Introduction
Cancer is a leading cause of death worldwide,
accounting for approximately 10 million deaths in 2020 (1). Breast, lung, colorectal, prostate,
skin (non-melanoma) and stomach are among the most frequent types
of cancer worldwide (1). The
increasing number of cancer cases and deaths annually, the
inefficacy of the strategies to prevent this disease and the
adverse effects of the therapeutic approaches, have led an
increasing number of studies to search for alternative and more
effective approaches (1).
According to the World Health Organization, strategies to reduce
cancer risk include not using tobacco, maintaining a healthy body
weight, eating a healthy diet (with fruits and vegetables), doing
physical exercise regularly, avoiding harmful use of alcohol,
minimizing exposure to ionizing radiation and reducing exposure to
air pollution (1).
Plants have been used by humans since primitive
times for food and medicines (2).
Due to the presumable adverse effects of synthetic food additives
on human health, in some cases resulting from antagonist synergies
between the different synthetic compounds, and the increased
consumer perception of this problem, there is a growing interest in
obtaining natural extracts from human diet compounds (3).
Species of the genus Quercus spp., also known
as oak, are a group of deciduous and evergreen trees of the family
Fagaceae, which comprises around 600 species worldwide. They are
widely distributed in temperate forests of the northern hemisphere
and tropical climatic areas (4–6).
These species may be found in the basin Mediterranean (Algeria,
France, Italy, Morocco, Portugal, Spain and Tunisia), Asia, and
North America (7). These trees,
abundant in Southern Europe and in the Alentejo region in Portugal,
are the basis of the sustainable agriculture system called
‘Montado’ (Portugal) or ‘Dehesa’ (Spain), a traditional
silvo-pastoral land use system characterized by low density trees
combined with agriculture or pastoral activities (8). Portuguese oak (Quercus
faginea), holm oak (Quercus ilex or Quercus
rotundifolia) and cork oak (Quercus suber) are the most
common members of the Fagaceae family in Portugal (9). Almost all parts of the plants of the
genus Quercus spp., including fruit, bark and leaves,
display numerous medicinal properties. Therefore, they have been
used in folk medicine in numerous countries and by numerous tribes
to treat conditions, including asthma, colitis, diarrhea,
furuncles, gonorrhea, hemorrhoids, labor pains, mucosa
inflammation, obesity and stomatitis (10–13).
Acorn is a fruit of the trees of the genus
Quercus spp. It is nutritionally considered a rich product,
with a higher nutritional value than cereals (14–17).
The acorn is an excellent source of minerals (calcium, magnesium,
phosphorus and potassium), unsaturated fatty acids (oleic acid) and
essential fatty acids (linoleic and linolenic acids) (18–20).
Starch is the largest nutrient component of acorns (about 55%)
(21). Moreover, acorns contain
numerous biologically active compounds such as flavonoids, phenolic
acids, and tannins, which are important in the human diet to
maintain an adequate level of antioxidants (4,6,22–29)
and consequently to prevent certain diseases, such as heart
diseases, diabetes and cancer. The acorn was used as a food stuff
in the Mesolithic era and constitutes more than half of the diet of
native people in the North American West Coast (30–36).
In the northeast of the Iberian Peninsula, the acorn was used raw,
boiled, roasted and like coffee, and used to make oil, soup,
mush/porridge, cake, bread and coffee-like beverages (30–36).
The oil obtained from the acorn is a nutritious cooking oil similar
to oils obtained from avocado, cotton, olive and peanut (17).
The pharmacological effects of acorn include
antioxidant, antimicrobial, anti-inflammatory, antidiabetic,
hepatoprotective, anti-obesity, anticancer and
anti-neurodegenerative effects, which has promoted its use on both
food and medicine (3,37,38).
Although it was an important ingredient in the past, it has been
under-appreciated in modern times, and it is still far from being
as widely used as other nuts. Acorn consumption declined over the
last few centuries and it is now mostly associated with livestock
feeding, to increase the nutritional value of meat and meat
products (4,14,17).
It should be noted that the high content of tannins is a problem
that may limit the acorn consumption. These compounds belong to the
group of polyphenols that cause bitterness and form complexes with
proteins, polysaccharides and metal ions, reducing its
digestibility and absorption (39,40).
When they complex with salivary proteins the astringency caused can
be very unpleasant, which may compromise its acceptance and
consumption (21).
The Holm oak (Quercus ilex) produces the
sweetest acorns when compared with other oak species due to a lower
level of tannins; due to this, the Holm oak acorns are
traditionally made into flour to mix with wheat and other cereals
(9,41). This flour is a valuable source of
macroelements (calcium, magnesium and potassium), microelements
(copper, iron, manganese and zinc), vitamins from complex B,
tocopherols, dietary fiber, unsaturated fatty acids (linoleic acid)
and antioxidant substances (polyphenols) (22,42–46),
improving the nutritional and sensorial characteristics of the
final product within which it is used (47–51).
Due to the absence of gluten proteins, this flour has recently been
used for the production of gluten-free foods, adequate for people
with gluten intolerance, such as patients with celiac disease
(36,40,52,53).
Furthermore, due to its unique characteristics, acorn flour can be
considered an adjuvant for cancer prevention and therapy, adding
value to the product (22).
An up-to-date assessment of the literature on the
benefits of Quercus spp. extracts for cancer prevention and
treatment might promote further research to validate its use.
Therefore, the presented study performed a systematic review and
meta-analysis of studies which had investigated the effects of
Quercus spp. extracts in cancer prevention and
treatment.
