Introduction
The human liver exerts vital functions, including
absorbing metabolites from the intestine, regulating glucose and
lipid metabolism and biotransforming xenobiotics. The liver is one
of the few organs that can regenerate itself following partial
ablation or liver damage. Although hepatocytes have extensive
regenerative capacity, there are many diseases in which this
capacity is insufficient to compensate for the loss of hepatocytes
and liver function (1–3). Over the past three decades, liver
regeneration has been studied extensively and several regulatory
pathways have been identified, which provided regenerative
alternatives to the liver and ensured the return of its mass and
function to the size needed for the body (4–7).
Previous studies involving humans and rodents have demonstrated
that fish oil (FO), which is rich in omega-3 polyunsaturated fatty
acids (n-3 PUFAs), ameliorates the degree of liver injury and
improves liver function (8,9).
However, the mechanism by which dietary supplementation of FO
improves liver function remains elusive.
Liver regeneration involves increased cell
proliferation and successful hepatocyte polarization. As the main
epithelial cells in the liver, hepatocyte polarization, including
tight junctional, cytoskeletal and intracellular trafficking
components, is essential for the regenerative liver function. The
tight junction, a polarized structure, forms a seal between cells,
separating the basolateral and apical membrane domains when
epithelial cells acquire polarity (10). Bile salt export pump (BSEP) is a
member of the ABC superfamily of efflux transporter proteins, and
is crucial in the efflux of bile salts (11). Inhibition of BSEP may lead to
accumulation of cytotoxic bile salts and eventually have severe
consequences such as intrahepatic cholestasis (12).
AMP-activated protein kinase (AMPK), which is a
cellular metabolic sensor, has been demonstrated to be involved in
liver regeneration (13,14). Generally, AMPK activation switches
off ATP-consuming processes and switches on ATP-generating
pathways. Following partial hepatectomy (PH), hepatocyte
proliferation is associated with increased AMPK phosphorylation.
Deletion of AMPK delays liver proliferation by affecting the G1/S
transition phase (14). An
additional function of AMPK activation is also evident on
hepatocyte polarization (15). In
collagen sandwich cultures of rat hepatocytes, AMPK regulated bile
canalicular network formation and maintenance (13). AMPK activation and canalicular
network formation are associated with BSEP trafficking (15).
Based on these previous studies, a hypothesis was
formed that oral supplementation with FO may be able to induce AMPK
activation and regulate hepatocyte polarization markers, thereby
promoting the recovery of liver function during PH.
Materials and methods
Animal experiments
A total of 120 ICR mice (n=24/group, n=6/time
interval; male; 25–30 g; 8–15 weeks old) obtained from the Animal
Experimental Center, Nanjing Drum Tower Hospital, Medical School of
Nanjing University (Nanjing, China) were used for simple 70% PH
experiments. Mice were housed under conditions of 60% humidity, a
temperature of 18–22°C, a 12 h light/dark cycle and free access to
food and water. Mice were fasted overnight prior to PH. Anesthesia
was induced with chloral hydrate (10%; 350 mg/kg) by
intraperitoneal (i.p.) injection. Following laparotomy, the median
and left liver lobes were surgically removed resulting in 70% PH.
Mice were sacrificed via carbon dioxide inhalation asphyxia to
collect blood and liver specimens prior to PH (days −2 and 0) and
following PH (days 1, 2, 3 and 5), and the recovery of liver mass
was estimated by the liver-to-body weight ratios. The experimental
design was examined and approved by the Experimental Animal Ethics
Committee of the Nanjing Drum Tower Hospital, Medical School of
Nanjing University (Nanjing, China).
Experimental groups
All animals were divided randomly into 5 groups
(n=24 per group): i) Sham; ii) Control; iii) Compound C (Selleck
Chemicals, Houston, TX, USA), which is the AMPK inhibitor
dorsomorphin; iv) FO [comprising 40% docosahexaenoic acid (DHA) and
40% eicosapentaenoic acid (EPA); Wuhan Shengtianyu Biotechnology,
Wuhan, China]; and v) Compound C + FO. Mice in the Sham group had
laparotomy and exposure of the liver, but no PH. Mice in the
Control group had 70% PH without any treatment. Mice in the
Compound C group had 70% PH and treatment with Compound C (8 mg/kg)
by i.p. injection daily, beginning half an hour prior to the
operation until the day prior to the indicated time. Mice in the FO
group had 70% PH and treatment with FO (12 ml/kg) by oral gavage
daily, beginning at 2 days prior to the operation until the day
prior to the indicated time. Mice in the Compound C + FO group had
70% PH and treatment with FO (12 ml/kg) by oral gavage daily from 2
days prior the operation until the day prior to the indicated time
and Compound C (8 mg/kg) by i.p. injection daily from half an hour
prior to the operation until the day prior to the indicated
time.
Western blotting
A total of 50 mg liver tissues were lysed in cold
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing 1:100 volume of
phenylmethylsulfonyl fluoride. Following lysis at 4°C for 1 h, cell
lysates were centrifuged at 14,000 × g for 5 min at 4°C. The total
protein concentration in the supernatant was determined by
Bicinchoninic Acid assay (Beyotime Institute of Biotechnology).
Proteins were separated by 10% SDS-PAGE (30 µg/lane) and
transferred to a nitrocellulose membrane at 100 V for 60 min.
