Introduction
Acute lung injury (ALI) and acute respiratory
distress syndrome, which is an intricate cascade process that
develops from ALI, are multifactorial clinical disorders with a
high incidence that frequently cause acute respiratory failure and
subsequent death (1,2). The major characteristic of ALI is an
exaggerated inflammatory response, which may lead to alveolar
capillary destruction, respiratory failure and even multiple organ
failure (3,4). ALI can be caused by several factors;
for example, aspiration of low-pH gastric fluid (acid aspiration)
can induce an inflammatory pulmonary reaction associated with
neutrophilic infiltration and alveolar epithelial damage, termed
aspiration pneumonitis, which may be followed by infection
(5). In addition, sepsis,
transfusion, trauma and other factors may lead to ALI (6). Several animal models, such as sepsis
or mechanical ventilation-induced acute lung injury, have been
established to explore the mechanism and treatment of ALI (7,8).
LPS is a component of the cell walls of
gram-negative bacteria (9). LPS
causes microvascular lung injury, increases macrophage and
neutrophil presence, and induces pulmonary inflammation in animals
and humans (10). The feasibility
of analyzing the pathogenesis of ALI with an in vitro ALI
model induced by LPS has been verified in previous studies
(11,12). Zhang et al (13) have reported that amygdalin
treatment decreases inflammatory cell infiltration and the release
of inflammatory cytokines, and represses the activation of nuclear
factor-κB (NF-κB) and NLR family pyrin domain containing 3 in
LPS-induced ALI mice, exerting a protective effect. Pedrazza et
al (14) have demonstrated
that decreases in inflammation, oxidative damage and the formation
of neutrophil extracellular traps are observed in an LPS-induced
ALI model following treatment with mesenchymal stem cells, as well
as an increased survival curve. Mechanical ventilation (MV) is a
necessary treatment for patients with respiratory dysfunction
(15,16). However, the ventilation effect and
oxygenation provided by MV and the negative side effects should be
considered when using MV (17).
Lung injury caused by MV has attracted increasing attention from
the medical community (18). The
occurrence of such lung injury is not only associated with high
airway pressure, but also with ventilation capacity, ventilation
mode and inhaled oxygen concentration (19,20).
Chian et al (21) have
demonstrated that an inflammatory reaction and NF-κB activation are
implicated in ventilator-induced lung injury, and treatment with an
inhibitory peptide of NF-κB (SN50) reduces such lung injury.
Despite previous advances, the specific mechanisms underlying
LPS-induced and ventilator-induced ALI remain unclear.
In clinical practice, a number of patients with
sepsis-related lung injury often require the support of a
ventilator, and MV may further aggravate lung injury, resulting in
a potential exacerbation of the condition or an additional
condition in the patients (22).
However, the differences between LPS-induced lung injury and
MV-induced lung injury, as well as the effects of the combination
of the two means of inducing lung injury have not been studied
previously. To assess this, the gene expression dataset GSE18341 of
ALI in adult C57BL/6 mice was used to analyze the underlying
mechanisms in the present study. The genes that were differentially
expressed in response to LPS, MV and LPS + MV treatment were
screened, followed by functional enrichment analysis, construction
of interaction networks, and prediction of transcription factors
(TFs) and small molecule drugs. The results of the present study
may provide novel insight into a theoretical basis for the study
and treatment of ALI.
Materials and methods
Dataset
The gene expression dataset GSE18341 of ALI in adult
C57BL/6 mice caused by inhaling LPS, MV and LPS combined with MV
were downloaded from the Gene Expression Omnibus (GEO; http://ncbi.nlm.nih.gov/geo/) (23). A total of 4 groups of data in the
dataset were selected to analyze the possible mechanisms and key
genes of ALI induced by mechanical ventilation and LPS, including
differential, Venn diagram, enrichment, protein-protein interaction
(PPI) network and transcription factor (TF) prediction analysis, as
well as drug target gene interaction network construction, in order
to verify the associated genes and signaling pathways.
Data source and preprocessing
To clarify the differences and relationship between
LPS- and MV-induced lung injury, as well as the effects of the
combination of the two factors on lung tissue, a total of 16
samples were divided into 4 groups (n=4 samples/group): i) MV group
(treated with MV for 2 h); ii) LPS group (treated with inhaled LPS,
then spontaneous breathing for 2 h); iii) MV + LPS group (treated
with inhaled LPS, then MV for 2 h); and iv) control group
(untreated, spontaneously breathing). The data were collected on
the GPL1261 [Mouse430_2] Affymetrix Mouse Genome 430 2.0 Array. The
raw data were preprocessed using the RMA method in the affy package
(Version 1.50.0; http://bioconductor.org/packages/release/bioc/html/affy.html)
used with R (version 3.3.3) (24),
including background correction, normalization and expression
calculation. The probes were matched to gene symbols based on the
annotations file, and probes without matched gene symbols were
removed, whereas the mean expression value was selected as the
final expression value when multiple probes matched to one gene
symbol. The data were selected to analyze the possible mechanism
and key genes of ALI induced by MV and LPS, and the identified
genes were verified using reverse transcription-quantitative
(RT-q)PCR.
Differential expression and Venn
analysis
Differential expression analysis of MV vs. control,
LPS vs. control, MV + LPS vs. control, MV + LPS vs. MV and MV + LPS
vs. LPS was performed using the limma package (version 3.10.3;
www.bioconductor.org/packages/2.9/bioc/html/limma.html)
to obtain the corresponding P-value and log fold change (FC); then,
the adjusted P-value (adj.P) was obtained using the Benjamini and
Hochberg test. The differentially expressed genes (DEGs) were
screened with a threshold of adj.P<0.05 and |log FC|>0.585.
The DEGs in each compared group were used to perform the Venn
analysis. Specifically, the overlapping genes in the MV vs.
control, MV + LPS vs. Control and MV + LPS vs. LPS groups were
considered to be genes associated with MV. Similarly, the
overlapping genes in LPS vs. control, MV + LPS vs. control and MV +
LPS vs. MV were considered to be genes associated with LPS.
Notably, the DEGs in MV + LPS vs. control were considered to
comprise the gene response to LPS combined with MV. The Venn
diagrams were plotted using an online tool (http://bioinformatics.psb.ugent.be/webtools/Venn/).
Functional enrichment analysis
ClusterProfiler (version 3.8.1; bioconductor.org/packages/release/bioc/html/clusterProfiler.html)
in R was used to perform functional enrichment analysis of the
genes associated with MV, LPS or the gene response to LPS combined
with MV, including identification of the biological processes in
Gene Ontology (GO_BP) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathways. The terms with P<0.05 and an enriched gene
count ≥2 were selected as significantly enriched results.
PPI network
The interactions between coding proteins of DEGs
associated with MV, LPS or the gene response to LPS combined with
MV were predicted using Search Tool for the Retrieval of
Interacting Genes/Proteins (version 10.0; string-db.org/) with the following parameters:
Species, Mus musculus; and PPI score, 0.9, highest confidence. The
PPI networks associated with MV, LPS and MV + LPS were visualized
using Cytoscape (version 3.4.0, chianti.ucsd.edu/cytoscape-3.4.0/). Subsequently,
CytoNCA (version 2.1.6, apps.cytoscape.org/apps/cytonca) was used to analyze
the topological properties of the nodes in the PPI networks, and
the parameter was set to ‘without weight’.
Prediction of TFs
The TFs of the DEGs were predicted using TRRUST
(version 2, www.grnpedia.org/trrust/). The TF-gene interaction
pairs with P-values <0.05 were selected to construct the
regulatory network using Cytoscape.
Drug-gene interaction prediction
Drug targets of the DEGs were predicted using The
Drug Gene Interaction database (dgidb.org/search_interactions) with the parameter ‘FDA
approved’. Drug-gene interaction pairs with references and known
interaction type were selected, and drug-gene interaction networks
were visualized using Cytoscape.