Materials and methods
Study protocol
The internationally accepted preferred reporting
items for systematic reviews and meta-analysis (PRISMA) guidelines
(54) were followed for all steps
in the present study.
Data sources and search strategy
An electronic literature search was performed in
four different databases [PubMed, Cochrane Library, Literatura
Latino-Americana e do Caribe em Ciências da Saúde (LILACS), Web of
Science and Science Direct] to identify studies which investigated
the effects of Quercus spp. extract on cancer prevention or
treatment. Keywords were searched by combining Medical Subject
Headings with text terms related to cancer and oak as follows:
‘quercus’, ‘acorn’, ‘oncology’, ‘cancer’, ‘disease’, ‘extract’ and
‘flour’, in English, Portuguese and French. The terms were searched
in these three languages and the papers were also reviewed in these
languages. Previously published systematic reviews and references
cited in the retrieved articles were also assessed to identify
potentially eligible studies. Boolean operators (AND, OR) or its
meta and truncation were used when appropriate to increase the
number of relevant papers. The search results were exported to a
Microsoft Excel sheet and duplicate papers were removed.
Study selection and inclusion
criteria
The review included full text articles published in
English, Portuguese or French. The articles were reviewed for
inclusion through scanning of the titles and abstracts for
relevance. Articles deemed important (i.e. articles with
information concerning the effects of Quercus spp. extract
in cancer using in vitro or in vivo approaches) or
for which decisions were difficult to exclude were retained for
full-text review. In vitro studies or in vivo
randomized controlled trials with humans and animals with any kind
of cancer were considered. It was confirmed that all studies were
approved by the relevant Ethics Committee and that patient consent
was obtained, when applicable. Studies with humans of any gender,
race or ethnicity and all settings (i.e., local communities,
nursing homes and hospitals) worldwide were considered. Similarly,
studies with animals from any species, race/strain or gender were
considered. All studies included a clarification of the exposure
(i.e., dose, consumption duration and frequency) and evaluated at
least one health outcome (i.e., body composition, tumor development
or cytotoxicity). Conference abstracts, reviews, trial protocols,
book chapters, studies without control group, case reports, and
commentaries or opinions were excluded. There was no restriction
based on the year of publication or sample size. The inclusion and
exclusion criteria were summarized in Table I.
 | Table I.Literature search strategy. |
Table I.
Literature search strategy.
| Parameter | Inclusion
criteria | Exclusion
criteria |
|---|
| Article type | Full text | Conference
abstracts, reviews, trial protocols, book chapters, studies without
control group, case reports, and commentaries or opinions |
| Language | English,
Portuguese, French |
|
| Study design | In vitro
studies, in vivo randomized controlled trial, Ethics
Committee approval and patient consent |
|
| Sample | Humans from any
gender, race or ethnicity and animals from any species,
race/strain, or gender |
|
| Exposure | Quercus
spp., dose, consumption, duration and frequency |
|
| Outcomes | Body composition,
tumor development, cytotoxicity |
|
Data extraction
The studies were randomly distributed by the
researchers. Each of them analyzed the papers and met to discuss
them. All eligible studies were reviewed and each study was given
an identifying code. Information on the sample (number of subjects
or animals, age and/or cell line), exposure (oak species, dose,
duration and frequency), groups (treated and control group) and
outcomes (body condition, tumor development/size/volume and
cytotoxicity) was extracted from the relevant studies.
Risk of bias and study quality
assessment
The risk of bias was assessed using the revised
version of the Cochrane risk of bias tool (RoB 2.0), which consists
of a fixed set of bias categories (55). A total of five specific categories
were evaluated as ‘high risk of bias’, ‘low risk of bias’ or ‘some
concerns’ to establish the overall risk of bias as follows: i) bias
arising from the randomization process; ii) bias due to deviation
from intended interventions; iii) bias due to missing outcome data;
iv) bias in the measurement of the outcome; and v) bias in the
selection of reported results. The researchers could also present
personal judgment about the risk of bias in each category, which
was reflected in the overall judgment value.
Statistical analysis
All outcomes were meta-analyzed, using Comprehensive
Meta-Analysis (CMA; version 2) software (https://www.meta-analysis.com/), when at least two
studies provided data. Outcomes, measured as the mean change from
baseline to endpoint of the intervention study between the
treatment and placebo groups, were considered continuous variables.
P<0.05 was deemed to indicate a statistically significant
difference.