Following blocking in TBS + 0.1% Tween-20 (TBST) buffer containing
5% bovine serum albumin (BSA; Absin Bioscience Inc., Shanghai,
China) at 37°C for 1 h, the membranes were incubated in TBST
containing 2% BSA and antibodies against Occludin (cat. no.
ab64482; 1:1,000; Abcam, Cambridge, UK), Claudin-3 (cat. no.
sc-17662; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), tight junction protein ZO-1 (ZO-1; cat. no. sc-10804;
1:1,000; Santa Cruz Biotechnology, Inc.), phosphorylated (p)-AMPK
(cat. no. sc-33524; 1:1,000; Santa Cruz Biotechnology, Inc.), AMPK
(cat. no. sc-33524; 1:1,000; Santa Cruz Biotechnology, Inc.) and
BSEP (cat. no. sc-74500; 1:1,000; Santa Cruz Biotechnology, Inc.)
at 37°C for 1 h. An antibody against β-actin (cat. no. 4970;
1:5,000; Cell Signaling Technology, Inc., Danvers, MA, USA) was
used as an internal control. The membranes were washed three times
with TBST and incubated with the following secondary antibodies at
37°C for 1 h: Goat anti-rabbit horseradish peroxidase
(HRP)-conjugated antibody (cat. no. BA1055; 1:5,000; Boster
Biological Technology, Pleasanton, CA, USA), HRP-goat anti-mouse
antibody (cat. no. BA1051; 1:5,000; Boster Biological Technology)
and HRP-rabbit anti-goat antibody (cat. no. BA1060; 1:5,000; Boster
Biological Technology). Following three washes with TBST, the
target proteins were detected using an Enhanced Chemiluminescence
kit (WBULS0500; EMD Millipore, Billerica, MA, USA). Densitometry
analysis was performed using the Intel iPP 6.0 software (Intel
Corporation, Santa Clara, CA, USA).
Immunohistochemical assays
Paraffin-embedded liver tissue sections (~4 µm) were
fixed in 4% paraformaldehyde at room temperature for 24 h,
deparaffinized and dehydrated through graded ethanol. The sections
were washed 3 times with PBS for 5 min each, and then treated with
3% hydrogen peroxide for 10 min at room temperature to block the
endogenous peroxidase activity. The sections were subsequently
washed 3 times with PBS for 5 min each. Following blocking with 5%
BSA for 30 min at 37°C, sections were incubated with primary
antibodies against Occludin, ZO-1, BESP or Ki-67 (1:200 dilution in
PBS) overnight and then with a HRP-conjugated anti-rabbit
immunoglobulin G secondary antibody (cat. no. BA1055; 1:100; Boster
Biological Technology) for 1 h at room temperature. Finally, the
sections were incubated with 3,3-diaminobenzidine reagent for 10
min, counterstained with hematoxylin, dehydrated and mounted for
inverted phase contrast microscopy (CK30 OLYMPUS; Olympus
Corporation, Tokyo, Japan) analysis.
Serum biochemical parameters
Serum expression levels of alanine aminotransferase
(ALT), aspartate aminotransferase (AST), total bilirubin (TBIL),
albumin (ALB) and C-reactive protein (CRP) were determined by the
Laboratory of Biochemistry, Nanjing Drum Tower Hospital, Medical
School of Nanjing University (Nanjing, China).
Statistical analysis
GraphPad Prism software version 5.01 (GraphPad
Software, Inc., La Jolla, CA, USA) and PASW statistics version 18.0
(SPSS, Inc., Chicago, IL, USA) were used for statistical analyses.
Results are expressed as the mean ± standard deviation. One-way
analysis of variance followed by the Least Significant Difference
post hoc test was used to detect the statistically significant
variations between groups. Each experiment was performed in
triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Perioperative oral supplementation
with FO improves liver function through AMPK activation following
PH
Serum levels of ALT, AST, TBIL, ALB and CRP were
evaluated at 1, 2, 3 and 5 days following PH to evaluate
postoperative hepatic function (Table
I). Following PH, the serum levels of ALT and AST in the
Control mice were significantly increased compared with Sham
(P<0.05; Table I), followed by
a gradual trend to be restored to normal over time. When PH was
accompanied by perioperative FO supplementation, the restoration of
serum ALT and TBIL levels was significantly faster than those in
the Control group (P<0.05 from day 2 and day 3 post-PH,
respectively; Table I). To further
examine the mechanism involved in the effects of perioperative FO
supplementation on liver function, AMPK was inhibited by i.p.
injection of Compound C. The levels of ALT and AST were
significantly higher in the Compound C group compared with the
Control group on day 2 post-PH, with or without perioperative FO
supplementation (P<0.05; Table
I). These results indicated that liver function improvement
following PH in mice with perioperative oral FO supplementation may
be mediated through AMPK signaling.
 | Table I.Serum biochemical parameters following
partial hepatectomy. |
Table I.
Serum biochemical parameters following
partial hepatectomy.
