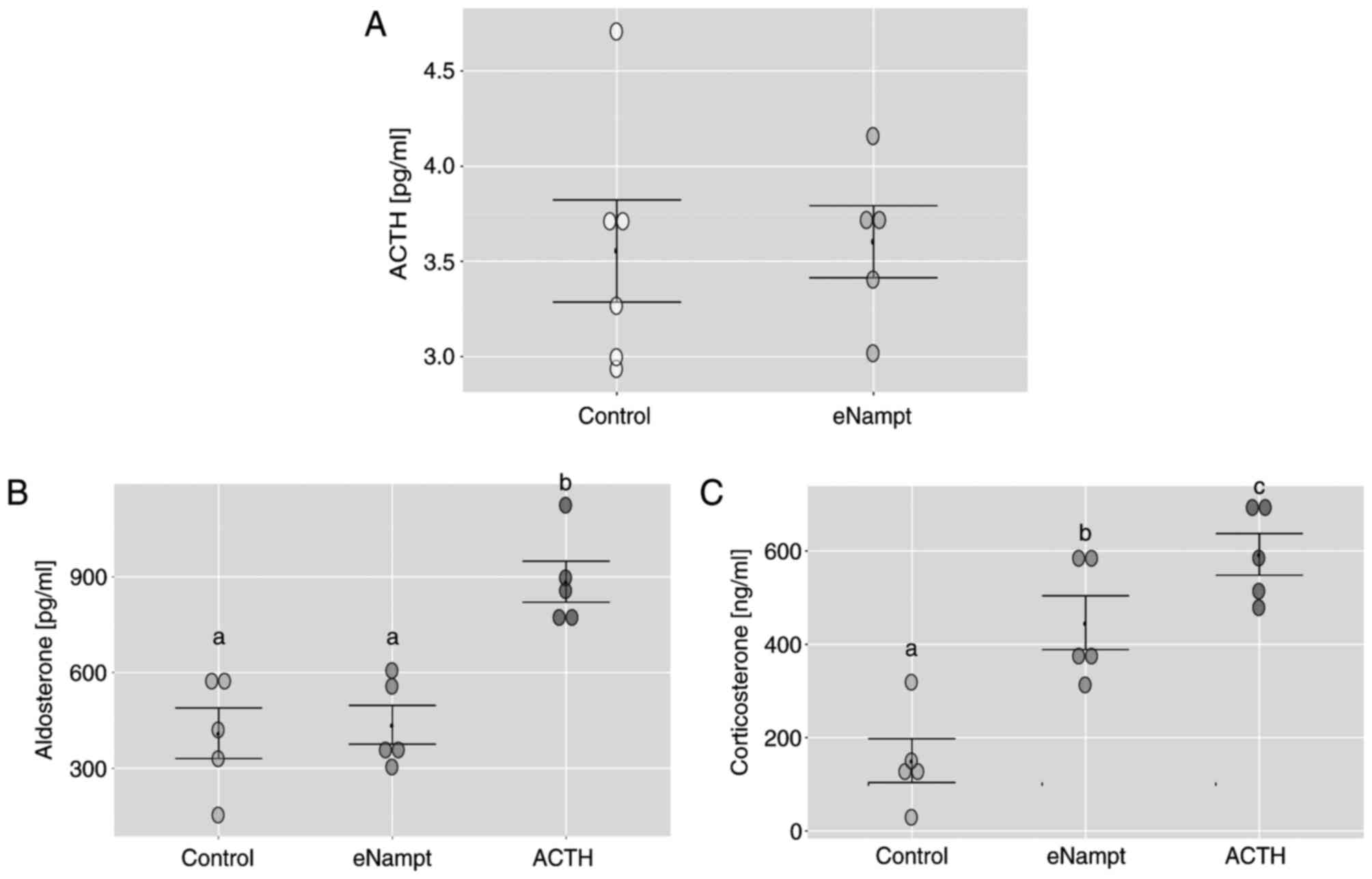
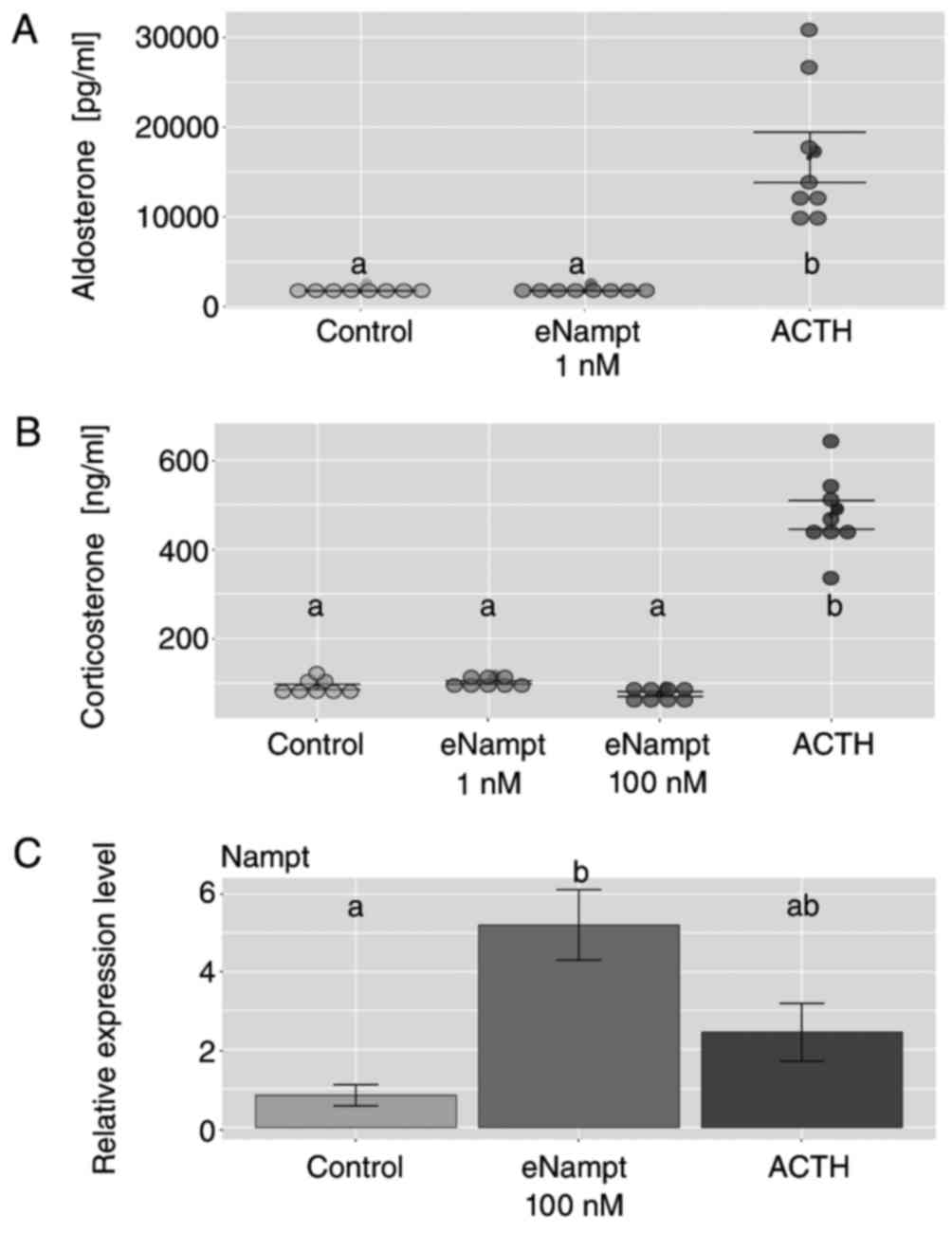
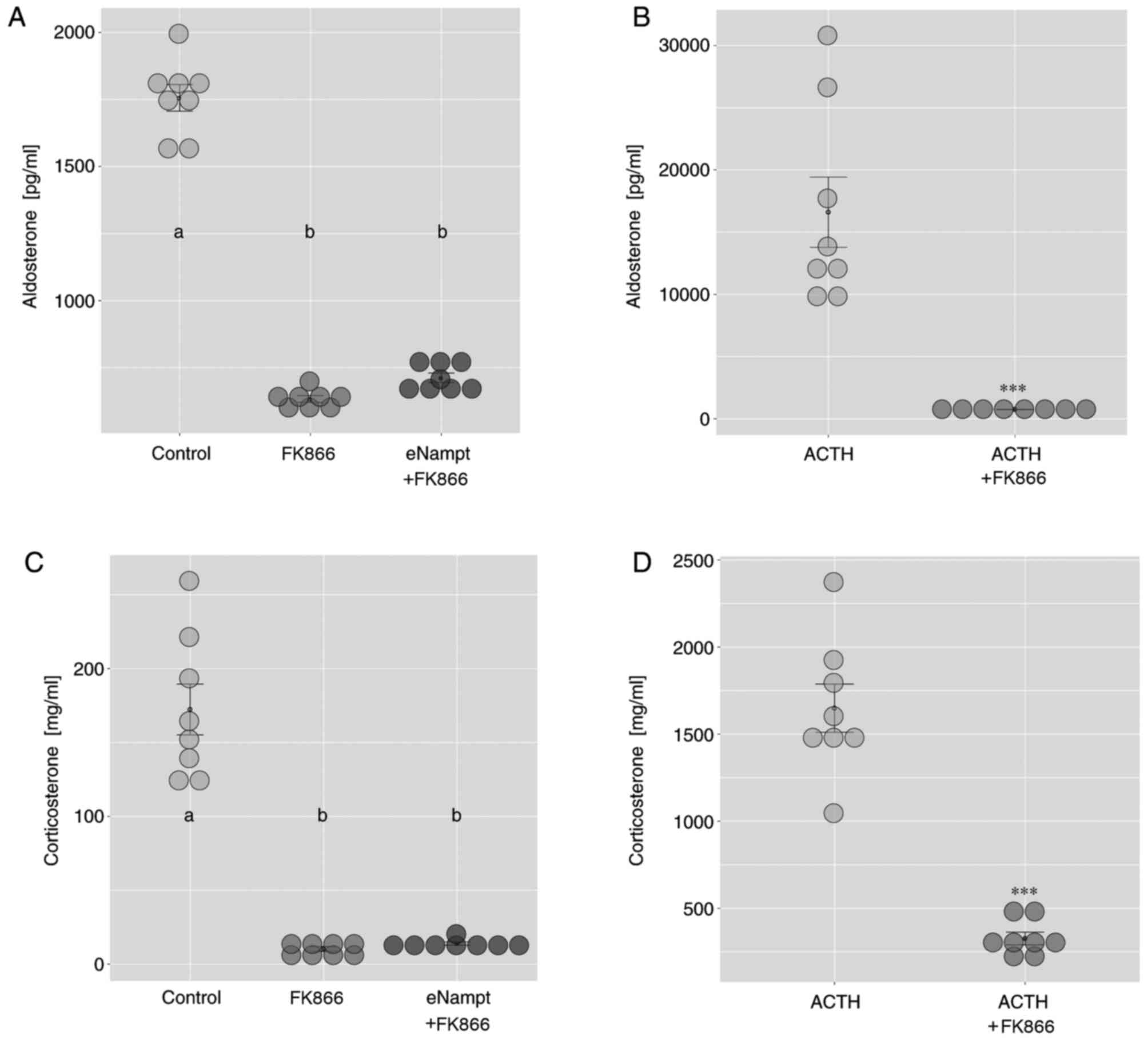
|
1
|
Samal B, Sun Y, Stearns G, Xie C, Suggs S
and McNiece I: Cloning and characterization of the cDNA encoding a
novel human pre-B-cell colony-enhancing factor. Mol Cell Biol.
14:1431–1437. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Araki T, Sasaki Y and Milbrandt J:
Increased nuclear NAD biosynthesis and SIRT1 activation prevent
axonal degeneration. Science. 305:1010–1013. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Revollo JR, Grimm AA and Imai S: The NAD
biosynthesis pathway mediated by nicotinamide
phosphoribosyltransferase regulates Sir2 activity in mammalian
cells. J Biol Chem. 279:50754–50763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bogan KL and Brenner C: Nicotinic acid,
nicotinamide, and nicotinamide riboside: A molecular evaluation of