Experimental animals
A total of 28 male C57BL/6 mice (age, 7–9 weeks;
weight, 24–28 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. Mice were randomly divided into four groups. 28
mice were randomly divided into four groups. Mice were housed in an
antigen- and virus-free room at 25°C with 40–70% humidity and ad
libitum access to food and water under a natural day/night
cycle. All animal experiments were approved by the Ethics Committee
of Experimental Animals of Shanghai Jiaotong University School of
Medicine. Based on the results of the bioinformatics analysis, the
expression levels of the identified genes associated with MV, LPS
or MV + LPS response were verified in mice in four different
groups. For the MV group, after being anesthetized with
intraperitoneal injection of ketamine (75 mg/kg) and xylazine (10
mg/kg), the mice were orotracheally intubated with 20 G arterial
cannule (BD Pharmingen, San Jose, CA), and mechanically ventilated
(Inspira, Harvard Apparatus, Boston, MA) with 20 ml/kg at 70
breaths per minute for 2 h. For the LPS group, after anesthesia,
mice were administered a single intratracheal dose of purified LPS
extracted from the membrane of Escherichia coli 0111:B4
(Sig-maeAldrich, St. Louis, MO, USA) at 5 mg/kg in a total volume
of 50 µl for 2 h. For MV + LPS group, after anesthesia, mice were
treated with 5 mg/kg LPS and then were ventilated with 20 ml/kg
tidal volume for 2 h. Control mice underwent incubation but
breathed spontaneously. Lungs were harvested at the time points
indicated.
RT-qPCR verification
Total RNA was isolated from the lung tissues using
TRIzol® reagent (cat. no. 9109; Takara Bio, Inc.), and
the RNA concentration and quality were determined by detecting
absorbance at a wavelength of 260 nm. Subsequently, reverse
transcription (25°C for 10 min, 42°C for 1 h, 85°C for 10 min, 4°C
hold) of total RNA was performed using PrimeScript™ RT Master mix
(cat. no. RR036A; Takara Bio, Inc.), followed by qPCR using Power
SYBR® Green PCR Master mix (Roche Diagnostics) with the
following thermocycling conditions: 50°C for 2 min; 95°C for 10
min; followed by 40 cycles of 95°C for 10 sec and 58–62°C for 30
sec. The melting curve was analyzed between 60 and 95°C at an
incremental rate of 0.5°C/10 sec. The primer sequences are
presented in Table SI. β-actin
was measured as an internal control. The relative expression levels
of genes were calculated using the 2−ΔΔCq method
(25). RT-qPCR was repeated 6
times to obtain statistical values.
ELISA verification
The left upper lung tissues were homogenized in PBS,
centrifuged for 10 min at 4,000 × g at 4°C and sonicated in 1 ml
PBS containing protease inhibitors (2 mM phenylmethylsulfonyl
fluoride and 1 µg/ml each antipain, leupeptin, and pepstatin A).
IL-6 (cat. no. F10830), C-X-C motif chemokine ligand (CXCL)2 (cat.
no. F11170), CXCL3 (cat. no. F10244), CXCL10 (cat. no. F10933) and
TNF-α (cat. no. F11630) protein levels in lung tissue homogenates
were measured using commercially available ELISA kits (Shanghai
Westang Bio-Tech Co., Ltd.) according to the manufacturer's
protocol. ELISAs were repeated 7 times to obtain statistical
values.
Lung histopathological
examination
Lung tissues were fixed using 10% formalin for 24 h
at room temperature and embedded in paraffin for histopathological
analysis; 4-µm sections were cut and stained with hematoxylin and
eosin (H&E). The total staining of each slide was scored by two
blinded expert lung pathologists. The criteria for scoring lung
inflammation were set as previously described (26): 0, normal tissue; 1, minimal
inflammatory change; 2, mild to moderate inflammatory changes (no
obvious damage to the lung architecture); 3, moderate inflammatory
injury (thickening of the alveolar septae); 4, moderate to severe
inflammatory injury (formation of nodules or areas of pneumonitis
that distorted the normal architecture); and 5, severe inflammatory
injury with total obliteration of the field. Histopathological
examination was repeated 7 times.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical significance was calculated using a Student's
t-test or one-way ANOVA followed by the Bonferroni's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS software
(version 16.0.1; SPSS, Inc.).
Results
Data preprocessing
A 21,499-gene expression matrix was obtained
according to the method described above, and principal component
analysis (PCA) was performed based on this gene expression matrix.
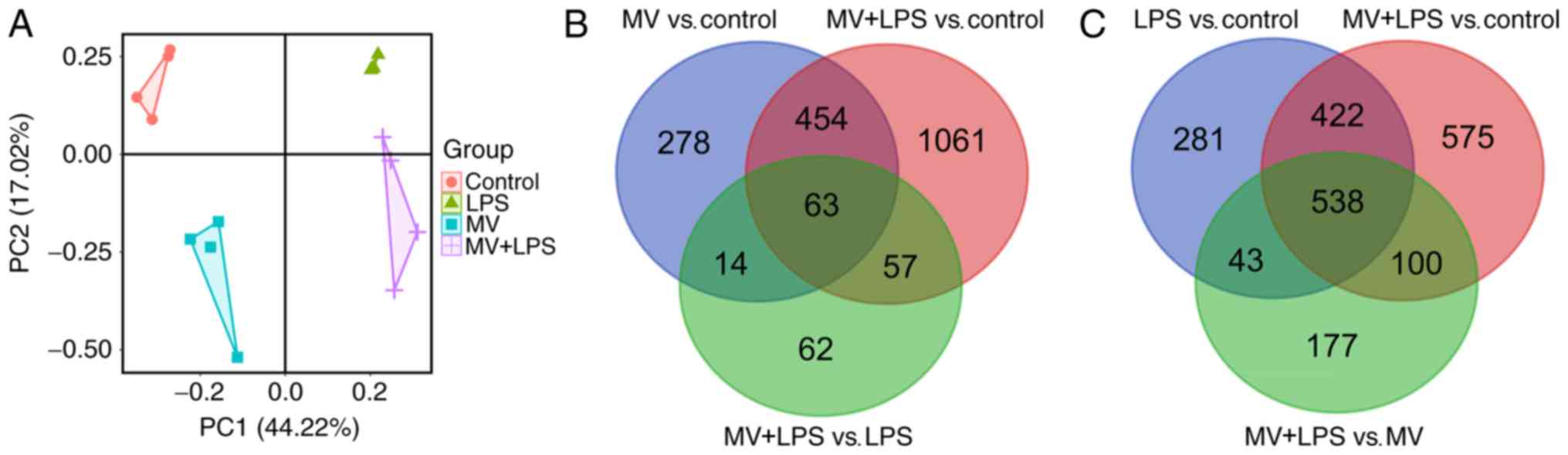
As presented in Fig. 1A, the
samples from each group were clustered, suggesting that LPS, MV and
MV + LPS had notable effects on lung injury.
Differential expression and Venn
diagram analysis
A total of 809, 1,284, 1,635, 858 and 196 DEGs were
identified between MV vs. control, LPS vs. control, MV + LPS vs.
control, MV + LPS vs. MV and MV+LPS vs. LPS, respectively (Table I). The 1,635 genes that were
differentially expressed in samples with MV + LPS treatment
compared with the control were considered to be the genes
responsive to LPS combined with MV. Following Venn diagram
analysis, 63 overlapping DEGs among MV vs. control, MV + LPS vs.
control and MV + LPS vs. LPS were selected as the genes associated
with MV (Fig. 1B). Similarly, the
538 overlapping DEGs among LPS vs. control, MV + LPS vs. control
and MV + LPS vs. MV were considered to be the genes associated with
LPS (Fig. 1C).
 | Table I.Results of DEG analysis. |
Table I.
Results of DEG analysis.
| DEGs | LPS vs.
control | MV vs. control | MV + LPS vs.
control | MV + LPS vs.
LPS | MV + LPS vs.