| Serum parameter | Day | Sham | Control | FO | Compound C | Compound C + FO |
|---|
| ALT (U/l) | 1 | 28.4±2.2 |
1,353.0±644.7a |
1,253.4±530.9a |
1,910.0±499.8a |
2,198.7±390.3a–c |
|
| 2 | 28.0±1.7 |
177.4±46.4a |
112.4±19.9a,b |
241.5±47.8a,b |
236.2±32.7a,b |
|
| 3 | 26.6±1.2 |
65.0±15.4a | 36.2±4.8b |
77.9±23.9a |
78.3±24.9a |
|
| 5 | 27.8±1.2 |
54.0±10.4a | 29.0±7.8b |
66.5±24.9a | 39.4±9.6c |
| AST (U/l) | 1 | 92.8±3.4 |
2,344.0±738.1a |
1,744.9±705.1a,b |
2,597.0±316.3a |
2,186.2±243.5a |
|
| 2 | 90.1±2.2 |
243.7±57.4a |
226.0±42.6a |
500.4±69.4a,b |
313.3±25.8a–c |
|
| 3 | 93.3±2.2 |
133.3±13.6a |
134.8±30.0a |
170.1±52.1a,b |
155.4±21.3a |
|
| 5 | 91.3±2.2 |
120.8±38.4a | 101.2±13.3 |
135.0±29.2a |
139.1±22.1a |
| TBIL (µmol/l) | 1 |
1.1±0.4 | 15.9±20.0 | 4.3±1.1 | 18.7±26.6 | 6.4±2.5 |
|
| 2 |
1.6±0.2 | 2.7±0.2 | 2.0±0.4 |
6.3±2.9a,b |
4.1±1.0a,c |
|
| 3 |
1.3±0.4 |
3.4±0.2a |
1.3±0.2b |
2.6±0.1a,b |
1.6±0.3a–c |
|
| 5 |
1.6±0.3 |
2.0±0.2a |
0.9±0.2b | 2.0±1.7 | 1.2±0.2 |
| ALB (g/l) | 1 | 26.9±1.5 | 26.5±1.1 | 26.3±1.1 | 25.4±1.4 | 25.6±1.0 |
|
| 2 | 26.5±1.2 | 26.5±1.1 | 25.1±1.8 | 25.4±1.0 | 25.5±1.0 |
|
| 3 | 27.1±1.0 | 26.5±1.6 | 26.6±1.5 | 26.0±1.6 | 25.9±1.4 |
|
| 5 | 26.6±1.3 | 26.2±1.7 | 26.0±1.4 | 25.5±1.4 | 25.3±0.7 |
| CRP (mg/l) | 1 |
1.4±0.2 | 1.5±0.1 | 1.5±0.8 | 1.8±0.5 | 1.7±0.4 |
|
| 2 |
1.6±0.4 | 1.9±0.6 | 1.4±0.8 | 2.1±0.3 | 2.1±0.2 |
|
| 3 |
1.5±0.4 | 2.1±0.7 | 2.0±0.8 |
2.3±0.4a | 1.7±0.6 |
|
| 5 |
1.3±0.3 | 2.1±0.9 | 2.4±1.1 |
4.9±1.8a,b |
2.3±0.6c |
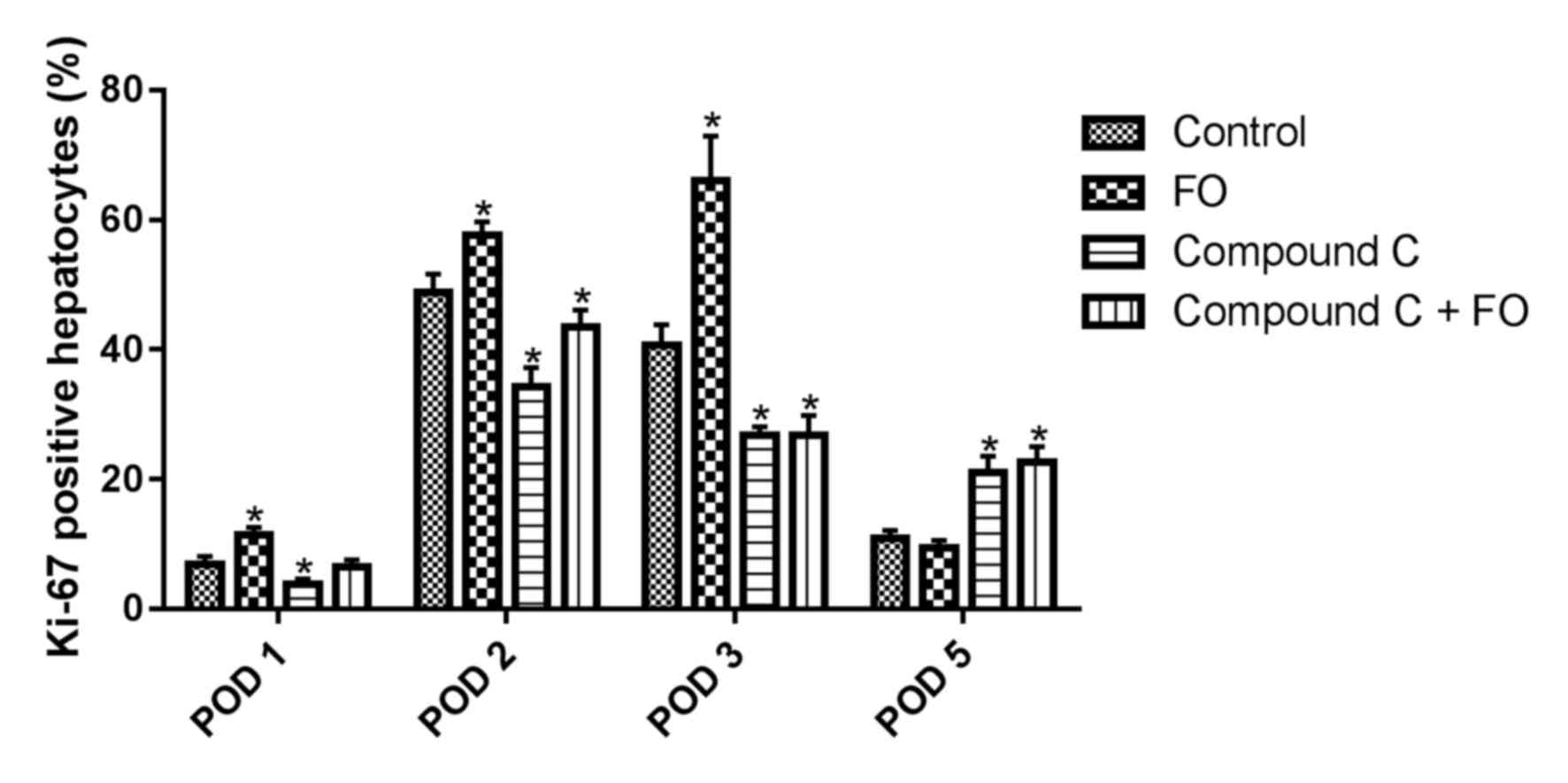
To investigate the mechanisms of FO supplementation
on improving liver function through AMPK activation, hepatocyte
proliferation was examined following PH by immunohistochemical
staining for the Ki-67 proliferation marker. The proportion of
Ki-67 positive hepatocytes in the livers of the FO group was
significantly increased compared with the Control group on days 1,
2 and 3 following PH (P<0.05; Fig.