Results
Study selection
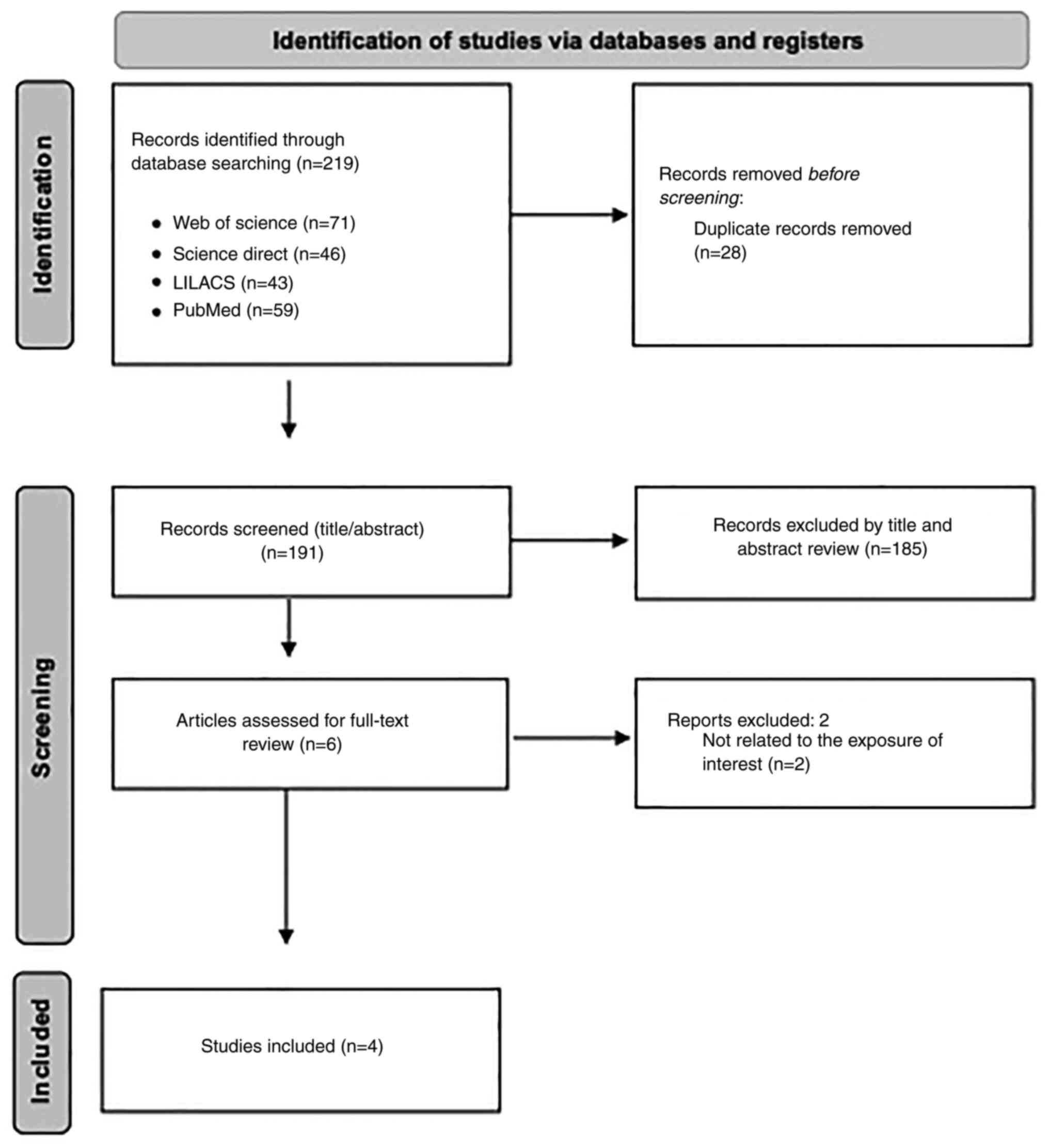
The PRISMA flow diagram summarized the study
selection process (Fig. 1). The
search identified a total of 219 articles: 71 from Web of Science,
46 from Science Direct, 43 from LILACS and 59 from PubMed. After
removing duplicates (n=28) and screening the remaining articles
(n=191) by title and abstract, 185 were excluded as irrelevant. Of
the six articles assessed by full-text review, two were excluded
because they were not related to the exposure of interest. Finally,
a total of four studies were included in the systematic review:
three in vitro studies (56–58)
and one mixed study with an in vivo and in vitro
approach (59) (Table II). Assessment of the risk of
bias, i.e. a critical analysis in which the strengths and the
limitations of the studies were assessed, was performed in this
review, and the risk was considered low.
 | Table II.Summary of the studies evaluating the
effects of Quercus spp. extracts in cancer, included in the
present meta-analysis. |
Table II.
Summary of the studies evaluating the
effects of Quercus spp. extracts in cancer, included in the
present meta-analysis.
| ID | First authors,
year | Issue | Cells/animals | Groups | Age |
Species/compound |
Dose/concentration | Duration | Outcomes | (Refs.) |
|---|
| 1 | Eggenschwiler et
al, 2006 | Breast cancer | Human breast cancer
cell lines: MCF-7, Kpl-1 and Mfm-223 | - | - | Quercus spp.
extract | 0.01–0.1 mg/ml | 48 h | Proliferation
reduction of 12% for MCF-7 cells, 63% for Kpl-1 and 70% for
Mfm-223 | (56) |
| 2 | Heydari and
Rashidipour, 2015 | Breast cancer | Human breast cancer
MCF-7 cells | - | - | Extract of
Quercus ilex acorns containing silver nanoparticles Silver
nanoparticles | Silver
nanoparticles: 10, 20, 30, 40 and 50 µg/ml Extract and silver
nanoparticles: 0.02, 0.03, 0.04 and 0.05 µg/ml | 24 h | The cytotoxic
effects of nanoparticles increased in the presence of the
extract | (57) |
| 3 | Moreno-Jimenez
et al, 2015 | Colorectal
cancer | Human colorectal
adenocarcinoma HT-29 cells | - | - | Lyophylized extract
from Quercus sideroxyla, Quercus durifolia, and Quercus
eduardii | 0.050, 0.075,
0.100, 0.125 and 0.150 mg/ml | 3 h | Q.
sideroxyla decreased COX-2 and IL-8 expression Q.
