmonary interstitial and alveolar
cavity edema caused by the infiltration of inflammatory cells
(1). The primary clinical
manifestations of ALI include acute respiratory distress,
refractory hypoxemia and non-cardiogenic pulmonary edema. ALI may
progress into acute respiratory distress syndrome (ARDS) (2).
ALI and ARDS are the most common complications in
patients with a large-area deep burn, particularly when combined
with an inhalation injury, shock and delayed resuscitation
(3). ALI and ARDS cause body
hypoxia, act on various organs and systems of the body, and cause
necrosis and dysfunction of tissues, resulting in the development
of multiple organ dysfunction syndrome and death. An American
epidemiological survey demonstrated that, among patients with
severe burns to >30% of the total body area, ARDS is the most
common complication, with 26.7–45% incidence and a 40–60% mortality
rate (4). ALI and ARDS are the
principal diseases threatening the health of severely burned
patients, and impose a heavy financial burden on individuals,
families and society (2).
NACHT, LRR and PYD domains-containing protein 3
(NLRP3) inflammasomes may be activated by a range of exogenous and
endogenous stimuli. Infection with Sendai virus, influenza virus,
adenovirus, Saccharomyces cerevisiae, Candida
albicans and certain bacteria, including Staphylococcus
aureus, Listeria monocytogenes and Shigella
flexneri may induce the activation of the NLRP3 inflammasome
(5,6). In certain cases, specific microbial
components may trigger the activation of the NLRP3 inflammasome to
cause ALI (5,7).
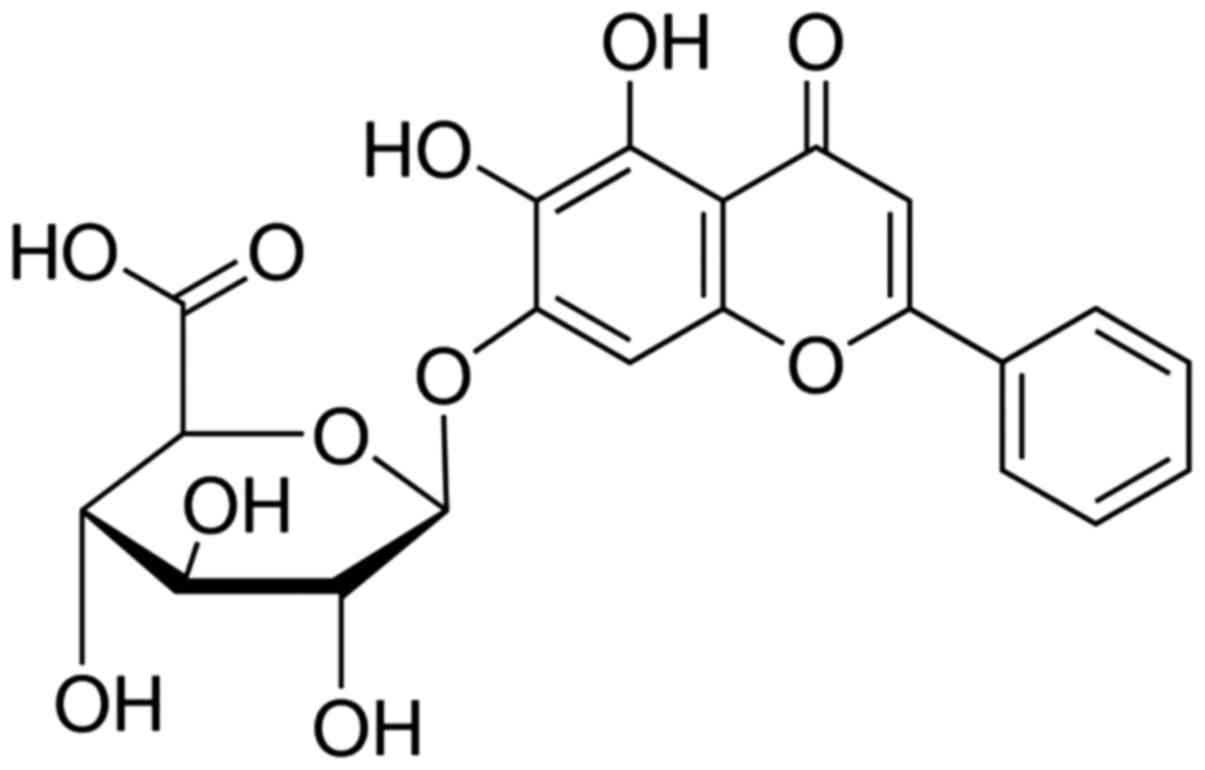
Baicalin, additionally termed
5,6,7-trihydroxyflavone (Fig. 1)
or baiceli, belongs to a group of flavonoids and is most readily
synthesized by the plant species Scutellaria baicalensis
(8). Baicalin is a glycoside
compound synthesized by the combination of baicalein with a
molecule of glucuronic acid, which are also synthesized in skullcap
plants. When baicalein enters the body of an animal, it is rapidly
transformed into baicalin and other metabolites in the blood. Since
the oral absorption of baicalin is challenging, it translocates
into the blood via the intestinal tract, aided by enzymatic
hydrolysis into baicalein, and is rapidly transformed into
baicalinin in vivo. Baicalein has been receiving an
increased amount of attention from researchers due to its
versatility (9). In the present
study, a possible mechanism underlying the neuroprotective effects
of baicalin against ALI was investigated.
