Introduction
As the lifespan of human beings extends due to
medical and economic developments, the worldwide elderly population
is increasing (1,2). Due to this, the frequency of patients
with age-related osteoporosis and mortality from osteoporotic
fractures is also rapidly increasing (3). Bone is a dynamic tissue that
undergoes destruction and recreation throughout a human's lifespan
and requires a balance of osteoclasts and osteoblasts (4). Osteoporosis occurs when the excessive
activity of osteoclasts, responsible for bone destruction,
outbalances the activity of osteoblasts, responsible for bone
formation (5). Osteoporosis is
asymptomatic, but even a relatively small impact can cause
fractures in patients, leading to a serious deterioration in the
quality of life (6). Osteoporosis
treatment typically involves bisphosphonates and selective estrogen
receptor modulators (SERMs) (7).
However, these treatments have adverse side effects, with long-term
use of bisphosphonate causing mandibular osteonecrosis and
cardiovascular disease, and SERM treatment known to cause serious
side effects, such as breast cancer (8,9).
Therefore, the development of alternative therapeutic agents from
natural products with fewer side effects is essential.
Osteoclasts are formed from hematopoietic precursors
of the monocyte/macrophage lineage (10) and are responsible for bone
resorption. The cytokine, receptor activator of nuclear factor-κB
ligand (RANKL), is crucial for osteoclastogenesis (11). The binding of RANKL to the cell
surface receptor, RANK, triggers a signaling cascade that involves
TNF receptor associated factor 6 (TRAF6), ultimately leading to the
activation of essential transcription factors for
osteoclastogenesis, including nuclear factor of activated T cells 1
(NFATc1) and c-Fos (12–14). The activation of NFATc1 induces
osteoclastogenesis-related markers, such as tartrate-resistant acid
phosphatase (TRAP), carbonic anhydrase type II (CA2) and ATPase H+
transporting V0 subunit d2 (ATP6v0d2) (15–17).
Therefore, inhibition of the RANK-RANKL signaling pathway is a
major therapeutic target for osteoporosis.
Gleditsiae fructus (GF) is the dried or
immature fruit of Gleditsia sinensis Lam. and has been
traditionally used for the management of inflammation and injury.
GF is abundant in triterpenoid saponins, a class of glycosides
widely found in the plant realm (18). Saponins possess noteworthy
properties including anti-inflammatory, analgesic, antioxidant and
hydrogen peroxide scavenging effects (19). Moreover, saponins play a key role
in promoting cell regeneration, particularly in keratinocytes.
Amongst the saponins, echinocystic acid and oleanolic acid are
known to be active components of GF (20), with echinocystic acid displaying
anti-inflammatory and antioxidant effects (21,22),
and oleanolic acid demonstrating anti-inflammatory and antiallergic
effects through inhibition of the transcriptional regulation of
MAPKs and NF-κB (23,24). Oleanolic acid has also been shown
to inhibit osteoclastogenesis and bone loss (25). The expression of inflammatory
cytokines is an osteoclast activation factor and has been reported
to be one of the causes of metabolic bone disease (26). Therefore, it was inferred that GF
could be effective for the treatment of osteoporosis. However, to
the best of our knowledge, the effect of GF on osteoclast
differentiation and postmenopausal osteoporosis has not yet been
elucidated. Therefore, the present study aimed to investigate the
effect of GF on osteoclastogenesis, identify the
NFATc1/c-fos-mediated osteoclastogenesis pathway and confirm the
promoting effect of GF on osteoblasts. Additionally, the inhibitory
effect of GF on bone density reduction was evaluated and the
mechanism underlying the anti-osteoporosis effect of GF in the
ovariectomy (OVX) model was elucidated.
Materials and methods
Reagents
Dulbecco's Modified Eagle's Medium (DMEM) and
Dulbecco's PBS (DPBS) were acquired from Welgene, Inc. α-Minimum
essential medium (α-MEM) and penicillin/streptomycin (P/S) were
acquired from Gibco (Thermo Fisher Scientific, Inc.). Fetal bovine
serum (FBS) was purchased from Atlas Biologicals, Inc. RANKL was
purchased from PeproTech EC Ltd. Cell Counting Kit-8 (CCK-8) was
acquired from Dojindo Laboratories, Inc. TRAP staining kits,
bicinchoninic acid (BCA) solution, 17b-estradiol (E2),
alendronate (ALN) and 4′,6-diamidino-2-phenylindole (DAPI) were
obtained from Sigma-Aldrich (Merck KGaA). Acti-stain™ 488
Fluorescent Phalloidin was obtained from Cytoskeleton, Inc. Osteo
assay strip well plates were acquired from Corning, Inc. PCR
primers were purchased from GenoTech Corp. Primary and secondary
antibodies were as follows: β-actin (sc-8432; Santa Cruz
Biotechnology, Inc.), NFATc1 (cat. no. 556602; BD Biosciences),
c-Fos (cat. no. sc-447; Santa Cruz Biotechnology, Inc.), matrix
metalloprotease 9 (MMP-9; cat. no. ab38898; Abcam), cathepsin K
(CTK; cat. no. ab19027; Abcam), peroxidase AffiniPure Goat
Anti-Mouse IgG (cat. no. 115-035-062; Jackson ImmunoResearch
Laboratories, Inc.) and peroxidase AffiniPure Goat Anti-Rabbit IgG
(cat. no. 115-035-144; Jackson ImmunoResearch Laboratories,
Inc.).
Preparation of GF ethanol extract
GF (100 g) purchased from Omni Herb, Co., Ltd. was
immersed in a liter of 30% ethanol at 4°C for 3 weeks and sonicated
(40 kHz) once every 2 days at room temperature for 1 h. The
resulting mixture was filtered using no. 3 filter paper (Whatman
plc; Cytiva), and the filtrate was concentrated using a rotary
evaporator. Finally, the concentrated extract was freeze-dried into
a powder with a yield of 15.9% (15.9 g powder). The resulting
powder was stored at −20°C until further use.
Cell culture and cytotoxicity
measurement
RAW 264.7 cells were obtained from The Korean Cell
Line Bank (Korean Cell Line Research Foundation) and MC3T3-E1 cells
from the American Type Culture Collection (cat. no. ATCC-CRL-2593).