eduardii decreased TNFα expression Q. durifolia
increased nuclear factor-κB and tumor necrosis factor α COX-2, IL-8
and IL-10 levels decreased in all treatments | (59) |
|
|
|
| 68 male
Sprague-Dawley rats 1,2-dimethylhydrazine (DMH)-induced colon
carcinogenesis | Control (n=6) DMH
(n=17) DMH + Q. sideroxyla (n=15) DMH + Q. durifolia
(n=15) DMH + Q. eduardii | 5 weeks | Oak infusion of
Quercussideroxyla, Quercus durifolia, and Quercus
eduardii | 1% (w/v), oral
infusion | 26 weeks | Body weight and
food consumption were similar among groups Q.
sideroxyla-treated group had a lower mean number and
multiplicity, (n=15) and β-catenin protein level in adenocarcinomas
Q. eduardii and Q. durifolia had no protective effects |
|
| 4 | Amessis-Ouchemoukh
et al, 2017 | Glioblastoma | Human glioblastoma
U87 cells |
|
| Extract of holm oak
acorns from Quercus ilex L. | 25, 100 and 250
µg/ml | 15 min 30 min 60
min | Reactive oxygen
species inhibition and cell viability reduction in a concentration
dependent manner | (58) |
Study characteristics and
outcomes
A summary of the characteristics and outcomes of the
studies included in this systematic review was presented in
Table II. The studies were
published between 2006 and 2017. In the in vitro studies
breast, colorectal and glioblastoma cancer cell lines were used.
The mixed (in vitro and in vivo) study, was published
in 2015 and assessed the effects of oak infusion in 68 female
Sprague-Dawley rats of five weeks of age (59). The MCF-7 human breast cancer cell
line was used in both in vitro studies which evaluated the
role of Quercus spp. extract in breast cancer (56,57).
One of the studies used two cell lines in addition to the MCF-7
cell line, the Kpl-1 and Mfm-223 cell lines (56). The concentration of the extract as
well as the time of exposure was different among in vitro
studies. The concentrations ranged from 10 and 250 µg/ml, while the
time of exposure ranged from 15 min to 48 h. A cytotoxic activity
was reported in all in vitro studies, along with decreased
protein levels of cyclooxygensase (COX)-2, interleukin (IL)-8 and
IL-10, and inhibition of reactive oxygen species (ROS) in certain
studies. In the in vivo study, the animals were exposed to
the carcinogen 1,2-dimethylhydrazine to induce colorectal
carcinogenesis and were provided with a 1% oral infusion of
Quercus sideroxyla, Quercus durifolia or Quercus
eduardii, for 26 weeks. The group treated with Quercus
sideroxyla presented a lower mean number and multiplicity of
colorectal tumors, and lower β-catenin protein level in
adenocarcinomas, when compared with animals not treated with the
infusion. Where qualitative data was obtained from <2 studies,
meta-analysis was not performed.
Discussion
Cancer is a leading cause of death worldwide, and it
is associated with several risk factors, including lifestyle
(1). At present, the human diet is
changing. The Food and Agriculture Organization has underlined the
importance of re-designing food production systems based on the
fundamentals of the circular economy, with the aim of upgrading the
efficient use of resources, giving new use to underexploited
properties (60). The current
global food system must adapt to the expected growth of the world
population to ~9 billion individuals by 2050 and the effects of
climate changes. This adaptation will probably include an increased
consumption of edible wild foods, due to their richness in
micronutrients and bioactive compounds, besides providing a
cost-effective and sustainable way of improving calorific food
security (22). Moreover,
epidemiological research has indicated that a regular ingestion of
plant polyphenols increases protection against different types of
cancer, cardiovascular diseases, diabetes, osteoporosis and
neurodegenerative diseases (61,62).
The present systematic review analyzed the effects of
Quercus spp. extracts in cancer prevention and
treatment.
The effects of Quercus spp. extract have been
previously assessed in certain diseases beyond cancer, including
diabetes mellitus, neurodegenerative diseases, allergic asthma,
gastric ulcer, colitis, vaginitis, acne vulgaris and episiotomy
wounds. Its antimicrobial effects along with antiangiogenic
potential have also been assessed (Table SI).
The number of studies in the cancer field are
limited, and only three in vitro studies and one mixed study
involving in vitro and in vivo approach have been
previously performed addressing the role of Quercus spp.
extract in cancer development, to the best of our knowledge. These
studies were included in the present review and the data was
extracted accordingly. The findings demonstrated that
Quercus spp. extract had positive effects in breast and
colorectal cancer cell lines, reducing cell viability and
decreasing protein levels of COX-2, IL-8, IL-10, as well as ROS. In
the in vivo study, the Quercus spp. extract did not
affect body weight or food consumption, and decreased the mean
number and multiplicity of tumors, and the protein expression level
of β-catenin in colorectal adenocarcinomas. Due to an insufficient
number of studies focusing on the effects of Quercus spp. in
cancer, a meta-analysis was not performed. Overall, direct
comparison with other systematic reviews is challenging due to
methodological variations. However, in line with the results of the
present study, earlier reviews reported potential positive effects
of Quercus spp. extract in cancer development.
Despite the frequent use of Quercus spp. in
folk medicine since ancient times, the present analysis identified
few studies which addressed the role of this plant in cancer
treatment. To the best of our knowledge, the present study is the
first systematic review to evaluate the effects of Quercus
spp. extract on cancer development. This review was based on the
best current standards for systematic reviews and meta-analysis.
The Cochrane risk of bias tool was used for individual randomized
controlled trials and the reporting of this review was based on the
PRISMA statement. The multidisciplinary team was composed of
relevant experts with a long experience in research, literature
search and scientific writing.
