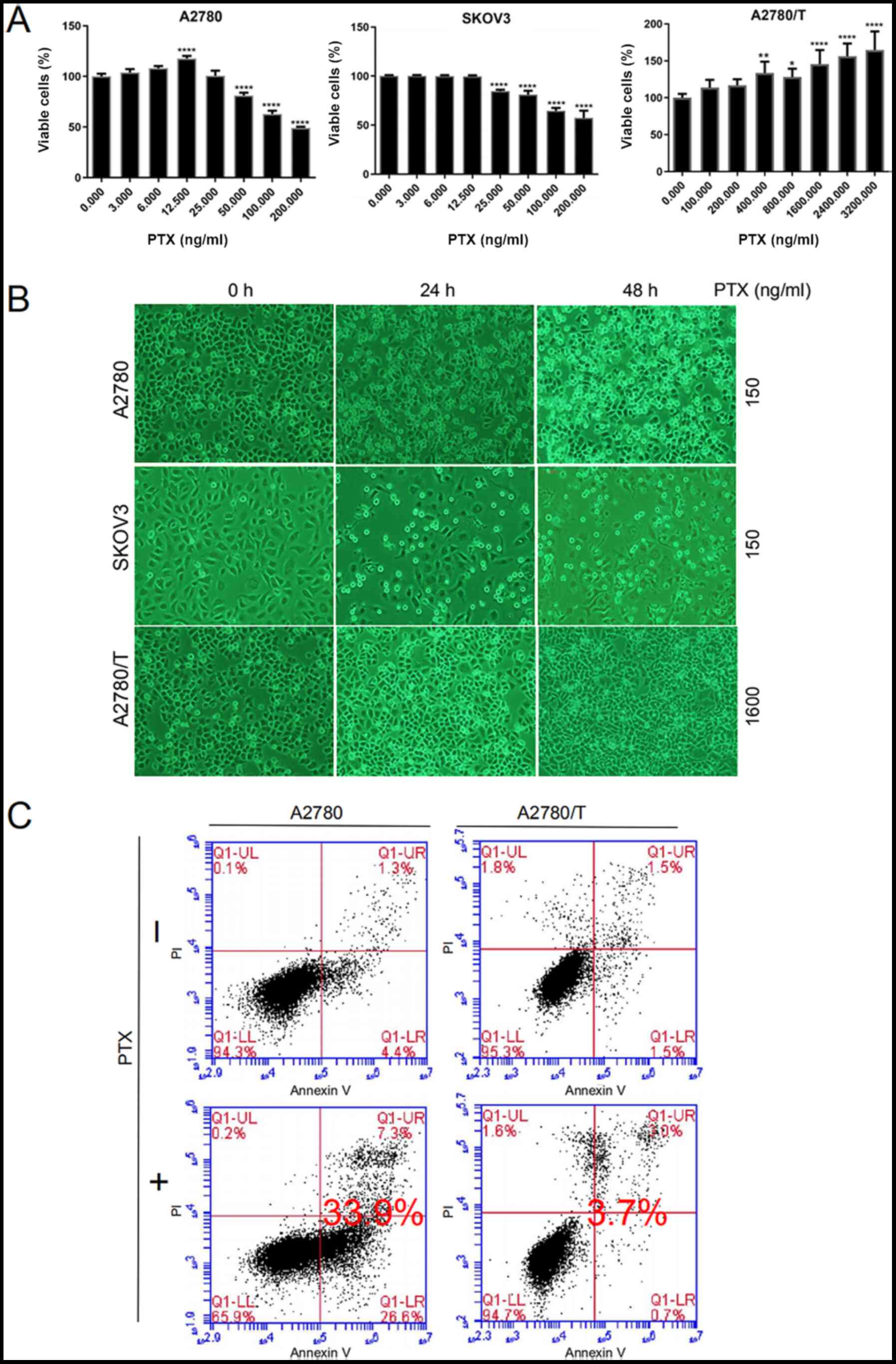
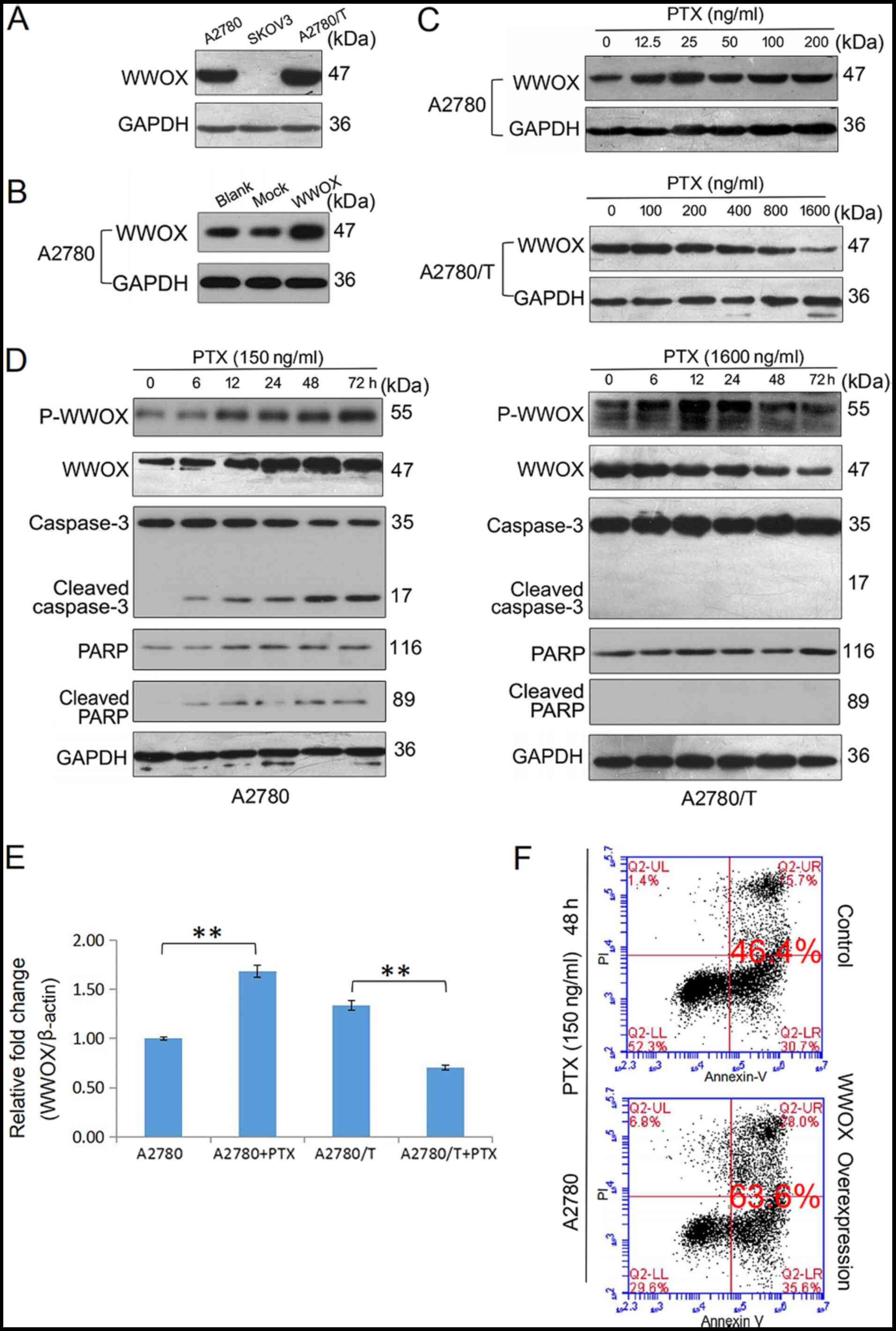
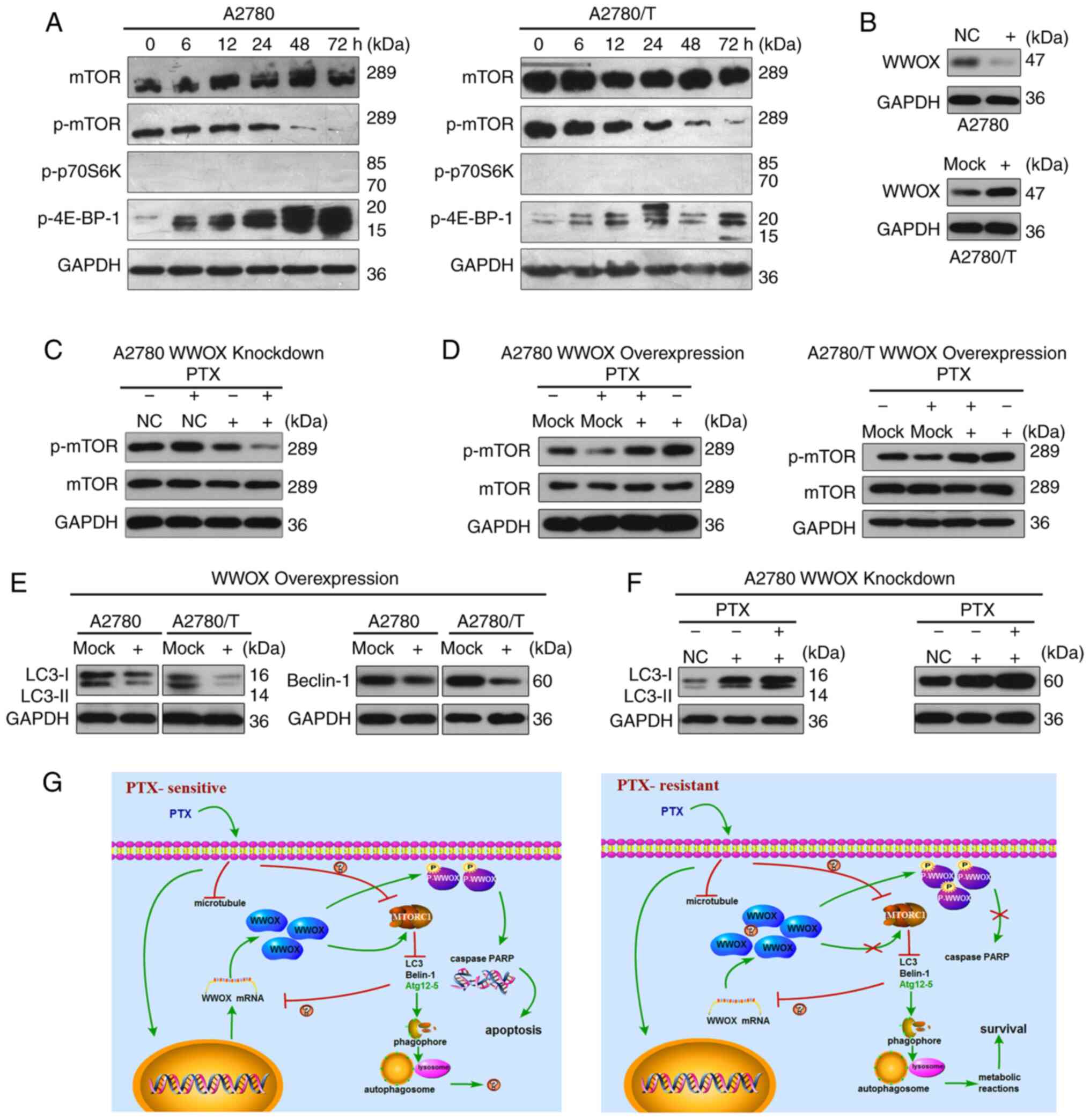
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mozzetti S, Ferlini C, Concolino P,
Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E,
Ranelletti FO, Ferrandina G, et al: Class III beta-tubulin
overexpression is a prominent mechanism of paclitaxel resistance in
ovarian cancer patients. Clin Cancer Res. 11:298–305.
2005.PubMed/NCBI
|
|
3
|
Veldhoen RA, Banman SL, Hemmerling DR,
Odsen R, Simmen T, Simmonds AJ, Underhill DA and Goping IS: The
chemotherapeutic agent paclitaxel inhibits autophagy through two
distinct mechanisms that regulate apoptosis. Oncogene. 32:736–746.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee Y, Na J, Lee MS, Cha EY, Sul JY, Park
JB and Lee JS: Combination of pristimerin and paclitaxel additively
induces autophagy in human breast cancer cells via ERK1/2
regulation. Mol Med Rep. 18:4281–4288. 2018.PubMed/NCBI
|
|
5
|
Zhan L, Zhang Y, Wang W, Song E, Fan Y, Li
J and Wei B: Autophagy as an emerging therapy target for ovarian
carcinoma. Oncotarget. 7:83476–83487. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hale AN, Ledbetter DJ, Gawriluk TR and
Rucker EB III: Autophagy: Regulation and role in development.