MV |
|---|
| Upregulated | 688 | 187 | 824 | 121 | 721 |
| Downregulated | 596 | 622 | 811 | 75 | 137 |
| Total | 1284 | 809 | 1635 | 196 | 858 |
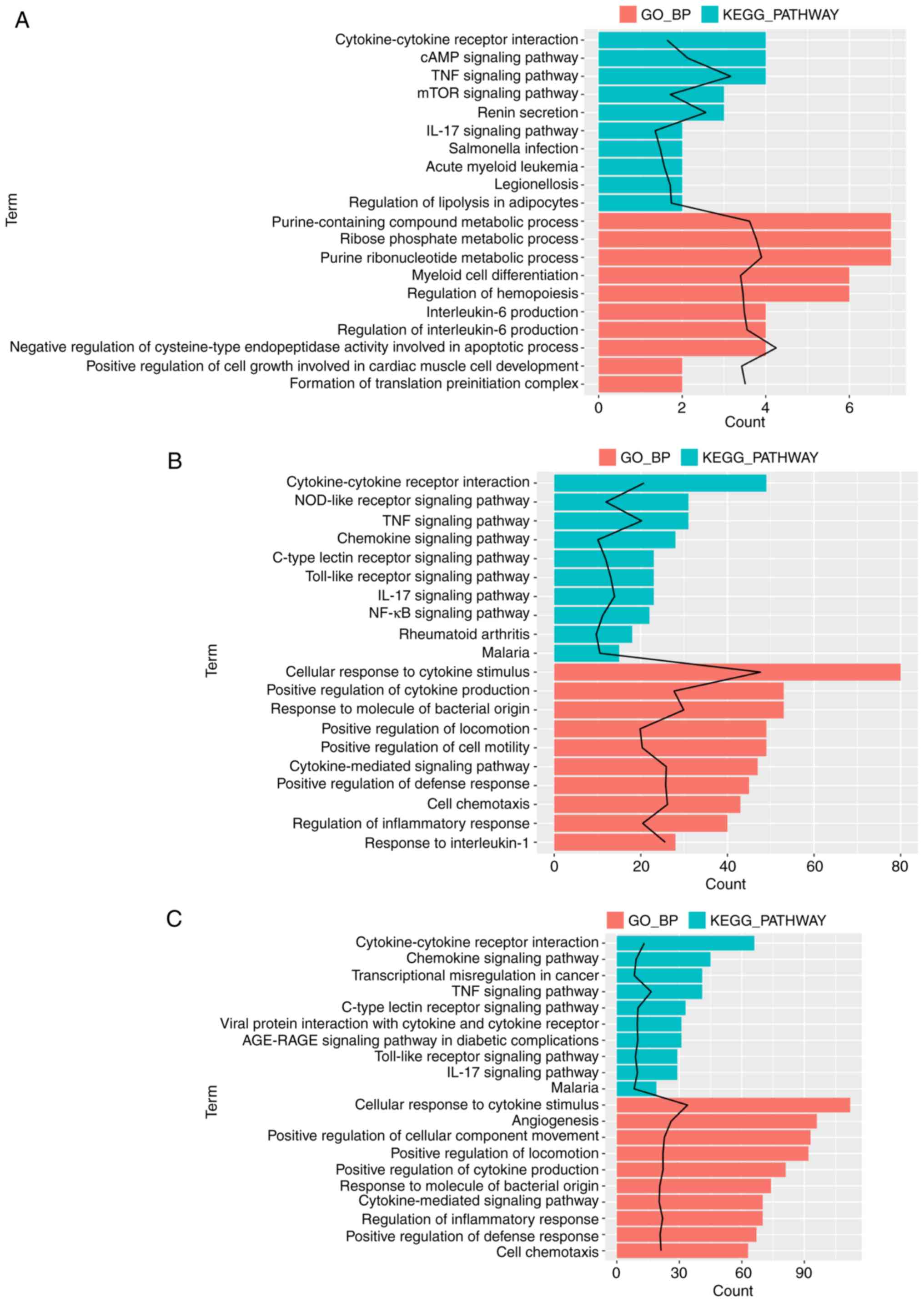
Functional enrichment analysis
Enrichment analysis was performed for the
aforementioned DEGs associated with MV, LPS or the gene response to
LPS combined with MV to determine their functions. The gene set
associated with MV was significantly enriched in genes associated
with 110 GO_BP terms and 12 KEGG pathways; among these, the GO_BP
term ‘negative regulation of cysteine-type endopeptidase activity
involved in apoptotic process’ was the most significant (Fig. 2A). The gene sets associated with
LPS were significantly enriched in genes associated with 268 GO_BP
terms and 70 KEGG pathways (Fig.
2B), and the MV + LPS gene set was significantly enriched in
genes associated with 339 GO_BP terms and 90 KEGG pathways
(Fig. 2C). The term ‘cellular
response to cytokine stimulus’ was the most significant in the DEGs
associated with LPS and MV + LPS. Fig.
2 demonstrates the top 10 significantly enriched GO_BP terms
and KEGG pathways arranged by P-value. These DEG sets were all
significantly enriched in genes associated with the GO_BP terms and
KEGG pathways involved in the inflammatory response, including the
‘TNF signaling pathway’, ‘IL-17 signaling pathway’, ‘cellular
response to cytokine stimulus’ and ‘regulation of the inflammatory
response’, amongst others (Fig.
2).
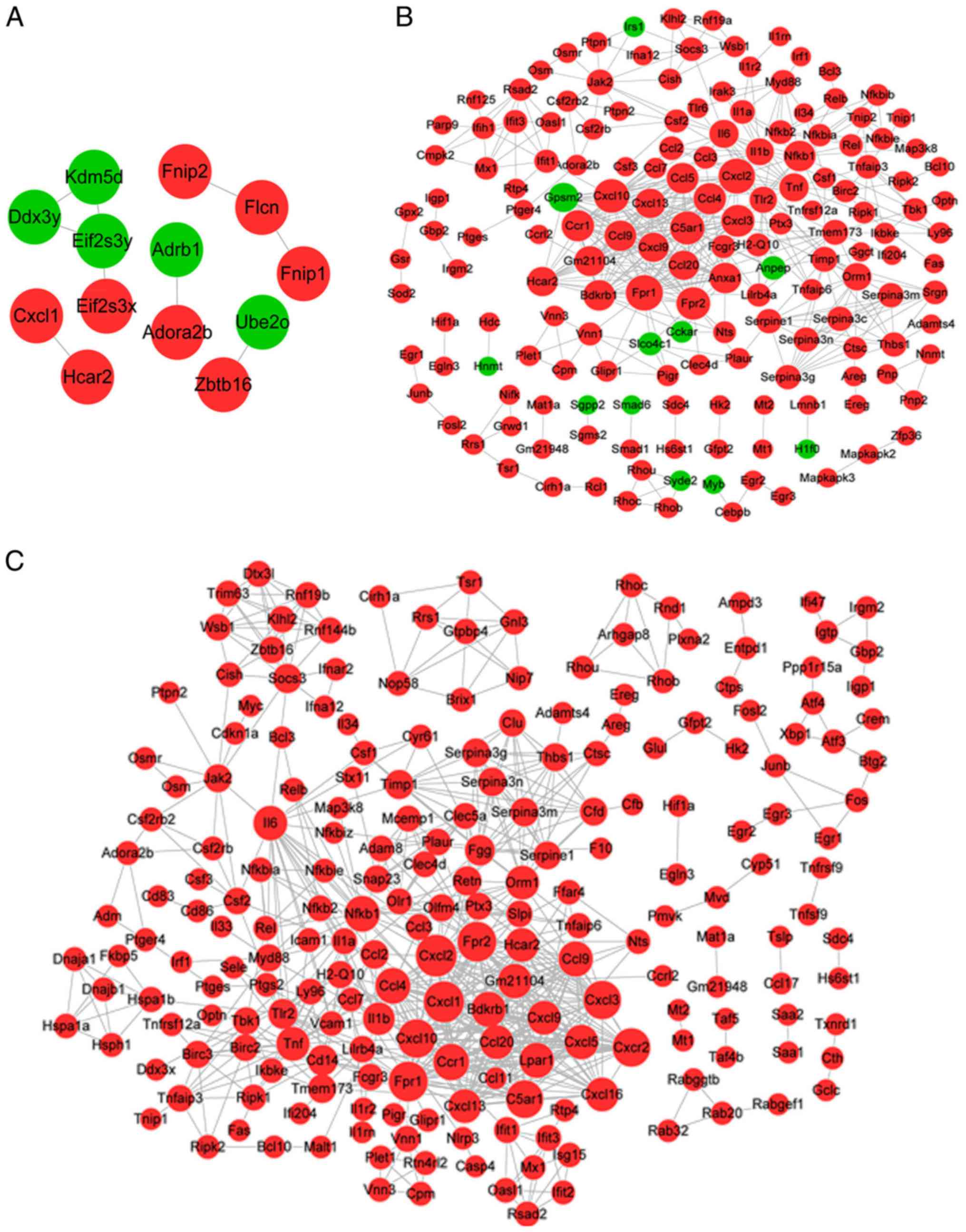
Construction of the PPI network
For the genes associated with MV, the PPI network
contained 13 genes and 9 interactions, among which 8 genes were
upregulated and 5 were downregulated (Fig. 3A). For example, adenosine A2b
receptor (ADORA2B), zinc finger and BTB domain containing 16
(ZBTB16) and hydroxycarboxylic acid receptor 2 (HCAR2) were
upregulated.
For the genes associated with LPS, the PPI network
comprised 166 genes, including 11 downregulated and 155 upregulated
genes, and 472 interactions (Fig.
3B). The topological properties of the nodes (top 20, arranged
by score) are listed in Table II.