There were certain limitations of the present
systematic review. The literature search was limited to Portuguese,
English and French language reports. Due to the limited number of
studies within the inclusion criteria, meta-analysis was not
performed. The authors of the included studies were not contacted
for further details. However, the findings could still provide
insights into the effects of Quercus spp. extract on cancer
development and lead to the development of new studies in the
field.
The findings of the present study suggested that
Quercus spp. extract was safe and demonstrated positive
effects on colorectal cancer development. Despite this, it should
be noted that a small number of studies and low sample sizes posed
a challenge in drawing solid conclusions. Therefore, more studies
with different cancer cell lines and animal models, including
chemically-induced models and xenograft models using cell lines
evaluated in vitro, are required to assess the efficacy of
the acorn extracts in numerous types of cancer. Furthermore, the
effects of acorn flour, incorporated in the diet, in an animal
model of chemically-induced mammary cancer should be evaluated in a
future study.
Supplementary Material
Supporting Data
Acknowledgments
Not applicable.
Funding
The present study was supported by National Funds by Portuguese
Foundation for Science and Technology, under the project nos.
UIDB/04033/2020 and LA/P/0126/2020, and the PhD grant no.
2020.07675.BD.
Availability of data and materials
The datasets used and/or produced during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PAO, BMF, CVN, AA, MJP, HV, AIRNAB and AIFR
performed the analysis of the papers, and were involved in the
manuscript writing and data confirmation. AIFR was responsible for
the study design and manuscript submission. AIFR and PAO confirmed
the authenticity of all the raw data. All authors read and approved
the version of the manuscript submitted.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO), . Cancer
Fact Sheet 2021. WHO; Geneva: 2021
|
|
2
|
Bharti VK, Malik JK and Gupta RC: Chapter
52-Ashwagandha: Multiple health benefits. Nutraceuticals. Academic
Press; pp. 865–880. 2021, View Article : Google Scholar
|
|
3
|
Dogan A, Celik I and Kaya MS: Antidiabetic
properties of lyophilized extract of acorn (Quercus brantii
Lindl.) on experimentally STZ-induced diabetic rats. J
Ethnopharmacol. 176:243–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cantos E, Espín JC, López-Bote C, de la
Hoz L, Ordóñez JA and Tomás-Barberán FA: Phenolic compounds and
fatty acids from acorns (Quercus spp.), the main dietary
constituent of free-ranged Iberian pigs. J Agric Food Chem.
51:6248–6255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tejerina D, García-Torres S, Cabeza de
Vaca M, Vázquez FM and Cava R: Acorns (Quercus rotundifolia
Lam.) and grass as natural sources of antioxidants and fatty
acids in the ‘montanera’ feeding of Iberian pig: Intra- and
inter-annual variations. Food Chem. 124:997–1004. 2011. View Article : Google Scholar
|
|
6
|
Sánchez-Burgos JA, Ramírez-Mares MV,
Larrosa MM, Gallegos-Infante JA, González-Laredo RF, Medina-Torres
L and Rocha-Guzmán NE: Antioxidant, antimicrobial,
antitopoisomerase and gastroprotective effect of herbal infusions
from four Quercus species. Ind Crops Prod. 42:57–62. 2013.
View Article : Google Scholar
|
|
7
|
Taib M, Bouyazza L and Lyoussi B: Acorn
Oil: Chemistry and functionality. J Food Qual. 20202020.
|
|
8
|
Azeda C, Guiomar N, Godinho S, Medeiros JP
and Pinto-Correia T: The ambiguous role of agri-environment-climate
measures in the safeguarding of high nature value farming systems:
The case of the montado in portugal. Agric Ecosyst Environ.
319:1075622021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toumi L and Lumaret R: Allozyme
characterisation of four Mediterranean evergreen oak species.
Biochem Syst Ecol. 29:799–817. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moon HR, Chung MJ, Park JW, Muk CHO, Choi
DJ, Kim SM, Chun MH, Kim IB, Kim SO, Jang SJ and Park YI:
Antiasthma effects through anti-inflammatory action of acorn
(Quercus Acutissima Carr.) in vitro and in vivo. J Food
Biochem. 37:108–118. 2013. View Article : Google Scholar
|
|
11
|
Kim H, Song MJ and Potter D: Medicinal
efficacy of plants utilized as temple food in traditional Korean
Buddhism. J Ethnopharmacol. 104:32–46. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bahador N and Baserisalehi M: The effect
of Quercus castaneifolia extract on pathogenic enteric
bacteria. Anaerobe. 17:358–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deryabin DG and Tolmacheva AA:
Antibacterial and anti-quorum sensing molecular composition derived
from quercus cortex (Oak bark) extract. Molecules.
20:17093–17108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Al-Rousan WM, Ajo RY, Al-Ismail KM, Attlee
A, Shaker RR and Osaili TM: Characterization of acorn fruit oils
extracted from selected mediterranean Quercus species.