NAD+ precursor vitamins in human nutrition. Annu Rev Nutr.
28:115–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Houtkooper RH, Cantó C, Wanders RJ and
Auwerx J: The secret life of NAD+: An old metabolite controlling
new metabolic signaling pathways. Endocr Rev. 31:194–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen H, Wang S, Zhang H, Nice EC and Huang
C: Nicotinamide phosphoribosyltransferase (Nampt) in
carcinogenesis: New clinical opportunities. Expert Rev Anticancer
Ther. 16:827–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoon MJ, Yoshida M, Johnson S, Takikawa A,
Usui I, Tobe K, Nakagawa T, Yoshino J and Imai S: SIRT1-mediated
eNAMPT secretion from adipose tissue regulates hypothalamic NAD+
and function in mice. Cell Metab. 21:706–717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka T and Nabeshima Y:
Nampt/PBEF/Visfatin: A new player in beta cell physiology and in
metabolic diseases? Cell Metab. 6:341–343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Revollo JR, Korner A, Mills KF, Satoh A,
Wang T, Garten A, Dasgupt B, Sasaki Y, Wolberger C, Townsend RR, et
al: Nampt/PBEF/Visfatin regulates insulin secretion in beta cells
as a systemic NAD biosynthetic enzyme. Cell Metab. 6:363–375. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hallschmid M, Randeva H, Tan BK, Kern W
and Lehnert H: Relationship between cerebrospinal fluid visfatin
(PBEF/Nampt) levels and adiposity in humans. Diabetes. 58:637–640.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brunetti L, Recinella L, Di Nisio C,
Chiavaroli A, Leone S, Ferrante C, Orlando G and Vacca M: Effects
of visfatin/PBEF/NAMPT on feeding behaviour and hypothalamic
neuromodulators in the rat. J Biol Regul Homeost Agents.