Materials and methods
Animals and experimental design
Healthy adult female Sprague-Dawley rats (200–230 g;
8–10 weeks old) were acquired from Animal Experiment of Shandong
University (Shandong, China) and fed a standard animal diet with
food and tap water ad libitum and housed at 23–25°C, 55–60%
humidity under a 12 h light/dark cycle. Rats were acclimatized to
their environment for at least 1 week prior to the experiment. A
total of 30 rats were randomly allocated into one of three groups:
i) The sham group (n=6); ii) the burn group (n=12); and iii) the
burn + baicalin group (n=12). The rats in the burn + baicalin group
were treated with 80 mg/kg of baicalin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for one week. The rats in sham group were
treated with normal saline. The present study was approved by the
Animal Ethical and Welfare Committee of 401 Hospital of People's
Liberation Army (Qingdao, China).
Burn procedure
Rats were intraperitoneally anesthetized with sodium
pentobarbital (30 mg/kg), shaved on the dorsal and lateral surfaces
and secured on a constructed template device. Hot water (100–95°C)
was poured on the dorsal surface of the rat skin for 10 sec to
induce burns. Full-thickness dermal burns averaged 30% of the total
body surface area.
Lung wet weight to dry weight (W/D)
ratio
The upper left parts of lungs were harvested
following the sacrifice of rats under anesthesia. The lungs were
weighed to record the wet weight and dried in an oven at 75°C for
48 h to record the dry weight. The W/D ratio was calculated as
dry/wet weight ×100.
Western blotting analysis
Lung tissue samples were lysed using
radioimmunoprecipitation (Beyotime Institute of Biotechnology,
Haimen, China) assay lysis buffer at 4°C for 30 min and, following
centrifugation at 12,000 × g for 10 min at 4°C, the supernatant was
collected and the protein concentration determined using a
bicinchoninic acid assay. A total of 50 µg total protein was
incubated at 100°C for 10 min and separated on an 8–12% SDS-PAGE
gel, blotted onto a polyvinylidene fluoride membrane (EMD
Millipore, Billerica, MA, USA), and probed overnight at 4°C with
anti-NLRP3 (1:500, sc-66846; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), caspase-1 (1:500, sc-622; Santa Cruz
Biotechnology, Inc.), nuclear factor-κB (NF-κB, 1:500; sc-109,
