Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic
disorder, and its prevalence significantly increases with age
(1,2). Hyperglycemia is a prominent
characteristic of T2DM, which results in secondary
pathophysiological alterations in multiple organs, including the
heart, lung and liver (3–5). Approximately 70% of patients with
T2DM have fatty liver and exhibit a more severe course of liver
fibrosis (6).
Hepatic fibrosis is characterized by the aberrant
production and deposition of extracellular matrix (ECM) proteins,
which leads to hepatic parenchyma and liver structure damage
(7). Epithelial-to-mesenchymal
transition (EMT) has been described as a principal mechanism for
the deposition of ECM in liver, renal and pulmonary fibrosis injury
models (8,9). Furthermore, adult hepatocytes serve a
key role in the process of hepatic fibrogenesis via EMT (10,11).
EMT is a dynamic biological process that induces epithelial cells
to lose their typical properties and acquire the phenotypic traits
of mesenchymal cell or fibroblast markers. EMT contributes to
tissue repair, in addition to adversely causing organ fibrosis,
including that of the kidney (12,13).
Of the multiple stimuli involved in liver fibrosis,
metastasis-associated protein MTA3 (MTA3) represses the
transcription of Snail family transcriptional repressor 1 (Snail),
an E-cadherin (E-cad) transcription activator by binding to its
promoter region, although a Snail/MTA3 complex has not yet been
observed (14,15). Further studies on the effects of
MTA3 on EMT will assist in identifying novel therapeutic targets
for liver fibrosis.
MicroRNAs (miRNAs/miRs) are endogenous, non-coding,
single-stranded RNAs that mediate post transcriptional repression
through complementary nucleotide sequence-specific binding to
3′-untranslated regions (3′-UTRs) of target mRNA, resulting in the
degradation or inhibition of translation, and in some cases the
destruction of the target mRNA (16,17).
miRNAs participate in a wide range of pathophysiological processes,
including cell proliferation, apoptosis, development and
differentiation (18–21). Previous studies have demonstrated
that dysregulated miRNA expression and function are essential
components in the development and progression of liver fibrosis.
For example, the inhibition of miR-21 ameliorates liver fibrosis in
schistosoma japonicum infections (22). Furthermore, miRNAs have been
demonstrated to be involved in the EMT. For example, downregulation
of miR-26a induces EMT by directly targeting high mobility group
protein HMGI-C (4). Based on these
observations, miRNAs may describe novel drug targets in liver
fibrosis diagnosis, prevention and therapy.
Previous evidence has suggested that miR-32
regulates phosphatase and tensin homolog (PTEN) expression and
serves a critical role in cell proliferation, migration and
invasion in hepatocellular carcinoma and colorectal carcinoma
(23,24). miR-32 negatively regulates mothers
against decapentaplegic homology (Smad)7 expression and contributes
to peribiliary fibrosis caused by Clonorchis sinensis
infection (18). However, the
detailed role of miR-32 in EMT, specifically in liver fibrosis,
remains unknown. The present study was designed to investigate
miR-32 expression under hyperglycemic conditions and evaluate its
role in high glucose (HG)-induced liver fibrosis. The underlying
mechanisms responsible for fibrosis and progression inhibition were
assessed in the present study, and miR-32 and MTA3 were identified
as potential therapeutic targets in liver fibrosis treatment.
Materials and methods
Establishment of a diabetic model
In total, 20 healthy 5-month-old male Wistar rats
(180–220 g) were obtained from the Experimental Animal Center of
Harbin Medical University (Harbin, China) and subjected to a 12/12
h light-dark cycle with standard animal room conditions
(temperature, 22±1°C; humidity, 55±5%), with food and water
available ad libitum. Rats were randomly divided into
control and diabetic model (T2DM) groups. Non-diabetic rats were
fed a standard diet. Rats with T2DM were gavaged with a high-fat
diet (2 ml/day), which contained lard (20%), cholesterol (5%),
sucrose (5%), glucose (5%) and salt (6%) emulsified in 20% Tween-80
with 30% propylene glycol in distilled water. After 2 weeks,
diabetic rats were intraperitoneally injected with 35 mg/kg/day
streptozotocin (STZ; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
in 0.1 M citrate buffer (pH 4.3) for 3 days, once a day. Following
this, fasting blood glucose (FBG) levels were measured. FBG>16.7
nmol/l was considered to indicate a successfully established
diabetic model.
Histopathological and morphometric
analyses
Histopathological alterations and collagen
distribution were evaluated by hematoxylin and eosin (H&E) and
Masson's trichrome staining. The livers of rats in the control and
T2DM groups were quickly excised and fixed in 4% paraformaldehyde
for 24 h at 4°C ad subsequently embedded in paraffin. The samples
were cut into 5-µm-thick cross-sections and stained with H&E
and Masson's trichrome reagent to assess the degree of fibrosis, as
previously described (15,25). The degree of fibrosis was
quantified with ImagePro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA).
Cell culture and transfection
AML12 cells were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China) and
cultured in a 1:1 mixture of Dulbecco's modified Eagle's
medium/Ham's F-12 medium (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), containing 5 µg/ml pre-mixed ITS (insulin,
transferrin and sodium selenite; Sigma-Aldrich; Merck KGaA), 40
ng/ml dexamethasone (Sigma-Aldrich; Merck KGaA) and 10% fetal
bovine serum (HyClone; GE Healthcare Life Sciences). The AML12
cells were maintained at 37°C with 5% CO2 and 95% air.
When the culture reached 60% confluence, following culturing in
serum-free medium for 6 h, the cells were transiently transfected
with miRNA-32 mimics (5′-UAUUGCACAUUACUAAGUUGCA-3′), anti-miRNA
oligonucleotide (AMO)-32 (5′-UGCAACUUAGUAAUGUGCAAUA-3′) or negative
control (5′-UUUGUACUACACAAAAGUACUG-3′) (NC; Guangzhou RiboBio Co.,
Ltd., Guangzhou, China) at a concentration of 100 nM. X-tremeGENE
siRNA Transfection reagent (cat. no. 04476093001; Roche Diagnostics
GmbH, Mannheim, Germany) was used as a transfection vehicle.
MTA3-overexpressing pcDNA3.1 (100 nM; IBSBIO,
Shanghai, China) and the NC (100 nM; empty pcDNA3.1 plasmid) were
transfected into AML12 cells using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's instructions.
Following incubation for 6 h, the culture medium was
replaced with fresh medium, as described above, low glucose (LG;
1,000 mg/l) or HG (6,000 mg/l) medium for 24 h at 37°C. Protein
and/or RNA was subsequently extracted from cells for further
experimentation.
Identification of target gene
The potential target genes of miR-32 were predicted
by TargetScanHuman Release 7.2 (http://www.targetscan.org/vert_72/), miRanda.org (http://miranda.org.uk/) and miRDB (http://www.mirdb.org/).
Luciferase reporter assays
Luciferase reporters containing the wild-type or
mutated 3′-UTR of MTA3 were constructed by using psi-CHECK2 vectors
(Promega Corporation, Madison, WI, USA). When the culture reached
90% confluence, the 293T (Cell Bank of Chinese Academy of Sciences,
Shanghai, China) cells were seeded in a 24-well plate. When the
293T cells reached 60–70% confluence in a 24-well plate, the 3′-UTR
luciferase vectors (100 mg) were co-transfected with miR-32 mimics,
AMO-32 or NC using Lipofectamine® 2000 reagent,
according to the manufacturer's protocol. Renilla luciferase
reporters (10 ng) were used as an internal control. Following 48 h
of transfection, luciferase activity was examined using the
Dual-Luciferase Reporter assay system (Promega Corporation),
according to the manufacturer's protocol.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from rat liver tissues or from AML12 cells
was lysed using 1 ml TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The extracted RNA was reverse transcribed into cDNA using a
High-Capacity cDNA RT kit (cat. no. 4368814; Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The plates were incubated for 15 min at 16°C, 1 h at
37°C, 5 min at 85°C and finally maintained at 4°C. A SYBR Green PCR
Master Mix kit (cat. no. 4309155; Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to quantify the relative levels of
E-cad, α-smooth muscle actin (SMA), vimentin, MTA3, Snail and
miR-32. GAPDH or U6 were used as an internal control. The cDNA
samples were amplified in 96-well plates for 10 min at 95°C,
followed by 40 cycles of 15 sec at 95°C, 30 sec at 60°C and 30 sec
at 72°C and finally maintained at 4°C. The relative expression of
the miRNA and mRNA were determined by the Cq (2−∆∆Cq)
method (26). qPCR was performed
on a ABI 7500 FAST Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The sequences of the primers used are
presented in Table I.
