Introduction
Osteoarthritis (OA) is the most common degenerative
joint disease affecting the articular cartilage worldwide, and
causes pain, stiffness and decreased mobility, thus resulting in a
reduced quality of life (1,2). It has been demonstrated that 10–18% of
adults >60 years of age suffer from OA, and the number of
patients with OA is estimated to increase to >130 million
worldwide by the year 2050 (3).
Factors, such as genetics, age, sex, body mass index, nutrition and
joint leptin levels are related to OA (3–5).
Initially, OA was considered to be a disease of the cartilage;
however, it was subsequently demonstrated to be a complex disease
mediated by inflammation (6).
Additionally, high cholesterol levels have been found to be closely
related to OA (7). Currently, an
increasing number of studies are focusing on cholesterol metabolism
and inflammation in OA.
The phosphoinositide 3-kinase (PI3K)/AKT/mammalian
target of rapamycin (mTOR) signaling pathway is a common signaling
pathway that plays a role in the progression of a number of
diseases, and therefore may also be involved in the development of
OA. As previously reported, the inhibition of the PI3K/AKT/mTOR
signaling pathway can attenuate the inflammatory response and
promote autophagy in articular chondrocytes in rats (8). The increment of PI3K/AKT signaling
activation has been demonstrated to be associated with the
degeneration of articular cartilage (9). Thus, the inhibition of the
PI3K/AKT/mTOR signaling pathway has been viewed as a treatment
strategy for OA (10).
Aside from the inflammatory response, high
cholesterol levels are another important risk factor of OA
(11). High levels of low-density
lipoprotein cholesterol (LDL-C) accelerate the incidence of OA in
humans and mice (12,13). The molecular mechanisms are
associated with the cholesterol 25-hydroxylase (CH25H)/cytochrome
P450 family 7 subfamily B polypeptide 1 (CYP7B1)/RAR-related orphan
receptor α axis. In psoriasis, it has been reported that the
activation of the PI3K/AKT/mTOR signaling pathway plays a vital
role in increasing cholesterol levels (14). Another study demonstrated that
insulin-induced cholesterol uptake, lipid droplet content and
apolipoprotein B secretion in CaCo-2 cells were associated with the
PI3K/AKT/mTOR signaling pathway (15). For this reason, the expression
levels of cholesterol-related proteins and proteins in the
PI3K/AKT/mTOR signaling pathway were measured in the present
study.
Chinese herbal medicine can regulate the
PI3K/AKT/mTOR signaling pathways by attenuating interleukin
(IL)-1β-induced apoptosis and extracellular matrix (ECM) catabolism
in OA (16). Chinese herbal
medicine is frequently used as a therapeutic strategy for OA.
Herbs, such as Clematis, Nigella sativa, Radix Angelica
sinensis, Salvia miltiorrhiza and the components purified from
these herbs have been reported to attenuate the development of OA
or even to function as therapies for OA (17–20).
Scutellarin is an active flavonoid isolated from the Chinese
traditional herb Erigeron breviscapus, which has been found
to possess several functions, such as anti-inflammatory and
antioxidant activities (21), and
it has also been reported to exert effects on various types of
tumors, such as malignant melanoma and bladder cancer (22,23).
The effects of scutellarin in OA have been partially
studied; however, its mechanism of action is complex. The present
study investigated the function of scutellarin in regulating the
release of inflammatory cytokines, the expression of collagen- and
cholesterol-related proteins, as well as its effects on the
PI3K/AKT/mTOR signaling pathway. IL-1β was used to stimulate human
osteosarcoma cells (SW1353) to induce OA in vitro, and the
effects of scutellarin with or without a PI3K inhibitor (LY294002)
on IL-1β-induced SW1353 cells were then observed. The results
revealed that scutellarin inhibited the expression of IL-6, and
regulated the expression levels of collagen-related proteins,
collagen II (Col2), SRY-box 9 (SOX9) and matrix metalloproteinase
(MMP)13, and cholesterol-related proteins, CH25H, CYP7B1,
apolipoprotein A-1 (APOA-1) and adenosine triphosphate-binding
cassette transporter A1 (ABCA1). As the expression levels of AKT,
phosphorylated (p)-AKT, mTOR and p-mTOR were suppressed by
scutellarin treatment, it was thus suggested that scutellarin
regulates OA in vitro by inhibiting the PI3K/AKT/mTOR
signaling pathway.