RAW 264.7 cells were cultured in DMEM supplemented with 10% FBS and
1% P/S and subcultured every 2 days in a humidified incubator
(Thermo Fisher Scientific, Inc.) at 37°C and 5% CO2.
MC3T3-E1 cells were cultured in α-MEM (without ascorbic acid),
supplemented with 10% FBS and 1% P/S and subcultured every 3 days
in a humidified incubator at 37°C and 5% CO2. To
evaluate the cytotoxicity of GF, 5,000 RAW 264.7 and 5,000 MC3T3-E1
cells were seeded in 96-well plates and allowed to stabilize for 24
h. Various concentrations of GF (0, 5, 10, 20 and 40 µg/ml) were
then added to the cells and the cells were incubated for a further
24 h. After treatment, 20 µl CCK-8 solution was added to each well
and the plates were incubated for an additional 2 h. Cell viability
was determined by measuring the absorbance at 450 nm using an
enzyme-linked immunosorbent assay (ELISA) plate reader. The results
were expressed as a percentage of the untreated cell control and a
survival rate ≤90% compared with the control was considered
indicative of toxicity.
TRAP staining and TRAP activity
To evaluate the inhibitory effect of GF on
osteoclast formation, RAW 264.7 cells were seeded at a density of
5,000 cells/well in 96-well plates and incubated for 24 h. The
cells were then treated with 100 ng/ml RANKL, which is an
osteoclastogenesis-inducing cytokine, and various concentrations of
GF (0, 5, 10, 20 and 40 µg/ml) for 5 days with medium changes on
days 2 and 4. After fixation with 4% formalin for 1 h at the room
temperature, TRAP staining was performed for 1 h at the room
temperature and TRAP (+) cells with ≥3 nuclei were counted using
ImageJ software v1.46 (National Institutes of Health) under an
inverted microscope (magnification, ×100). In addition, the
activity of TRAP in the culture medium was measured by mixing an
equal volume of TRAP solution (4.93 mg p-nitrophenyl phosphate in
750 ml 0.5 M acetate solution and 150 ml tartrate acid solution)
with the medium on day 5 and then incubated for 1 h. Finally, the
reaction was stopped with NaOH and the absorbance at 405 nm was
measured using an ELISA plate reader.
Pit formation and filamentous actin
(F-actin) ring formation assays
To confirm the effect of GF on the inhibition of
bone resorption ability, 5,000 RAW 264.7 cells were seeded into
osteo assay strip well plates and stabilized for 24 h at 37°C. The
cells were treated with RANKL (100 ng/ml) and various
concentrations of GF (0, 5, 10, 20 and 40 µg/ml) for 5 days at
37°C. The culture medium was exchanged on days 2 and 4 with the
same medium. Then, the cells were removed using 4% sodium
hypochlorite. The area of the pit in the plate was measured using
ImageJ software v1.46 and the area in the plate absorbed by
osteoclasts was expressed as a percentage of the total area.
To determine the effect of GF on actin ring
formation, 5,000 RAW 264.7 cells were seeded into 96-well plates
and stabilized for 24 h. The cells were treated with RANKL (100
ng/ml) and various concentrations of GF (0, 5, 10, 20 and 40 µg/ml)
for 5 days at 37°C. The culture medium was exchanged on days 2 and
4 with the same medium. After the formation of osteoclasts, the
cells were fixed using 4% paraformaldehyde for 10 min at room
temperature and then permeabilized with 0.1% Triton™ X-100 in PBS
for 5 min at room temperature. The cells were then incubated with
Acti-stain™ 488 fluorescent phalloidin and DAPI in the dark for 30
min at room temperature. The actin rings were visualized using a
fluorescence microscope (magnification, ×200) and the number of
actin rings was quantified using ImageJ software v1.46.
Western blot analysis
RAW 264.7 cells were seeded at a density of
5×105 cells per 60 mm dish and allowed to stabilize for
24 h. The cells were then treated with RANKL (100 ng/ml) and
different concentrations of GF (0, 5, 10, 20 and 40 µg/ml) for 24 h
to confirm the effect of GF on transcription factors related to
osteoclastogenesis. After treatment, the cells underwent a DPBS
wash, and the total protein was extracted from the cells using RIPA
buffer (50 mM Tris-Cl, 150 mM NaCl, 1% NP-40, 0.5% sodium
deoxycholate and 0.1% SDS) containing protease and phosphatase
inhibitors (1:100; Sigma-Aldrich; Merck KGaA), to obtain a
comprehensive protein profile. The protein concentration was
quantified using the BCA assay and 30 µg protein/lane was separated
by SDS-PAGE on 10% gels, before being transferred onto
nitrocellulose membranes. The membranes were blocked with TBS-0.5%
Tween 20 containing 5% skim milk to minimize non-specific protein
binding for 1 h at room temperature, and then incubated with
primary antibodies overnight at 4°C (NFATc1, 1:1,000; c-Fos, 1:200;
β-actin, 1:1,000) and then with secondary antibodies (1:10,000) for
1 h at room temperature. Finally, the bands were visualized using
ECL solution (Cytiva) and their density was measured using ImageJ
v1.46. The protein expression levels were then normalized to those
of the loading control (β-actin).
Reverse transcription-PCR
(RT-PCR)
To confirm the effect of GF on
osteoclastogenesis-related transcription factors, 2×105
RAW 264.7 cells were seeded in 6-well plates and stabilized for 24
h. Cells were treated with RANKL (100 ng/ml) and various
concentrations of GF (0, 5, 10, 20 and 40 µg/ml) for 4 days. Next,
the cells were washed with DPBS three times and total RNA was
extracted using TRIzol® reagent (Takara Bio, Inc.). The
NanoDrop 2.0 spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.) was utilized to quantify 2 µg of extracted
RNA. Subsequently, cDNA was synthesized in accordance with the
manufacturer's protocol provided by the RT kit (Invitrogen; Thermo
Fisher Scientific, Inc.), followed by its amplification with Taq
polymerase (Kapa Biosystems; Roche Diagnostics). The PCR conditions
were as follows: Denaturation for 30 sec at 94°C, annealing for 30
sec at 53–58°C and extension for 30 sec at 72°C. Primer sequences
and number of cycles for each gene are listed in Table I. mRNA expression levels were
normalized to that of the loading control (β-actin). The RT-PCR
results were confirmed using a 2% agarose gel stained with SYBR
Green (1:10,000; Invitrogen; Thermo Fisher Scientific, Inc.). The
density of the bands was measured using ImageJ v1.46.
 | Table I.Primer sequences for reverse
transcription PCR. |
Table I.