 | Table I.Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Species | Direction | Sequence
(5′-3′) |
|---|
| Collagen-1 | Mouse | F |
GAGCGGAGAGTACTGGATCG |
|
|
| R |
TACTCGAACGGGAATCCATC |
|
| Rat | F |
CAGCCCAAAGTGTGTGAGAA |
|
|
| R |
TGTGATGTTGGCCGTGTTAT |
| E-cadherin | Mouse | F |
CAAGGACAGCCTTCTTTTCG |
|
|
| R |
AGCTCTGGGTTGGATTCAGA |
|
| Rat | F |
TCGGAGCATGTGAAGAACAG |
|
|
| R |
TGGCAGAACTGCATATTTCG |
| α-SMA | Mouse, rat | F |
CCACCGCAAATGCTTCTAAGT |
|
|
| R |
GGCAGGAATGATTTGGAAAGG |
| Vimentin | Mouse | F |
GATCAGCTCACCAACGACAA |
|
|
| R |
GGATTCCACTTTCCGTTCAA |
|
| Rat | F |
TCAGCTCACCAATGACAAGG |
|
|
| R |
GCTCCTGGATCTCTTCATCG |
| MTA3 | Mouse | F |
GGATTTGGCATATGTCCCTA |
|
|
| R |
ATATGGCTGAGCCGAAGAGA |
|
| Rat | F |
CATTGGTCTATGACCCCTCATTG |
|
|
| R |
GTCGATCCGTAAGTGGGCTAT |
| Snail | Mouse | F |
CTTGTGTCTGCACGACCTGT |
|
|
| R |
CTTCACATCCGAGTGGGGTTT |
|
| Rat | F |
TGCACATCCGAAGCCACA |
|
|
| R |
TCTTCACATCCGAGTGGGTCTG |
| GAPDH | Mouse, rat | F |
AAGAAGGTGGTGAAGCAGGC |
|
|
| R |
TCCACCACCCAGTTGCTGTA |
| miR-32 | Mouse, rat | F |
GCCACGCTATTGCACATTACTA |
|
|
| R |
TATCCAGTGCGTGTCGTGGAGT |
| U6 | Mouse, rat | F |
GCTTCGGCAGCACATATACTAAAAT |
|
|
| R |
CGCTTCACGAATTTGCGTGTCAT |
Western blotting
Protein samples were obtained from liver tissues and
AML12 cells using radioimmunoprecipitation assay (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) lysis buffer
supplemented with protease inhibitors. Following centrifugation at
12,000 × g for 15 min at 4°C, the supernatant was collected and
quantified using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology, Haimen, China). For the western blot
analysis, 100 µg each protein sample was separated by SDS-PAGE (10%
gels), transferred to nitrocellulose membranes and blocked for 2 h
with 5% non-fat milk at room temperature. Subsequently, the samples
were incubated at 4°C overnight with primary antibodies against
E-cad (1:1,000; cat. no. ab76055; Abcam, Cambridge, MA, USA),
vimentin (1:1,000; cat. no. 7431; Cell Signaling Technology, Inc.,
Danvers, MA, USA), α-SMA (1:100; cat. no. ab7817; Abcam), MTA3
(1:1,000; cat. no. ab176346; Abcam), Snail (1:500; cat. no.
ab82846; Abcam), GAPDH (1:1,000; cat. no. TA-08; ZhongShanJinQiao,
Inc., Beijing, China) and collagen-1 (Col-1; 1:1,000, cat. no.
ab34710; Abcam) in PBS. Membranes were incubated with a
fluorescence-conjugated anti-rabbit immunoglobulin G secondary
antibody (1:10,000; cat. no. 926-32211; LI-COR Biosciences,
Lincoln, NE, USA) at room temperature for 1 h. The immunoreactivity
were detected and quantified using an Odyssey Infrared Imaging
System (LI-COR Biosciences) with Odyssey Software (LI-COR
Biosciences; version 3.0).
Immunofluorescence staining
For immunofluorescence staining, AML12 cells were
fixed with 4% paraformaldehyde in PBS for 30 min at room
temperature and then treated with 1% bovine serum albumin (cat. no.
A-9647; Sigma-Aldrich; Merck KGaA) and 0.4% Triton X-100 (Beijing
Solarbio Science & Technology Co., Ltd.) in PBS at room
temperature for 2 h. Following blocking with 10% goat serum (cat.
no. AR0009; Wuhan Boster Biological Technology Ltd., Wuhan, China),
the cells were incubated with primary antibodies against E-cad
(1:250; cat. no. ab76055; Abcam) and vimentin (1:100; cat. no.
7431; Cell Signaling Technology, Inc.) in PBS overnight at 4°C. A
secondary antibody conjugated with Alexa Fluor 594 (1:500; cat. no.
A-11032; Invitrogen; Thermo Fisher Scientific, Inc.) in PBS was
added and incubated with the cells for 1 h at 37°C. Nuclei were
stained using DAPI (1:500; Beyotime Institute of Biotechnology) for
20 min at room temperature. Immunofluorescence was examined using a
confocal laser-scanning microscope (magnification, ×200; Olympus
Corporation, Tokyo, Japan).
Measurement of collagen content
Total collagen content was detected with a Biocolor
Collagen Assay kit (cat. no. S1000; Biocolor Ltd., Carrickfergus,
UK) according to the manufacturer's protocol. Briefly, lysates (100
µl) were collected from AML12 cells under HG conditions after 24 h.
Sircol dye reagent (1 ml) binding to collagen was added to each
sample and the solution was mixed at 4°C for 30 min. Following
centrifugation, 1 ml of the alkali reagent was added to each tube
to dissolve the sediment. Subsequently, 200 µl of the sample was
transferred to a each well of a 96-well plate to measure the
absorbance at 540 nm. Total collagen (mg) was calculated using a
linear calibration curve generated from standards and normalized to
the total protein amount (mg) in each lysate.
Cell viability assay
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used for the detection of
cell viability. AML12 cells (60% confluence) were cultured with HG
for 24 h. Subsequently, 10% CCK-8 solution was added to the cell
culture medium and incubated for 1 h. The optical density was
determined at 450 nm on a microplate reader and viability rates
were calculated.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Each experiment was replicated three times independently.
Differences between groups were analyzed by one-way analysis of
variance followed by Tukey's multiple-comparisons test. Comparisons
between two groups were performed using Student's t-test.
Statistical analysis was performed using GraphPad Prism version 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Epithelial-mesenchymal transition
participates in liver fibrosis induced by hyperglycemia
A T2DM rat model was established, accompanied by
hyperglycemia. Rats with T2DM exhibited significantly increased
blood glucose levels, water intake, blood triglycerides (TG), total
cholesterol (TC) and low-density lipoprotein (LDL), as well as
decreased body weight and high-density lipoprotein (HDL; Table II). These markers indicated that
the T2DM rat model was successfully established in the present
study, similar to a previous study (4). It was observed that Col-1 protein and
mRNA levels were significantly increased in the T2DM group
(Fig. 1A and B). H&E and
Masson's trichrome staining of the liver tissues revealed a
broadened hepatic portal area, infiltrative inflammatory cells
fibers spreading from the portal area and periportal fibrosis in
the T2DM group (Fig. 1C). These
findings indicated that hyperglycemia induced liver fibrosis, as
previously described (6).