Materials and methods
Reagents
Scutellarin was purchased from Shanghai Yuanye
Bio-Technology Co., Ltd. The Cell Counting Kit-8 (CCK-8) was
obtained from Vazyme Biotech Co., Ltd. LY294002 was purchased from
Sigma-Aldrich (Merck KGaA). IL-1β was obtained from Novoprotein
Scientific, Inc. RIPA lysate was purchased from Beyotime Institute
of Biotechnology. Primary antibodies against Col2 (1:2,000; cat.
no. ab34712), SOX9 (1:1,000; cat. no. ab185966), MMP13 (1:1,000;
cat. no. ab51072), CH25H (1:500; cat. no. ab133933), CYP7B1
(1:1,000; cat. no. ab138497), ABCA1 (1:200; cat. no. ab18180),
APOA-1 (1:500; cat. no. ab75114), PI3K (1:1,000; cat. no. ab32089),
AKT (1:500; cat. no. ab8805), p-AKT (1:500; cat. no. ab38449), mTOR
(1:1,000; cat. no. ab32028) and p-mTOR (1:500; cat. no. ab84400),
and a goat anti-rabbit secondary antibody (1:5,000; cat. no.
ab6721) were purchased from Abcam. The primary antibodies and
secondary antibodies were used according to the manufacturers
instructions.
Cell culture and treatment
SW1353 cells (The Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences) were cultured in
Dulbeccos modified Eagles medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 units/ml penicillin and 100 µg/ml
streptomycin. The cells were maintained at 37°C in 5%
CO2. To assess cell viability, SW1353 cells
(7×103 cells/well) were cultured in 96-well plates
overnight and then treated with increasing concentrations of
scutellarin (0, 5, 10, 20, 40, 80 and 100 µmol/l) for 48 h at 37°C.
For the second part of the experiment, SW1353 cells were
pre-treated with 80 µmol/l scutellarin for 2 h, following which
IL-1β (10 ng/ml) was added to the cells for 48 h at 37°C. The dose
of IL-1β was used as reported by Xue et al (8) and Liu et al (24). Subsequently, 10 µl CCK-8 reagent was
added to each well for a further 2 h of incubation, according to
the manufacturers instructions. The absorbance value was assessed
using a microplate reader (BioTek Epoch; BioTek Instruments, Inc.)
at 450 nm. To inhibit the PI3K/AKT signaling pathway, serum-starved
(0.5% FBS) cells were pre-treated with scutellarin (80 µmol/l) for
2 h and LY294002 (25 µg/ml) for 1 h, and then exposed to IL-1β (10
ng/ml) for 48 h. The test was repeated three times.
ELISA for conditioned medium
The expression levels of C-reactive protein (CRP),
tumor necrosis factor (TNF)-α and IL-6 in conditioned medium
(collected from culture supernatant) were measured using CRP (cat.
no. ZC-31853; ZCi Bio), TNF-α (cat. no. ZC-35733; ZCi Bio) and IL-6
(cat. no. ZC-32446; ZCi Bio) ELISA kits following the manufacturers
instructions.
Western blot analysis
RIPA buffer containing 0.1% protease inhibitor was
used to homogenize the cell samples, and cell lysates were
centrifuged at 13,680 × g for 15 min at 4°C, and the supernatants
were then collected for protein detection. Total protein
concentrations were measured using a BCA kit (Thermo Fisher
Scientific, Inc.). Equal amounts of protein (20 µg per lane for
cell samples) were separated via 10% SDS-PAGE and then transferred
onto PVDF membranes. The membranes were then blocked in 5% skimmed
milk at room temperature for 2 h. After washing in TBST three
times, the immunoblots were incubated with the following primary
antibodies (3% BSA dilution): Col2, SOX9, MMP13, CH25H, CYP7B1,
ABCA1, APOA-1, PI3K, AKT, p-AKT, mTOR and p-mTOR (details of
antibody dilutions were described in the ‘Reagents’ section)
overnight at 4°C. The membranes were then incubated with goat
anti-rabbit IgG secondary antibody (1:5,000, 1% BSA dilution) for 2
h at room temperature. The membranes were washed with TBST again
and then detected with ECL (EMD Millipore). The band sizes were
quantified using Scion Image 4.0 software (Scion Corporation).
Protein expression was normalized relative to β-actin as a loading
control. The final results are expressed as ‘fold changes’ in
comparison with the control group.
Statistical analyses
GraphPad Prism v.6.0 (GraphPad Software, Inc.) was
used to analyze the data generated in the charts in this
experiment. All data are presented as the mean ± SEM, and all
experiments were performed three times. Differences between two
groups were statistically analyzed using an unpaired, two-tailed
Students t-test. Differences among three groups were analyzed by
one-way analysis of variance followed by Tukeys post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of scutellarin on SW1353 cell
viability
To analyze the effects of scutellarin on human
cartilage cells, the SW1353 cell line was used in the present
study. A CCK-8 assay was used to measure the viability of SW1353
cells. As shown in Fig. 1A, no cell
cytotoxicity was observed when the concentration of scutellarin was
<80 µmol/l; however, cell viability was reduced when the
concentration of scutellarin was >80 µmol/l. IL-1β was used to
establish the model of OA using SW1353 cells; treatment of
IL-1β-induced SW1353 cells with scutellarin significantly increased
cell viability (Fig. 1B).