26:295–302. 2012.PubMed/NCBI
|
|
12
|
Iwen KA, Senyaman O, Schwartz A, Drenckhan
M, Meier B, Hadaschik D and Klein J: Melanocortin crosstalk with
adipose functions: ACTH directly induces insulin resistance,
promotes a pro-inflammatory adipokine profile and stimulates UCP-1
in adipocytes. J Endocrinol. 196:465–472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dammer EB and Sewer MB: Phosphorylation of
CtBP1 by cAMP-dependent protein kinase modulates induction of CYP17
by stimulating partnering of CtBP1 and 2. J Biol Chem.
283:6925–6934. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reverchon M, Rame C, Bunel A, Chen W,
Froment P and Dupont J: VISFATIN (NAMPT) improves In Vitro
IGF1-induced steroidogenesis and IGF1 receptor signaling through
SIRT1 in bovine granulosa cells. Biol Reprod. 94:542016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paschke L, Zemleduch T, Rucinski M,
Ziolkowska A, Szyszka M and Malendowicz LK: Adiponectin and
adiponectin receptor system in the rat adrenal gland: Ontogenetic
and physiologic regulation, and its involvement in regulating
adrenocortical growth and steroidogenesis. Peptides. 31:1715–1724.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trejter M, Hochol A, Tyczewska M,
Ziolkowska A, Jopek K, Szyszka M, Malendowicz LK and Rucinski M:
Visinin-like peptide 1 in adrenal gland of the rat. Gene expression
and its hormonal control. Peptides. 63:22–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hinson JP, Kapas S, Orford CD and Vinson
GP: Vasoactive intestinal peptide stimulation of aldosterone
secretion by the rat adrenal cortex may be mediated by the local
release of catecholamines. J Endocrinol. 133:253–258. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malendowicz LK, Nussdorfer GG, Warchol JB,
Markowska A, MacChi C, Nowak KW and Butowska W: Effects of
neuromedin-K on the rat hypothalamo-pituitary-adrenal axis.
Neuropeptides. 29:337–341. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malendowicz LK, Rebuffat P, Nussdorfer GG
and Nowak KW: Corticotropin-inhibiting peptide enhances aldosterone
secretion by dispersed rat zona glomerulosa cells. J Steroid
Biochem Mol Biol. 67:149–152. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rucinski M, Ziolkowska A, Szyszka M and
Malendowicz LK: Cerebellin and des-cerebellin exert ACTH-like
effects on corticosterone secretion and the intracellular signaling
pathway gene expression in cultured rat adrenocortical cells-DNA
microarray and QPCR studies. Int J Mol Med. 23:539–546.
2009.PubMed/NCBI
|
|
21
|
Benito-Martin A, Ucero AC, Izquierdo MC,
Santamaria B, Picaroste B, Carrasco S, Lorenzo O, Ruiz-Ortega M,
Egido J and Ortiz A: Endogenous NAMPT dampens chemokine expression
and apoptotic responses in stressed tubular cells. Biochim Biophys
Acta. 1842:293–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ziolkowska A, Rucinski M, Tyczewska M and
Malendowicz LK: Orexin B inhibits proliferation and stimulates
specialized function of cultured rat calvarial osteoblast-like
cells. Int J Mol Med. 22:749–755. 2008.PubMed/NCBI
|
|
23
|
Rucinski M, Ziolkowska A, Szyszka M,
Hochol A and Malendowicz LK: Evidence suggesting that ghrelin
O-acyl transferase inhibitor acts at the hypothalamus to inhibit
hypothalamo-pituitary-adrenocortical axis function in the rat.