Primer sequences for reverse
transcription PCR.
| Gene name | Primer sequence
(5′-3′) | Tm, °C | No. of cycles | Accession no. |
|---|
| NFATc1
(Nfatc1) | Forward:
TGCTCCTCCTCCTGCTGCTC | 58 | 32 | NM_198429.2 |
|
| Reverse:
CGTCTTCCACCTCCACGTCG |
|
|
|
| c-Fos
(Fos) | Forward:
ATGGGCTCTCCTGTCAACAC | 58 | 33 | NM_010234.3 |
|
| Reverse:
GGCTGCCAAAATAAACTCCA |
|
|
|
| TRAP
(Acp5) | Forward:
ACTTCCCCAGCCCTTACTACCG | 58 | 30 | NM_007388.3 |
|
| Reverse:
TCAGCACATAGCCCACACCG |
|
|
|
| RANK
(Tnfrsf11a) | Forward:
AAACCTTGGACCAACTGCAC | 53 | 32 | NM_009399.3 |
|
| Reverse:
ACCATCTTCTCCTCCCGAGT |
|
|
|
| MMP-9
(Mmp9) | Forward:
CGACTTTTGTGGTCTTCCCC | 58 | 30 | NM_013599.4 |
|
| Reverse:
TGAAGGTTTGGAATCGACCC |
|
|
|
| CA2
(Ca2) | Forward:
CTCTCAGGACAATGCAGTGCTGA | 58 | 32 | NM_001357334.1 |
|
| Reverse:
ATCCAGGTCACACATTCCAGCA |
|
|
|
| OSCAR
(Oscar) | Forward:
CTGCTGGTAACGGATCAGCTCCCCAGA | 53 | 35 | NM_001290377.1 |
|
| Reverse:
CCAAGGAGCCAGAACCTTCGAAACT |
|
|
|
| ATP6v0d2
(Atp6v0d2) | Forward:
ATGGGGCCTTGCAAAAGAAATCTG | 58 | 30 | NM_175406.3 |
|
| Reverse:
CGACAGCGTCAAACAAAGGCTTGTA |
|
|
|
| DC-STAMP
(Dcstamp) | Forward:
TGGAAGTTCACTTGAAACTACGTG | 63 | 30 | NM_001289506.1 |
|
| Reverse:
CTCGGTTTCCCGTCAGCCTCTCTC |
|
|
|
| β-actin
(Actb) | Forward:
TTCTACAATGAGCTGCGTGT | 58 | 30 | NM_008084.3 |
|
| Reverse:
CTCATAGCTCTTCTCCAGGG |
|
|
|
Animals and OVX model
A total of 48 Sprague Dawley 11-week-old female rats
were obtained from Nara Biotech (mean weight 240±10 g) and the
animals were stabilized in a new environment for 1 week. All
animals were raised in a specific pathogen-free environment,
including a temperature of 20–24°C and humidity of 50–60%, under a
12 h light/dark cycle. All animals had ad libitum access to
food and water and the animal experiments were approved by The
Kyung Hee University Institutional Animal Care and Use Committee
(Seoul, Republic of Korea; approval no. KHSASP-21-185). The
postmenopausal osteoporosis model was induced by OVX. For this, all
animals were subjected to deep anesthesia in 100% oxygen with 5%
isoflurane before undergoing bilateral OVX. The concentration of
isoflurane was maintained at 2–3% during surgery. Animals in the
sham group underwent the same surgical procedure with identical
stress levels but without bilateral OVX. After surgery, 4 mg/kg
gentamycin was injected for 3 days to prevent infection at the
wound site. The animal groups (n=8/group) comprised the following:
i) Sham group, animals subjected to sham operation and orally
administered distilled water; ii) OVX group, animals subjected to
OVX and orally administered distilled water; iii) E2
group, animals subjected to OVX and orally administered 100 µg/kg
E2; iv) ALN group, animals subjected to OVX and orally
administered 3 mg/kg ALN; v) low GF (GF-L) group, animals subjected
to OVX and orally administered 21.147 mg/kg GF; and vi) high GF
(GF-H) group, animals subjected to OVX and orally administered
135.34 mg/kg GF. The concentration of administered GF was
determined as per the following procedure. In Korean medicine, the
average amount of GF consumed by a 60 kg adult per day is 8 g
(27). The yield of GF is 15.9%.
The calculated dose to be administered in the GF-L group was 21.147
mg/kg. It is known that the metabolism of rats is 6.4× faster than
that of humans (28). Therefore,
the GF-H group was administered 135.34 mg/kg. In the present study,
two drugs were used as positive controls to compare the
anti-osteoporotic effects of GF. The first is E2, which
is commonly used to treat osteoporosis in postmenopausal women. The
second is ALN, which is a bisphosphonate agent used to treat
osteoporosis (29), preventing
bone destruction and increasing bone thickness. The change in body
weight of animals was measured once a week and drug administration
was performed for a total of 8 weeks. The health status of the rats
was checked twice daily. The humane endpoints of this study were
set as follows: i) Inability to eat, ii) rapid weight loss of 20%
or more in 1 week, iii) immobility. During the experiment, no
abnormal changes in health were observed in all of the rats. After
8 weeks, all animals were sacrificed by lethal cardiac puncture up
to 10 ml (30), under deep
anesthesia in 100% oxygen and 5% isoflurane. Subsequently, after
confirming that the hearts of all animals had completely stopped,
cervical dislocation was performed.
Biochemical assays using rat
serum
Following whole blood extraction and reaction in an
serum separator tube for 30 min at room temperature, the serum was
separated through centrifugation (14,310 × g, 4°C, 10 min) and TRAP
activity of the serum was assessed as aforementioned. The
expression of alkaline phosphatase (ALP), aspartate
aminotransferase (AST) and alanine transferase (ALT) was analyzed
by DkKorea, an animal serum analysis company.