Formyl peptide receptor (FPR)1, FPR2, CXCL2, CXCL3, CXCL10 and
nuclear factor κB subunit 1 (NFKB1), tumor necrosis factor (TNF)
and interleukin 6 (IL6) were identified as the hub nodes in the PPI
network as their degree, betweenness and closeness scores were all
in the top 20.
 | Table II.Top 20 genes in the PPI network of
genes associated with lipopolysaccharide treatment. |
Table II.
Top 20 genes in the PPI network of
genes associated with lipopolysaccharide treatment.
| A, Top 20 genes
based on degree |
|---|
|
|---|
| Gene | Degree |
|---|
| Fpr1a | 27 |
| Cxcl2a | 26 |
| Cxcl10a | 24 |
| C5ar1 | 23 |
| Fpr2a | 22 |
| Cxcl3a | 21 |
| Ccr1 | 20 |
| Ccl4 | 20 |
| Bdkrb1 | 19 |
| Ccl9 | 19 |
| Anxa1 | 19 |
| Ccl5 | 19 |
| Nfkb1a | 19 |
| Gm21104 | 18 |
| Gpsm2 | 17 |
| Hcar2 | 17 |
| Cxcl13 | 17 |
| Ccl20 | 17 |
| Il6a | 15 |
| Tnfa | 15 |
|
| B, Top 20 genes
based on betweenness |
|
| Gene |
Betweenness |
|
| Il6a | 3,750.5457 |
| Cxcl10a | 2,844.6838 |
| Nfkb1a | 2,505.5789 |
| Tnfa | 2,152.0164 |
| Ifit1 | 1,969.5000 |
| Fpr1a | 1,809.1663 |
| Cxcl2a | 1,700.6371 |
| Orm1 | 1,651.6079 |
| Jak2 | 1,479.5116 |
| Tlr2 | 1,104.2716 |
| Socs3 | 1,070.4786 |
| Csf2 | 1,046.3145 |
| Cxcl3a | 813.3837 |
| Il1b | 801.5933 |
| Vnn1 | 690.0000 |
| Timp1 | 662.3790 |
| Tnfaip3 | 609.1126 |
| Fpr2a | 561.2064 |
| Tmem173 | 525.8387 |
| Tbk1 | 519.1886 |
|
| C, Top 20 genes
based on closeness |
|
| Gene |
Closeness |
| Cxcl2a | 0.02045 |
| Il6a | 0.02043 |
| Cxcl10a | 0.02042 |
| Tnfa | 0.02040 |
| Nfkb1a | 0.02039 |
| Ccl5 | 0.02037 |
| Cxcl3a | 0.02036 |
| Tlr2 | 0.02035 |
| Il1b | 0.02035 |
| Ccl4 | 0.02035 |
| Fpr1a | 0.02034 |
| Ccl2 | 0.02032 |
| C5ar1 | 0.02032 |
| Ccr1 | 0.02031 |
| Fpr2a | 0.02030 |
| Bdkrb1 | 0.02029 |
| Ccl9 | 0.02029 |
| Anxa1 | 0.02029 |
| Gm21104 | 0.02028 |
| Orm1 | 0.02028 |
For the gene response to LPS combined with MV, the
PPI network contained 216 genes and 679 interactions, and all the
genes were upregulated (Fig. 3C).
Table III displays the top 20
nodes; CXCL2, CXCL3, CXCL10, CXCL11, FPR1, FPR2, TNF, IL6, NFKB1
and orosomucoid 1 (ORM1) were the hub nodes in the PPI network as
their degree, betweenness and closeness scores were all in the top
20. Notably, most of the hub genes in the LPS and MV + LPS PPI
networks were consistent, with the exception of CXCL11 and
ORM1.
 | Table III.Top 20 genes in the PPI network of
genes associated with lipopolysaccharide treatment and mechanical
ventilation. |
Table III.
Top 20 genes in the PPI network of
genes associated with lipopolysaccharide treatment and mechanical
ventilation.
| A, Top 20 genes
based on degree |
|---|
|
|---|
| Gene | Degree |
|---|
| Cxcl2a | 31 |
| Cxcl1a | 30 |
| Fpr1a | 28 |
| Fpr2a | 28 |
| Cxcl3a | 26 |
| Cxcr2 | 26 |
| Cxcl10a | 26 |
| C5ar1 | 25 |
| Ccr1 | 23 |
| Ccl4 | 22 |
| Nfkb1a | 22 |
| Bdkrb1 | 21 |
| Lpar1 | 21 |
| Ccl9 | 21 |
| Gm21104 | 20 |
| Cxcl5 | 20 |
| Il6a | 20 |
| Orm1a | 19 |
| Tnfa | 19 |
| Cxcl16 | 19 |
|
| B, Top 20 genes
based on betweenness |
|
| Gene |
Betweenness |
|
| Il6a | 6,930.810 |
| Cxcl10a | 3,350.056 |
| Nfkb1a | 3,270.180 |
| Tlr2 | 2,781.188 |
| Tnfa | 2,772.925 |
| Socs3 | 2,672.574 |
| Fpr1a | 2,494.769 |
| Il1b | 2,181.053 |
| Cxcl2a | 2,124.261 |
| Ifit1 | 2,034.400 |
| Jak2 | 2,021.862 |
| Fpr2a | 1,942.306 |
| Cxcl1a | 1,859.115 |
| Csf2 | 1,650.456 |
| Orm1a | 1,555.060 |
| Hspa1b | 1,473.000 |
| Fgg | 1,185.236 |
| Vnn1 | 1,184.000 |
| Tbk1 | 801.417 |
| Cxcl3a | 750.258 |
|
| C, Top 20 genes
based on closeness |
|
| Gene |
Closeness |
|
| Il6a | 0.01541 |
| Cxcl2a | 0.01541 |
| Cxcl1a | 0.01539 |
| Cxcl10a | 0.01538 |
| Nfkb1a | 0.01538 |
| Tnfa | 0.01538 |
| Tlr2 | 0.01536 |
| Il1b | 0.01536 |
| Cxcl3a | 0.01535 |
| Ccl4 | 0.01535 |
| Fpr1a | 0.01534 |
| Ccl2 | 0.01534 |
| Cxcr2 | 0.01534 |
| C5ar1 | 0.01534 |
| Ccl3 | 0.01533 |
| Cxcl5 | 0.01532 |
| Ccr1 | 0.01532 |
| Fpr2a | 0.01532 |
| Orm1a | 0.01531 |
| Bdkrb1 | 0.01531 |
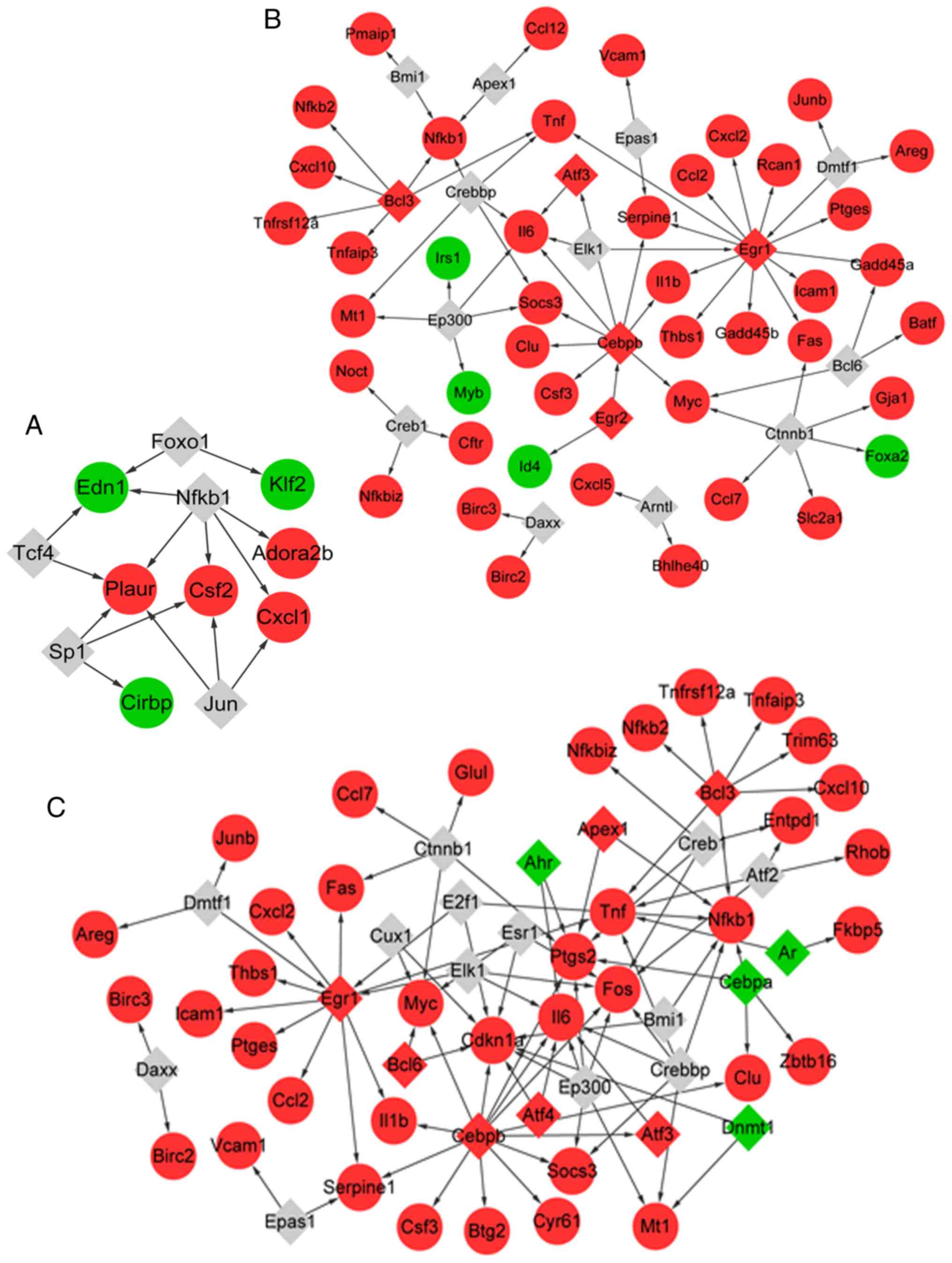
Prediction of TFs
The TFs of the genes associated with MV were
predicted. In total, 15 TF-target pairs were obtained (Fig. 4A), among which five TFs (such as
NFKB1) were predicted to target seven genes (such as ADORA2B).