1). On day 5 following PH, there was no difference between the
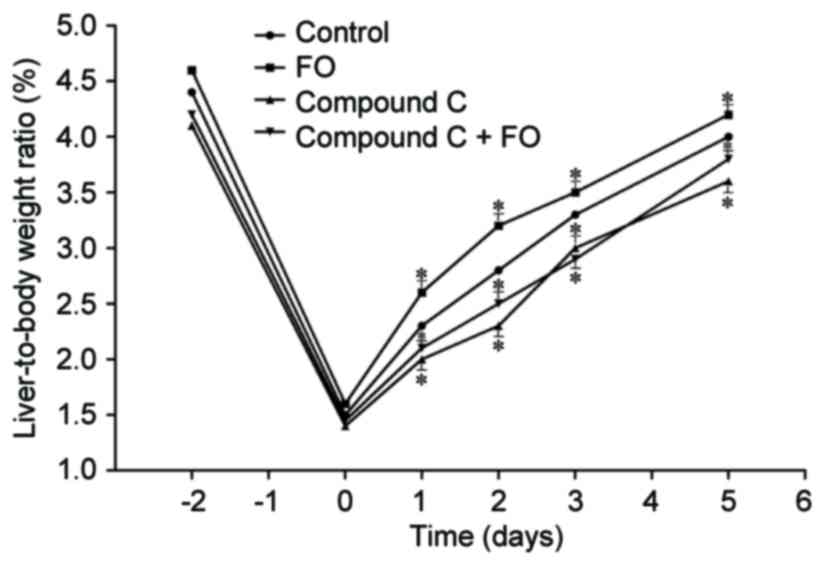
FO group and the Control group (P>0.05; Fig. 1). Measurement of the liver-to-body
weight ratio reflected the results of hepatocyte proliferation.
Perioperative oral supplementation with FO significantly increased
postoperative liver-to-body weight ratio in mice undergoing PH
compared with Control (P<0.05; Fig.
2). To further examine the mechanism involved in the effects of
perioperative FO supplementation on hepatocyte proliferation, AMPK
was inhibited by use of Compound C by i.p. injection. The
proportion of Ki-67 positive hepatocytes was significantly
decreased in the livers of the Compound C-treated group compared
with the Control group from day 1 to 3 following PH, which was not
resolved by perioperative oral supplementation with FO (P<0.05
vs. Control; Fig. 1). The
discrepant results of proliferation at day 5 may be explained in
that the AMPK inhibitor may have delayed the process of hepatocyte
proliferation and FO treatment was unable to reverse it, which was
consistent with the liver-to-body weight ratio. The liver-to-body
weight ratio was also decreased in the Compound C group compared
with the Control group from day 1 to 5 following PH, and treatment
with perioperative FO supplementation did not improve it
(P<0.05; Fig. 2). These data
suggested that perioperative oral supplementation with FO promoted
hepatocyte proliferation in mice following PH and this effect was
mediated through AMPK signaling.
Perioperative oral supplementation
with FO promotes the expression of tight junction and BSEP proteins
through AMPK activation following PH
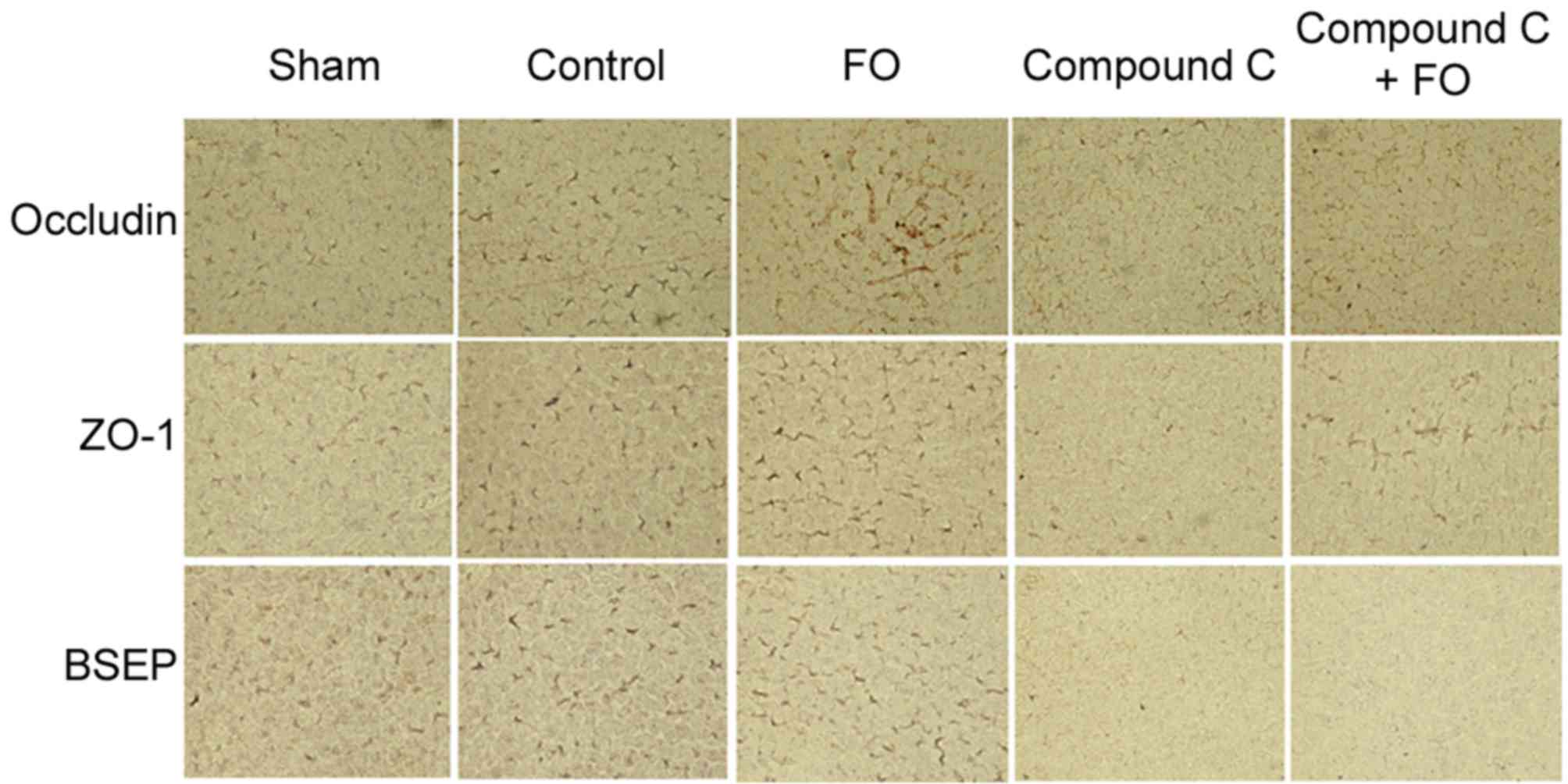
To further investigate the effects of perioperative
FO supplementation on liver regeneration following PH, the
expression levels hepatocyte polarization markers were examined.