Microcomputed tomography (Micro-CT)
analysis
The femurs of all animals were harvested 8 weeks
after surgery and immersed in 10% neutral buffered formalin (NBF)
at room temperature for 24 h to fix the bone tissue. Bone mass and
microstructure were then evaluated by micro-CT using SkyScan1176
(Skyscan; Bruker Corporation) under the following conditions: An
aluminum filter of 0.5 mm, 8.9 µm pixel size and a rotation angle
of 180° with rotation steps of 0.4°. The analyzed bone parameters
included bone mineral density (BMD), bone volume/total volume
(BV/TV), trabecular thickness (Tb.Th) and trabecular separation
(Tb.Sp).
Histological analysis
The femurs of all animals were harvested 8 weeks
after surgery and fixed in 10% NBF at room temperature for 24 h.
Subsequently, the femurs were demineralized in
ethylenediaminetetraacetic acetic acid (pH 7.4) for 3 weeks and
embedded in paraffin. Tissue sections of 5 µm thickness were
obtained using a Zeiss rotary microtome and the sections were
air-dried for 1 day. Hematoxylin-eosin (H&E) staining was used
to analyze histomorphological changes. In brief, paraffin-embedded
tissue sections were subjected to deparaffinization using xylene
and ethanol, followed by counterstaining with eosin for 10 sec and
hematoxylin for 5 min. For immunohistochemistry (IHC) staining,
initially, the paraffin-embedded tissue underwent deparaffinization
using a combination of xylene and ethanol. After that, antigen
retrieval was performed using proteinase K (0.4 mg/ml) at 37°C for
30 min. Subsequently, 0.3% H2O2 (w/v) in
Mt-OH was added at room temperature for 30 min to inhibit the
activity of endogenous peroxidases, and 5% goat serum (Gibco;
Thermo Fisher Scientific, Inc.) in PBS was used to prevent the
binding of non-specific proteins for 1 h at room temperature. The
tissues were incubated overnight at 4°C with primary antibodies
NFATc1 (diluted 1:100) and CTK (diluted 1:100). The sections were
then incubated with the secondary antibody (cat. no. BA-1000;
Vector Laboratories, Inc.; Maravai LifeSciences) for 1 h at room
temperature. The signal was detected using the ABC kit (Vector
Laboratories, Inc.; Maravai LifeSciences) at room temperature for
30 min and visualized with 3,3-diaminobenzidine (DAB solution; cat.
no. SK-4100; Vector Laboratories, Inc.; Maravai LifeSciences) at
room temperature for 10 min. The stained tissues were analyzed
using a light microscope (Olympus Corporation) and the trabecular
area and positive cells were measured using ImageJ software
v1.46.
Alizarin red S staining
Alizarin red S staining was conducted to investigate
the impact of GF on osteoblast formation and the formation of
calcified nodules. A 6-well plate was seeded with 3×105
MC3T3-E1 cells and incubated for 24 h. In order to differentiate
MC3T3-E1 cells into osteoblasts, the cells were treated with
ascorbic acid (A.A; 25 µg/ml), β-glycerol phosphate (B.G.P; 10 mM)
and various concentrations of GF (0, 5, 10, 20 and 40 µg/ml) at
37°C for 2 weeks. The culture medium was replaced every 3 days.
Once calcified nodules were visible on the plate, the medium was
removed and the cells were washed three times with cold PBS. The
cells were then fixed with 80% ethanol at 4°C for 1 h and stained
with alizarin red S dye at room temperature for 30 min. Finally,
images of the plate were captured using a digital camera (Canon,
Inc.). The degree of dye staining was measured by extracting the
dye using 10% (v/w) cetylpyridinium chloride in sodium phosphate
and measuring the absorbance at 570 nm.
High-performance liquid chromatography
analysis
Echinocystic acid and oleanolic acid are active
ingredients of GF (20) and were
purchased from Sigma-Aldrich (Merck KGaA). To analyze the GF
extract, the absorbance was measured using a UV detector (2695 HPLC
system with 2996 PDA detector; Waters Corp.), the flow rate was 1
ml and the operating time was 45 min using an Xbridge C18 (250×4.6
mm, 5 µm; Waters Corp.) column at the room temperature. The sample
insertion volume was 10 µl. Solvent A of the mobile phase was
acetonitrile and solvent B was water with 0.1% formic acid. The
gradient program was as follows. 0 min, 5% B; 0–30 min, 5–50% B;
30–60 min, 50–95% B.
Statistical analysis
All data are presented as the mean ± standard error
of mean. All experiments were repeated at least 3 times.
Comparisons were conducted with one-way ANOVA and Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
GraphPad PRISM v5.01 (Dotmatics).