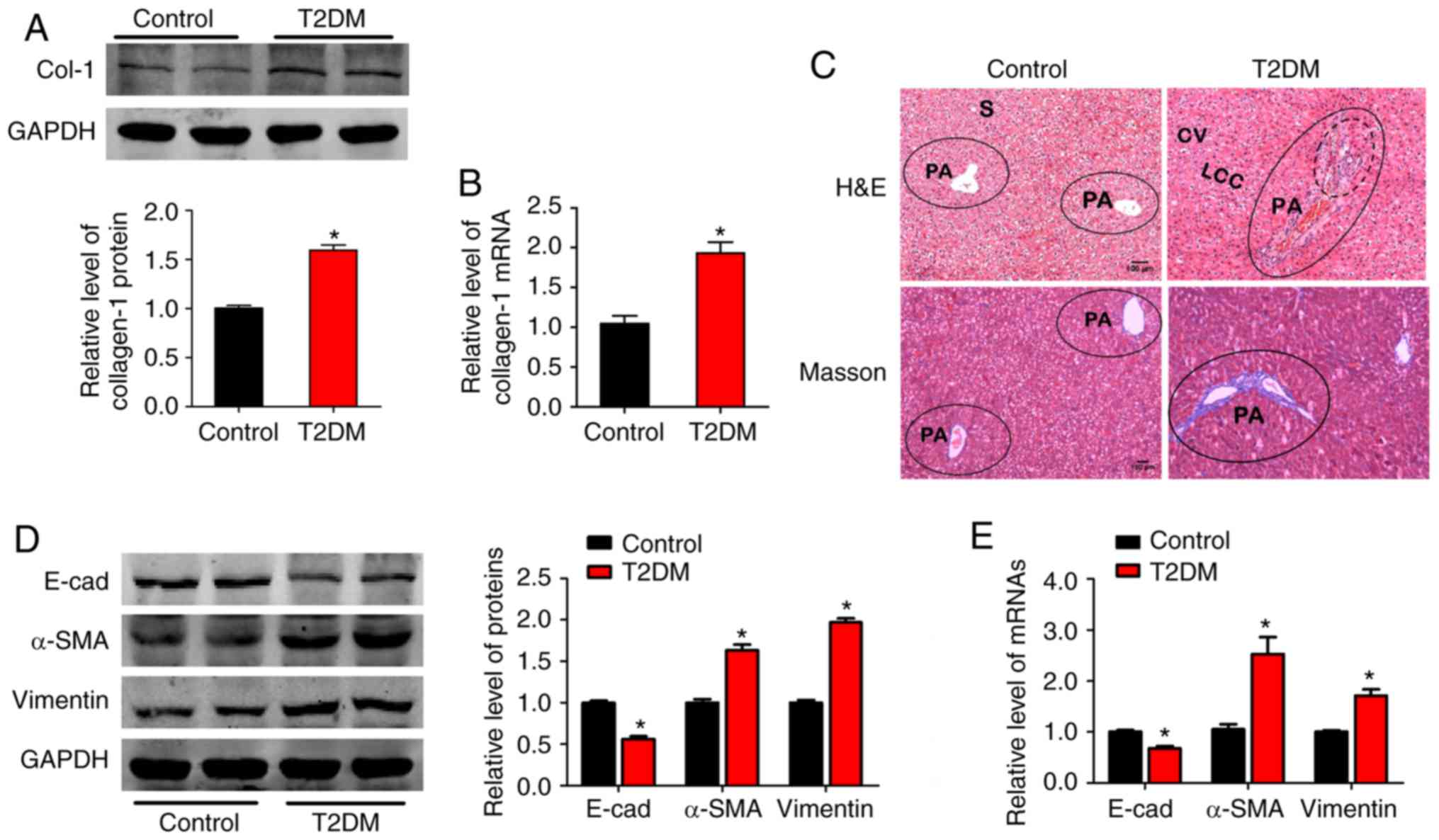 | Figure 1.Epithelial-mesenchymal transition
participates in liver fibrosis induced by hyperglycemia. (A)
Relative Col-1 protein and (B) mRNA expression in liver tissues of
the control and T2DM rats (n=5/group). (C) Histology was assessed
by H&E staining and fibrillar collagen deposition was assessed
by Masson's trichrome staining (magnification, ×100). Dotted lines
denote infiltration of inflammatory cells, solid lines and blue
color indicates tissue fibrosis in the portal area (n=5/group). (D)
Effects of hyperglycemia on expression of epithelial marker E-cad,
mesenchymal markers α-SMA and vimentin by western blot and (E)
reverse transcription-quantitative polymerase chain reaction
(n=5/group). GAPDH was used as loading control. *P<0.05 vs.
control group. Col-1, collagen-1; T2DM, type 2 diabetes mellitus;
CV, central vein; LCC, hepatic cell cords; S, hepatic sinusoid; PA,
portal area; E-cad, E-cadherin; α-SMA, α-smooth muscle actin;
H&E, hematoxylin and eosin. |
 | Table II.Rat characteristics in the control
and diabetic groups, indicating the successfully established
diabetic rat model. |
Table II.
Rat characteristics in the control
and diabetic groups, indicating the successfully established
diabetic rat model.
| Parameter | Control | Diabetic model |
|---|
| Food intake, g | 20.9±0.1 | 22.3±0.2 |
| Water intake,
ml | 68.2±1.0 |
122.0±0.4a |
| Body weight, g | 288.2±8.6 |
226.1±3.2a |
| Blood glucose,
mmHg | 7.0±0.2 |
16.2±0.3a |
| TC, mmol/l | 3.4±0.1 |
10.0±0.4a |
| TG, mmol/l | 0.8±0.1 |
5.2±0.6a |
| LDL, mmol/l | 1.7±0.1 |
4.2±0.1a |
| HDL, mmol/l | 1.0±0.1 |
0.6±0.1a |
To evaluate whether EMT was involved in liver
fibrosis, alterations in EMT marker expression were detected.
E-cad, an epithelial marker, was markedly decreased. However,
mesenchymal markers, including α-SMA and vimentin, increased in
expression at the protein and mRNA level in rats with T2DM,
compared with the control group (Fig.
1D and E). These results indicated that mesenchymal phenotypes
may be involved in liver fibrosis induced by hyperglycemia.
HG induces the epithelial-mesenchymal
transition in AML12 cells
To investigate the effects of HG on EMT in AML12
cells, Col-1, E-cad, α-SMA and vimentin protein (Fig. 2A) and mRNA (Fig. 2B) expression was evaluated. It was
observed that Col-1, α-SMA and vimentin expression was
significantly upregulated in the HG group, whereas E-cad expression
was markedly downregulated, compared with the LG and control groups
(Fig. 2A and B). Collagen content
was significantly increased in HG-treated AML12 cells (Fig. S1). Normal AML12 cells exhibited a
typical epithelial phenotype with a polygonal morphology and tight
arrangement. However, exposure to HG for 24 h decreased E-cad
(Fig. 2C) and increased vimentin
(Fig. 2D) protein expression,
compared with the control group, as determined by
immunofluorescence. These alterations in E-cad and vimentin
expression were in line with the aforementioned results.
Hyperglycemic culture exposes cells to a hypertonic
microenvironment (27), and cell
viability under these conditions was assessed by CCK8 assays. As
presented in Fig. S2, HG
decreased cell viability compared with the control. These data
suggested that HG may have induced EMT in AML12 cells.