Autophagy. 9:951–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang C, Yang Y, Sun L, Wang J, Jiang Z, Li
Y, Liu D, Sun H and Pan Z: Baicalin reverses radioresistance in
nasopharyngeal carcinoma by downregulating autophagy. Cancer Cell
Int. 20:352020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tyutyunyk-Massey L and Gewirtz DA: Roles
of autophagy in breast cancer treatment: Target, bystander or
benefactor. Semin Cancer Biol. 66:155–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao K, Yin J and Dong J: Deregulated WWOX
is involved in a negative feedback loop with microRNA-214-3p in
osteosarcoma. Int J Mol Med. 38:1850–1856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan H, Yu N and Tong J: Effects of
5-Aza-2′-deoxycytidine on the methylation state and function of the
WWOX gene in the HO-8910 ovarian cancer cell line. Oncol Lett.
6:845–849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo W, Wang G, Dong Y, Guo Y, Kuang G and
Dong Z: Decreased expression of WWOX in the development of
esophageal squamous cell carcinoma. Mol Carcinog. 52:265–274. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Liu J, Ren Y and Liu P: Roles of the
WWOX in pathogenesis and endocrine therapy of breast cancer. Exp
Biol Med (Maywood). 240:324–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baykara O, Demirkaya A, Kaynak K, Tanju S,
Toker A and Buyru N: WWOX gene may contribute to progression of
non-small-cell lung cancer (NSCLC). Tumour Biol. 31:315–320. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Del MS, Husanie H, Iancu O, Abu-Odeh M,
Evangelou K, Lovat F, Volinia S, Gordon J, Amir G, Stein J, et al:
WWOX and p53 dysregulation synergize to drive the development of
osteosarcoma. Cancer Res. 76:6107–6117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aderca I, Moser CD, Veerasamy M, Bani-Hani
AH, Bonilla-Guerrero R, Ahmed K, Shire A, Cazanave SC, Montoya DP,
Mettler TA, et al: The JNK inhibitor SP600129 enhances apoptosis of
HCC cells induced by the tumor suppressor WWOX. J Hepatol.
49:373–383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang NS, Doherty J, Ensign A, Lewis J,
Heath J, Schultz L, Chen ST and Oppermann U: Molecular mechanisms
underlying WOX1 activation during apoptotic and stress responses.
Biochem Pharmacol. 66:1347–1354. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai CW, Lai FJ, Sheu HM, Lin YS, Chang
TH, Jan MS, Chen SM, Hsu PC, Huang TT, Huang TC, et al: WWOX
suppresses autophagy for inducing apoptosis in methotrexate-treated
human squamous cell carcinoma. Cell Death Dis. 4:e7922013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong W, Mao J, Yang Y, Yuan J, Chen J, Luo
Y, Lai T and Zuo L: Mechanisms of mTOR and autophagy in human
endothelial cell infected with dengue Virus-2. Viral Immunol.
33:61–70. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jia J, Abudu YP, Claude-Taupin A, Gu Y,
Kumar S, Choi SW, Peters R, Mudd MH, Allers L, Salemi M, et al:
Galectins control MTOR and AMPK in response to lysosomal damage to
induce autophagy. Autophagy. 15:169–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Munson MJ and Ganley IG: MTOR, PIK3C3, and
autophagy: Signaling the beginning from the end. Autophagy.
11:2375–2376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
simplehttps://www.atcc.org/products/all/HTB-77.aspx#characteristics
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using Real-Time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu BS, Tan JW, Zhu GH, Wang DF, Zhou X and
Sun ZQ: WWOX induces apoptosis and inhibits proliferation of human
hepatoma cell line SMMC-7721. World J Gastroenterol. 18:3020–3026.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lo JY, Chou YT, Lai FJ and Hsu LJ:
Regulation of cell signaling and apoptosis by tumor suppressor
WWOX. Exp Biol Med (Maywood). 240:383–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ekizoglu S, Bulut P, Karaman E, Kilic E
and Buyru N: Epigenetic and genetic alterations affect the WWOX
gene in head and neck squamous cell carcinoma. PLoS One.