Similarly, 17 TFs were predicted to target 46 genes associated with
LPS, comprising 67 TF-target pairs (Fig. 4B). A total of five out of the 17
TFs were differentially expressed in samples with LPS treatment vs.
control, including early growth response 1 (EGR1), activating TF 3
(ATF3) and BCL3 transcription coactivator (BCL3).
In addition, a total of 24 TFs were predicted to
target 40 genes that responded to LPS combined with MV, which
comprised 91 TF-target pairs (Fig.
4C). In total, 11 out of the 24 TFs were differentially
expressed in samples from animals treated with MV + LPS, including
aryl hydrocarbon receptor (AHR) and androgen receptor (AR).
Prediction of drug-gene
interactions
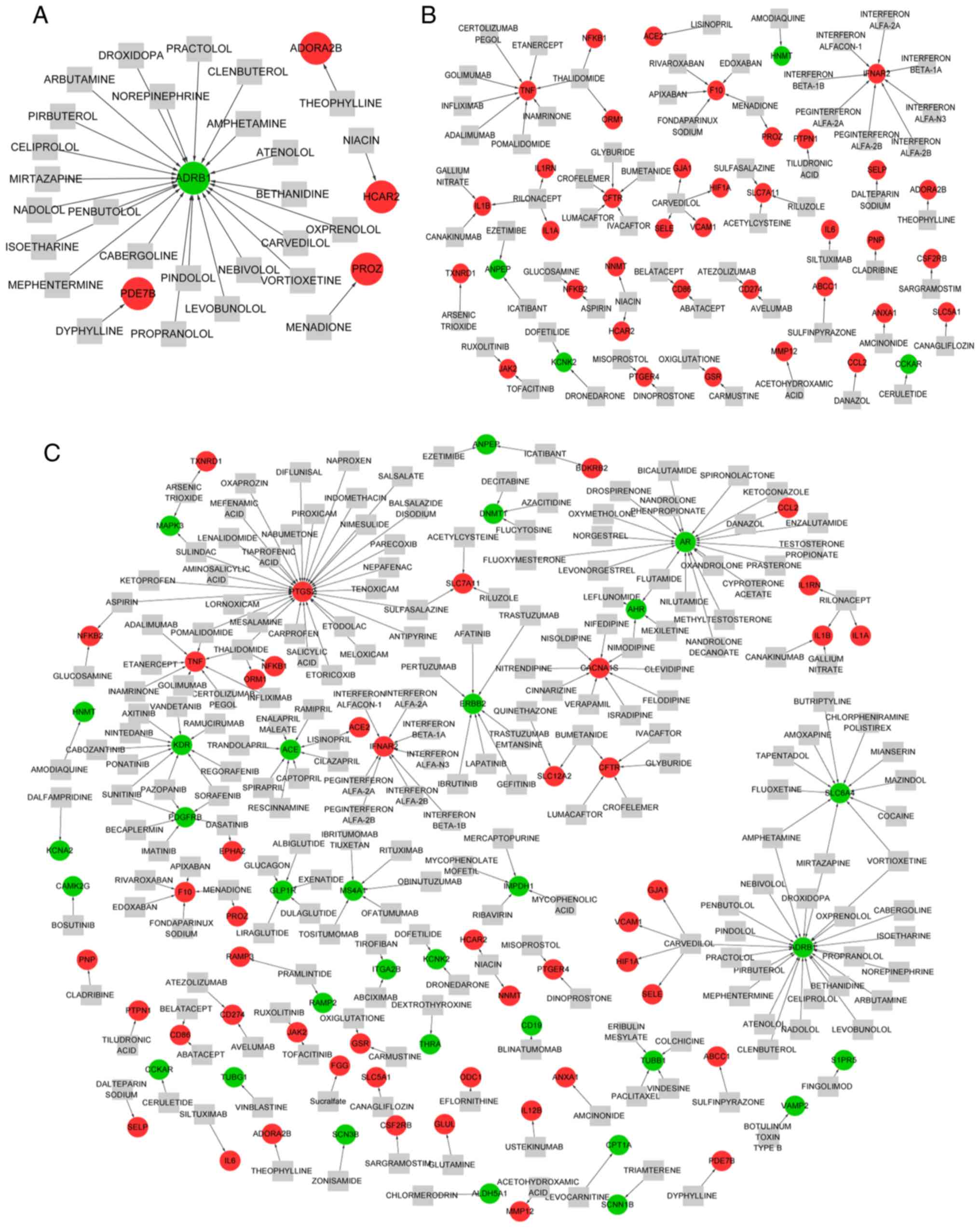
For the genes associated with MV, 27 drugs were
predicted to target 5 genes, including 4 upregulated and 1
downregulated gene (Fig. 5A). A
total of 23 drugs were identified to target adrenoceptor β1
(ADRB1), and most of these were agonists of ADRB1 such as
norepinephrine (Table SII).
Of the genes associated with LPS, 40 genes (36
upregulated and 4 downregulated genes) were targeted by 65 drugs,
and the drug-gene network consisted of 74 drug-gene interactions
(Fig. 5B). For example,
glucosamine and aspirin were identified to be antagonists of NFKB2.
Thalidomide was an antagonist of NFKB1, and theophylline was an
antagonist of ADORA2B (Table
SII).
A total of 218 drugs were predicted to target 78
genes that responded to LPS combined with MV, and the drug-gene
network comprised 248 drug-gene interactions (Fig. 5C). For example, niacin was
predicted to be an agonist of HCAR2, whereas siltuximab was
identified to be an inhibitor, antibody and antagonist of IL6
(Table SII).