Grasas y Aceites. 64:554–560. 2013. View Article : Google Scholar
|
|
15
|
Özcan T: Characterization of Turkish
Quercus L. taxa based on fatty acid compositions of the acorns. J
Am Oil Chem Soc. 84:653–662. 2007. View Article : Google Scholar
|
|
16
|
Özcan T: Total protein and amino acid
compositions in the acorns of Turkish Quercus L. Taxa. Genet Resour
Crop Evol. 53:419–429. 2006. View Article : Google Scholar
|
|
17
|
Vinha AF, Costa ASG, Barreira JCM, Pacheco
R, Beatriz M and Oliveira PP: Chemical and antioxidant profiles of
acorn tissues from Quercus spp.: Potential as new industrial
raw materials. Ind Crop Prod. 94:143–151. 2016. View Article : Google Scholar
|
|
18
|
Salajpal K, Karolyi D, Đikić M, Kantura V,
Kiš G and Sinjeri Ž: Influence of acorn intake on blood lipid
profile and longisimus muscle characteristics of black slavonian
pig. Acta Agric Slov. 2:99–107. 2008.
|
|
19
|
Sekeroglu N, Ozkutlu F and Kilic E:
Mineral composition of acorn coffees. Indian J Pharm Educ Res. 51
(Suppl):S504–S507. 2017. View Article : Google Scholar
|
|
20
|
Li S, Zhou Y, Liu M, Zhang Y and Cao S:
Nutrient composition and starch characteristics of Quercus
glandulifera Bl. seeds from China. Food Chem. 185:371–376.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taib M and Bouyazza L: Composition,
physicochemical properties, and uses of acorn starch. J Chem.
2021:99885702021. View Article : Google Scholar
|
|
22
|
Vinha AF, Barreira JCM, Costa ASG and
Oliveira MBPP: A New Age for Quercus spp. Fruits: Review on
nutritional and phytochemical composition and related biological
activities of acorns. Compr Rev Food Sci Food Saf. 15:947–981.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh A and Bisht M: Evaluation of
in-vitro antioxidant potential and in-vivo hepatoprotective
activity of root extract of Quercus oblongata D. DON. J Drug
Deliv Ther. 8:152–161. 2018. View Article : Google Scholar
|
|
24
|
Rocha-Guzmán NE, Gallegos-Infante JA,
González-Laredo RF, Reynoso-Camacho R, Ramos-Gómez M, Garcia-Gasca
T, Rodríguez-Muñoz E, Guzmán-Maldonado SH, Medina-Torres L and
Lujan-García BA: Antioxidant activity and genotoxic effect on HeLa
cells of phenolic compounds from infusions of Quercus
resinosa leaves. Food Chem. 115:1320–1325. 2009. View Article : Google Scholar
|
|
25
|
Huang J, Wang Y, Li C, Wang X and He X:
Triterpenes isolated from acorns of Quercus serrata var.
brevipetiolata exert anti-inflammatory activity. Ind Crops Prod.
91:302–309. 2016. View Article : Google Scholar
|
|
26
|
Gezici S and Sekeroglu N: Neuroprotective
potential and phytochemical composition of acorn fruits. Ind Crops
Prod. 128:13–17. 2019. View Article : Google Scholar
|
|
27
|
Xu J, Cao J, Yue J, Zhang X and Zhao Y:
New triterpenoids from acorns of Quercus liaotungensis and
their inhibitory activity against α-glucosidase, α-amylase and
protein-tyrosine phosphatase 1B. J Funct Foods. 41:232–239. 2018.
View Article : Google Scholar
|
|
28
|
Lei Y, Huang Y, Wang Y and He X: Potential
anti-neuroinflammatory triterpenoids isolated from Chinese acorns
(Quercus serrata var. brevipetiolata). J Funct Foods.
50:18–25. 2018. View Article : Google Scholar
|
|
29
|
Biesalski HK, Dragsted LO, Elmadfa I,
Grossklaus R, Müller M, Schrenk D, Walter P and Weber P: Bioactive
compounds: Definition and assessment of activity. Nutrition.
25:1202–1205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rakić S, Petrović S, Kukić J, Jadranin M,
Tešević V, Povrenović D and Šiler-Marinković S: Influence of
thermal treatment on phenolic compounds and antioxidant properties
of oak acorns from Serbia. Food Chem. 104:830–834. 2007. View Article : Google Scholar
|
|
31
|
Rakić S, Povrenović D, Tešević V, Simić M
and Maletić R: Oak acorn, polyphenols and antioxidant
activity in functional food. J Food Eng. 74:416–423. 2006.
View Article : Google Scholar
|
|
32
|
García-Gómez E, Pérez-Badia R, Pereira J
and Puri RK: The Consumption of acorns (from Quercus spp.)
in the Central West of the Iberian Peninsula in the 20th Century.
Econ Bot. 71:256–268. 2017. View Article : Google Scholar
|
|
33
|
Tantray YR, Wani M and Hussain A: Genus
Quercus: An Overview. Int J Adv Res Sci Eng. 6:1880–1886. 2017.
|
|
34
|
Makhlouf FZ, Squeo G, Difonzo G, Faccia M,
Pasqualone A, Summo C, Barkat M and Caponio F: Effects of storage
on the oxidative stability of acorn oils extracted from three
different Quercus species. J Sci Food Agric. 101:131–138.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Makhlouf FZ, Squeo G, Barkat M, Trani A
and Caponio F: Antioxidant activity, tocopherols and polyphenols of
acornoil obtained from Quercus species grown in Algeria. Food Res
Int. 114:208–213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Polimac M and Komlenic D: Acorn
flour-naturally gluten free. Proceedings of the 8th International
Congress Flour-Bread '15 [and] 10th Croatian Congress of Cereal
Technologists. Josip Juraj Strossmayer University of Osijek,
Faculty of Food Technology Osijek; Osijek: pp. 177–181. 2016
|
|
37
|
González JA, García-Barriuso M and Amich
F: Ethnobotanical study of medicinal plants traditionally used in
the Arribes del Duero, western Spain. J Ethnopharmacol.