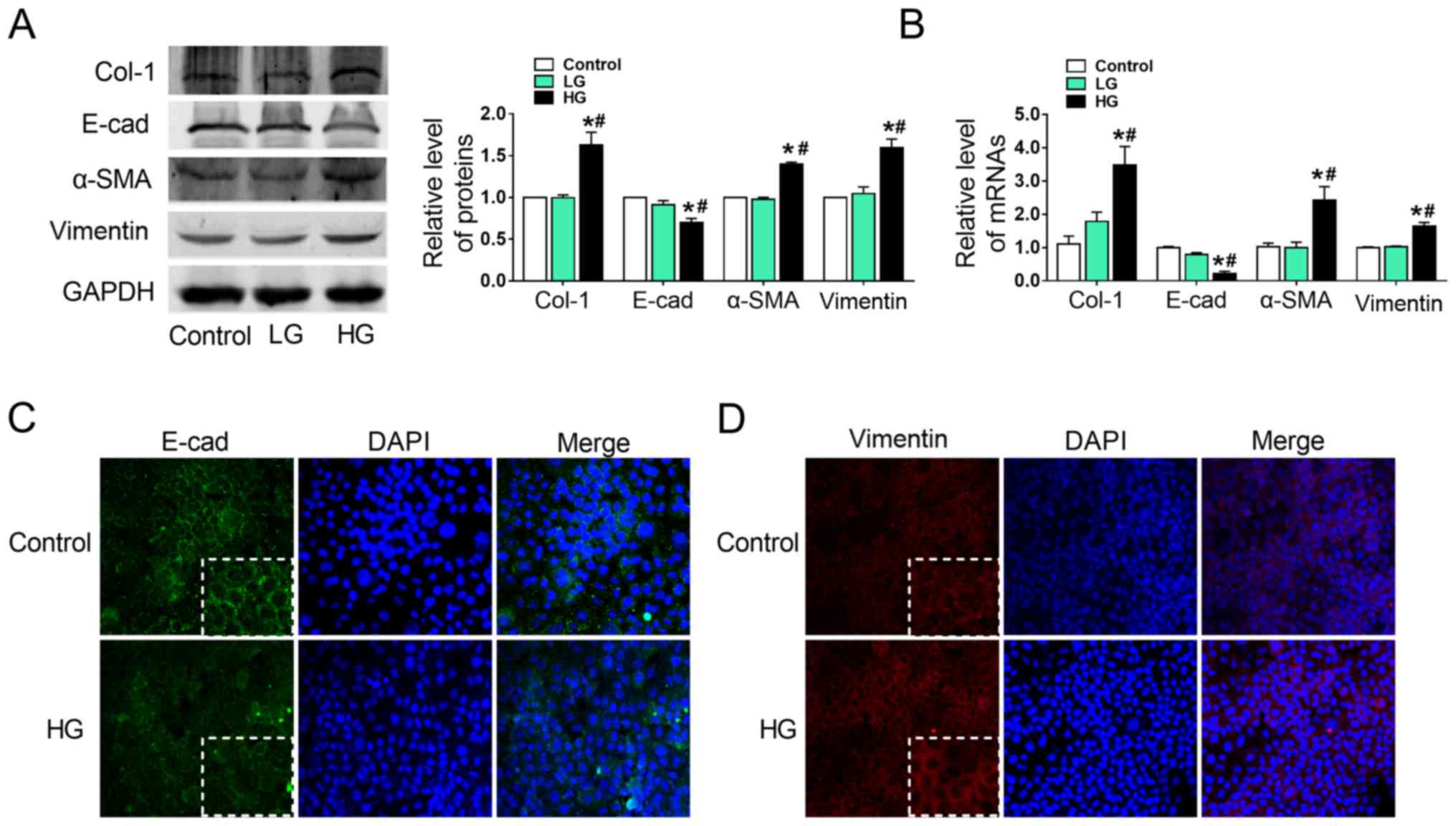 | Figure 2.HG induces epithelial-mesenchymal
transition in AML12 cells. (A) Alterations in Col-1, E-cad, α-SMA
and vimentin protein expression in HG-treated AML12 cells was
detected by western blotting, with representative blots on the left
and relative quantification analysis on the right. (B) Relative
mRNA expression of Col-1, E-cad, α-SMA and vimentin in HG-treated
AML12 cells. GAPDH was used as an internal control. (C and D)
Immunofluorescence images showing the location of EMT markers E-cad
and vimentin in the control and HG-treated groups, with DAPI
nuclear staining in blue, (C) E-cad in green and (D) vimentin in
red (magnification, ×200). *P<0.05 vs. control;
#P<0.05 vs. LG groups. E-cad, E-cadherin; α-SMA,
α-smooth muscle actin; Col-1, collagen-1; HG, high glucose (6,000
mg/l); LG, low glucose (1,000 mg/l). |
miR-32 overexpression contributes to
liver fibrosis progression and metastasis in AML12 cells
As the role of miR-32 and EMT in liver fibrosis
remains unclear, miR-32 expression in liver tissue obtained from
the T2DM group and in HG-treated AML12 cells was assessed by
RT-qPCR. The results demonstrated that miR-32 was significantly
upregulated in the liver tissue of the T2DM group (Fig. 3A) and in HG-treated AML12 cells
(Fig. 3B), compared with the
corresponding control groups. To investigate whether miR-32 was
associated with EMT in liver fibrosis, cells were transfected with
miR-32 mimics or AMO-32. Transfection efficiency was confirmed by
RT-qPCR (Fig. 3C). It was observed
that overexpression of miR-32 increased the protein (Fig. 3D) and mRNA (Fig. 3E) expression of Col-1, α-SMA and
vimentin, and markedly downregulated E-cad expression (Fig. 3D and E). Additionally, collagen
content was significantly increased following overexpression of
miR-32 (Fig. S1). These results
were supported and confirmed by immunofluorescent staining analysis
for E-cad (Fig. 3F) and vimentin
(Fig. 3G).
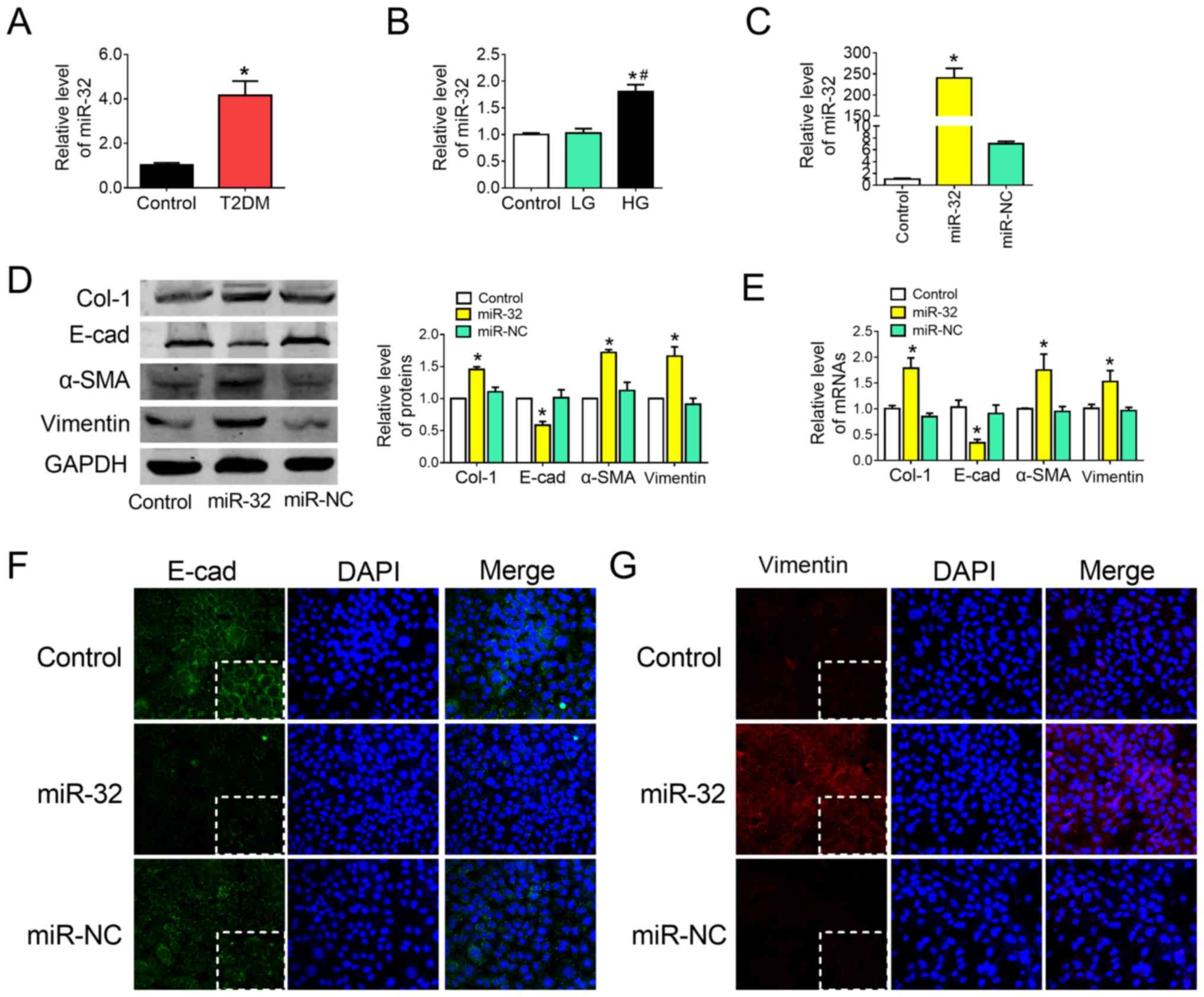 | Figure 3.Overexpression of miR-32 contributes
to liver fibrosis progression and metastasis in AML12 cells. (A)
miR-32 levels were measured by RT-qPCR in the liver tissues of the
control and rats with T2DM (n=4/group). (B) miR-32 expression was
measured by RT-qPCR in HG-treated AML12 cells. *P<0.05 vs.
control group; #P<0.05 vs. LG group. (C) miR-32
expression was measured by RT-qPCR in hepatocytes treated with
miR-32 mimics (n=5/group). (D) Effect of miR-32 on Col-1, E-cad,
α-SMA and vimentin protein expression, with representative blots on
the left and the relative quantification analysis on the right
(n=5/group). (E) Relative mRNA expression of Col-1, E-cad, α-SMA
and vimentin was detected by RT-qPCR (n=5/group). GAPDH was used as
an internal control. (F and G) Immunofluorescence images
highlighting the location of EMT markers E-cad and vimentin, with
DAPI nuclear staining in blue, (F) E-cad in green and (G) vimentin
in red (magnification, ×200). *P<0.05 vs. control group. E-cad,
E-cadherin; α-SMA, α-smooth muscle actin; Col-1, collagen-1;
miR-32, microRNA-32 mimic; miR-NC, microRNA-32 mimic NC; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
miR-32 knockdown inhibits liver
fibrosis under HG conditions
It was investigated whether decreased miR-32
suppressed liver fibrosis by affecting EMT under hyperglycemic
conditions. First, AMO-32 transfection efficiency was confirmed by
RT-qPCR (Fig. 4A). It was observed
that miR-32 inhibition decreased Col-1, α-SMA and vimentin at the
protein (Fig. 4B) and mRNA
(Fig. 4C) level in HG-treated
AML12 cells. However, levels of E-cad were markedly up-regulated
under miR-32 inhibition. In addition, a Sircol collagen assay
suggested that collagen content was significantly decreased by
miR-32 inhibition (Fig. S1).