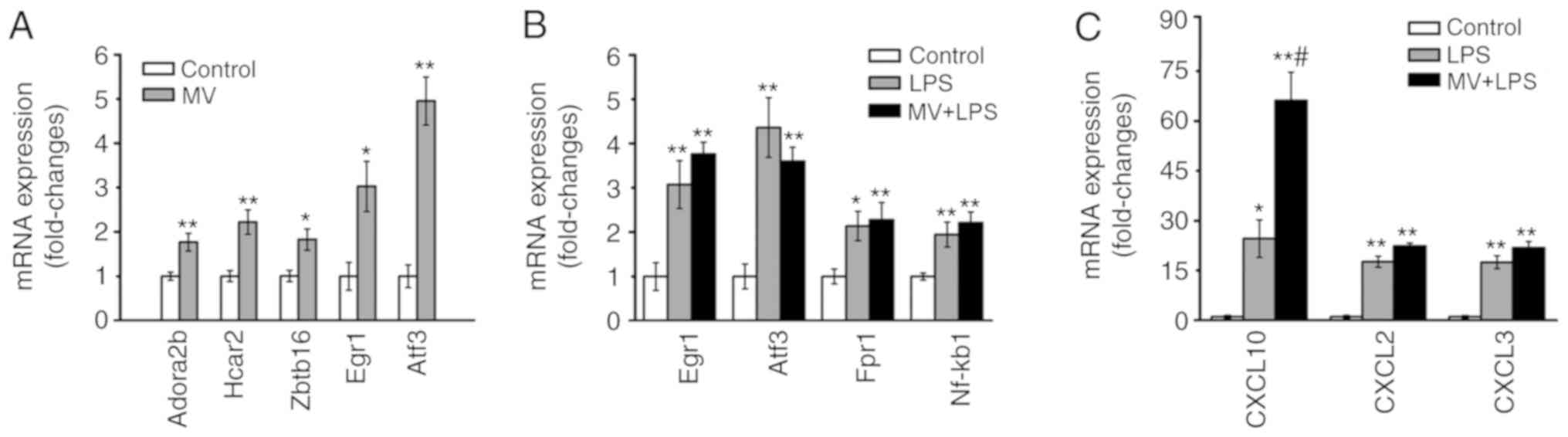
Expression of the verified genes
According to the results of the bioinformatics
analyses, the genes ADORA2B, HCAR2 and ZBTB16, and the TFs EGR1 and
ATF3 were selected for further verification as they were
significantly upregulated in the mechanical ventilation group. The
RT-qPCR analysis demonstrated that expression levels of ADORA2B,
HCAR2, ZBTB16, EGR1 and ATF3 were upregulated in the lung tissues
obtained from mice in the MV group compared with those in the
control group (P<0.05 or P<0.01; Fig. 6A). In the LPS and MV + LPS groups,
CXCL10, CXCL2, CXCL3, FPR1, IL-6 and NFKB1 were identified as the
key nodes in the PPI network, and EGR1, ATF3 and BCl3 were
identified as the key TFs; thus, a number of their encoding genes
were chosen for verification according to the results of the
bioinformatics analysis. The expression levels of EGR1, ATF3, FPR1,
NFKB1 CXCL10, CXCL2 and CXCL3 were increased in the lung tissues of
mice in the LPS and MV + LPS groups compared with those in the
control group (P<0.05 or P<0.01; Fig. 6B and C).
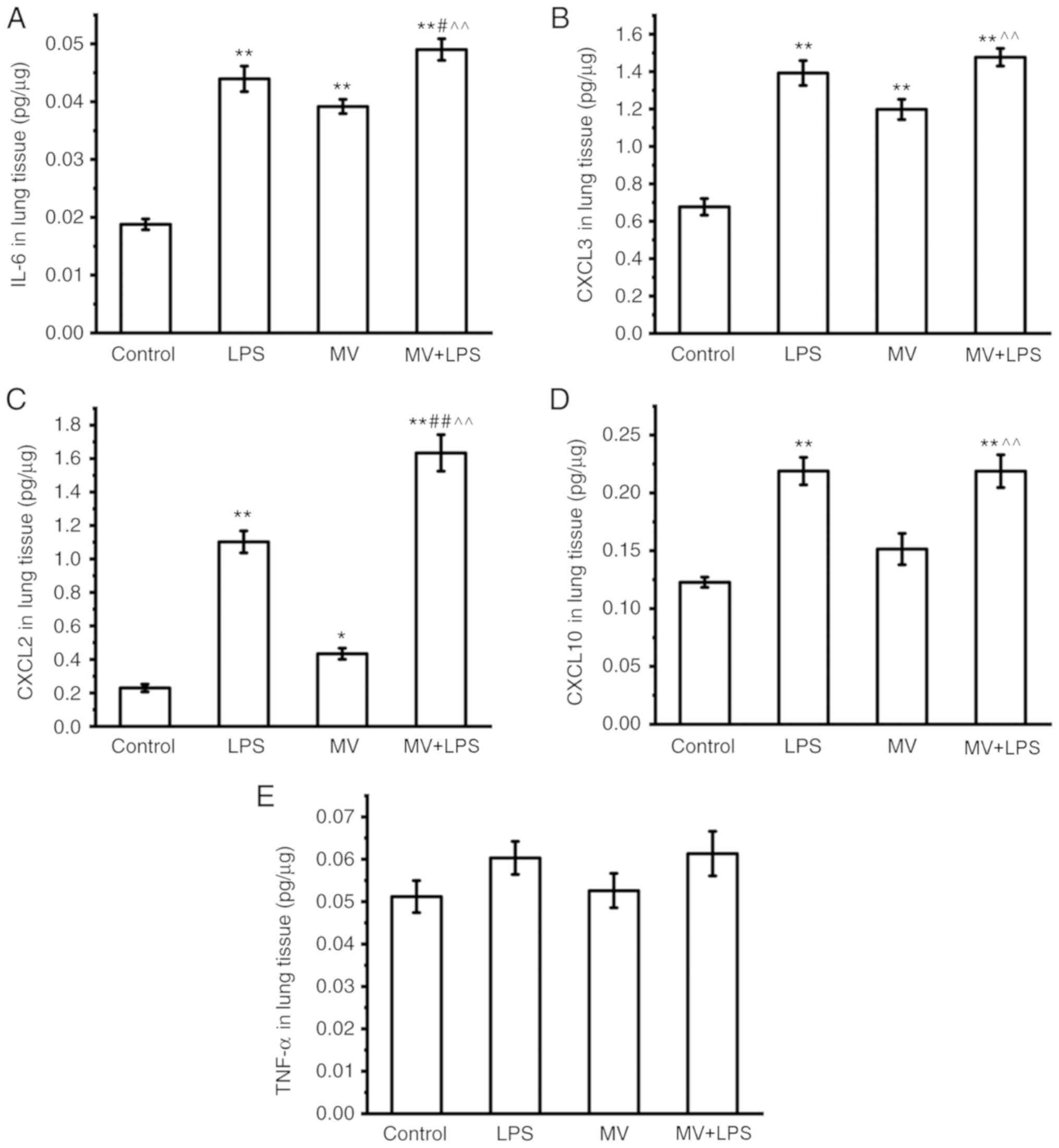
ELISA was used to further confirm the changes at the
protein expression level of the selected genes. As presented in
Fig. 7, the protein levels of
IL-6, CXCL3 and CXCL2 in mouse lung tissues were increased in the
MV, LPS and MV + LPS groups compared with those in the control
group (P<0.05 or P<0.01; Fig.
7A-C). The protein levels of CXCL10 were increased in the LPS
and MV + LPS group, but not in the MV group compared with those in
the control group (P<0.01; Fig.
7D). No significant changes were observed in the levels of
TNF-α in each group (Fig. 7E).