Scutellarin affects the release of
inflammatory cytokines, and the expression of collagen- and
cholesterol-related proteins
Western blot analysis was performed to investigate
whether scutellarin inhibits the release of inflammatory cytokines,
attenuates the degradation of collagen by regulating
collagen-related proteins, and reduces the level of cholesterol by
regulating cholesterol-related proteins. In the present study
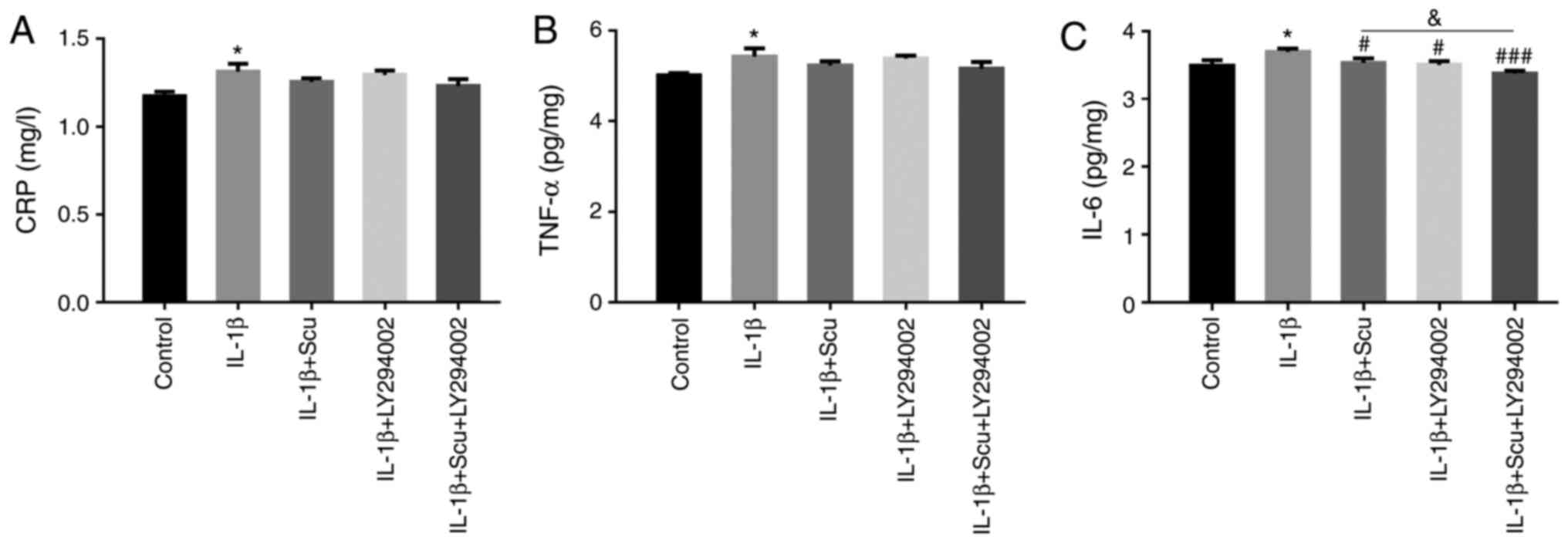
(Fig. 2), the protein expression
levels of CRP, TNF-α and IL-6 were measured. The results revealed
that CRP, TNF-α and IL-6 expression was increased by IL-1β;
however, only the expression of IL-6 was significantly inhibited by
scutellarin treatment (Fig.
2C).
The degradation of collagen is another feature of
OA. As demonstrated in Fig. 3, the
expression levels of Col2 and SOX9 were significantly downregulated
by IL-1β, whereas MMP13 expression was upregulated. Scutellarin
combined with IL-1β significantly increased the expression of Col2
and SOX9 (Fig. 3A-C), and
significantly decreased the expression of MMP13 compared with the
IL-1β group (Fig. 3D).
As aforementioned, high cholesterol level is another
risk factor for OA. The present study measured the expression of
CH25H, CYP7B1, ABCA1 and APOA-1 in SW1535 cells (Fig. 4). In the IL-1β-induced group, the
expression levels of CH25H and CYP7B1 significantly increased, and
those of ABCA1 and APOA-1 significantly decreased compared with the
control group. Following treatment with scutellarin in the
IL-1β-induced cell model, CH25H and CYP7B1 expression levels were
significantly downregulated (Fig. 4B
and C). By contrast, ABCA1 and APOA-1 expression levels
significantly increased (Fig. 4D and
E).