131:343–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leporatti ML and Ivancheva S: Preliminary
comparative analysis of medicinal plants used in the traditional
medicine of Bulgaria and Italy. J Ethnopharmacol. 87:123–142. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Łuczaj Ł, Adamczak A and Duda M: Tannin
content in acorns (Quercus spp.) from Poland. Dendrobiology.
72:103–111. 2014. View Article : Google Scholar
|
|
40
|
Szabłowska E and Tańska M: Acorn flour
properties depending on the production method and laboratory baking
test results: A review. Compr Rev Food Sci Food Saf. 20:980–1008.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Adonizio AL, Downum K, Bennett BC and
Mathee K: Anti-quorum sensing activity of medicinal plants in
southern Florida. J Ethnopharmacol. 105:427–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rybicka I and Gliszczyńska-Świgło A:
Minerals in grain gluten-free products. The content of calcium,
potassium, magnesium, sodium, copper, iron, manganese, and zinc. J
Food Compos Anal. 59:61–67. 2017. View Article : Google Scholar
|
|
43
|
Rybicka I and Gliszczynska-Swiglo A:
Gluten-free flours from different raw materials as the source of
Vitamin B1, B2, B3 and B6. J Nutr Sci Vitaminol (Tokyo).
63:125–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Akcan T, Gökçe R, Asensio M, Estévez M and
Morcuende D: Acorn (Quercus spp.) as a novel source of oleic
acid and tocopherols for livestock and humans: Discrimination of
selected species from Mediterranean forest. J Food Sci Technol.
54:3050–3057. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Correia PR, Leitão AE and Beirão-da-Costa
ML: Effect of drying temperatures on chemical and morphological
properties of acorn flours. Int J Food Sci Technol. 44:1729–1736.
2009. View Article : Google Scholar
|
|
46
|
Silva S, Costa EM, Borges A, Carvalho AP,
Monteiro MJ and Pintado MME: Nutritional characterization of acorn
flour (a traditional component of the Mediterranean
gastronomical folklore). J Food Meas Charact. 10:584–588. 2016.
View Article : Google Scholar
|
|
47
|
Hrusková M, Svec I and Kadlcíková I:
Effect of chestnut and acorn flour on wheat/wheat-barley flour
properties and bread quality. Int J Food Stud. 8:41–57. 2019.
View Article : Google Scholar
|
|
48
|
Pasqualone A, Makhlouf FZ, Barkat M,
Difonzo G, Summo C, Squeo G and Caponio F: Effect of acorn flour on
the physico-chemical and sensory properties of biscuits. Heliyon.
5:e022422019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Švec I, Hrušková M and Kadlčíková I:
Features of flour composites based on the wheat or wheat-barley
flour combined with acorn and chestnut. Croat J food Sci Technol.
10:89–97. 2018. View Article : Google Scholar
|
|
50
|
Kiumarsi M, Shahbazi M, Yeganehzad S,
Majchrzak D, Lieleg O and Winkeljann B: Relation between
structural, mechanical and sensory properties of gluten-free bread
as affected by modified dietary fibers. Food Chem. 277:664–673.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Naqash F, Gani A, Gani A and Masoodi FA:
Gluten-free baking: Combating the challenges-A review. Trends Food
Sci Technol. 66:98–107. 2017. View Article : Google Scholar
|
|
52
|
Korus A, Gumul D, Krystyjan M, Juszczak L
and Korus J: Evaluation of the quality, nutritional value and
antioxidant activity of gluten-free biscuits made from corn-acorn
flour or corn-hemp flour composites. Eur Food Res Technol.
243:1429–1438. 2017. View Article : Google Scholar
|
|
53
|
Korus J, Witczak M, Ziobro R and Juszczak
L: The influence of acorn flour on rheological properties of
gluten-free dough and physical characteristics of the bread. Eur
Food Res Technol. 240:1135–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sterne JAC, Savović J, Page MJ, Elbers RG,
Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge
SM, et al: RoB 2: A revised tool for assessing risk of bias in
randomised trials. BMJ. 366:l48982019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Eggenschwiler J, Patrignani A, Wagner U,
Rehrauer H, Schlapbach R, Rist L, Ramos MH and Viviani A: Gene
expression profiles of different breast cancer cells compared with
their responsiveness to fermented mistletoe (Viscum album
L.) extracts Iscador from oak (Quercus), pine
(Pinus), white fir (Abies) and apple tree
(Malus) in vitro. Arzneimittelforschung. 56:483–496.
2006.PubMed/NCBI
|
|
57
|
Heydari R and Rashidipour M: Green
synthesis of silver nanoparticles using extract of oak fruit hull
(jaft): Synthesis and in vitro cytotoxic effect on mcf-7 cells. Int
J Breast Cancer. 2015:8467432015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Amessis-Ouchemoukh N, Ouchemoukh S,
Meziant N, Idiri Y, Hernanz D, Stinco CM, Rodríguez-Pulido FJ,
Heredia FJ, Madani K and Luis J: Bioactive metabolites involved in
the antioxidant, anticancer and anticalpain activities of Ficus
carica L., Ceratonia siliqua L. and Quercus ilex
L. extracts. Ind Crops Prod. 95:6–17. 2017. View Article : Google Scholar
|
|
59
|
Moreno-Jimenez MR, Trujillo-Esquivel F,
Gallegos-Corona MA, Reynoso-Camacho R, González-Laredo RF,
Gallegos-Infante JA, Rocha-Guzmán NE and Ramos-Gomez M:
Antioxidant, anti-inflammatory and anticarcinogenic activities of
edible red oak (Quercus spp.) infusions in rat colon
carcinogenesis induced by 1,2-dimethylhydrazine. Food Chem Toxicol.