Santa Cruz Biotechnology, Inc.) matrix metalloproteinase-9 (MMP-9,
1:500, sc-10737; Santa Cruz Biotechnology, Inc.) and GAPDH
(1:2,000, sc-25778; Santa Cruz Biotechnology, Inc.) antibodies
following blocking with 5%-non-fat milk in TBST for 1 h at 37°C.
Subsequently, the membrane was incubated with an anti-rabbit
horseradish peroxidase-conjugated secondary antibody (1:2,000,
sc-2030; Santa Cruz Biotechnology, Inc.) at 37°C for 1 h. The
membrane was washed and detected using an electrochemiluminescence
plus detection kit (GE Healthcare, Chicago, IL, USA) and analyzed
using Image_Lab_3.0 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Measurement of inflammation and
oxidative stress
The serum concentrations of tumor necrosis factor-α
(TNF-α, H052), interleukin (IL)-8 (H008), −1β (H002), −18 (H015),
myeloperoxidase (MPO, A044), malondialdehyde (MDA, A003-1) and
superoxide dismutase (SOD, A001-1-1) were measured using ELISA kits
for rats (Nanjing Jiangcheng Bioengineering Institute, Nanjing
China), according to the manufacturer's protocols.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Differences between two groups were analyzed using the
Student's t-test, and between more than two groups by one-way
analysis of variance followed by a Tukey post-hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
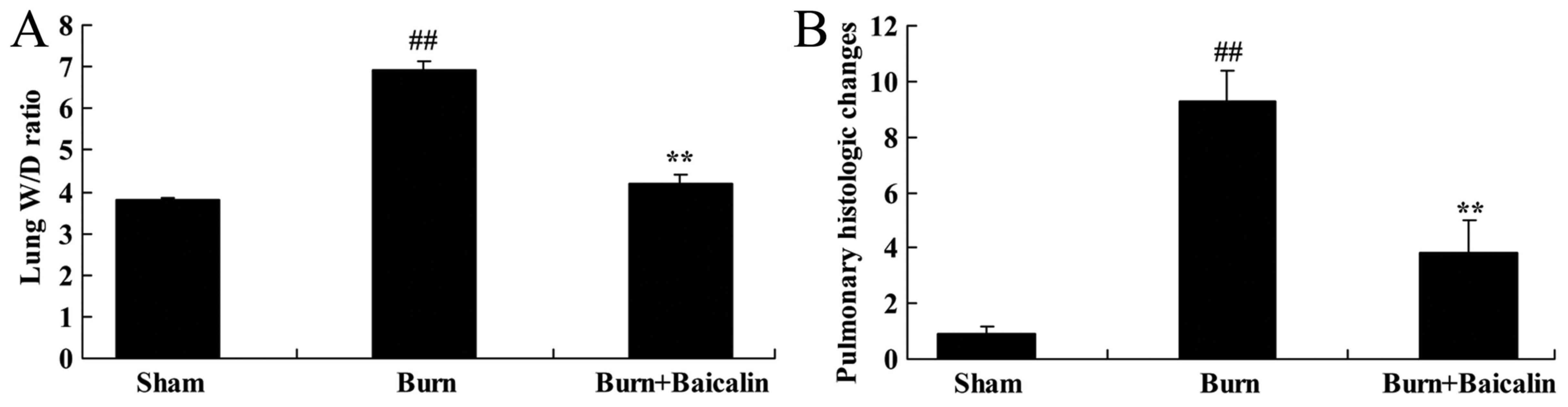
Effects of baicalin on ALI
To identify the effects of baicalin on burn-induced
remote ALI, lung W/D ratio and the pulmonary histological
alterations were measured in the present study. The lung W/D ratio
and pulmonary histological alterations in burn-induced remote ALI
model were increased compared with the sham group (Fig. 2). Treatment with baicalin
significantly decreased the lung W/D ratio and improved the
pulmonary histological alterations compared with the burn group
(Fig. 2).
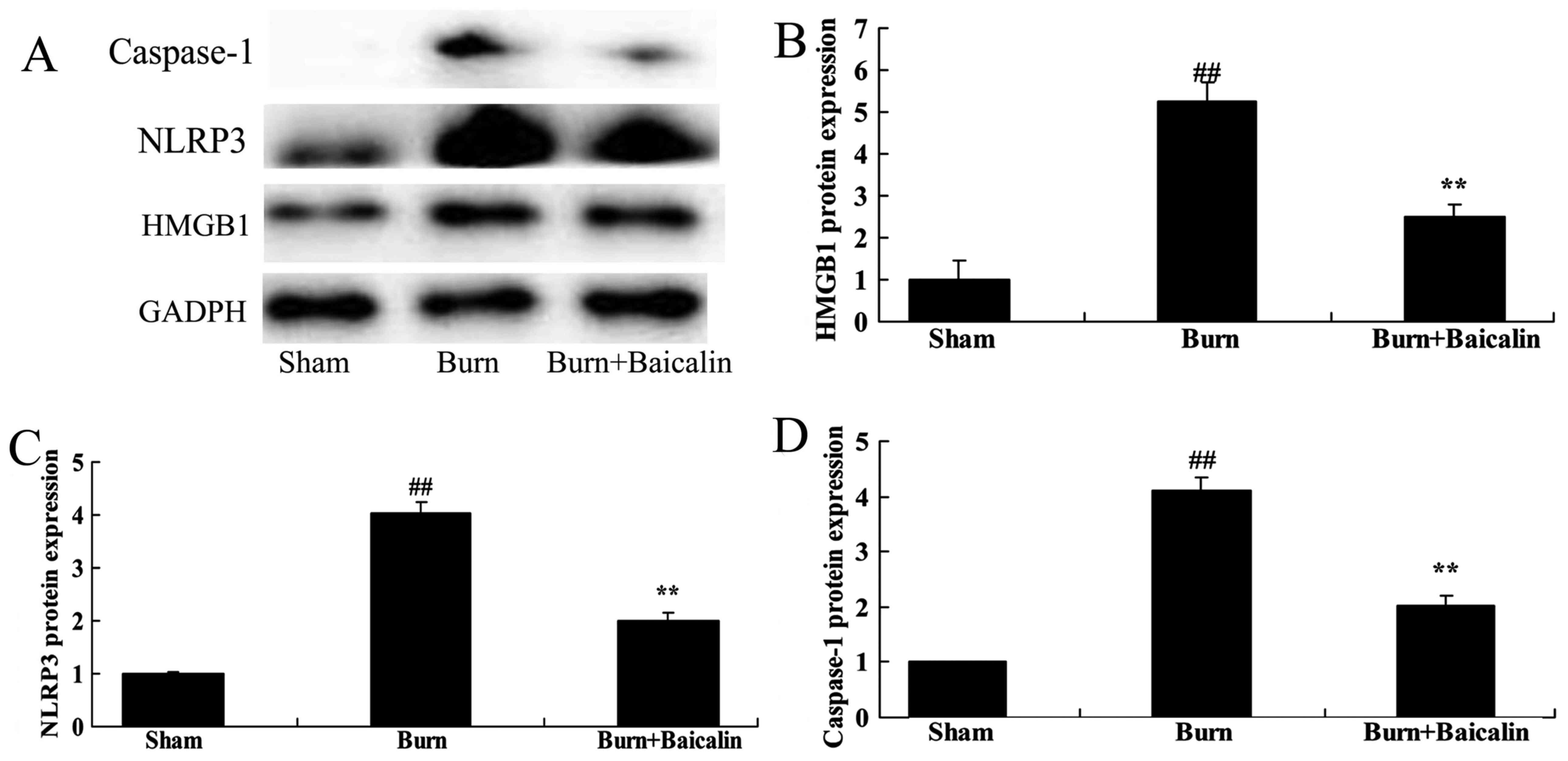
Effects of baicalin on high mobility
group protein B1 (HMGB1) and NLRP3 expression in ALI
The present study evaluated the effects of baicalin
on burn-induced remote ALI. HMGB1 protein expression was measured
using western blot analysis. As presented in Fig. 3, there was a significant increase
in HMGB1, NLRP3 and caspase-1 protein expression in the
burn-induced remote ALI model, compared with the sham group.