Polarization was evaluated by western blot analysis and
immunohistochemistry for the expression of epithelial
differentiation markers Occludin, Claudin-3 and ZO-1, and the
hepatocyte marker BSEP. Phosphorylation levels of AMPK were also
evaluated as a marker of AMPK signaling activation. The ratio of
p-AMPK/total AMPK and the protein expression levels of Occludin,
Claudin-3, ZO-1 and BSEP were gradually increased between day 1 and
day 5 following PH in the Control group (Figs. 3 and 4). Perioperative oral supplementation
with FO further enhanced this change (Figs. 3 and 4). To evaluate the effects of AMPK
signaling on tight junction and BSEP protein expression, AMPK was
inhibited by i.p. injection with Compound C (Fig. 5). The level of p-AMPK expression
was reduced in the Compound C group compared with the Control group
(P<0.05; Fig. 5A and B), as was
expression of Occludin, Claudin-3, ZO-1 and BSEP; however, the
level of p-AMPK expression was still reduced in the Compound C+FO
group compared with the Control group between days 2 and 5
(P<0.05; Fig. 5). In
conclusion, the results demonstrated that perioperative FO oral
supplementation promoted the expression of hepatocyte polarization
markers in mice following PH and this effect was probably mediated
through AMPK activation.
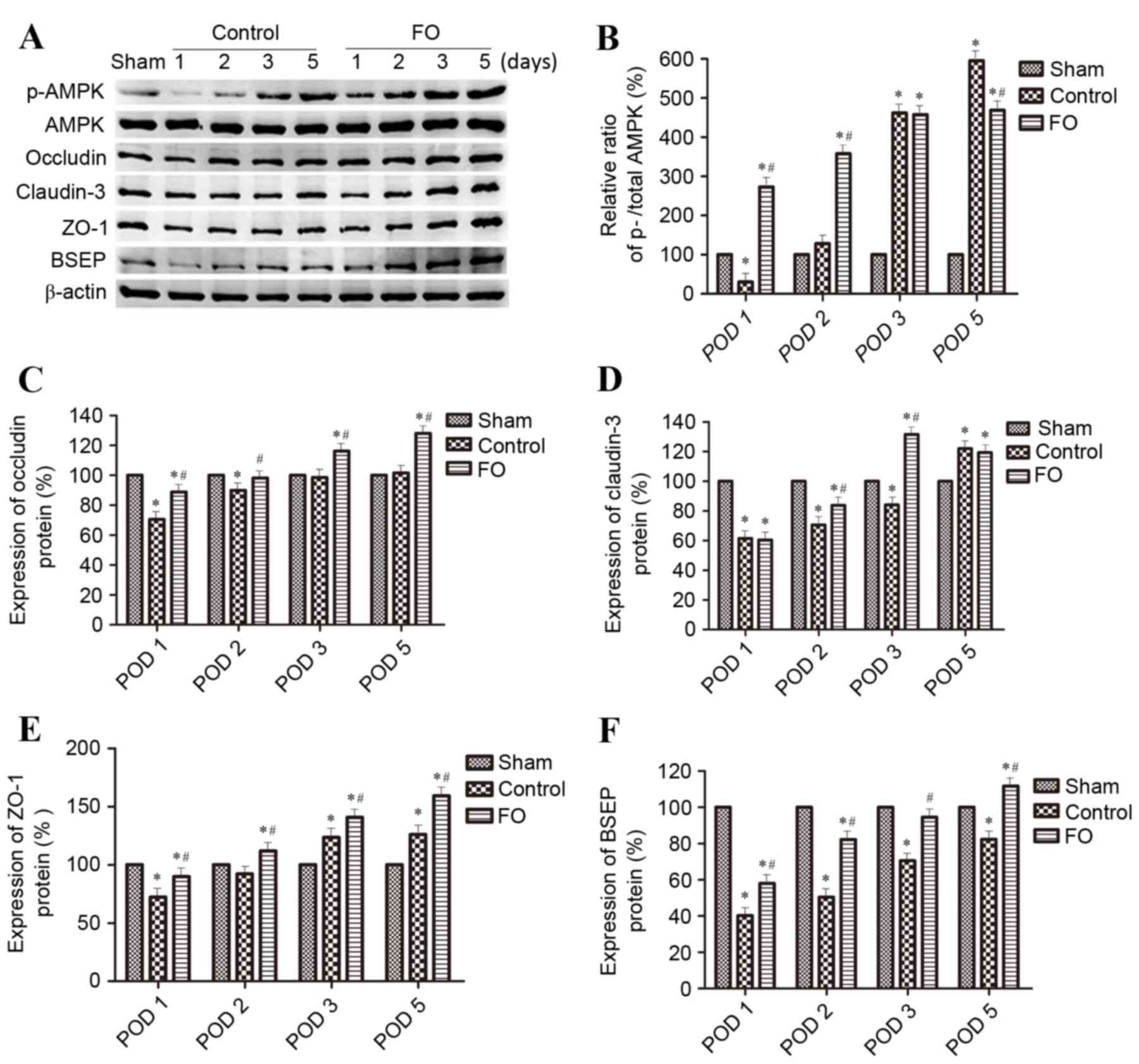 | Figure 3.Effects of perioperative FO oral
supplementation on hepatocyte polarization protein marker
expression levels following partial hepatectomy. (A) Protein
expression levels of p-AMPK, AMPK, Occludin, Claudin-3, ZO-1 and
BSEP were determined by western blotting in Sham, Control and
FO-treated mice on days 1, 2, 3 and 5 following partial
hepatectomy; β-actin was used as an internal Control.