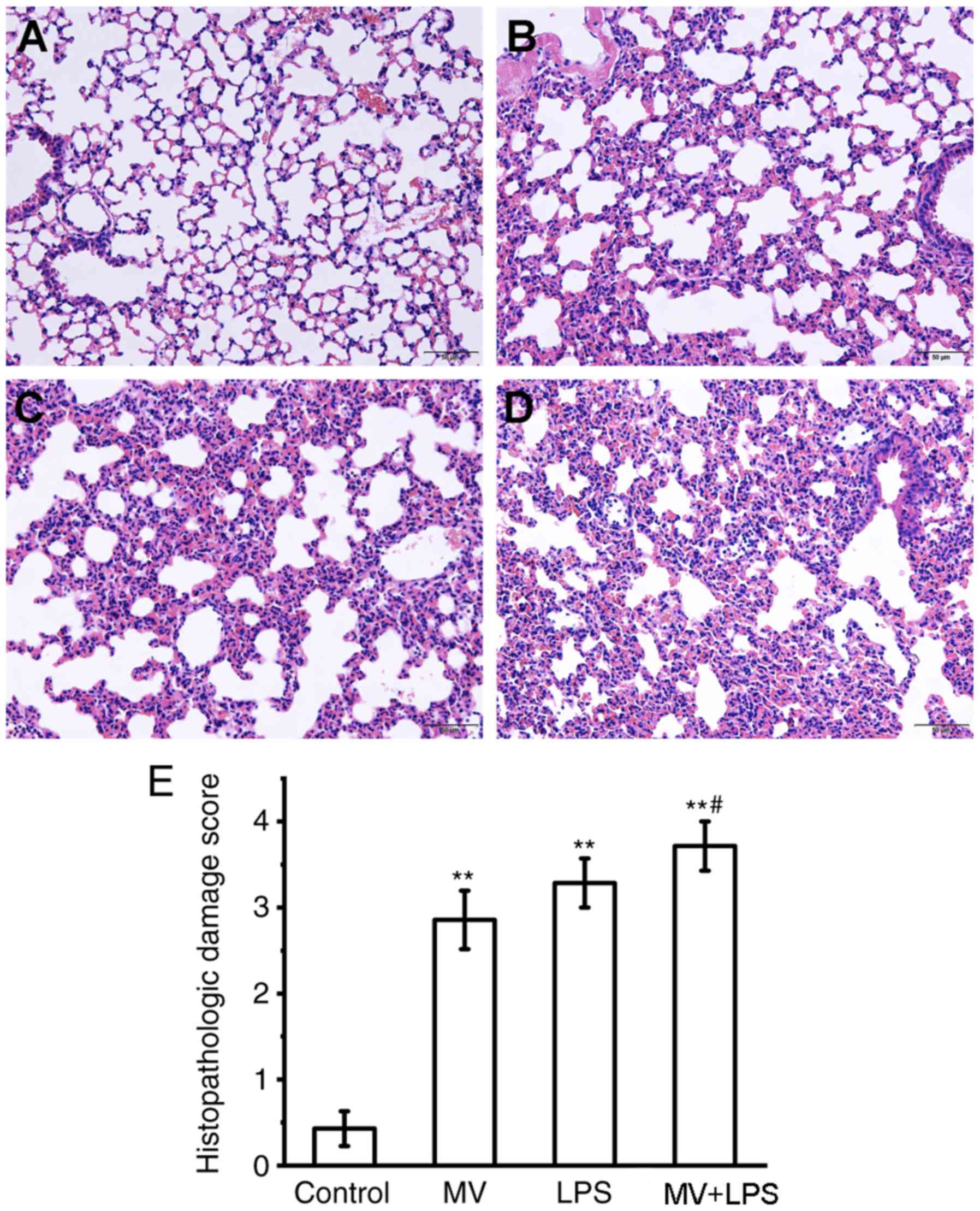
Histological evaluation of lung tissue. Sections of
mouse lung tissue were stained with H&E and scored by
histopathological analysis. Histological analysis of lung tissue
sections revealed that MV, LPS or LPS combined with MV all induced
diffuse interstitial edema, alveolar thickening and marked
decreases in alveolar air space, as well as lung recruitment of
leukocyte and a high histopathological damage score compared with
those in the control group (P<0.01; Fig. 8). Additionally, compared with the
MV group, MV + LPS treatment further aggravated lung injury
(P<0.05 vs. MV group; Fig.
8E).
Discussion
In the present study, a total of 63, 538 and 1,635
DEGs were identified as associated with MV, LPS, and MV + LPS,
respectively. The MV DEG set was significantly enriched in genes
associated with ‘negative regulation of cysteine-type endopeptidase
activity involved in apoptotic process’ and ‘purine ribonucleotide
metabolic process’. The LPS and MV + LPS DEG sets were
significantly enriched in genes associated with ‘cellular response
to cytokine stimulus’, ‘response to molecule of bacterial origin’
and ‘cell chemotaxis’. Notably, these three groups of genes were
significantly enriched in the ‘TNF signaling pathway’ and ‘IL-17
signaling pathway’. Li et al (27) have reported that LPS-induced ALI
may be attenuated by IL-17 via inhibition of the expression of
extracellular signal-regulated kinase 1/2 and NF-κB. Patel et
al (28) have indicated that
TNF induces dysfunction of the alveolar epithelia through induction
of death signaling, and blocking such signaling results in
favorable effects in ALI. These findings suggest that treatment of
the inflammatory response is shared amongst the different
treatments, although certain differences are present between MV and
LPS treatment. In the present study, the MV + LPS group exhibited
the highest number of DEGs, which was greater than the sum of each
group alone, indicating that MV may enhance the effects of LPS on
gene expression. Similar views were proposed in a previous study by
Chen et al (11). Chen
et al (11) reported that
the MV + LPS group generated the most DEGs, suggesting that MV is
able to augment the influence of LPS on gene expression. In
addition, in the present study, the chemokines of the CXC subfamily
(including CXCL2, CXCL3 and CXCL10), EGR1 and ATF3 were upregulated
in the LPS and MV + LPS groups compared with the control group,
whereas ADORA2B, ZBTB16, HCAR2, EGR1 and ATF3 were differentially
expressed in the MV group both in the bioinformatics analysis and
in vivo.
Chemokines are typical basic heparin-binding
proteins with a molecular weight of 7–10 kDa that perform crucial
roles in the migration, recruitment and recirculation of leukocytes
(29). Chemokines are secreted
through stimulation of the resident lung cells by inflammatory
mediators and bacterial products, and are retained at inflammatory
sites by matrix heparin sulfate proteoglycans, forming a gradient
of chemokines toward inflammatory lesions (30). In ALI, based on the chemokine
gradients, inflammatory cells are recruited to the lung, including
neutrophils, macrophages and mononuclear cells, which together with
chemokines have been identified to serve important roles in the
pathogenesis of ALI (30). The
levels of chemokines are increased in the lungs of ALI animal
models, and the severity of lung injury can be reduced by
neutralizing chemokines or their corresponding receptors (31,32).
For instance, Wang et al (31) have demonstrated that influenza
A-induced ALI can be attenuated by treatment with a monoclonal
antibody against CXCL10. Chen et al (10) have suggested that rutin exhibits a
protective effect against LPS-induced ALI by decreasing macrophage
inflammatory protein 2α (also termed CXCL2) levels and inactivating
matrix metalloproteinase 9. Yang et al (33) have demonstrated that tephrosin
exerts a favorable effect against sepsis-induced ALI by
downregulating the expression of intracellular adhesion molecule 1
and CXCL2. C-C motif chemokine ligand 2 (CCL2), also termed MCP1,
was also significantly upregulated in the LPS and MV + LPS groups
in the present study. A previous study has reported high levels of
CCL2 in H7N9 virus-induced ALI in mice and in infected lung
tissues, and that the injury is attenuated in CCL2-deficient mice
(34). The results of the present
study were consistent with the aforementioned previous reports. In
the drug-gene prediction, danazol was predicted to be an inhibitor
of CCL2. Although there are no studies that focus on the effect of
danazol on CCL2 in ALI, it has been reported that danazol can
directly inhibit the expression of CCL2 in endometrial epithelial
cells in a dose-dependent manner (35,36).
This may provide novel ideas and a theoretical basis for the study
and treatment of ALI.
ADORA2B encodes the adenosine A2b receptor, also
termed A2BAR, which is a G protein-coupled receptor (37). The expression of ADORA2B was
upregulated in the MV group compared with that in the control group
in the present study. Eckle et al (38) have previously demonstrated that the
pulmonary adenosine level is increased in mice treated with MV
compared with the control group, and myeloid and pulmonary A2BAR
signaling mediates the inflammatory response of ventilator-induced
lung injury; however, pulmonary A2BARs weaken the
alveolar-capillary barrier and improve alveolar fluid transport,
suggesting that A2BAR is a potential therapeutic target in ALI.
A2BAR may serve roles in the inflammatory response and airway wall
remodeling in asthma, suggesting that A2BAR antagonists may be a
potential new therapeutic approach (39). Theophylline has been predicted to
be an antagonist of A2BAR in previous studies (39,40).
The mechanisms behind the protective effects of A2BAR remain
unclear and require further investigation.
In conclusion, the similarities and differences
between ALI induced by different treatments were analyzed in the
present study. The gene response to MV was significantly enriched
in urine ribonucleotide metabolism-related processes, whereas the
gene response to LPS and LPS+MV was significantly enriched in
‘cellular response to cytokine stimulus’ and ‘cell chemotaxis’. The
involvement in the inflammatory response was shared between the
DEGs identified in the MV and LPS-induced ALI groups. In addition,
MV may enhance the effect of LPS on gene expression. The results of
the present study provide novel insight and a theoretical basis for
the study and treatment of ALI.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by grants from The Hospital
Foundation of Xin Hua Hospital (grant nos. 15YJ04 and 15YJ14) and
The National Natural Science Foundation of China (grant no.
81901991).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WD designed the study and analyzed the
bioinformatics data. ZF conducted the verification experiment. YZ
was responsible for data acquisition and data analysis, and
participated in the animal modeling experiment. ZR conducted
statistical analysis. LJ designed the study, and made substantial
contributions in drafting the manuscript and revising it critically
for important intellectual content. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of Experimental Animals of Shanghai Jiaotong University
School of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Wang C, Zeng L, Zhang T, Liu J and Wang W:
Casticin inhibits lipopolysaccharide-induced acute lung injury in
mice. Eur J Pharmacol. 789:172–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng M, Sang W, Chen S, Chen R, Zhang H,
Xue F, Li Z, Liu Y, Gong Y, Zhang H and Kong X: 4-PBA inhibits
LPS-induced inflammation through regulating ER stress and autophagy
in acute lung injury models. Toxicol Lett. 271:26–37. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niu X, Liu F, Li W, Zhi W, Zhang H, Wang X
and He Z: Cavidine ameliorates lipopolysaccharide-induced acute
lung injury via NF-κB signaling pathway in vivo and in vitro.