80:144–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gustavsson J, Cederberg C, Sonesson U, Van
Otterdijk R and Meybeck A: Global food losses and food waste. Food
Agric Organ United Nations; 2011
|
|
61
|
Manach C, Milenkovic D, Van de Wiele T,
Rodriguez-Mateos A, de Roos B, Garcia-Conesa MT, Landberg R, Gibney
ER, Heinonen M, Tomás-Barberán F and Morand C: Addressing the
inter-individual variation in response to consumption of plant food
bioactives: Towards a better understanding of their role in healthy
aging and cardiometabolic risk reduction. Mol Nutr Food Res.
61:16005572017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fraga CG, Croft KD, Kennedy DO and
Tomás-Barberán FA: The effects of polyphenols and other bioactives
on human health. Food Funct. 10:514–528. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Määttä J, Haapakoski R, Lehto M, Leino M,
Tillander S, Husgafvel-Pursiainen K, Wolff H, Savolainen K and
Alenius H: Immunomodulatory effects of oak dust exposure in a
murine model of allergic asthma. Toxicol Sci. 99:260–266. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Custódio L, Patarra J, Alberício F, Neng
NR, Nogueira JMF and Romano A: Extracts from Quercus sp.
acorns exhibit in vitro neuroprotective features through inhibition
of cholinesterase and protection of the human dopaminergic cell
line SH-SY5Y from hydrogen peroxide-induced cytotoxicity. Ind Crops
Prod. 45:114–120. 2013. View Article : Google Scholar
|
|
65
|
Yarani R, Mansouri K, Mohammadi-Motlagh
HR, Mahnam A and Emami Aleagha MS: In vitro inhibition of
angiogenesis by hydroalcoholic extract of oak (Quercus
infectoria) acorn shell via suppressing VEGF, MMP-2, and MMP-9
secretion. Pharm Biol. 51:361–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Azizi S, Pirbalouti AG and Amirmohammadi
M: Effect of Hydro-alcoholic extract of persian oak (Quercus
brantii) in experimentally gastric ulcer. Iran J Pharm Res.
13:967–974. 2014.PubMed/NCBI
|
|
67
|
Mandal A and Bishayee A: Mechanism of
breast cancer preventive action of pomegranate: Disruption of
estrogen receptor and Wnt/β-Catenin signaling pathways. Molecules.
20:22315–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Soto-García M, Rosales-Castro M,
Escalona-Cardoso GN and Paniagua-Castro N: Evaluation of
hypoglycemic and genotoxic effect of polyphenolic bark extract from
quercus sideroxyla. Evid Based Complement Alternat Med.
2016:40326182016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Aleebrahim-Dehkordy E, Rafieian-kopaei M,
Amini-Khoei H and Abbasi S: In vitro evaluation of antioxidant
activity and antibacterial effects and measurement of total
phenolic and flavonoid contents of quercus brantii L. Fruit
Extract. J Diet. (Suppl 16):408–416. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Barmak MJ, Menshadi AD, Mahmoudi R and
Bardania H: Evaluation of the effect of the internal layer of oak
fruit (jaft) extract on the prevention of gastric ulcers caused by
stress in male rats. J Med Life. 11:2252018. View Article : Google Scholar
|
|
71
|
Tayel AA, El-Sedfy MA, Ibrahim AI and
Moussa SH: Application of Quercus infectoria extract as a
natural antimicrobial agent for chicken egg decontamination. Rev
Argent Microbiol. 50:391–397. 2018.PubMed/NCBI
|
|
72
|
Yin P, Wang Y, Yang L, Sui J and Liu Y:
Hypoglycemic Effects in Alloxan-Induced Diabetic Rats of the
Phenolic Extract from Mongolian Oak Cups Enriched in Ellagic Acid,
Kaempferol and Their Derivatives. Molecules. 30:10462018.
View Article : Google Scholar
|
|
73
|
Castejón ML, Rosillo MÁ, Villegas I,
Sánchez-Hidalgo M, Hadidi L, Zaidi F and Alarcón-De-La-Lastra C:
Quercus ilex extract ameliorates acute TNBS-induced colitis
in rats. Planta Med. 85:670–677. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Askari SF, Jahromi BN, Dehghanian A, Zarei
A, Tansaz M, Badr P, Azadi A and Mohagheghzadeh A: Effect of a
novel herbal vaginal suppository containing myrtle and oak gall in
the treatment of vaginitis: A randomized clinical trial. Daru.
28:603–614. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kim M, Yin J, Hwang IH, Park DH, Lee EK,
Kim MJ and Lee MW: Anti-Acne vulgaris effects of pedunculagin from
the leaves of Quercus mongolica by anti-inflammatory
activity and 5α-reductase inhibition. Molecules. 25:21542020.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zibanejad S, Miraj S and Kopaei MR:
Healing effect of Quercus persica and Lawsonia
inermis ointment on episiotomy wounds in primiparous women. J
Res Med Sci. 25:2020.PubMed/NCBI
|
|
77
|
Xu J, Fu C, Li T, Xia X, Zhang H, Wang X
and Zhao Y: Protective effect of acorn (Quercus liaotungensis
Koidz) on streptozotocin-damaged MIN6 cells and type 2 diabetic
rats via p38 MAPK/Nrf2/HO-1 pathway. J Ethnopharmacol.
266:1134442021. View Article : Google Scholar : PubMed/NCBI
|