Peptides. 35:149–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rucinski M, Albertin G, Spinazzi R,
Ziolkowska A, Nussdorfer GG and Malendowicz LK: Cerebellin in the
rat adrenal gland: Gene expression and effects of CER and
[des-Ser1]CER on the secretion and growth of cultured
adrenocortical cells. Int J Mol Med. 15:411–415. 2005.PubMed/NCBI
|
|
25
|
Rucinski M, Spinazzi R, Ziolkowska A,
Nussdorfer GG and Malendowicz LK: Effects of beacon on the rat
pituitary-adrenocortical axis response to stress. Int J Mol Med.
16:297–299. 2005.PubMed/NCBI
|
|
26
|
Rucinski M, Tortorella C, Ziolkowska A,
Nowak M, Nussdorfer GG and Malendowicz LK: Steroidogenic acute
regulatory protein gene expression, steroid-hormone secretion and
proliferative activity of adrenocortical cells in the presence of
proteasome inhibitors: In vivo studies on the regenerating rat
adrenal cortex. Int J Mol Med. 21:593–597. 2008.PubMed/NCBI
|
|
27
|
Tyczewska M, Rucinski M, Ziolkowska A,
Szyszka M, Trejter M, Hochol-Molenda A, Nowak KW and Malendowicz
LK: Enucleation-induced rat adrenal gland regeneration: Expression
profile of selected genes involved in control of adrenocortical
cell proliferation. Int J Endocrinol. 2014:1303592014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tyczewska M, Rucinski M, Ziolkowska A,
Trejter M, Szyszka M and Malendowicz LK: Expression of selected
genes involved in steroidogenesis in the course of
enucleation-induced rat adrenal regeneration. Int J Mol Med.
33:613–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jopek K, Celichowski P, Szyszka M,
Tyczewska M, Milecka P, Malendowicz LK and Rucinski M:
Transcriptome Profile of rat adrenal evoked by gonadectomy and
testosterone or estradiol replacement. Front Endocrinol (Lausanne).
8:262017.PubMed/NCBI
|
|
30
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT–PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Owens K, Park JH, Schuh R and Kristian T:
Mitochondrial dysfunction and NAD(+) metabolism alterations in the
pathophysiology of acute brain injury. Transl Stroke Res.
4:618–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang XQ, Lu JT, Jiang WX, Lu YB, Wu M,
Wei EQ, Zhang WP and Tang C: NAMPT inhibitor and metabolite protect
mouse brain from cryoinjury through distinct mechanisms.
Neuroscience. 291:230–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
López FJ and Negro-Vilar A: Estimation of
endogenous adrenocorticotropin half-life using pulsatility
patterns: A physiological approach to the evaluation of secretory
episodes. Endocrinology. 123:740–746. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kleine Bernhard RWG: Hormones and the
Endocrine System. Springer; New York, NY: 2016, View Article : Google Scholar
|
|
35
|
Dampney RA and Horiuchi J: Functional
organisation of central cardiovascular pathways: Studies using
c-fos gene expression. Prog Neurobiol. 71:359–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoffman GE, Smith MS and Verbalis JG:
c-Fos and related immediate early gene products as markers of
activity in neuroendocrine systems. Front Neuroendocrinol.
14:173–213. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hasmann M and Schemainda I: FK866, a
highly specific noncompetitive inhibitor of nicotinamide
phosphoribosyltransferase, represents a novel mechanism for
induction of tumor cell apoptosis. Cancer Res. 63:7436–7442.
2003.PubMed/NCBI
|
|
38
|
Hara N, Yamada K, Shibata T, Osago H and
Tsuchiya M: Nicotinamide phosphoribosyltransferase/visfatin does
not catalyze nicotinamide mononucleotide formation in blood plasma.
PLoS One. 6:e227812011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ratajczak J, Joffraud M, Trammell SA, Ras
R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud
ME, et al: NRK1 controls nicotinamide mononucleotide and
nicotinamide riboside metabolism in mammalian cells. Nat Commun.
7:131032017. View Article : Google Scholar
|
|
40
|
Carbone F, Liberale L, Bonaventura A,
Vecchie A, Casula M, Cea M, Monacelli F, Caffa I, Bruzzone S,
Montecucco F and Nencioni A: Regulation and function of
extracellular nicotinamide phosphoribosyltransferase/visfatin.