Similar results were observed in immunofluorescence staining
results for E-cad (Fig. 4D) and
vimentin (Fig. 4E). The results
indicated that decreased miR-32 expression may inhibit liver
fibrosis by affecting EMT in HG-treated AML12 cells.
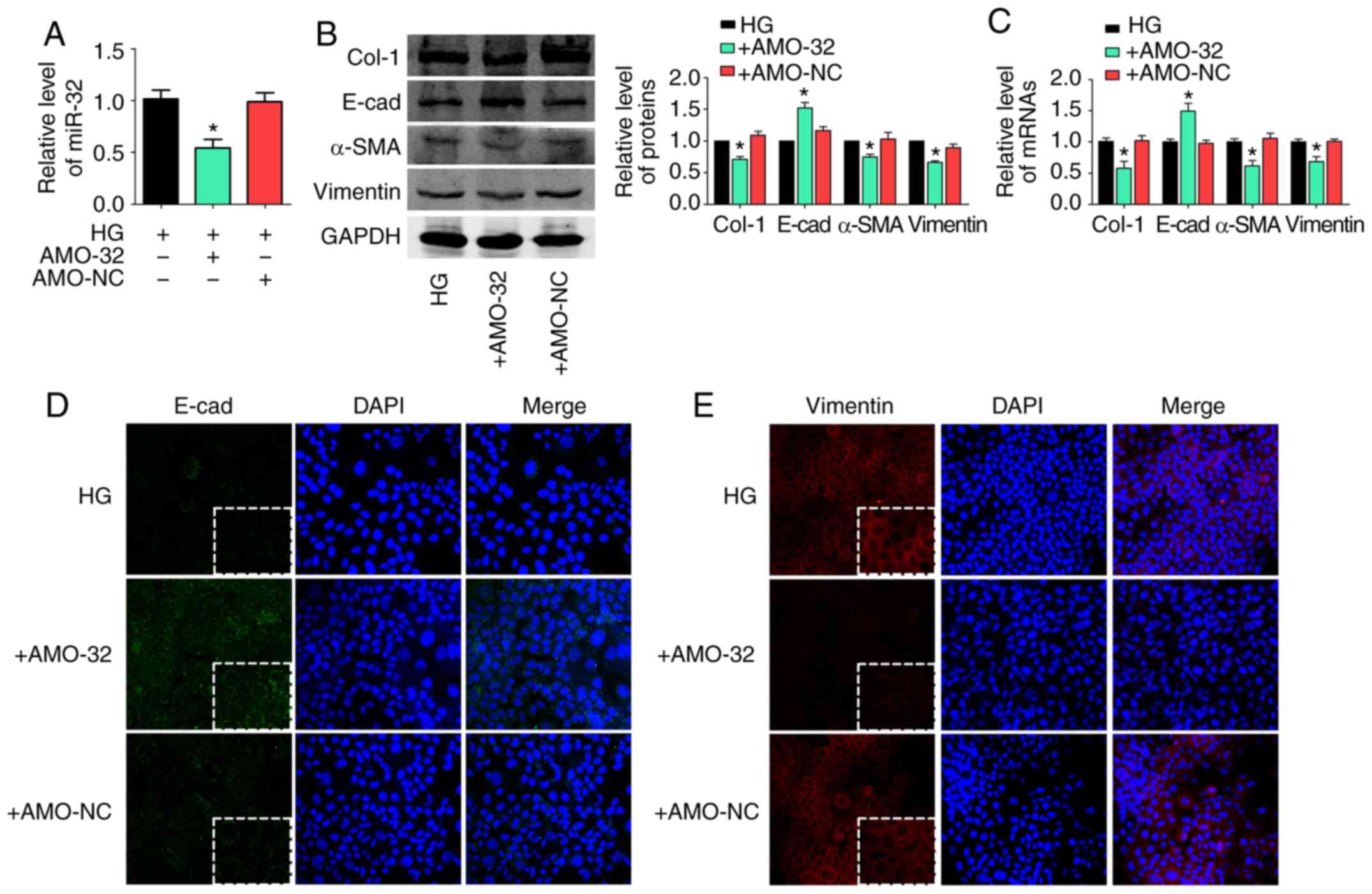 | Figure 4.miR-32 knockdown inhibits liver
fibrosis under HG conditions. (A) miR-32 expression was measured by
RT-qPCR following transfection with AMO-32 under hyperglycemic
conditions in AML12 cells (n=5/group). (B) Effects of miR-32
inhibition on Col-1, E-cad, α-SMA and vimentin protein expression
in HG-treated AML12 cells, with representative blots on the left
and the relative quantification analysis on the right (n=5/group).
(C) Relative mRNA expression levels of Col-1, E-cad, α-SMA and
vimentin were detected by RT-qPCR (n=5/group). GAPDH was used as an
internal control. (D and E) Immunofluorescence images highlighting
the location of EMT markers E-cad and vimentin, with DAPI nuclear
staining in blue, (D) E-cad in green and (E) vimentin in red
(magnification, ×200). *P<0.05 vs. HG group. E-cad, E-cadherin;
α-SMA, α-smooth muscle actin; Col-1, collagen-1; AMO-32, antisense
inhibitor of miR-32; AMO-NC, negative control for AMO-32; HG: high
glucose (6,000 mg/l); RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
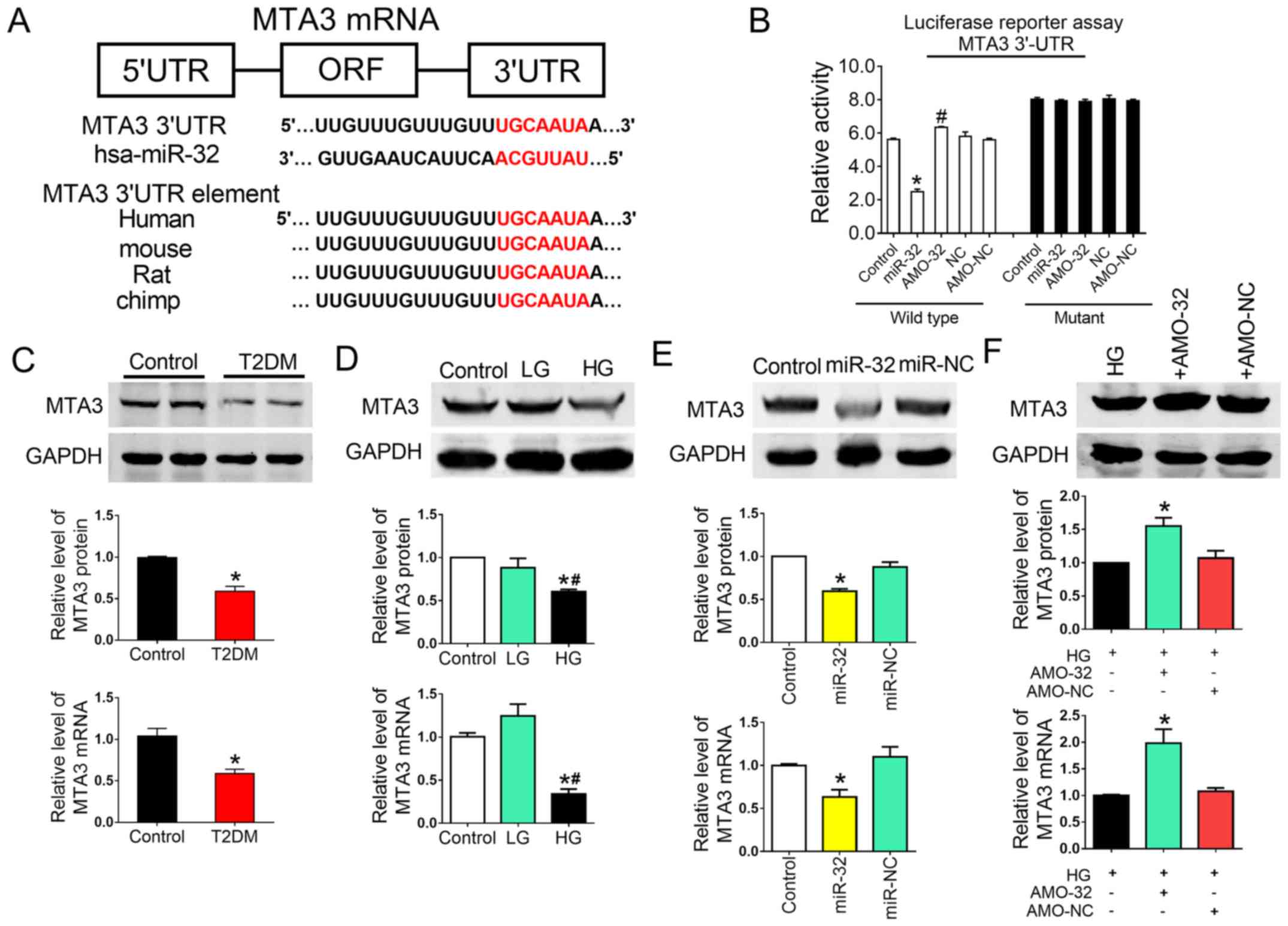
Targeting of MTA3 by miR-32 has a
direct functional effect on liver fibrosis under HG conditions
To explore the molecular mechanisms by which miR-32
functioned in the progression and metastasis of liver fibrosis, the
TargetScan miRNA database was scanned to determine potential gene
targets. Various targets were predicted, including MTA3, CXXC-type
zinc finger protein 5 and bone morphogenetic protein 5. It was
speculated that those involved in EMT may be relevant targets
influencing the biological function of miR-32. Of these genes, MTA3
represses the transcription of Snail by binding to the promoter
region of Snail. Snail contributes to the repression of E-cad
transcription (28). It has been
suggested that MTA3 is the major regulator and upstream protein in
EMT.