Results
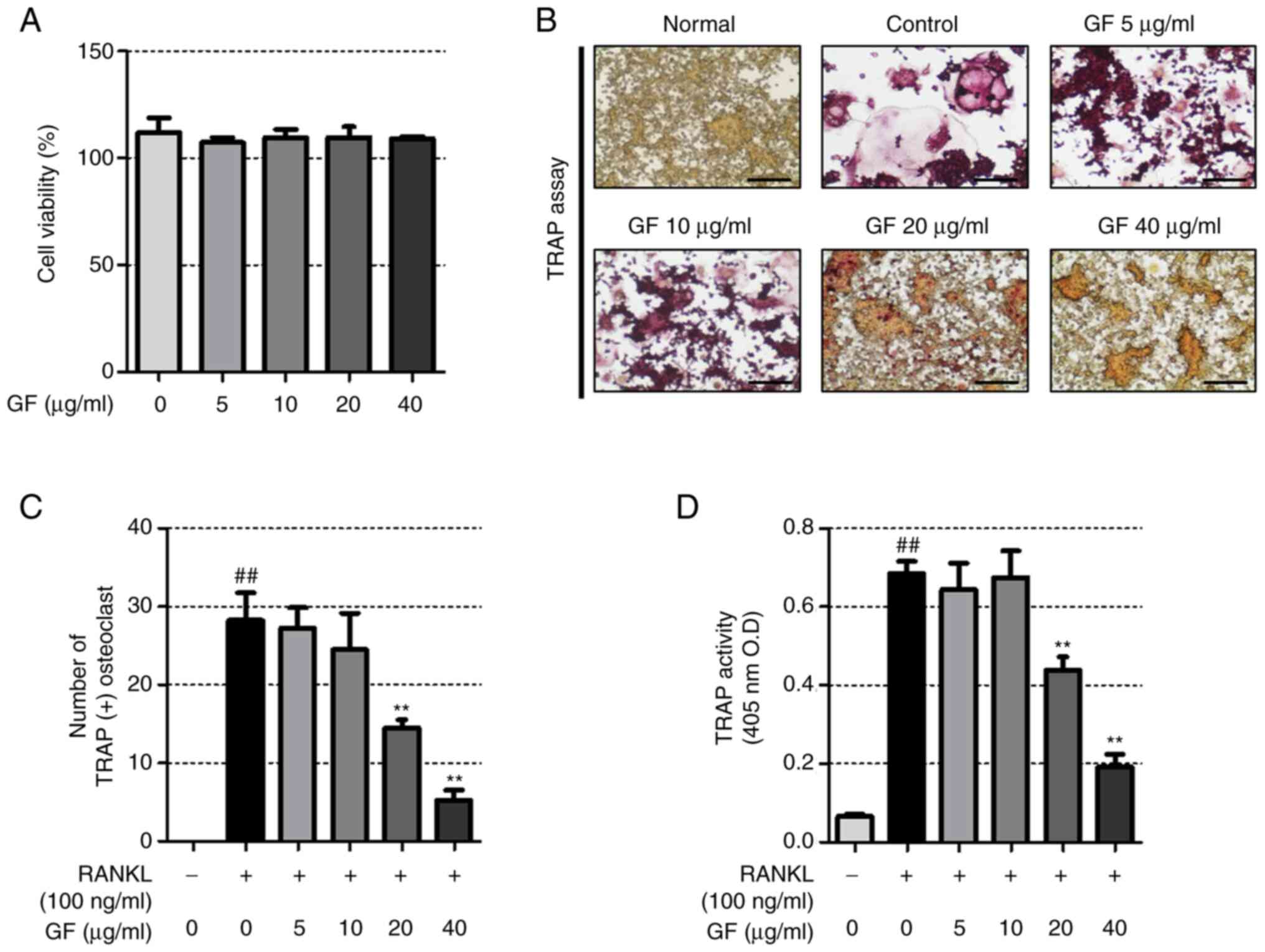
GF inhibits RANKL-induced
osteoclastogenesis
To determine the effect of GF on RAW 264.7 cell
cytotoxicity, a CCK-8 assay was performed that showed that GF did
not induce RAW 264.7 cell cytotoxicity (Fig. 1A). The impact of GF on
osteoclastogenesis in RAW 264.7 cells was also investigated by
analyzing TRAP activity and performing TRAP staining. The results
showed that RANKL-treated cells had a greater number of TRAP (+)
cells and higher TRAP activity compared with untreated cells, while
GF reduced both the number of TRAP (+) cells and TRAP activity in a
dose-dependent manner (Fig.
1B-D).
GF suppresses RANKL-induced bone
resorption and F-actin formation
To investigate the impact of GF on
osteoclastogenesis and bone resorption, experiments on F-actin ring
and pit formation were conducted. The results, as illustrated in
Fig. 2A and C, showed that
RANKL-treated cells had a higher pit area compared with the
untreated cells and GF dose-dependently decreased the pit area.
Furthermore, RANKL-treated cells displayed more F-actin rings than
the untreated cells, but GF inhibited both the number and structure
of F-actin rings, as depicted in Fig.
2B and D.
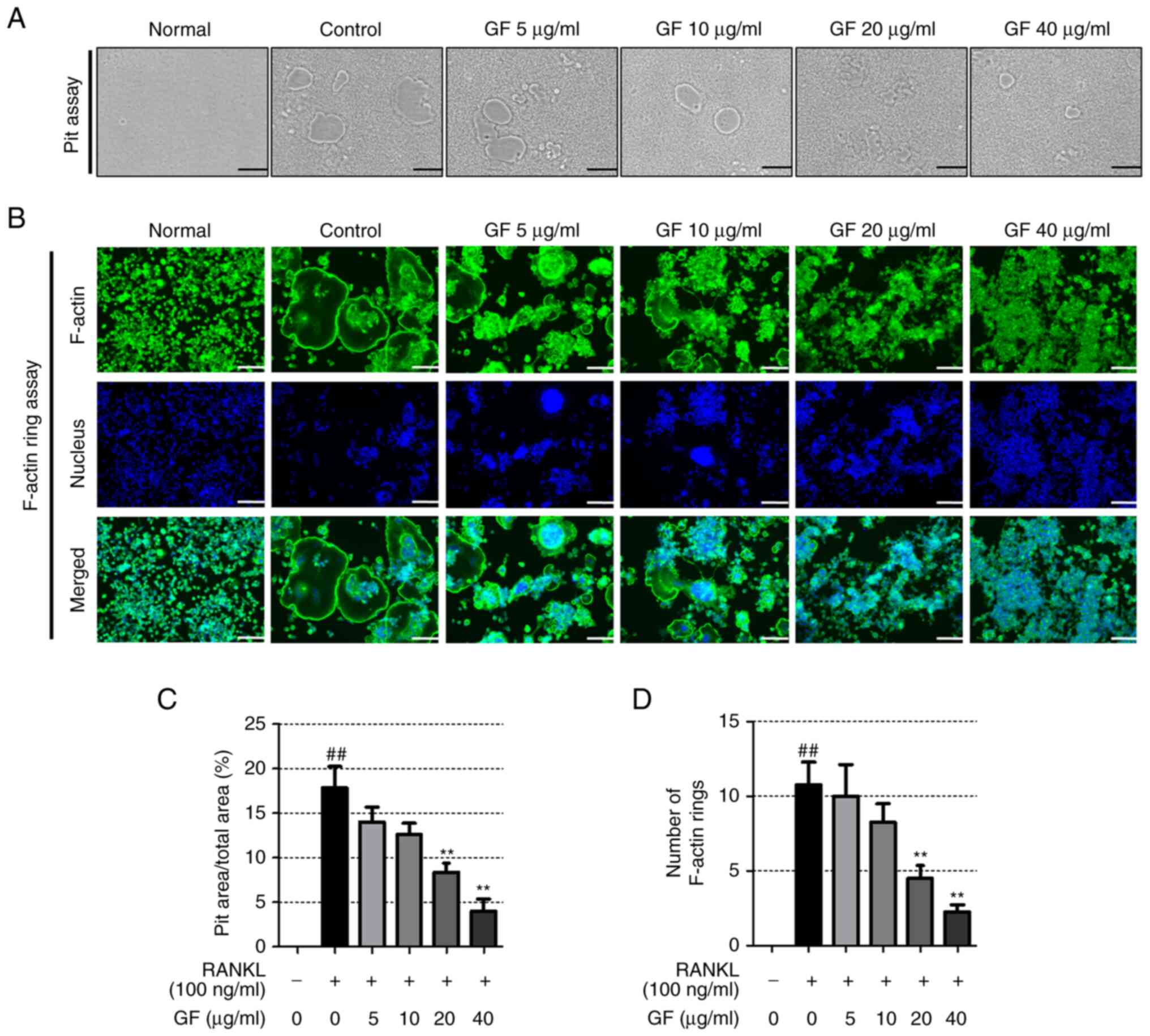 | Figure 2.Effect of increasing doses of GF on
pit formation and F-actin ring formation. (A) Images of the formed
pit were captured using an optical inverted microscope
(magnification, ×100, scale bar, 200 µm). (B) Images of the F-actin
ring were captured using an immunofluorescence microscope
(magnification, ×100, scale bar, 200 µm). (C) The area of the pit
and (D) the number of F-actin rings were counted using ImageJ
software. All experiments were repeated at least 3 times and the
results are expressed as the mean ± SEM. Statistical significance
was verified by one-way ANOVA and Tukey's post hoc test.