Quantification of (B) p-AMPK:AMPK ratio, (C) Occludin, (D)
Claudin-3, (E) ZO-1 and (F) BSEP levels relative to β-actin. Data
are presented as the mean ± standard deviation; *P<0.05 vs.
Sham; #P<0.05 vs. Control. AMPK, AMP-activated
protein kinase; BSEP, bile salt export pump; FO, fish oil; p,
phosphorylated; POD, postoperative day; ZO-1, tight junction
protein ZO-1. |
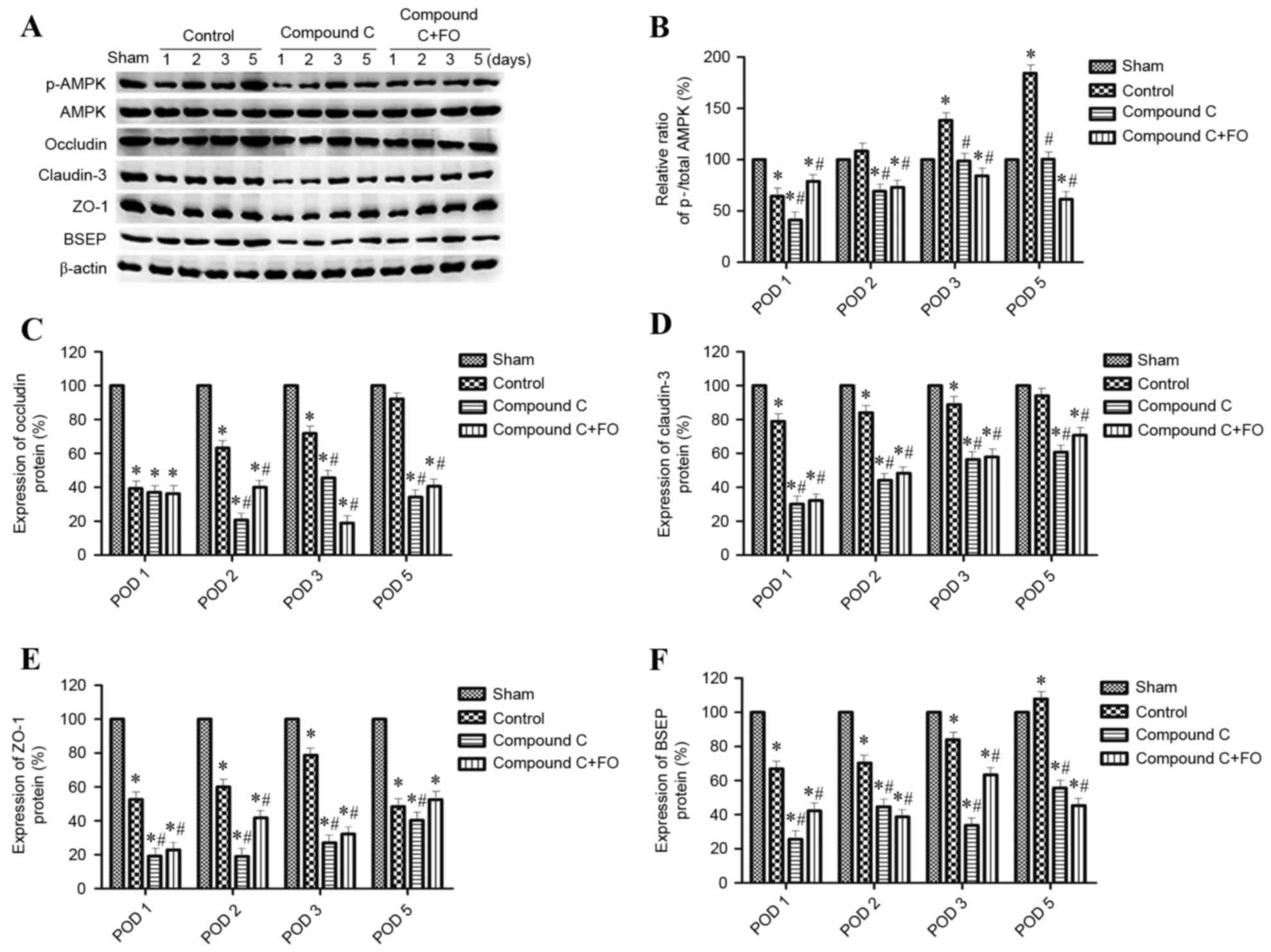 | Figure 5.Effects of AMPK inhibition on
expression hepatocyte polarization marker proteins following
partial hepatectomy. (A) Protein expression levels of p-AMPK, AMPK,
Occludin, Claudin-3, ZO-1 and BSEP were determined by western
blotting in Sham, Control, Compound C and Compound C + FO mice on
days 1, 2, 3 and 5 following partial hepatectomy; β-actin was used
as an internal control. Quantification of (B) p-AMPK:AMPK ratio,
and (C) Occludin, (D) Claudin-3, (E) ZO-1 and (F) BSEP expression
levels relative to β-actin. Data are presented as the mean ±
standard deviation; *P<0.05 vs. Sham; #P<0.05 vs.