To obtain further direct evidence that MTA3 was a
target of miR-32, a binding site for miR-32 was identified on the
MTA3 gene (Fig. 5A). In addition,
luciferase reporter gene assays demonstrated that miR-32
significant suppressed luciferase activity, when using a vector
carrying the wild-type 3′-UTR of MTA3, and a mutation in this
binding site attenuated the action of miR-32 (Fig. 5B). Therefore, it was concluded that
the inserted MTA3 fragment was a target of miR-32.
Based on the observations of the anti-fibrotic
functions of decreased miR-32 expression and the bioinformatics
analysis, the effects of miR-32 on MTA3 expression was
investigated. MTA3 expression in the liver tissue and AML12 cells
was assessed. Protein (Fig. 5C)
and mRNA (Fig. 5D) expression of
MTA3 was significantly decreased in liver tissues of diabetic rats
and in HG-treated AML12 cells, suggesting that MTA3 may be involved
in hyperglycemia-induced liver fibrosis. Western blotting and
RT-qPCR were performed to determine the effects of miR-32 on
endogenous MTA3 protein and mRNA expression in AML12 cells. It was
observed that the overexpression of miR-32 in AML12 cells markedly
downregulated MTA3 protein and mRNA (Fig. 5E).
To confirm whether miR-32 regulated liver fibrosis
through MTA3 changes in MTA3 protein and mRNA levels were assessed
following miR-32 knockdown. In addition, EMT markers in MTA3
overexpressing HG-treated AML12 cells were evaluated. It was
observed that miR-32 knockdown by AMO-32 increased MTA3 protein and
mRNA levels compared with control and miR-NC cells (Fig. 5F). In addition, E-cad mRNA and
protein levels were markedly increased by MTA3 overexpression.
However, MTA3 overexpression decreased α-SMA and vimentin protein
and mRNA levels at increased MTA3 levels in HG-treated AML12 cells
(Fig. S3A and B). The
transfection efficiency of MTA3-overexpressing plasmid was
confirmed by western blotting (Fig.
S3C). In conclusion, these findings indicated that miR-32
knockdown may ameliorate liver fibrosis progression and metastasis
via the regulation of MTA3 expression.
Downstream Snail acts as an indirect
target of miR-32
Previous studies have suggested that Snail, an
EMT-associated zinc finger transcription factor, is a
transcriptional target of MTA3 (29,30).
Therefore, it was hypothesized that Snail may be involved in the
inhibition of liver fibrosis induced by decreased miR-32
expression. In the present study, Snail mRNA and protein expression
was significantly increased in liver tissue of rats with T2DM and
in HG-treated AML12 cells, compared with their respective controls
(Fig. 6A and B). To further
explore whether miR-32 indirectly regulated the participation of
Snail in EMT, gain- and loss-of-function studies were performed.
Treating the cells with a miR-32 mimic reduced Snail expression, as
demonstrated by RT-qPCR. Furthermore, miR-32 overexpression
decreased MTA3 protein expression in AML12 cells, and miR-32
inhibition by AMO-32 reduced Snail protein and mRNA in HG-treated
AML12 cells (Fig. 6C and D).
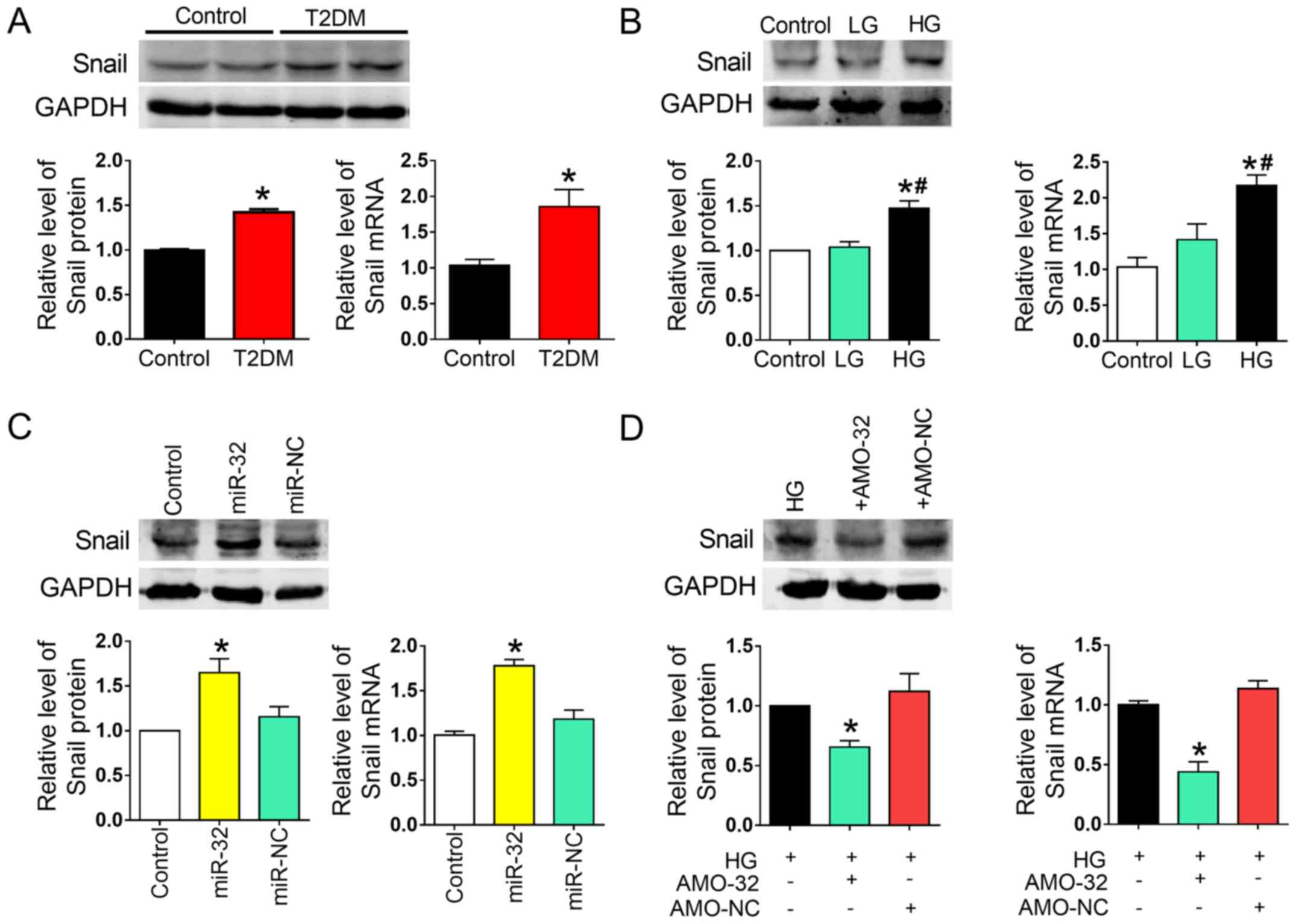 | Figure 6.Downstream Snail acts as an indirect
target of miR-32. (A) Relative protein and mRNA expression of Snail
in liver tissues of the control and rats with T2DM (n=5/group).