##P<0.01 vs. normal, untreated cells; **P<0.01 vs.
RANKL-induced cells. GF, Gleditsiae fructus; RANKL, receptor
activator of nuclear factor-κB ligand; F-actin, filamentous
actin. |
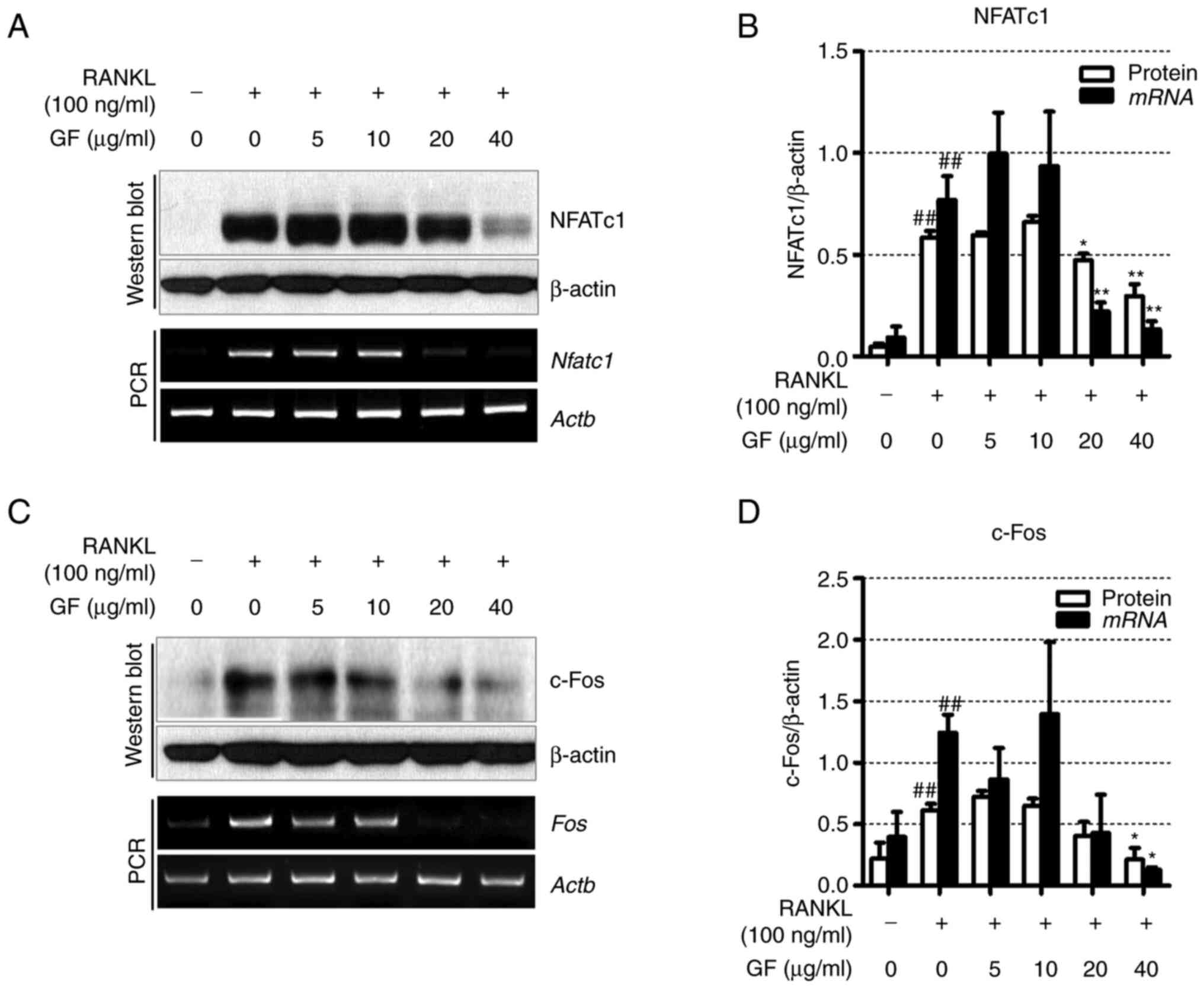
GF decreases the expression of
osteoclastogenesis-related transcription factors
To determine the effect of GF on
osteoclastogenesis-related transcription factors, western blotting
and RT-PCR were used. As shown in Fig.
3A and B, the RANKL-treated cells exhibited increased
expression of NFATc1 compared with the untreated cells. However, GF
significantly reduced the protein and mRNA levels of NFATc1 at
concentrations of 20 and 40 µg/ml. Furthermore, the results also
indicated that treatment with RANKL led to increased protein and
mRNA expression levels of c-Fos compared with the untreated cells.