Scutellarin suppresses the
PI3K/AKT/mTOR signaling pathway
In this experiment, the expression levels of AKT,
p-AKT, mTOR and p-mTOR, and the ratio of p-mTOR/mTOR were increased
by IL-1β (Fig. 5). Following
treatment with scutellarin however, the levels of these proteins
were significantly inhibited. Of note however, the expression of
PI3K and the ratio of p-AKT/AKT remained unaltered among the groups
(Fig. 5B and G). These results
suggested that scutellarin inhibited the PI3K/AKT/mTOR signaling
pathway.
 | Figure 5.PI3K/AKT/mTOR signaling pathway in
response to Scu treatment in IL-1β-induced SW1535 cells. (A)
Protein expression levels in the PI3K/AKT/mTOR signaling pathway
were determined by western blotting. (B) The expression of PI3K
exhibited no change following treatment with Scu and IL-1β. Scu
suppressed the IL-1β-induced upregulation of (C) AKT, (D) p-AKT,
(E) mTOR, (F) p-mTOR, (G) p-AKT/AKT and (H) p-mTOR/mTOR. Data are
presented as the mean ± SEM (n=3 in each group). *P<0.05,
***P<0.001 vs. control group; #P<0.05,
##P<0.01, ###P<0.001 vs. IL-1β group.
PI3K, phosphatidylinositol 3 kinase; p-, phosphorylated; mTOR,
mammalian target of rapamycin; Scu, scutellarin; IL-1β, interleukin
1β. |
Scutellarin and LY294002 treatment
affect the release of inflammatory cytokines, and the expression of
collagen- and cholesterol-related proteins
LY294002, as an inhibitor of PI3K, was used to
examine the effects of PI3K on the release of inflammatory
cytokines, and the regulation of collagen- and cholesterol-related
proteins following treatment with scutellarin. Firstly, the
expression levels of CRP, TNF-α and IL-6 were examined (Fig. 6), and only the level of IL-6 was
significantly reduced by scutellarin and LY294002 (Fig. 6C). When compared with the IL-1β +
scutellarin group, the addition of LY294002 significantly decreased
the expression of IL-6.
In regard to the collagen-related proteins (Fig. 7), the IL-1β-induced increase in
MMP13 expression was decreased by treatment with scutellarin and
LY294002, and the greatest decrease was observed in the group
treated with scutellarin and LY294002 in combination (Fig. 7D). By contrast, compared with the
IL-1β-induced model group, the expression of Col2 (Fig. 7B) and SOX9 (Fig. 7C) increased following treatment with
scutellarin and LY294002. When compared with the IL-1β + LY294002
group, the addition of scutellarin significantly increased the
expression of Col2 and SOX9. Thus, scutellarin and LY294002 can
partially restore the loss of Col2 and SOX9 caused by IL-1β.
The expression levels of cholesterol-related
proteins were also regulated by scutellarin and LY294002 (Fig. 8). The IL-1β-induced upregulation of
CH25H and CYP7B1 expression was reversed by scutellarin and
LY294002 (Fig. 8B and C), whereas
the IL-1β-induced downregulation of ABCA1 and APOA-1 expression was
reversed by a combination of scutellarin and LY294002 (Fig. 8D and E).
Inhibition of the PI3K/AKT/mTOR
signaling pathway by scutellarin and LY294002
To directly examine the effect of scutellarin and
LY294002 on the PI3K/AKT/mTOR signaling pathway, the expression
levels of PI3K, AKT, p-AKT, mTOR and p-mTOR were determined
(Fig. 9). The results of the
western blot analysis revealed that scutellarin and LY294002
affected the expression of AKT, mTOR, and the ratio of p-mTOR/mTOR,
but not that of PI3K and the ratio of p-AKT/AKT. In combination,
LY294002 and scutellarin decreased the IL-1β-induced upregulation
of AKT, mTOR and the ratio of p-mTOR/mTOR (Fig. 9C, E and F). Thus, this indicated
that scutellarin and LY294002 are able to inactivate the
PI3K/AKT/mTOR signaling pathway.
 | Figure 9.PI3K/AKT/mTOR signaling pathway in
response to Scu and LY294002 in IL-1β-induced SW1535 cells. (A)
Protein expression bands of PI3K, AKT, p-AKT, mTOR and p-mTOR. (B)
The protein expression of PI3K was not significantly different
among the groups. (C) Scu and LY294002 treatment inhibited the
expression of AKT. (D) The ratio of p-AKT/AKT was not significantly
different among the groups. (E) Scu and LY294002 treatment
inhibited the expression of mTOR. (F) Scu and LY294002 treatment
inhibited the ratio of p-mTOR/mTOR. ***P<0.001 vs. control
group; #P<0.05, ##P<0.01,
###P<0.001 vs. IL-1β group;
&&P<0.01. PI3K, phosphatidylinositol 3
kinase; p-, phosphorylated; mTOR, mammalian target of rapamycin;
Scu, scutellarin; IL-1β, interleukin 1β. |
Discussion
OA, as a chronic disease that results in disability
and a reduced quality of life, results from several different
etiologies (25). A number of
studies have been performed to elucidate the mechanisms responsible
and to identify novel treatment strategies for OA (2). Acetaminophen, a non-steroidal
anti-inflammatory drug, and corticosteroids as oral drugs, are
common pharmacological therapies for the management of OA. However,
they may cause toxicity when at high doses and are associated with
adverse reactions, such as gastric ulceration, kidney dysfunction
and increased bleeding under particular conditions (3,26).