*P<0.05 vs. control group. (B) Relative protein and mRNA levels
of Snail in AML12 cells treated with different concentrations of
glucose (n=5/group). *P<0.05 vs. control group;
#P<0.05 vs. LG group. (C) Relative protein and mRNA
expression of Snail in the control, miR-32 and miR-NC groups
(n=5/group). *P<0.05 vs. control group. (D) miR-32 knockdown
resulted in decreased Snail protein and mRNA expression in
HG-treated AML12 cells (n=5/group). *P<0.05 vs. HG group. T2DM,
type 2 diabetes mellitus; HG, high glucose (6,000 mg/l); LG, low
glucose (1,000 mg/l); miR-32, miR-32 mimic; AMO-32, antisense
inhibitor of miR-32; AMO-NC, negative control for AMO-32; Snail,
Snail family transcriptional repressor 1. |
Discussion
In the present study, it was observed that MTA3 was
decreased in liver tissues of rats with T2DM and in HG-treated
AML12 cells. A luciferase reporter assay demonstrated that miR-32
directly bound to the 3′-UTR of MTA3 and miR-32 overexpression in
AML12 cells suppressed MTA3 expression. In addition, it was
observed that Snail expression was significantly increased and
E-cad was decreased in the liver tissue of rats with T2DM and in
HG-treated AML12 cells. These results indicated that MTA3 may be
involved in regulating Snail and E-cad expression, and could have
an important role in EMT under hyperglycemic conditions.
Recently, miRNAs have emerged as important mediators
of translational control and as regulators of a wide range of
biological processes (31,32). Aberrantly expressed miRNAs are
often implicated in the pathogenesis of specific diseases,
including liver fibrosis (33,34).
Various miRNAs have been reported as fibrosis promoters and
potential prognostic markers, including miR-29 and miR-155
(35,36); however, no direct evidence was
available to support a role of miR-32 in liver fibrosis. In the
current study, it was demonstrated that miR-32 expression was
markedly increased in both streptozotocin-induced diabetic rats and
HG-treated AML12 cells, indicating a potential role of miR-32 in
liver fibrosis.
Previous studies have demonstrated that
hyperglycemia in diabetic patients stimulates HSC activation and
proliferation, and contributes to the development of hepatic
fibrosis (37,38). EMT is an indispensable mechanism in
embryogenesis, development and tissue remodeling, that contributes
to the progression of organ fibrosis and cancer (39). Evidence suggested that several
transcription factors, including overexpression of AmeloD resulted
in E-cadherin suppression and dysregulation of interferon
regulatory factor 5 regulated age-associated B cell formation by
binding to promoter regions of involved genes (40,41).
In particular, the transcriptional repressor Snail serves a
critical role in suppressing E-cad transcription by binding to the
CDH1 promoter (42,43). Additionally, studies have indicated
that miRNAs, including the miR-200 family, regulate the EMT through
overexpression-induced decreases in migration and invasion of
pancreatic cancer cells by targeting the EMT-inducer zinc finger
E-box binding homeobox 1 (44).
To illuminate the underlying molecular mechanisms by
which miR-32 participates in liver fibrosis progression, various
miRNA databases, including TargetScan, miRDB and miRanda, were
utilized to validate MTA3 as a target gene for miR-32 that was
conserved among various species. A previous study described that
MTA3, histone deacetylase 1, nucleosome-stimulated ATPase Mi2 and
the methyl-CpG binding protein-associated protein MBD3 form a
Mi2/NuRD transcriptional repression complex, which inhibits Snail
transcription by binding to its promoter region (14). Snail contributes to the repression
of E-cad transcription; however, a Snail-MTA3 complex has not been
identified thus far. Therefore, the current study investigated
whether MTA3 was involved in regulating Snail and E-cad expression
in diabetic rats and HG-treated AML12 cells.
In conclusion, the data from the current study
provided the first evidence that miR-32 was dysregulated in
HG-induced liver fibrosis and directly targeted MTA3. These
findings support the existence of a novel pathway based on miR-32
and MTA3-Snail-induced EMT that may regulate liver fibrosis.
Consequently, the pathological loss of miR-32 may ameliorate
hepatic fibrosis in T2DM and may provide a novel therapeutic
approach for conditions associated with EMT.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant nos. 81570399 and
81773735), the National Key Research and Development Program of
China-Traditional Chinese Medicine Modernization Research project
(grant no. 2017YFC1702003), Hei Long Jiang Outstanding Youth
Science Fund (grant no. JJ2017JQ0035) and Heilongjiang Provincial
Health and Family Planning Commission (grant no. 2018196).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YoZ, LW and QL participated in study design. QL, ZL,
YL, HC, YH, XK and YiZ performed the experiments. QL, ZL, YL and
YiZ analyzed the data. YoZ and LW contributed to the writing of the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All animal experimental protocols were based on the
regulations of the Ethics Committee of Harbin Medical University,
conducted in accordance with the Laboratory Animal Management
Regulations in China, and adhered to the Guide for the Care and Use
of Laboratory Animals published by the National Institutes of
Health (revised 2011).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gonçalves NP, Vægter CB, Andersen H,
Østergaard L, Calcutt NA and Jensen TS: Schwann cell interactions
with axons and microvessels in diabetic neuropathy. Nat Rev Neurol.
13:135–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niu HS, Chao PC, Ku PM, Niu CS, Lee KS and
Cheng JT: Amarogentin ameliorates diabetic disorders in animal
models. Naunyn Schmiedebergs Arch Pharmacol. 389:1215–1223. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li X, Du N, Zhang Q, Li J, Chen X, Liu X,
Hu Y, Qin W, Shen N, Xu C, et al: MicroRNA-30d regulates
cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic
cardiomyopathy. Cell Death Dis. 5:e14792014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang H, Gu Y, Li T, Zhang Y, Huangfu L,
Hu M, Zhao D, Chen Y, Liu S, Dong Y, et al: Integrated analyses
identify the involvement of microRNA-26a in epithelial-mesenchymal
transition during idiopathic pulmonary fibrosis. Cell Death Dis.
5:e12382014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhuo J, Zeng Q, Cai D, Zeng X, Chen Y, Gan
H, Huang X, Yao N, Huang D and Zhang C: Evaluation of type 2
diabetic mellitus animal models via interactions between insulin
and mitogenactivated protein kinase signaling pathways induced by a
high fat and sugar diet and streptozotocin. Mol Med Rep.
17:5132–5142. 2018.PubMed/NCBI
|
|
6
|
Bril F and Cusi K: Management of
nonalcoholic fatty liver disease in patients with type 2 diabetes:
A call to action. Diabetes Care. 40:419–430. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang H, Qin XJ, Li WP, Ma R, Wang T and
Li ZQ: Effects of Shu Gan Jian Pi formula on rats with carbon
tetrachlorideinduced liver fibrosis using serum metabonomics based
on gas chromatographytime of flight mass spectrometry. Mol Med Rep.
16:3901–3909. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li YK, Ma DX, Wang ZM, Hu XF, Li SL, Tian
HZ, Wang MJ, Shu YW and Yang J: The glucagon-like peptide-1 (GLP-1)
analog liraglutide attenuates renal fibrosis. Pharmacol Res.
131:102–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Li H, Zhang Z, Zheng J, Shi Y, Liu
J, Cao Y, Yuan X and Chu Y: Recombinant truncated TGF-β receptor II
attenuates carbon tetrachlorideinduced epithelialmesenchymal
transition and liver fibrosis in rats. Mol Med Rep. 17:315–321.
2018.PubMed/NCBI
|
|
10
|
Park JH, Yoon J, Lee KY and Park B:
Effects of geniposide on hepatocytes undergoing
epithelial-mesenchymal transition in hepatic fibrosis by targeting
TGFβ/Smad and ERK-MAPK signaling pathways. Biochimie. 113:26–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee WR, Kim KH, An HJ, Kim JY, Lee SJ, Han
SM, Pak SC and Park KK: Apamin inhibits hepatic fibrosis through
suppression of transforming growth factor β1-induced hepatocyte
epithelial-mesenchymal transition. Biochem Biophys Res Commun.