Conversely, treatment with 40 µg/ml GF resulted in a decrease in
c-Fos protein and mRNA expression levels compared with the
untreated cells (Fig. 3C and
D).
GF decreases the mRNA expression
levels of osteoclast-specific genes
To explore the effects of GF treatment on
osteoclast-specific genes, RT-PCR was performed. The results
revealed that RANKL-induced cells showed upregulation in the mRNA
expression of TRAP (Acp5), RANK (Tnfrsf11a), MMP-9
(Mmp9), CA2 (Ca2), osteoclast-associated receptor
(OSCAR; Oscar), ATP6vod2 (Atp6v0d2) and dendritic
cell-specific transmembrane protein (DC-STAMP; Dcstamp),
compared with the non-treated cells (Fig. 4A and B). However, GF treatment (20
and 40 µg/ml) resulted in a decrease in the mRNA expression of
TRAP, RANK, MMP-9, CA2, OSCAR, ATP6vod2 and DC-STAMP, compared with
the RANKL-treated cells.
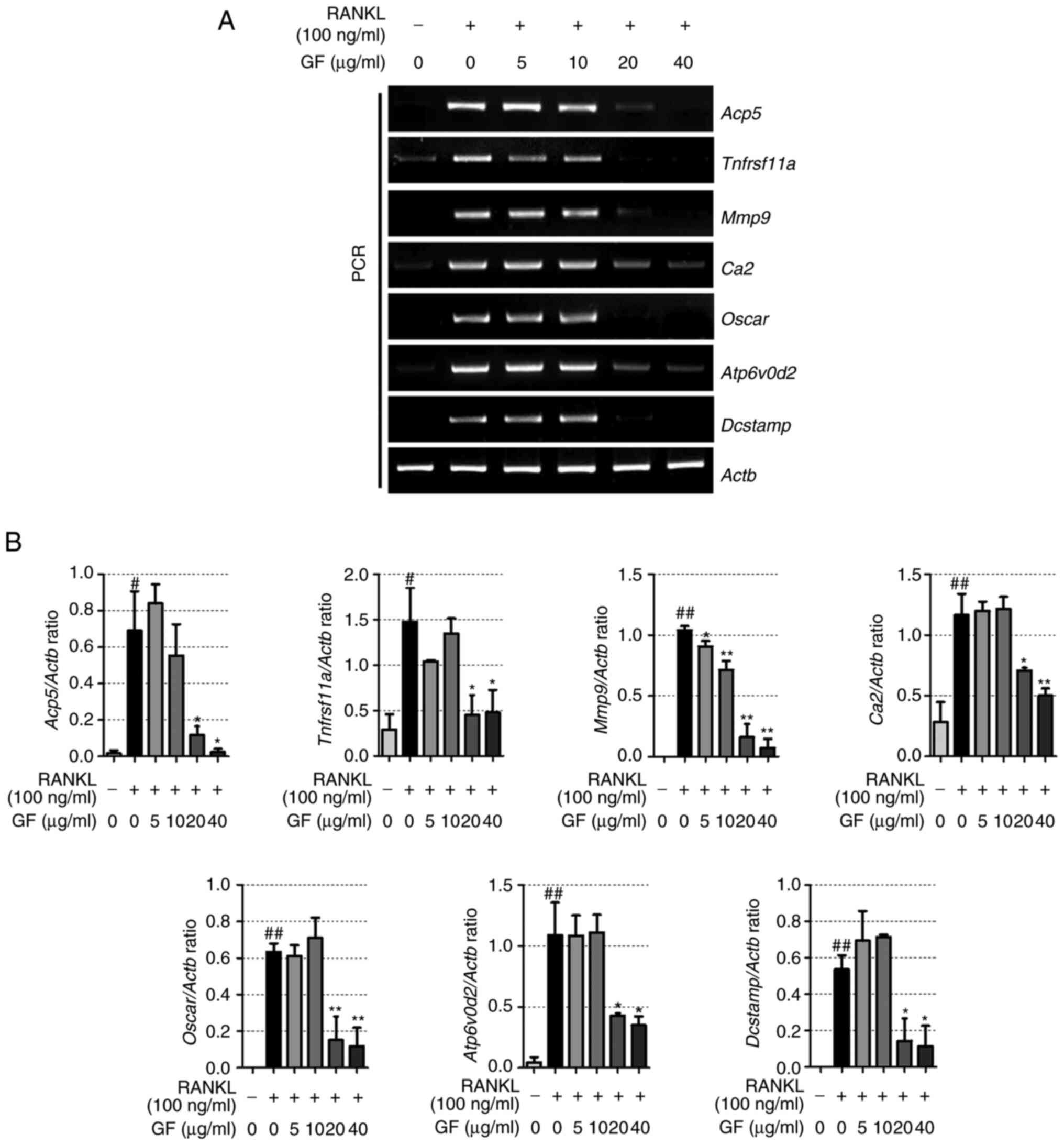 | Figure 4.Effect of increasing doses of GF on
the expression of genes related to osteoclastogenesis. (A) mRNA
expression of TRAP (Acp5), RANK (Tnfrsf11a), MMP-9
(Mmp9), CA2 (Ca2), OSCAR (Oscar), ATP6v0d2
(Atp6vod2) and DC-STAMP (Dcstamp) was verified by
reverse transcription-PCR. (B) Quantification of the expression of
each gene, standardized to β-actin. The experiment was repeated at
least 3 times, the results are expressed as the mean ± SEM.
Statistical significance was verified by one-way ANOVA and Tukey's
post hoc test. #P<0.05 and ##P<0.01 vs.
normal, untreated cells; *P<0.05 and **P<0.01 vs.