Treatment with baicalin significantly suppressed HMGB1, NLRP3 and
caspase-1 protein expression in burn-induced remote ALI rats
(Fig. 3).
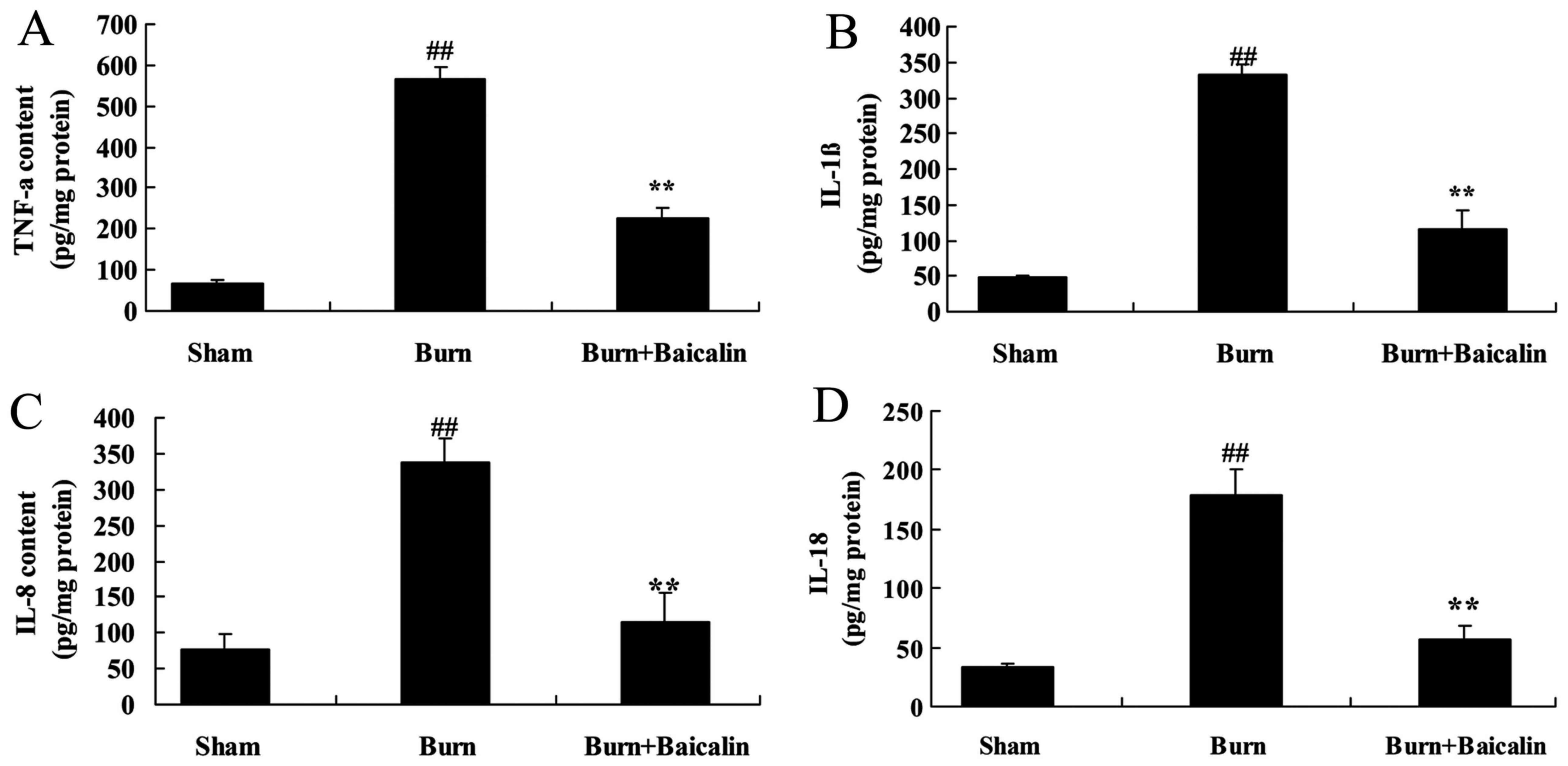
Effects of baicalin on inflammation in
ALI
Inflammatory mediators serve an important role in
burn-induced remote ALI. As demonstrated in Fig. 4, TNF-α IL-8, −1β and −18
concentrations in the serum of the burn group were significantly
increased compared with the sham group. The increased
concentrations of TNF-α, IL-8, −1β and −18 in the serum of the burn
group were significantly decreased in the burn + baicalin group
(Fig. 4).