Thus, the development of effective drugs with limited side effects
is of utmost importance. Recently, Chinese Traditional Medicine has
gained increasing attention. Effective components of Erigeron
breviscapus, including scutellarin, have been reported to
possess anti-inflammatory properties (27) and to restrain the development of
tumors (22). In the present study,
the effects of scutellarin on the PI3K/AKT/mTOR signaling pathway
were examined, and it was determined whether it can inhibit the
inflammatory response, and the expression of collagen- and
cholesterol-related proteins in the SW1353 cell model of OA.
OA is regarded as an inflammatory disease (6). Several inflammatory cytokines are
secreted during OA, and biomarkers of inflammation, including CRP
and TNF-α are elevated. IL-6 is an inflammatory cytokine that is
associated with systemic low-grade inflammation, and its expression
is also altered in OA (28).
Scutellarin has been reported to inhibit the expression of
inflammatory factors, such as IL-1β, IL-6 and TNF-α in
collagen-induced arthritis (29).
Another study reported that treatment with scutellarin
downregulated the expression levels of IL-1β, TNF-α and IL-6 in the
serum of osteoarthritis mice (30).
In the present study, it was found that only the expression of IL-6
was influenced by scutellarin. The levels of CRP and TNF-α were not
altered. Naderi et al (31)
measured the effects of Zingiber officinale on patients with
knee osteoarthritis, and no changes in the levels of CRP were found
within 3 months. Another study demonstrated that the expression of
CRP had no association with the incidence or progression of OA
(32). Besides, in the present
study, scutellarin failed to inhibit the IL-1β-induced increase in
TNF-α expression. In fact, unlike the chondroid cells isolated from
patients and in vivo animal models, the expression levels of
CRP, TNF-α and IL-6 were all only slightly increased by IL-1β in
SW1353 cells. This was a limitation of the present research.
Although the SW1353 cell line used to establish the cell model of
OA has been reported on by some previous research (33–35)
because the cell lines could provide enough and steady cells for
the research, the IL-1β-induced OA cell model used in the present
study cannot completely mimic the in vivo environment. This
may partly explain why there were no changes in the expression
levels of CRP and TNF-α in this study.
In OA, the expression of Col2 and SOX9 are
influenced by MMP13. As reported by Otero et al (36), the expression of ETS-related
transcription factor Elf-3 protein driven by MMP13 was increased in
OA-affected cartilage, and it inhibited SOX9-driven COL2A1 promoter
activity. Ouyang et al (37)
also found decreased expression of Col2 and SOX9, but increased
expression of MMP13 in OA. Thus, indicating that the expression of
MMP13 is enhanced, whereas that of Col2 and SOX9 is inhibited in
OA. Fisetin is a polyphenol extracted from fruit and vegetables,
and it can inhibit the IL-1β-induced upregulation of MMP13
expression and decrease the degradation of Col2 and SOX9 in OA
(38). Scutellarin has been
demonstrated to have similar functions; the mRNA expression of
MMP13 was found to be decreased, whereas Col2 expression was
increased by treatment with scutellarin in OA mouse models
(30). These findings were
supported by those of another study in human primary chondrocytes
(24). In the present study,
similar results were obtained. The expression of Col2 and SOX9
increased and the expression of MMP13 decreased by treatment with
scutellarin. These results suggested that scutellarin can attenuate
the degradation of cartilage in OA.
The function of scutellarin in regulating
cholesterol-related protein expression in the OA cell model was
determined in the present study. The results revealed that
scutellarin downregulated the expression of CH25H and CYP7B1 and
upregulated the expression of ABCA1 and APOA-1. As a metabolic
disorder disease (39), it has been
demonstrated that cholesterol metabolism is abnormal in OA with
increased levels of LDL (40).
Tsezou et al (41) reported
that APOA-1 and ABCA1 expression decreased in OA-affected
cartilage. On the other hand, the expression levels of CH25H and
CYP7B1 have been found to be increased, leading to high levels of
cholesterol (42). The findings of
the present study were consistent with these previous findings,
therefore demonstrating that scutellarin can decrease total
cholesterol levels and improve lipid metabolism (43,44).
The PI3K/AKT/mTOR signaling pathway has been found
to be associated with OA (22,45),
and the inhibition of this pathway can attenuate inflammation and
articular cartilage degeneration in OA (8–10). In
the present study, no significant changes were identified in the
protein expression of PI3K between the groups, which indicated that
IL-1β, scutellarin and LY294002 may not affect the expression of
PI3K. The present study also found that scutellarin inhibited the
expression of AKT, mTOR and p-mTOR, and the ratio of p-mTOR/mTOR in
the IL-1β-induced cells. The ratio of p-AKT/AKT may not have been
affected because the decrease in p-AKT expression may be caused by
the decrease in AKT expression and the decrease in AKT expression
essentially leads to the decrease in mTOR activity. It has been
revealed that the activation of PI3K/AKT signaling can degrade
chondrocytes and accelerate the progression of OA (8–10).