450:195–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lovisa S, LeBleu VS, Tampe B, Sugimoto H,
Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC,
Pentcheva-Hoang T, et al: Epithelial-to-mesenchymal transition
induces cell cycle arrest and parenchymal damage in renal fibrosis.
Nat Med. 21:998–1009. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujita N, Jaye DL, Kajita M, Geigerman C,
Moreno CS and Wade PA: MTA3, a Mi-2/NuRD complex subunit, regulates
an invasive growth pathway in breast cancer. Cell. 113:207–219.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin W, Du N, Zhang L, Wu X, Hu Y, Li X,
Shen N, Li Y, Yang B, Xu C, et al: Genistein alleviates pressure
overload-induced cardiac dysfunction and interstitial fibrosis in
mice. Br J Pharmacol. 172:5559–5572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du B, Wang X, Wu D, Wang T, Yang X, Wang
J, Shi X, Chen L and Zhang W: MicroRNA expression profiles identify
biomarkers for predicting the response to chemoradiotherapy in
rectal cancer. Mol Med Rep. 18:1909–1916. 2018.PubMed/NCBI
|
|
18
|
Huangfu L, Liang H, Wang G, Su X, Li L, Du
Z, Hu M, Dong Y, Bai X, Liu T, et al: miR-183 regulates autophagy
and apoptosis in colorectal cancer through targeting of UVRAG.
Oncotarget. 7:4735–4745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang T, Hu Y, Ju J, Hou L, Li Z, Xiao D,
Li Y, Yao J, Wang C, Zhang Y and Zhang L: Downregulation of miR-522
suppresses proliferation and metastasis of non-small cell lung
cancer cells by directly targeting DENN/MADD domain containing 2D.
Sci Rep. 6:193462016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Qin W, Zhang L, Wu X, Du N, Hu Y,
Li X, Shen N, Xiao D, Zhang H, et al: MicroRNA-26a prevents
endothelial cell apoptosis by directly targeting TRPC6 in the
setting of atherosclerosis. Sci Rep. 5:94012015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B,
Zhang Y, Xu C, Bai Y, Wang H, et al: The muscle-specific microRNA
miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1
and KCNJ2. Nat Med. 13:486–491. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sombetzki M, Loebermann M and Reisinger
EC: Vector-mediated microRNA-21 silencing ameliorates granulomatous
liver fibrosis in Schistosoma japonicum infection. Hepatology.
61:1787–1789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu W, Yang J, Feng X, Wang H, Ye S, Yang
P, Tan W, Wei G and Zhou Y: MicroRNA-32 (miR-32) regulates
phosphatase and tensin homologue (PTEN) expression and promotes
growth, migration, and invasion in colorectal carcinoma cells. Mol
Cancer. 12:302013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan SY, Chen MM, Li GM, Wang YQ and Fan
JG: miR-32 induces cell proliferation, migration, and invasion in
hepatocellular carcinoma by targeting PTEN. Tumour Biol.
36:4747–4755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J,
Li M, Zhao T, Yang H, Xu R, et al: Melatonin prevents endothelial
cell pyroptosis via regulation of long noncoding RNA
MEG3/miR-223/NLRP3 axis. J Pineal Res. 64:e124492018. View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zálešák M, BlaŽíček P, Pancza D, Gablovský
I, Štrbák V and Ravingerová T: Hyperosmotic environment blunts
effectivity of ischemic preconditioning against
ischemia-reperfusion injury and improves ischemic tolerance in
non-preconditioned isolated rat hearts. Physiol Res. 65:1045–1051.
2016.PubMed/NCBI
|
|
28
|
Kim NH, Cha YH, Lee J, Lee SH, Yang JH,
Yun JS, Cho ES, Zhang X, Nam M, Kim N, et al: Snail reprograms
glucose metabolism by repressing phosphofructokinase PFKP allowing
cancer cell survival under metabolic stress. Nat Commun.
8:143742017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong H, Guo H, Xie L, Wang G, Zhong X,
Khoury T, Tan D and Zhang H: The metastasis-associated gene MTA3, a
component of the Mi-2/NuRD transcriptional repression complex,
predicts prognosis of gastroesophageal junction adenocarcinoma.
PLoS One. 8:e629862013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dhasarathy A, Kajita M and Wade PA: The
transcription factor snail mediates epithelial to mesenchymal
transitions by repression of estrogen receptor-alpha. Mol
Endocrinol. 21:2907–2918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao D, Zhou T, Fu Y, Wang R, Zhang H, Li
M, Lin Y, Li Z, Xu C, Yang B, et al: MicroRNA-17 impairs glucose
metabolism in insulin-resistant skeletal muscle via repressing
glucose transporter 4 expression. Eur J Pharmacol. 838:170–176.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou T, Meng X, Che H, Shen N, Xiao D,
Song X, Liang M, Fu X, Ju J, Li Y, et al: Regulation of insulin
resistance by multiple miRNAs via targeting the GLUT4 signalling
pathway. Cell Physiol Biochem. 38:2063–2078. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsay HC, Yuan Q, Balakrishnan A, Kaiser M,
Möbus S, Kozdrowska E, Farid M, Tegtmeyer PK, Borst K, Vondran FWR,
et al: Hepatocyte-specific suppression of microRNA-221-3p mitigates
liver fibrosis. J Hepatol S0168-8278. 32635–32637. 2018.
|
|
34
|
Ji F, Wang K, Zhang Y, Mao XL, Huang Q,
Wang J, Ye L and Li Y: miR-542-3p controls hepatic stellate cell
activation and fibrosis via targeting BMP-7. J Cell Biochem.
120:4573–4581. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bala S, Csak T, Saha B, Zatsiorsky J,
Kodys K, Catalano D, Satishchandran A and Szabo G: The
pro-inflammatory effects of miR-155 promote liver fibrosis and
alcohol-induced steatohepatitis. J Hepatol. 64:1378–1387. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roderburg C, Urban GW, Bettermann K, Vucur
M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi
M, et al: Micro-RNA profiling reveals a role for miR-29 in human
and murine liver fibrosis. Hepatology. 53:209–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen E, Cen Y, Lu D, Luo W and Jiang H:
IL-22 inactivates hepatic stellate cells via downregulation of the
TGF-β1/Notch signaling pathway. Mol Med Rep. 17:5449–5453.
2018.PubMed/NCBI
|
|
38
|
Liu Z, Wang J, Xing W, Peng Y, Huang Y and
Fan X: Role of DDAH/ADMA pathway in TGF-β1-mediated activation of
hepatic stellate cells. Mol Med Rep. 17:2549–2556. 2018.PubMed/NCBI
|
|
39
|
Chang J, Hu S, Wang W, Li Y, Zhi W, Lu S,
Shi Q, Wang Y and Yang Y: Matrine inhibits prostate cancer via
activation of the unfolded protein response/endoplasmic reticulum
stress signaling and reversal of epithelial to mesenchymal
transition. Mol Med Rep. 18:945–957. 2018.PubMed/NCBI
|
|
40
|
He B, Chiba Y, Li H, de Vega S, Tanaka K,
Yoshizaki K, Ishijima M, Yuasa K, Ishikawa M, Rhodes C, et al:
Identification of the novel tooth-specific transcription factor
AmeloD. J Dent Res. 14:220345188082542018.
|
|
41
|
Manni M, Gupta S, Ricker E, Chinenov Y,
Park SH, Shi M, Pannellini T, Jessberger R, Ivashkiv LB and Pernis
AB: Regulation of age-associated B cells by IRF5 in systemic
autoimmunity. Nat Immunol. 19:407–419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Choi HJ, Park JH, Park M, Won HY, Joo HS,
Lee CH, Lee JY and Kong G: UTX inhibits EMT-induced breast CSC
properties by epigenetic repression of EMT genes in cooperation
with LSD1 and HDAC1. EMBO Rep. 16:1288–1298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu X, Ling M, Chen C, Luo F, Yang P, Wang
D, Chen X, Xu H, Xue J, Yang Q, et al: Impaired autophagic flux and
p62-mediated EMT are involved in arsenite-induced transformation of
L-02 cells. Toxicol Appl Pharmacol. 334:75–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, et al: The EMT-activator ZEB1 promotes tumorigenicity
by repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009. View Article : Google Scholar : PubMed/NCBI
|