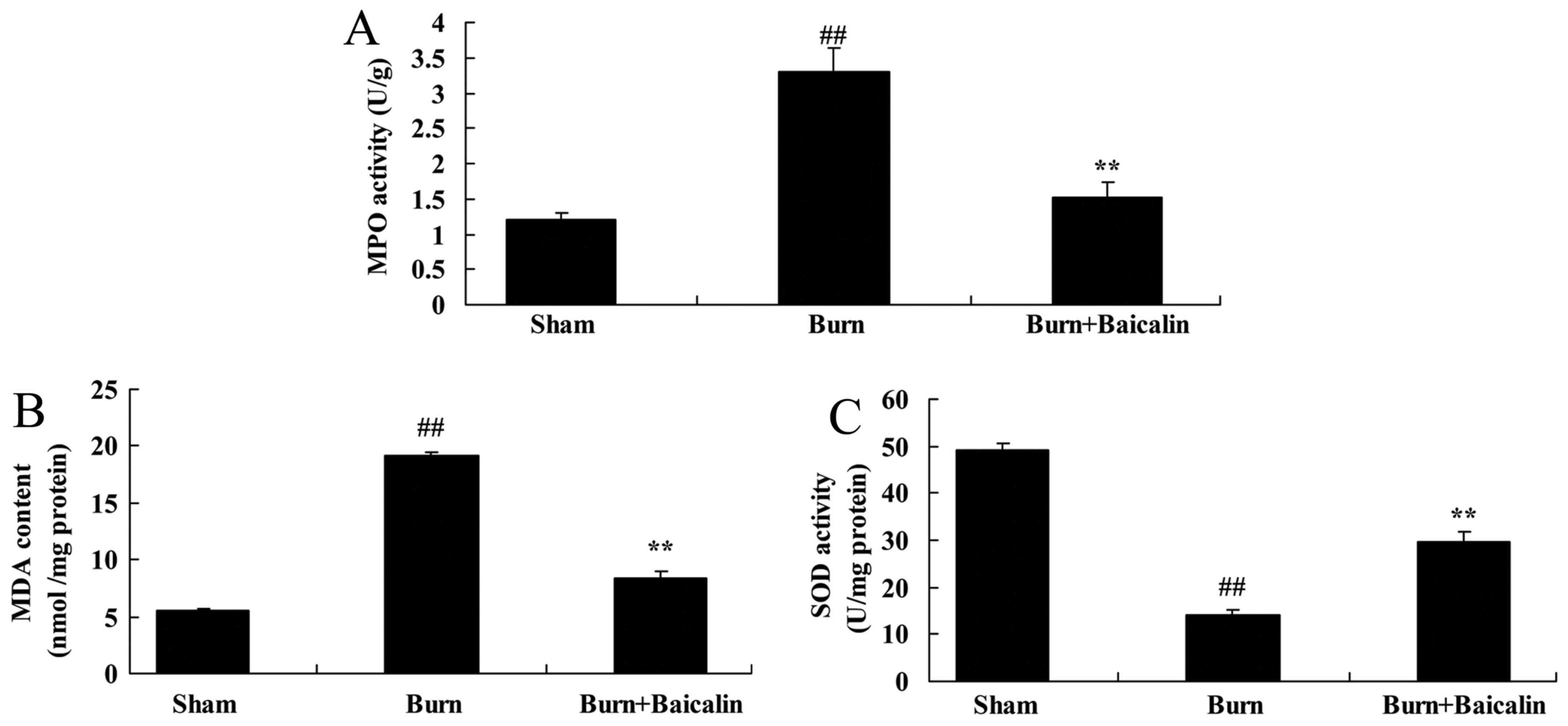
Effects of baicalin on the activity of
MPO and oxidative stress in ALI
In the burn-induced remote ALI model, MPO activity
and MDA content were increased, and SOD expression was decreased,
compared with the sham group (Fig.
5). By contrast, treatment with baicalin reduced MPO activity
and MDA content, and the increased SOD level in serum in the
treated ALI model group, compared with the untreated burn group
(Fig. 5).