Thus, these findings suggested that scutellarin can attenuate OA by
regulating the PI3K/AKT/mTOR signaling pathway.
In conclusion, the primary finding of the present
study was that scutellarin inhibited the activation of the
PI3K/AKT/mTOR signaling pathway, which alleviated cartilage
degradation by regulating relevant protein levels, and decreased
cholesterol levels by regulating cholesterol-related protein
expression in the OA cell model; however, it only affected the
release of the inflammatory cytokine, IL-6. These findings are
important for future studies investigating the function of
scutellarin in OA and they provide evidence that scutellarin could
be used as a latent medicine for OA. However, further studies are
required to confirm the pharmacological functions of scutellarin
in vivo and to explore the precise underlying
mechanisms.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
Application Foundation Fund of Science and Technology Department of
Sichuan Province (grant no. 2018JY0264), Innovative topics of
Chengdu Sport University (grant no. CX19C01) and Innovation team of
Chengdu Sport University (grant no. CXTD201805).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors contributions
SHJ, BXH and LRT designed the study. SHJ, PWL and
YLT performed the experiments. YTZ, XHL and MJW analyzed the data.
SHJ wrote the manuscript. All authors critically reviewed the
manuscript, and read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pereira D, Ramos E and Branco J:
Osteoarthritis. Acta Med Port. 28:99–106. 2015. View Article : Google Scholar
|
|
2
|
Nelson AE: Osteoarthritis year in review
2017: Clinical. Osteoarthritis Cartilage. 26:319–325. 2018.
View Article : Google Scholar
|
|
3
|
Saccomano SJ: Osteoarthritis treatment:
Decreasing pain, improving mobility. Nurse Pract. 43:49–55. 2018.
View Article : Google Scholar
|
|
4
|
Palazzo C, Nguyen C, Lefevre-Colau MM,
Rannou F and Poiraudeau S: Risk factors and burden of
osteoarthritis. Ann Phys Rehabil Med. 59:134–138. 2016. View Article : Google Scholar
|
|
5
|
Vina ER and Kwoh CK: Epidemiology of
osteoarthritis: Literature update. Curr Opin Rheumatol. 30:160–167.
2018. View Article : Google Scholar
|
|
6
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013. View Article : Google Scholar
|
|
7
|
Farnaghi S, Crawford R, Xiao Y and
Prasadam I: Cholesterol metabolism in pathogenesis of
osteoarthritis disease. Int J Rheum Dis. 20:131–140. 2017.
View Article : Google Scholar
|
|
8
|
Xue JF, Shi ZM, Zou J and Li XL:
Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of
articular chondrocytes and attenuates inflammatory response in rats
with osteoarthritis. Biomed Pharmacother. 89:1252–1261. 2017.
View Article : Google Scholar
|
|
9
|
Lin C, Shao Y, Zeng C, Zhao C, Fang H,
Wang L, Pan J, Liu L, Qi W, Feng X, et al: Blocking PI3K/AKT
signaling inhibits bone sclerosis in subchondral bone and
attenuates post-traumatic osteoarthritis. J Cell Physiol.
233:6135–6147. 2018. View Article : Google Scholar
|
|
10
|
Chen J, Crawford R and Xiao Y: Vertical
inhibition of the PI3K/Akt/mTOR pathway for the treatment of
osteoarthritis. J Cell Biochem. 114:245–249. 2013. View Article : Google Scholar
|
|
11
|
Farnaghi S, Crawford R, Xiao Y and
Prasadam I: Cholesterol metabolism in pathogenesis of
osteoarthritis disease. Int J Rheum. 20:131–140. 2017. View Article : Google Scholar
|
|
12
|
de Munter W, van der Kraan PM, van den
Berg WB and van Lent PL: High systemic levels of low-density
lipoprotein cholesterol: Fuel to the flames in inflammatory
osteoarthritis? Rheumatology. 55:16–24. 2016. View Article : Google Scholar
|
|
13
|
Beier F: Cholesterol and cartilage do not
mix well. Nat Rev Rheumatol. 15:253–254. 2019. View Article : Google Scholar
|
|
14
|
Varshney P and Saini N: PI3K/AKT/mTOR
activation and autophagy inhibition plays a key role in increased
cholesterol during IL-17A mediated inflammatory response in
psoriasis. Biochim Biophys Acta Mol Basis Dis. 1864((5 Pt A)):
1795–1803. 2018. View Article : Google Scholar
|
|
15
|
Fuentes M, Santander N and Cortés V:
Insulin increases cholesterol uptake, lipid droplet content, and
apolipoprotein B secretion in CaCo-2 cells by upregulating SR-BI
via a PI3K, AKT, and mTOR-dependent pathway. J Cell Biochem.