Control. AMPK, AMP-activated protein kinase; p, phosphorylated;
BSEP, bile salt export pump; FO, fish oil; POD, postoperative day;
ZO-1, tight junction protein ZO-1. |
Discussion
Results from the present study demonstrated that
oral supplementation with FO facilitated hepatocyte proliferation
and the expression of hepatocyte polarization markers through AMPK
activation, thereby improving liver function following PH. Serum
levels of liver enzymes ALT and AST, as well as TBIL, were
significantly decreased by perioperative FO oral supplementation,
indicating an improvement in liver function. As hepatocyte
polarization is essential for liver function, the hypothesis that
FO might promote polarization of liver parenchyma to improve liver
function was further tested. In addition, the role of AMPK
activation in the FO-mediated effect of decreasing liver injury and
improving liver function was examined. The present study revealed
that AMPK activation was essential for hepatocyte proliferation and
hepatocyte polarization marker expression following PH. AMPK
inhibition not only suppressed cell proliferation, but also delayed
cell polarization in the liver.
FO is rich in n-3 PUFA and has been demonstrated to
reduce liver enzyme expression levels and to improve liver function
in patients undergoing surgery (16). A meta-analysis performed by
Pradelli et al demonstrated that parenteral supplementation
with FO significantly reduced liver enzymes and improved liver
viability in both intensive care unit (ICU) and non-ICU patients
(9). Studies in humans and rodents
revealed that n-3 PUFA ameliorated the degree of liver injury
(8,17). Previous studies from our group have
also demonstrated that n-3 PUFA protected postoperative hepatic
function following 70% PH and prevented acute liver failure
following 90% hepatectomy in rats (18,19).
Therefore, dietary supplementation with FO has the potential to
reduce liver injury and improve liver function, which is consistent
with the findings of the present study. However, the exact
mechanism for this function of FO remained unclear.
The present study also demonstrated that liver
function, along with expression of hepatocyte polarization markers,
gradually recovered following PH. Induction of hepatocyte
polarization may reduce liver injury and improve liver viability,
and inhibition of hepatocyte polarization markers may delay the
recovery of liver function. Hepatocytes are the main epithelial
cells in the liver and they are regularly polarized (20). Hepatocyte polarization involves
formation of functionally distinct sinusoidal (basolateral) and
bile canalicular (apical) plasma membrane domains that are
separated by tight junction proteins, including Occludin, Claudin
and ZO-1 (21). BSEP is mainly
localized to the canalicular membrane of hepatocytes and serves a
crucial role in the disposition of conjugated bile salts from the
liver to the bile canaliculi (11). The inhibition of BSEP may lead to
the accumulation of cytotoxic bile salts and, eventually, to severe
consequences such as intrahepatic cholestasis. Cholestatic
hepatocytes cause deteriorated barrier function of tight junctions
(22). Therefore, hepatocyte
polarization is essential for biliary secretion. Loss of polarity
leads to cholestasis and liver damage (10).
In the present study, FO-mediated AMPK activation
induced the expression of tight junction and BSEP proteins. AMPK is
a sensor of cellular energy homeostasis that is important in energy
regulation and metabolism (13).
Previous studies have demonstrated that AMPK serves a role
downstream of liver kinase B1 (LKB1) to confer cell polarity
(23–25). In metazoan species, AMPK functions
not only in controlling metabolism, but also in regulating cell
structures (23). Activated LKB1
induces three major aspects of intestinal epithelial polarity in a
cell-autonomous way, including forming an apical brush border,
positioning junctional proteins around this brush border, and
correctly sorting the basolateral and apical plasma membrane
markers (24). In the polarized
renal epithelial cells, AMPK also regulates tight junction
assembly. Activation of AMPK facilitates tight junction assembly,
whereas expression of a dominant negative AMPK construct inhibits
it (25). A previous study
demonstrated that hepatocyte polarization, manifested by
canalicular network formation, is sequential and is associated with
activation of AMPK and LKB1 in collagen sandwich cultures of rat
hepatocytes (13). The canalicular
network formation was accelerated by activation of AMPK and LKB1
and was blocked by inhibition of AMPK or LKB1 (13). Another previous study demonstrated
that LKB1 regulates BSEP trafficking to the bile canalicular
membrane, canalicular network formation and hepatocyte polarization
(15).
The present study revealed that oral supplementation
with FO facilitated hepatocyte polarization by AMPK activation,
thereby improving liver function following PH. However, the
mechanism by which FO increases AMPK phosphorylation is not
understood. Previous studies have demonstrated that AMP initiates
the activation of AMPK by LKB1 (26,27).
In addition, it has been reported that cAMP accelerates hepatocyte
polarization via the LKB1/AMPK pathway (10,15).
Further studies will be required to address which of these factors
are induced by FO to regulate hepatocyte polarization. In addition,
n-3 PUFA, including EPA and DHA, are enriched in FO. Further
investigation will be required to examine in detail the
relationship between n-3 PUFA and AMPK activation.
In conclusion, the present study demonstrated that
oral supplementation with FO facilitated liver regeneration by AMPK
activation, thereby improving liver function following PH.
Acknowledgements
The authors acknowledge the financial support from
the National Natural Science Fund of China (grant no.
81470866).
Glossary
Abbreviations
Abbreviations:
|
FO
|
fish oil
|
|
n-3 PUFA
|
omega-3 polyunsaturated fatty acid
|
|
BSEP
|
bile salt export pump
|
|
AMPK
|
AMP-activated protein kinase
|
|
PH
|
partial hepatectomy
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
TBIL
|
total bilirubin
|
|
ALB
|
albumin
|
|
CRP
|
C-reactive protein
|
References
|
1
|
Ding BS, Cao Z, Lis R, Nolan DJ, Guo P,
Simons M, Penfold ME, Shido K, Rabbany SY and Rafii S: Divergent
angiocrine signals from vascular niche balance liver regeneration
and fibrosis. Nature. 505:97–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Willenbring H and Grompe M: A therapy for
liver failure found in the JNK yard. Cell. 153:283–284. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fausto N, Campbell JS and Riehle KJ: Liver
regeneration. Hepatology. 43 2 Suppl 1:S45–S53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wuestefeld T, Pesic M, Rudalska R, Dauch
D, Longerich T, Kang TW, Yevsa T, Heinzmann F, Hoenicke L, Hohmeyer
A, et al: A direct in vivo RNAi screen identifies MKK4 as a key
regulator of liver regeneration. Cell. 153:389–401. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taub R: Liver regeneration: From myth to
mechanism. Nat Rev Mol Cell Biol. 5:836–847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Michalopoulos GK: Principles of liver
regeneration and growth homeostasis. Compr Physiol. 3:485–513.