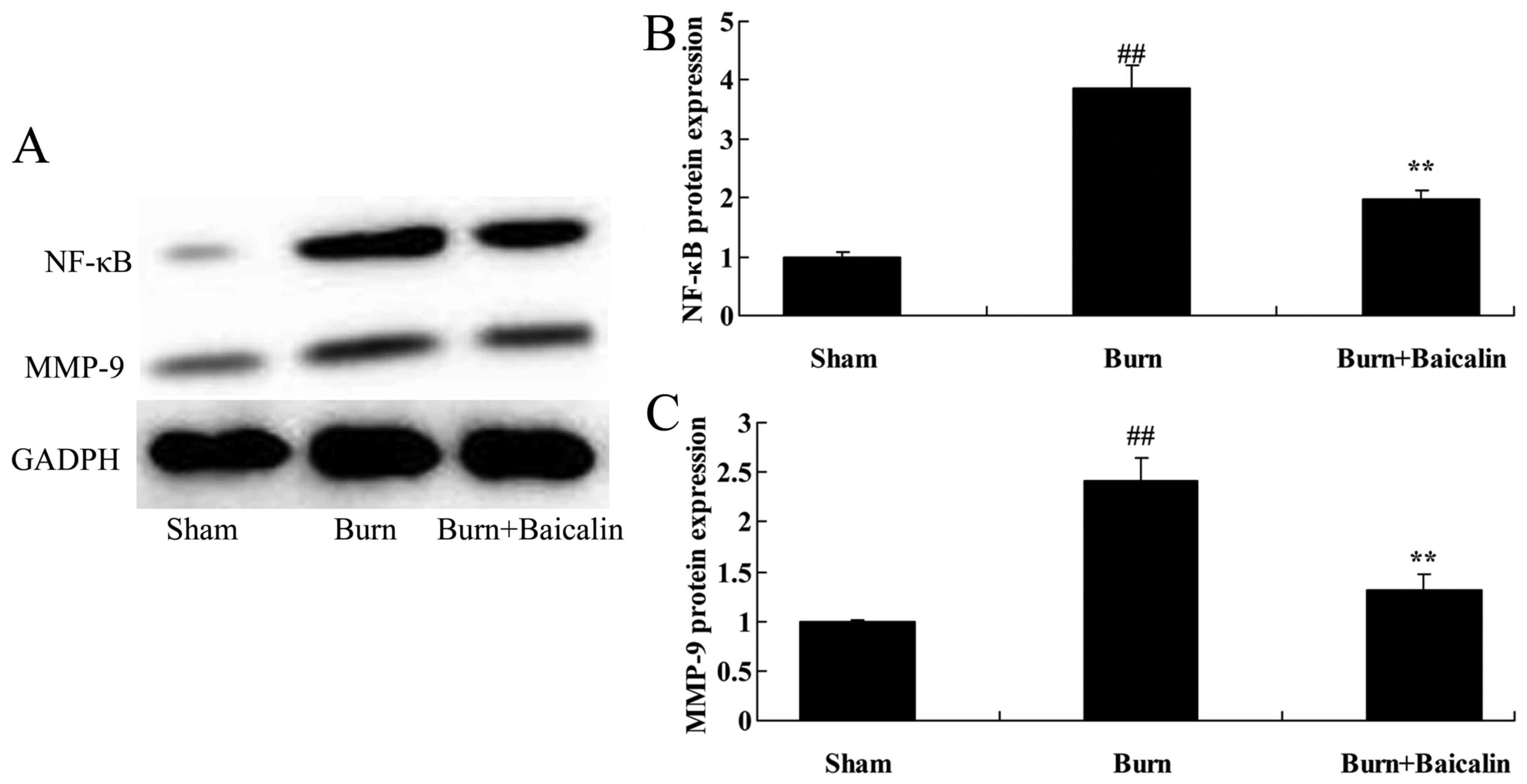
Effects of baicalin on NF-κB and MMP-9
protein expression in ALI
The effects of Baicalin on the regulation NF-κB and
MMP-9 protein expression in ALI were investigated. NF-κB and MMP-9
protein expression in the burn group were significantly increased
compared with the sham group (Fig.
6). Treatment with baicalin significantly suppressed the
expression of NF-κB and MMP-9 in the burn + baicalin group
(Fig. 6).
Discussion
ALI is caused by a variety of non-cardiogenic
pulmonary and extrinsic factors, including severe infection, burns,
shock, trauma, disseminated intravascular coagulation, aspiration
and other primary diseases (10).
In the case of multiple organ injuries, ALI develops earliest and
its incidence is greatest, making ALI the most common complication
of severe burns and delayed resuscitation (1). ALI causes hypoxia and leads to organ
damage and dysfunction of distant burn sites, which is one of the
leading causes of premature death in patients with burns (11). In the present study, baicalin
significantly reduced the lung W/D ratio, improved pulmonary
histological alterations and suppressed HMGB1 protein expression in
burn-induced remote ALI.