October 2–2018.(Online ahead of print). doi: 10.1002/jcb.27410.
|
|
16
|
Yang Y, Gu Y, Zhao H and Zhang S: Loganin
attenuates osteoarthritis in rats by inhibiting IL-1β-induced
catabolism and apoptosis in chondrocytes via regulation of
phosphatidylinositol 3-lkinases (PI3K)/Akt. Med Sci Monit.
25:4159–4168. 2019. View Article : Google Scholar
|
|
17
|
Li X, Wu D, Hu Z, Xuan J, Ding X, Zheng G,
Feng Z, Ni W and Wu A: The protective effect of ligustilide in
osteoarthritis: An in vitro and in vivo study. Cell Physiol
Biochem. 48:2583–2595. 2018. View Article : Google Scholar
|
|
18
|
Pan T, Cheng TF, Jia YR, Li P and Li F:
Anti-rheumatoid arthritis effects of traditional Chinese herb
couple in adjuvant-induced arthritis in rats. J Ethnopharmacol.
205:1–7. 2017. View Article : Google Scholar
|
|
19
|
Salimzadeh A, Ghourchian A, Choopani R,
Hajimehdipoor H, Kamalinejad M and Abolhasani M: Effect of an
orally formulated processed black cumin, from Iranian traditional
medicine pharmacopoeia, in relieving symptoms of knee
osteoarthritis: A prospective, randomized, double-blind and
placebo-controlled clinical trial. Int J Rheum Dis. 20:691–701.
2017. View Article : Google Scholar
|
|
20
|
Xu X, Lv H, Li X, Su H, Zhang X and Yang
J: Danshen attenuates osteoarthritis-related cartilage degeneration
through inhibition of NF-kappaB signaling pathway in vivo and in
vitro. Bio Cell Biol. 95:644–651. 2017. View Article : Google Scholar
|
|
21
|
Wang L and Ma Q: Clinical benefits and
pharmacology of scutellarin: A comprehensive review. Pharmacol
Ther. 190:105–127. 2018. View Article : Google Scholar
|
|
22
|
Li CY, Wang Q, Wang X, Li G, Shen S and
Wei X: Scutellarin inhibits the invasive potential of malignant
melanoma cells through the suppression epithelial-mesenchymal
transition and angiogenesis via the PI3K/Akt/mTOR signaling
pathway. Eur J Pharmacol. 858:1724632019. View Article : Google Scholar
|
|
23
|
Lv WL, Liu Q, An JH and Song XY:
Scutellarin inhibits hypoxia-induced epithelial-mesenchymal
transition in bladder cancer cells. J Cell Physiol.
234:23169–23175. 2019. View Article : Google Scholar
|
|
24
|
Liu F, Li L, Lu W, Ding Z, Huang W, Li YT,
Cheng C, Shan WS, Xu J, He W, et al: Scutellarin ameliorates
cartilage degeneration in osteoarthritis by inhibiting the
Wnt/β-catenin and MAPK signaling pathways. Int Immunopharmacol.
78:1059542020. View Article : Google Scholar
|
|
25
|
Lane NE, Shidara K and Wise BL:
Osteoarthritis year in review 2016: Clinical. Osteoarthritis
Cartilage. 25:209–215. 2017. View Article : Google Scholar
|
|
26
|
Zhang W, Ouyang H, Dass CR and Xu J:
Current research on pharmacologic and regenerative therapies for
osteoarthritis. Bone Res. 4:150402016. View Article : Google Scholar
|
|
27
|
Luo P, Zhang Z, Yi T, Zhang H, Liu X and
Mo Z: Anti-inflammatory activity of the extracts and fractions from
Erigeron multiradiatus through bioassay-guided procedures. J
Ethnopharmacol. 119:232–237. 2008. View Article : Google Scholar
|
|
28
|
Watt FE: Osteoarthritis biomarkers: Year
in review. Osteoarthritis Cartilage. 26:312–318. 2018. View Article : Google Scholar
|
|
29
|
Zhang L, Sun S, Li W, Zhang W, Wang X and
Yang SY: Effect of Scutellarin inhibits collagen-induced arthritis
through TLR4/NF-κB-mediated inflammation. Mol Med Rep.
16:5555–5560. 2017. View Article : Google Scholar
|
|
30
|
Wang W, Li J, Li F, Peng J, Xu M,
Shangguan Y, Li Y, Zhao Y, Qiu C, Qu R, et al: Scutellarin
suppresses cartilage destruction in osteoarthritis mouse model by
inhibiting the NF-κB and PI3K/AKT signaling pathways. Int
Immunopharmacol. 77:1059282019. View Article : Google Scholar
|
|
31
|
Naderi Z, Mozaffari-Khosravi H, Dehghan A,
Nadjarzadeh A and Huseini HF: Effect of ginger powder
supplementation on nitric oxide and C-reactive protein in elderly
knee osteoarthritis patients: A 12-week double-blind randomized
placebo-controlled clinical trial. J Tradit Complement Med.