10:e01153532015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Li P, Yang Z and Mo WN: Expression
of fragile histidine triad (FHIT) and WW-domain oxidoreductase gene
(WWOX) in nasopharyngeal carcinoma. Asian Pac J Cancer Prev.
14:165–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barth S, Glick D and Macleod KF:
Autophagy: Assays and artifacts. J Pathol. 221:117–124. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmitz KJ, Ademi C, Bertram S, Schmid KW
and Baba HA: Prognostic relevance of autophagy-related markers LC3,
p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients
with respect to KRAS mutational status. World J Surg Oncol.
14:1892016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li RF, Chen G, Ren JG, Zhang W, Wu ZX, Liu
B, Zhao Y and Zhao YF: The adaptor protein p62 is involved in
RANKL-induced autophagy and osteoclastogenesis. J Histochem
Cytochem. 62:879–888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Noureldein MH and Eid AA: Gut microbiota
and mTOR signaling: Insight on a new pathophysiological
interaction. Microb Pathog. 118:98–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan H, Tong J, Lin X, Han Q and Huang H:
Effect of the WWOX gene on the regulation of the cell cycle and
apoptosis in human ovarian cancer stem cells. Mol Med Rep.
12:1783–1788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin JT, Li HY, Chang NS, Lin CH, Chen YC
and Lu PJ: WWOX suppresses prostate cancer cell progression through
cyclin D1-mediated cell cycle arrest in the G1 phase. Cell Cycle.
14:408–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chiang MF, Chou PY, Wang WJ, Sze CI and
Chang NS: Tumor suppressor WWOX and p53 alterations and drug
resistance in glioblastomas. Front Oncol. 3:432013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Płuciennik E, Nowakowska M, Gałdyszyńska
M, Popęda M and Bednarek AK: The influence of the WWOX gene on the
regulation of biological processes during endometrial
carcinogenesis. Int J Mol Med. 37:807–815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baryła I, Styczeń-Binkowska E and Bednarek
AK: Alteration of WWOX in human cancer: A clinical view. Exp Biol
Med (Maywood). 240:305–314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hung PS, Chuang FJ, Chen CY, Chou CH, Tu
HF and Lo SS: miR-187* enhances SiHa cervical cancer cell
oncogenicity via suppression of WWOX. Anticancer Res. 40:1427–1436.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakayama S, Semba S, Maeda N, Aqeilan RI,
Huebner K and Yokozaki H: Role of the WWOX gene, encompassing
fragile region FRA16D, in suppression of pancreatic carcinoma
cells. Cancer Sci. 99:1370–1376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khawaled S, Suh SS, Abdeen SK, Monin J,
Distefano R, Nigita G, Croce CM and Aqeilan RI: WWOX inhibits
metastasis of triple-negative breast cancer cells via modulation of
miRNAs. Cancer Res. 79:1784–1798. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kimmelman AC and White E: Autophagy and
tumor metabolism. Cell Metab. 25:1037–1043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
O'Donovan TR, O'Sullivan GC and McKenna
SL: Induction of autophagy by drug-resistant esophageal cancer
cells promotes their survival and recovery following treatment with
chemotherapeutics. Autophagy. 7:509–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang SF, Wang XY, Fu ZQ, Peng QH, Zhang
JY, Ye F, Fu YF, Zhou CY, Lu WG, Cheng XD and Xie X: TXNDC17
promotes paclitaxel resistance via inducing autophagy in ovarian
cancer. Autophagy. 11:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li LQ, Xie WJ, Pan D, Chen H and Zhang L:
Inhibition of autophagy by bafilomycin A1 promotes chemosensitivity
of gastric cancer cells. Tumour Biol. 37:653–659. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Polishchuk EV, Merolla A, Lichtmannegger
J, Romano A, Indrieri A, Ilyechova EY, Concilli M, De Cegli R,
Crispino R, Mariniello M, et al: Activation of autophagy, observed
in liver tissues from patients with Wilson disease and from
ATP7B-deficient animals, protects hepatocytes from copper-induced
apoptosis. Gastroenterology. 156:1173–1189. 2019. View Article : Google Scholar : PubMed/NCBI
|