A recent study confirmed that the principal
pathological feature of ALI is the formation of protein-rich
pulmonary edema and a transparent membrane in the alveolar exudate,
caused by an increase in pulmonary capillary permeability, in
addition to diffuse alveolar capillary injury caused by
uncontrolled inflammation (12).
Various causes of injury that lead to the development of ALI
stimulate the binding of cell surface receptors by their ligands,
triggering signal transduction pathways. The signaling pathways
ultimately reach the cell nucleus and stimulate transcription
factors to initiate the expression of their target inflammatory
mediator genes, including cytokines and chemokines. The resultant
uncontrolled inflammation may lead to damage, apoptosis, and
mechanical ventilation of alveolar cells and lung capillaries,
resulting in lung abnormalities (13).
An uncontrolled inflammatory response is the leading
cause of ALI and, therefore, elucidation of the mechanism of action
of pulmonary inflammatory mediators in ALI is important for the
prevention and treatment of ALI (14). In the present study, baicalin
significantly decreased the TNF-α, and IL-8, −1β and −18
concentrations in the serum of rats with burn-induced remote ALI.
Liu et al (15)
hypothesized that baicalin attenuates inflammation by inhibiting
NF-κB in mice with ovalbumin-induced asthma.
An increase in MMP-9 expression in animal models and
clinical patients is a common pathophysiological manifestation of
lung injury caused by various factors and, therefore, MMP-9 was
hypothesized to serve an important role in the development of lung
injury (16,17). Analysis of candidate pathways
mediating MMP-9 upregulation in ALI demonstrated that an increased
level of MMP-9 in lung tissue corresponded with more severe lung
injury (16). In the present
study, treatment with baicalin significantly suppressed NF-κB and
MMP-9 protein expression in rats with burn-induced remote ALI. Yan
et al (9) suggested that
baicalin may attenuate pulmonary hypertension by downregulating the
p38 mitogen-activated protein kinase/MMP-9 pathway.
Inflammasomes are a class of macromolecules and
polyprotein complexes induced and assembled via the oligomerization
of domain-like receptors, and by activated nucleotide-binding in
the cytoplasm of cells (18).
Inflammasomes mediate the innate immune response (18). NLRP3 is a member of an inflammatory
cytokine family, an apoptosis-associated speck-like protein
containing a carboxy-terminal caspase recruiting domain and
caspase-1 precursors (19). NLRP3
is activated by the binding of pathogen-associated molecular
patterns or risk-associated molecular patterns to their ligands,
which induces NLRP3 inflammasome assembly and promotes
oligomerization. The resulting oligomerized pro-caspase-1 exhibits
self-enzymatic properties to form caspase-1, a biologically active
protein that promotes the maturation of IL-1β precursor and IL-18
precursor, additionally termed pro-IL-18, to generate biologically
active IL-1β and −18, which are subsequently secreted outside of
the cell to exert their biological effects (20). In the present study, baicalin
significantly attenuated NLRP3 inflammasome expression in rats with
burn-induced remote ALI. Fu et al (21) reported that baicalin suppressed
NLRP3 inflammasome and NF-κB signaling in Haemophilus
parasuis infection.
In conclusion, the present study identified that
baicalin protected against severe burn-induced remote ALI in rats
via modulation of the NLRP3 signaling pathway. The positive effects
of baicalin on burn-induced remote ALI make it a candidate for
application in therapeutic strategies.
Acknowledgements
The present study was supported by the Postdoctoral
Application Research Funded Program of Qingdao (grant no.
2015161).
References
|
1
|
Sun R, Li Y, Chen W, Zhang F and Li T:
Total ginsenosides synergize with ulinastatin against septic acute
lung injury and acute respiratory distress syndrome. Int J Clin Exp
Pathol. 8:7385–7390. 2015.PubMed/NCBI
|
|
2
|
Patel BK, Wolfe KS, Pohlman AS, Hall JB
and Kress JP: Effect of noninvasive ventilation delivered by helmet
vs face mask on the rate of endotracheal intubation in patients
with acute respiratory distress syndrome: A randomized clinical
trial. JAMA. 315:2435–2441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Z and Ni H: Prediction model for
critically ill patients with acute respiratory distress syndrome.
PLoS One. 10:e01206412015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah HA, Dritsaki M, Pink J and Petrou S:
Psychometric properties of Patient Reported Outcome Measures
(PROMs) in patients diagnosed with Acute Respiratory Distress
Syndrome (ARDS). Health Qual Life Outcomes. 14:152016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin N, Peng Z, Li B, Xia J, Wang Z, Yuan
J, Fang L and Lu X: Isoflurane attenuates
lipopolysaccharide-induced acute lung injury by inhibiting
ROS-mediated NLRP3 inflammasome activation. Am J Transl Res.