Inflammation. 40:1111–1122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Modrykamien AM and Gupta P: The acute
respiratory distress syndrome. Proc (Bayl Univ Med Cent).
28:163–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gramatté J, Pietzsch J, Bergmann R and
Richter T: Causative treatment of acid aspiration induced acute
lung injury-recent trends from animal experiments and critical
perspective. Clin Hemorheol Microcirc. 69:187–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fanelli V and Ranieri VM: Mechanisms and
clinical consequences of acute lung injury. Ann Am Thorac Soc. 12
(Suppl 1):S3–S8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao H, Zhao M, Wang Y, Li F and Zhang Z:
Glycyrrhizic acid prevents sepsis-induced acute lung injury and
mortality in rats. J Histochem Cytochem. 64:125–137. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang W, Luo F, Lu Q, Liu J, Li P, Wang X,
Fu Y, Hao K, Yan T and Ding X: The protective effect of Trillin
LPS-induced acute lung injury by the regulations of inflammation
and oxidative state. Chem Biol Interact. 243:127–134. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Zhao C, He W, Wang Z, Fang Q,
Xiao B, Liu Z, Liang G and Yang S: Discovery and evaluation of
asymmetrical monocarbonyl analogs of curcumin as anti-inflammatory
agents. Drug Des Devel Ther. 8:373–382. 2014.PubMed/NCBI
|
|
10
|
Chen WY, Huang YC, Yang ML, Lee CY, Chen
CJ, Yeh CH, Pan PH, Horng CT, Kuo WH and Kuan YH: Protective effect
of rutin on LPS-induced acute lung injury via down-regulation of
MIP-2 expression and MMP-9 activation through inhibition of Akt
phosphorylation. Int Immunopharmacol. 22:409–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Y, Zhou X and Rong L: Analysis of
mechanical ventilation and lipopolysaccharide induced acute lung
injury using DNA microarray analysis. Mol Med Rep. 11:4239–4245.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Bai C and Wang X: The value of the
lipopolysaccharide-induced acute lung injury model in respiratory
medicine. Expert Rev Respir Med. 4:773–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang A, Pan W, Lv J and Wu H: Protective
effect of Amygdalin on LPS-induced acute lung injury by inhibiting
NF-κB and NLRP3 signaling pathways. Inflammation. 40:745–751. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pedrazza L, Cunha AA, Luft C, Nunes NK,
Schimitz F, Gassen RB, Breda RV, Donadio MV, de Souza Wyse AT,
Pitrez PMC, et al: Mesenchymal stem cells improves survival in
LPS-induced acute lung injury acting through inhibition of NETs
formation. J Cell Physiol. 232:3552–3564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bouferrache K and Vieillard-Baron A: Acute
respiratory distress syndrome, mechanical ventilation, and right
ventricular function. Curr Opin Crit Care. 17:30–35. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gordo-Vidal F and Enciso-Calderón V: Acute
respiratory distress syndrome, mechanical ventilation and right
ventricular function. Med Intensiva. 36:138–142. 2012.(In Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nieman GF, Gatto LA, Bates JH and Habashi
NM: Mechanical ventilation as a therapeutic tool to reduce ARDS
incidence. Chest. 148:1396–1404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mekontso Dessap A, Boissier F, Charron C,
Bégot E, Repessé X, Legras A, Brun-Buisson C, Vignon P and
Vieillard-Baron A: Acute cor pulmonale during protective
ventilation for acute respiratory distress syndrome: Prevalence,
predictors, and clinical impact. Intensive Care Med. 42:862–870.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brochard L, Slutsky A and Pesenti A:
Mechanical ventilation to minimize progression of lung injury in
acute respiratory failure. Am J Respir Crit Care Med. 195:438–442.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sadowitz B, Jain S, Kollisch-Singule M,
Satalin J, Andrews P, Habashi N, Gatto LA and Nieman G: Preemptive
mechanical ventilation can block progressive acute lung injury.
World J Crit Care Med. 5:74–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chian CF, Chiang CH, Chuang CH, Liu SL and
Tsai AC: SN50, a cell-permeable-inhibitor of nuclear factor-κB,
attenuates ventilator-induced lung injury in an isolated and
perfused rat lung model. Shock. 46:194–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slutsky AS and Ranieri VM:
Ventilator-induced lung injury. N Engl J Med. 369:2126–2136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith LS, Gharib SA, Frevert CW and Martin
TR: Effects of age on the synergistic interactions between
lipopolysaccharide and mechanical ventilation in mice. Am J Respir
Cell Mol Biol. 43:475–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
The R Development Core Team, . R: A
language and environment for statistical computing. 2014.
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong WW, Liu YJ, Lv Z, Mao YF, Wang YW,
Zhu XY and Jiang L: Lung endothelial barrier protection by
resveratrol involves inhibition of HMGB1 release and HMGB1-induced
mitochondrial oxidative damage via an Nrf2-dependent mechanism.
Free Radic Biol Med. 88:404–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li TJ, Zhao LL, Qiu J, Zhang HY, Bai GX
and Chen L: Interleukin-17 antagonist attenuates lung inflammation
through inhibition of the ERK1/2 and NF-κB pathway in LPS-induced
acute lung injury. Mol Med Rep. 16:2225–2232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patel BV, Wilson MR, O'Dea KP and Takata
M: TNF-induced death signaling triggers alveolar epithelial
dysfunction in acute lung injury. J Immunol. 190:4274–4282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Puneet P, Moochhala S and Bhatia M:
Chemokines in acute respiratory distress syndrome. Am J Physiol
Lung Cell Mol Physiol. 288:L3–L15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bhatia M, Zemans RL and Jeyaseelan S: Role
of chemokines in the pathogenesis of acute lung injury. Am J Respir
Cell Mol Biol. 46:566–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang W, Yang P, Zhong Y, Zhao Z, Xing L,
Zhao Y, Zou Z, Zhang Y, Li C, Li T, et al: Monoclonal antibody
against CXCL-10/IP-10 ameliorates influenza A (H1N1) virus induced
acute lung injury. Cell Res. 23:577–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bao Z, Ye Q, Gong W, Xiang Y and Wan H:
Humanized monoclonal antibody against the chemokine CXCL-8 (IL-8)
effectively prevents acute lung injury. Int Immunopharmacol.
10:259–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Tian H and Huang X: Tephrosin
attenuates sepsis induced acute lung injury in rats by impeding
expression of ICAM-1 and MIP-2. Microb Pathog. 117:93–99. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lai C, Wang K, Zhao Z, Zhang L, Gu H, Yang
P and Wang X: C-C motif chemokine ligand 2 (CCL2) mediates acute
lung injury induced by lethal influenza H7N9 virus. Front
Microbiol. 8:5872017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boucher A, Lemay A and Akoum A: Effect of
hormonal agents on monocyte chemotactic protein-1 expression by
endometrial epithelial cells of women with endometriosis. Fertil
Steril. 74:969–975. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jolicoeur C, Lemay A and Akoum A:
Comparative effect of danazol and a GnRH agonist on monocyte
chemotactic protein-1 expression by endometriotic cells. Am J
Reprod Immunol. 45:86–93. 2015. View Article : Google Scholar
|
|
37
|
Vecchio EA, White PJ and May LT: The
adenosine A2B G protein-coupled receptor: Recent advances and
therapeutic implications. Pharmacol Ther. 198:20–33. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eckle T, Grenz A, Laucher S and Eltzschig
HK: A2B adenosine receptor signaling attenuates acute lung injury
by enhancing alveolar fluid clearance in mice. J Clin Invest.
118:3301–3315. 2008.PubMed/NCBI
|
|
39
|
Holgate ST: The Quintiles Prize Lecture
2004. The identification of the adenosine A2B receptor as a novel
therapeutic target in asthma. Br J Pharmacol. 145:1009–1015. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Philipp S, Yang XM, Cui L, Davis AM,
Downey JM and Cohen MV: Postconditioning protects rabbit hearts
through a protein kinase C-adenosine A2b receptor cascade.
Cardiovasc Res. 70:308–314. 2006. View Article : Google Scholar : PubMed/NCBI
|