6:199–203. 2015. View Article : Google Scholar
|
|
32
|
Kerkhof HJ, Bierma-Zeinstra SM,
Castano-Betancourt MC, de Maat MP, Hofman A, Pols HA, Rivadeneira
F, Witteman JC, Uitterlinden AG and van Meurs JB: Serum C reactive
protein levels and genetic variation in the CRP gene are not
associated with the prevalence, incidence or progression of
osteoarthritis independent of body mass index. Ann Rheum Dis.
69:1976–1982. 2010. View Article : Google Scholar
|
|
33
|
Xu X, Liu X, Yang Y, He J, Gu H, Jiang M,
Huang Y, Liu X and Liu L: Resveratrol inhibits the development of
obesity-related osteoarthritis via the TLR4 and PI3K/Akt signaling
pathways. Connect Tissue Res. 60:571–582. 2019. View Article : Google Scholar
|
|
34
|
Gao F and Zhang S: Salicin inhibits
AGE-induced degradation of type II collagen and aggrecan in human
SW1353 chondrocytes: Therapeutic potential in osteoarthritis. Artif
Cells Nanomed Biotechnol. 47:1043–1049. 2019. View Article : Google Scholar
|
|
35
|
Hong GU, Lee JY, Kang H, Kim TY, Park JY,
Hong EY, Shin YH, Jung SH, Chang HB, Kim YH, et al: Inhibition of
osteoarthritis-eelated molecules by isomucronulatol
7-O-β-d-glucoside and Ecliptasaponin A in IL-1β-stimulated
chondrosarcoma cell model. Molecules. 23:28072018.doi:
10.3390/molecules23112807. View Article : Google Scholar
|
|
36
|
Otero M, Peng H, Hachem KE, Culley KL,
Wondimu EB, Quinn J, Asahara H, Tsuchimochi K, Hashimoto K and
Goldring MB: ELF3 modulates type II collagen gene (COL2A1)
transcription in chondrocytes by inhibiting SOX9-CBP/p300-driven
histone acetyltransferase activity. Connect Tissue Res. 58:15–26.
2017. View Article : Google Scholar
|
|
37
|
Ouyang Y, Wang W, Tu B, Zhu Y, Fan C and
Li Y: Overexpression of SOX9 alleviates the progression of human
osteoarthritis in vitro and in vivo. Drug Des Devel Ther.
13:2833–2842. 2019. View Article : Google Scholar
|
|
38
|
Zheng W, Feng Z, You S, Zhang H, Tao Z,
Wang Q, Chen H and Wu Y: Fisetin inhibits IL-1β-induced
inflammatory response in human osteoarthritis chondrocytes through
activating SIRT1 and attenuates the progression of osteoarthritis
in mice. Int Immunopharmacol. 45:135–147. 2017. View Article : Google Scholar
|
|
39
|
Mobasheri A, Rayman MP, Gualillo O, Sellam
J, van der Kraan P and Fearon U: The role of metabolism in the
pathogenesis of osteoarthritis. Nat Rev Rheumatol. 13:302–311.
2017. View Article : Google Scholar
|
|
40
|
de Munter W, van den Bosch MH, Slöetjes
AW, Croce KJ, Vogl T, Roth J, Koenders MI, van de Loo FA, van den
Berg WB, van der Kraan PM, et al: High LDL levels lead to increased
synovial inflammation and accelerated ectopic bone formation during
experimental osteoarthritis. Osteoarthritis Cartilage. 24:844–855.
2016. View Article : Google Scholar
|
|
41
|
Tsezou A, Iliopoulos D, Malizos KN and
Simopoulou T: Impaired expression of genes regulating cholesterol
efflux in human osteoarthritic chondrocytes. J Orthop Res.
28:1033–1039. 2010. View Article : Google Scholar
|
|
42
|
Choi WS, Lee G, Song WH, Koh JT, Yang J,
Kwak JS, Kim HE, Kim SK, Son YO, Nam H, et al: The
CH25H-CYP7B1-RORα axis of cholesterol metabolism regulates
osteoarthritis. Nature. 566:254–258. 2019. View Article : Google Scholar
|
|
43
|
Li Q, Wu JH, Guo DJ, Cheng HL, Chen SL and
Chan SW: Suppression of diet-induced hypercholesterolemia by
scutellarin in rats. Planta Med. 75:1203–1208. 2009. View Article : Google Scholar
|
|
44
|
Fan H, Ma X, Lin P, Kang Q, Zhao Z, Wang
L, Sun D, Cheng J and Li Y: Scutellarin prevents nonalcoholic fatty
liver disease (NAFLD) and hyperlipidemia via PI3K/AKT-dependent
activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) in
rats. Med Sci Monit. 23:5599–5612. 2017. View Article : Google Scholar
|
|
45
|
Tang SL, Gao YL and Hu WZ: Scutellarin
inhibits the metastasis and cisplatin resistance in glioma cells.
OncoTargets Ther. 12:587–598. 2019. View Article : Google Scholar
|