8:2033–2046. 2016.PubMed/NCBI
|
|
6
|
Wang S, Zhao J, Wang H, Liang Y, Yang N
and Huang Y: Blockage of P2×7 attenuates acute lung injury in mice
by inhibiting NLRP3 inflammasome. Int Immunopharmacol. 27:38–45.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang W, Li M, He F, Bian Z, Liu J, He Q,
Wang X, Sun T and Zhu L: Dopamine D1 receptor agonist A-68930
inhibits NLRP3 inflammasome activation and protects rats from
spinal cord injury-induced acute lung injury. Spinal Cord.
54:951–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang LL, Xiao N, Liu J, Liu K, Liu B, Li P
and Qi LW: Differential regulation of baicalin and scutellarin on
AMPK and Akt in promoting adipose cell glucose disposal. Biochim
Biophys Acta. 1863:598–606. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan S, Wang Y, Liu P, Chen A, Chen M, Yao
D, Xu X, Wang L and Huang X: Baicalin attenuates hypoxia-induced
pulmonary arterial hypertension to improve hypoxic cor pulmonale by
reducing the activity of the p38 MAPK signaling pathway and MMP-9.
Evid Based Complement Alternat Med. 2016:25464022016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Craig TR, Duffy MJ, Shyamsundar M,
McDowell C, O'Kane CM, Elborn JS and McAuley DF: A randomized
clinical trial of hydroxymethylglutaryl-coenzyme a reductase
inhibition for acute lung injury (The HARP Study). Am J Respir Crit
Care Med. 183:620–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiu ZQ and Zhao K: Expression of ERCC1,
RRM1 and LRP in non-small cell lung cancers and their influence on
chemotherapeutic efficacy of gemcitabine concomitant with
nedaplatin. Asian Pac J Cancer Prev. 15:7303–7307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoeboer SH, Groeneveld AB, van der Heijden
M and Oudemans-van Straaten HM: Serial inflammatory biomarkers of
the severity, course and outcome of late onset acute respiratory
distress syndrome in critically ill patients with or at risk for
the syndrome after new-onset fever. Biomark Med. 9:605–616. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Onorati F, Santini F, Mariscalco G,
Bertolini P, Sala A, Faggian G and Mazzucco A: Leukocyte filtration
ameliorates the inflammatory response in patients with mild to
moderate lung dysfunction. Ann Thorac Surg. 92:111–121. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samransamruajkit R, Jiraratanawong K,
Siritantiwat S, Chottanapan S, Deelodejanawong J, Sritippayawan S,
Prapphal N and Poovorawan Y: Potent inflammatory cytokine response
following lung volume recruitment maneuvers with HFOV in pediatric
acute respiratory distress syndrome. Asian Pac J Allergy Immunol.
30:197–203. 2012.PubMed/NCBI
|
|
15
|
Liu J, Wei Y, Luo Q, Xu F, Zhao Z, Zhang
H, Lu L, Sun J, Liu F, Du X, et al: Baicalin attenuates
inflammation in mice with OVA-induced asthma by inhibiting NF-κB
and suppressing CCR7/CCL19/CCL21. Int J Mol Med. 38:1541–1548.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chao W, Deng JS, Huang SS, Li PY, Liang YC
and Huang GJ: 3,4-dihydroxybenzalacetone attenuates
lipopolysaccharide-induced inflammation in acute lung injury via
down-regulation of MMP-2 and MMP-9 activities through suppressing
ROS-mediated MAPK and PI3K/AKT signaling pathways. Int
Immunopharmacol. 50:77–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He W, Jiang J, Yu ZQ and Zhou JH: Novel
5-hydroxy, 5-substituted benzenesulfonamide
pyrimidine-2,4,6-triones attenuate lipopolysaccharide-induced acute
lung injury via inhibition of the gelatinases, MMP-2 and MMP-9.
Drug Dev Res. 77:251–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizushina Y, Shirasuna K, Usui F, Karasawa
T, Kawashima A, Kimura H, Kobayashi M, Komada T, Inoue Y, Mato N,
et al: NLRP3 protein deficiency exacerbates hyperoxia-induced
lethality through Stat3 protein signaling independent of
interleukin-1β. J Biol Chem. 290:5065–5077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo YP, Jiang L, Kang K, Fei DS, Meng XL,
Nan CC, Pan SH, Zhao MR and Zhao MY: Hemin inhibits NLRP3
inflammasome activation in sepsis-induced acute lung injury,
involving heme oxygenase-1. Int Immunopharmacol. 20:24–32. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukumoto J, Fukumoto I, Parthasarathy PT,
Cox R, Huynh B, Ramanathan GK, Venugopal RB, Allen-Gipson DS,
Lockey RF and Kolliputi N: NLRP3 deletion protects from
hyperoxia-induced acute lung injury. Am J Physiol Cell Physiol.
305:C182–C189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu S, Xu L, Li S, Qiu Y, Liu Y, Wu Z, Ye
C, Hou Y and Hu CA: Baicalin suppresses NLRP3 inflammasome and
nuclear factor-kappa B (NF-κB) signaling during Haemophilus
parasuis infection. Vet Res. 47:802016. View Article : Google Scholar : PubMed/NCBI
|