Compr Physiol. 7:603–621. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dupré SM, Burt DW, Talbot R, Downing A,
Mouzaki D, Waddington D, Malpaux B, Davis JR, Lincoln GA and Loudon
AS: Identification of melatonin-regulated genes in the ovine
pituitary pars tuberalis, a target site for seasonal hormone
control. Endocrinology. 149:5527–5539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
West A, Dupre SM, Yu L, Paton IR,
Miedzinska K, McNeilly AS, Davis JR, Burt DW and Loundon AS: Npas4
is activated by melatonin, and drives the clock gene Cry1 in the
ovine pars tuberalis. Mol Endocrinol. 27:979–989. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morgan PJ and Williams LM: The pars
tuberalis of the pituitary: A gateway for neuroendocrine output.
Rev Reprod. 1:153–161. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Reverchon M, Cornuau M, Cloix L, Rame C,
Guerif F, Royere D and Dupont J: Visfatin is expressed in human
granulosa cells: Regulation by metformin through AMPK/SIRT1
pathways and its role in steroidogenesis. Mol Hum Reprod.
19:313–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Diot M, Reverchon M, Rame C, Baumard Y and
Dupont J: Expression and effect of NAMPT (visfatin) on progesterone
secretion in hen granulosa cells. Reproduction. 150:53–63. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Diot M, Reverchon M, Rame C, Froment P,
Brillard JP, Brière S, Levêque G, Guillaume D and Dupont J:
Expression of adiponectin, chemerin and visfatin in plasma and
different tissues during a laying season in turkeys. Reprod Biol
Endocrinol. 13:812015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ocón-Grove OM, Krzysik-Walker SM,
Maddineni SR, Hendricks GL III and Ramachandran R: NAMPT (visfatin)
in the chicken testis: Influence of sexual maturation on cellular
localization, plasma levels and gene and protein expression.
Reproduction. 139:217–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kaur J, Ramanjaneya M, Chen J and Randeva
H: The adipokine, Pre-B cell colony enhancing factor
(PBEF)/visfatin, activates steroidogenic acute regulatory protein
(StAR) protein expression and steroid production in human
adrenocortical-H295R-cells via MAPK and PI3/AkT signalling
pathways. Endocrine Abstracts. 19:3142009.
|
|
49
|
Payne AH and Hales DB: Overview of
steroidogenic enzymes in the pathway from cholesterol to active
steroid hormones. Endocr Rev. 25:947–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hanukoglu I and Rapoport R: Routes and
regulation of NADPH production in steroidogenic mitochondria.
Endocr Res. 21:231–241. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roucher-Boulez F, Mallet-Motak D,
Samara-Boustani D, Jilani H, Ladjouze A, Souchon PF, Simon D, Nivot
S, Heinrichs C, Ronze M, et al: NNT mutations: A cause of primary
adrenal insufficiency, oxidative stress and extra-adrenal defects.
Eur J Endocrinol. 175:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Weinberg-Shukron A, Abu-Libdeh A, Zhadeh
F, Carmel L, Kogot-Levin A, Kamal L, Kanaan M, Zeligson S, Renbaum
P, Levy-Lahad E, et al: Combined mineralocorticoid and
glucocorticoid deficiency is caused by a novel founder nicotinamide
nucleotide transhydrogenase mutation that alters mitochondrial
morphology and increases oxidative stress. J Med Genet. 52:636–641.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Meimaridou E, Goldsworthy MG, Chortis V,
Fragoulis T, Foster PA, Arlt W, Cox RD and Metherell LA: OR24-2:
Role of Nicotinamide Nucleotide Transhydrogenase in the Control of
Steroidogenesis in Mouse Adrenals. Proceedings of Endocrine
Society's 98th Annual Meeting and Expo. Boston. 2016;
|
|
54
|
Buldak RJ, Gowarzewski M, Buldak L,
Skonieczna M, Kukla M, Polaniak R and Zwirska-Korczala K: Viability
and oxidative response of human colorectal HCT-116 cancer cells
treated with visfatin/eNampt in vitro. J Physiol Pharmacol.
66:557–566. 2015.PubMed/NCBI
|
|
55
|
Oita RC, Ferdinando D, Wilson S, Bunce C
and Mazzatti DJ: Visfatin induces oxidative stress in
differentiated C2C12 myotubes in an Akt- and MAPK-independent,
NFkB-dependent manner. Pflugers Arch. 459:619–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Song SY, Jung EC, Bae CH, Choi YS and Kim
YD: Visfatin induces MUC8 and MUC5B expression via p38
MAPK/ROS/NF-κB in human airway epithelial cells. J Biomed Sci.
21:492014. View Article : Google Scholar : PubMed/NCBI
|