2013.PubMed/NCBI
|
|
7
|
He VJ: Professor Norbert Hüser: The
function of immune cells for liver regeneration after partial
hepatectomy. Hepatobiliary Surg Nutr. 3:52–54. 2014.PubMed/NCBI
|
|
8
|
Svegliati-Baroni G, Candelaresi C,
Saccomanno S, Ferretti G, Bachetti T, Marzioni M, De Minicis S,
Nobili L, Salzano R, Omenetti A, et al: A model of insulin
resistance and nonalcoholic steatohepatitis in rats: Role of
peroxisome proliferator-activated receptor-alpha and n-3
polyunsaturated fatty acid treatment on liver injury. Am J Pathol.
169:846–860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pradelli L, Mayer K, Muscaritoli M and
Heller AR: n-3 fatty acid-enriched parenteral nutrition regimens in
elective surgical and ICU patients: A meta-analysis. Crit Care.
16:R1842012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu D, Wakabayashi Y, Lippincott-Schwartz J
and Arias IM: Bile acid stimulates hepatocyte polarization through
a cAMP-Epac-MEK-LKB1-AMPK pathway. Proc Natl Acad Sci USA. 108:pp.
1403–1408. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
International Transporter Consortium, ;
Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X,
Dahlin A, Evers R, Fischer V, et al: Membrane transporters in drug
development. Nat Rev Drug Discov. 9:215–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woods A, Heslegrave AJ, Muckett PJ, Levene
AP, Clements M, Mobberley M, Ryder TA, Abu-Hayyeh S, Williamson C,
Goldin RD, et al: LKB1 is required for hepatic bile acid transport
and canalicular membrane integrity in mice. Biochem J. 434:49–60.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu D, Wakabayashi Y, Ido Y,
Lippincott-Schwartz J and Arias IM: Regulation of bile canalicular
network formation and maintenance by AMP-activated protein kinase
and LKB1. J Cell Sci. 123:3294–3302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Merlen G, Gentric G, Celton-Morizur S,
Foretz M, Guidotti JE, Fauveau V, Leclerc J, Viollet B and
Desdouets C: AMPKα1 controls hepatocyte proliferation independently
of energy balance by regulating Cyclin A2 expression. J Hepatol.
60:152–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Homolya L, Fu D, Sengupta P, Jarnik M,
Gillet JP, Vitale-Cross L, Gutkind JS, Lippincott-Schwartz J and
Arias IM: LKB1/AMPK and PKA control ABCB11 trafficking and
polarization in hepatocytes. PLoS One. 9:e919212014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stehr SN and Heller AR: Omega-3 fatty acid
effects on biochemical indices following cancer surgery. Clin Chim
Acta. 373:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu XH, Wu YF, Qiu YD, Jiang CP and Ding
YT: Liver-protecting effects of omega-3 fish oil lipid emulsion in
liver transplantation. World J Gastroenterol. 18:6141–6147. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan XP, Wang S, Yang Y and Qiu YD: Effects
of n-3 polyunsaturated fatty acids on rat livers after partial
hepatectomy via LKB1-AMPK signaling pathway. Transplant Proc.
43:pp. 3604–3612. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu YD, Wang S, Yang Y and Yan XP: Omega-3
polyunsaturated fatty acids promote liver regeneration after 90%
hepatectomy in rats. World J Gastroenterol. 18:3288–3295.
2012.PubMed/NCBI
|
|
20
|
Grosse B, Degrouard J, Jaillard D and
Cassio D: Build them up and break them down: Tight junctions of
cell lines expressing typical hepatocyte polarity with a varied
repertoire of claudins. Tissue Barriers. 1:e252102013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Son S, Kojima T, Decaens C, Yamaguchi H,
Ito T, Imamura M, Murata M, Tanaka S, Chiba H, Hirate K and Sawada
N: Knockdown of tight junction protein claudin-2 prevents bile
canalicular formation in WIF-B9 cells. Histochem Cell Biol.
131:411–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kojima T, Yamamoto T, Murata M, Chiba H,
Kokai Y and Sawada N: Regulation of the blood-biliary barrier:
Interaction between gap and tight junctions in hepatocytes. Med
Electron Microsc. 36:157–164. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JH, Koh H, Kim M, Kim Y, Lee SY,
Karess RE, Lee SH, Shong M, Kim JM, Kim J and Chung J:
Energy-dependent regulation of cell structure by AMP-activated
protein kinase. Nature. 447:1017–1020. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baas AF, Kuipers J, van der Wel NN, Batlle
E, Koerten HK, Peters PJ and Clevers HC: Complete polarization of
single intestinal epithelial cells upon activation of LKB1 by
STRAD. Cell. 116:457–466. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Li J, Young LH and Caplan MJ:
AMP-activated protein kinase regulates the assembly of epithelial
tight junctions. Proc Natl Acad Sci USA. 103:pp. 17272–17277. 2006;
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang YL, Guo H, Zhang CS, Lin SY, Yin Z,
Peng Y, Luo H, Shi Y, Lian G, Zhang C, et al: AMP as a low-energy
charge signal autonomously initiates assembly of AXIN-AMPK-LKB1
complex for AMPK activation. Cell Metab. 18:546–555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gowans GJ, Hawley SA, Ross FA and Hardie
DG: AMP is a true physiological regulator of AMP-activated protein
kinase by both allosteric activation and enhancing net
phosphorylation. Cell Metab. 18:556–566. 2013. View Article : Google Scholar : PubMed/NCBI
|