RANKL-induced cells. GF, Gleditsiae fructus; RANKL, receptor
activator of nuclear factor-κB ligand; TRAP, tartrate-resistant
acid phosphatase; RANK, receptor activator of nuclear factor-κB;
MMP-9, matrix metalloproteinase-9; CA2, carbonic anhydrase type II;
OSCAR, osteoclast associated Ig-like receptor; ATP6v0d2, ATPase H+
transporting V0 subunit d2; DC-STAMP, dendritic cell-specific
transmembrane protein; Actb, β-actin. |
GF does not significantly affect the
weight of postmenopausal osteoporotic rats and has a beneficial
impact on changes in bone metabolism markers in serum
To further demonstrate the suppressive effect of GF
on osteoclast differentiation, formation and bone resorption, an
OVX-induced rat model was used. As shown in Fig. 5A, compared with the sham group, the
OVX group showed a significant increase in body weight from 3
weeks. The ALN and GF groups did not show significant changes in
body weight compared with the OVX group. Uterine weight was
significantly reduced in the OVX group compared with the sham group
(Fig. 5B). In the E2
and ALN groups, there was a significant increase in uterine weight
compared with that of the OVX group. However, the GF groups showed
no changes in uterine weight compared with the sham group.
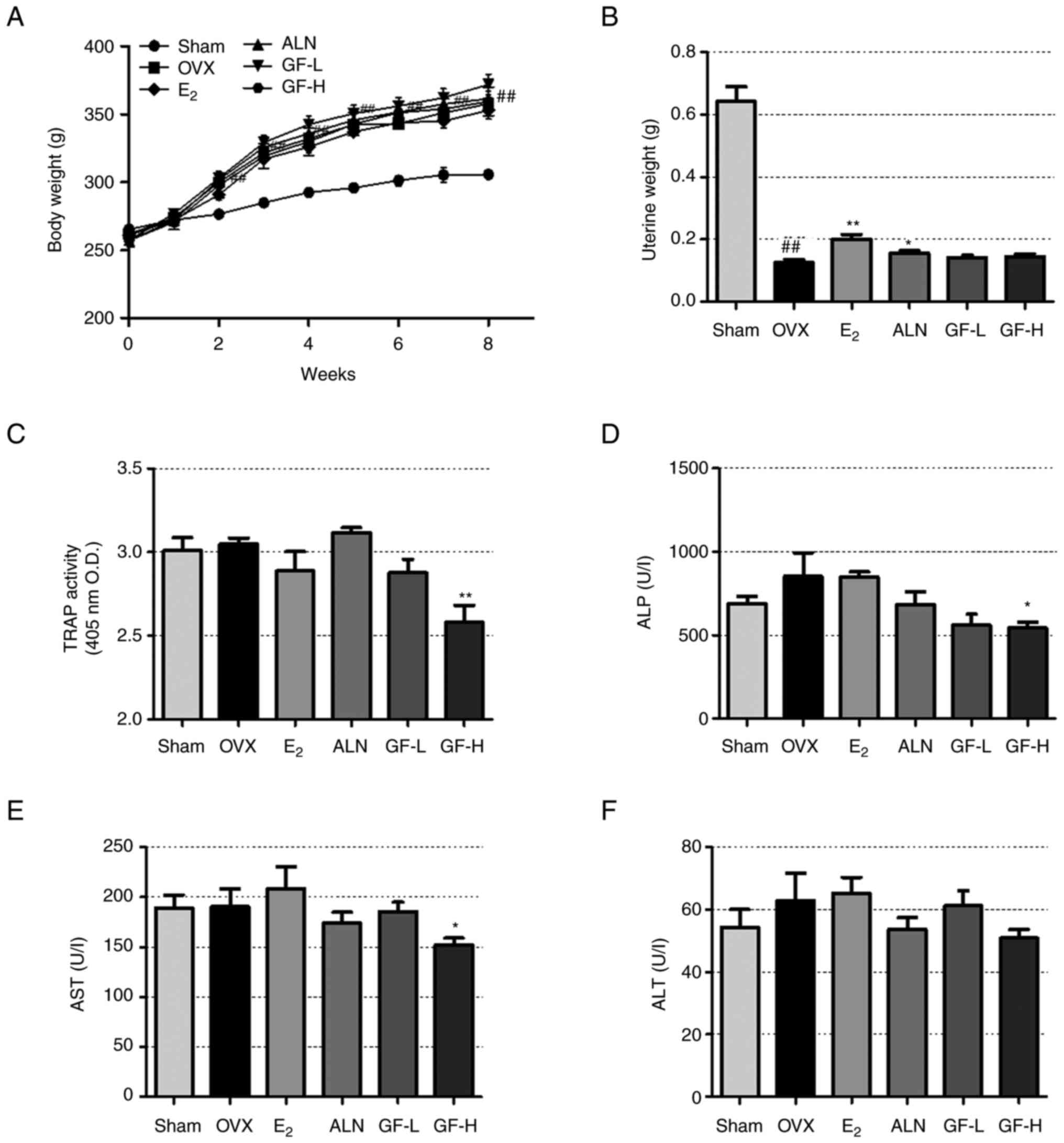 | Figure 5.Effect of GF, E2 and ALN
in the rat model of postmenopausal osteoporosis. (A) Animal body
weights were measured weekly and (B) uterine weights were measured
on the day of sacrifice. (C) TRAP activity, and (D) ALP, (E) AST
and (F) ALT levels in serum. The results are expressed as the mean
± SEM (n=8). Statistical significance was verified by one-way ANOVA
and Tukey's post hoc test. ##P<0.01 vs. the sham
group; *P<0.05 and **P<0.01 vs. the OVX group. GF,
Gleditsiae fructus; OVX, ovariectomy (rats); TRAP,
tartrate-resistant acid phosphatase; ALP, alkaline phosphatase;
AST, aspartate aminotransferase; ALT, alanine transaminase;
E2, 17β-estradiol; ALN, alendronate; GF-L, 16.9 mg/kg
GF; GF-H, 108.16 mg/kg GF; O.D., optical density. |
Next, serum analysis was performed to confirm the
effect of GF on bone formation and bone resorption markers.
Compared with the sham group, the OVX group exhibited a slight
increase in TRAP activity (not significant), as indicated in
Fig. 5C. The E2 and
GF-L groups demonstrated a reduction in TRAP activity compared with
the OVX group, but this difference was not significant. The ALN
group had no significant change in TRAP activity. In the GF-H
group, TRAP activity was significantly decreased compared with that
in the OVX group. In addition, the ALP level increased in the OVX
group compared with the sham group, although the difference was not
statistically significant (Fig.
5D). The E2 group did not show a significant change
in ALP levels, while the ALN and GF-L groups demonstrated lower ALP
levels compared with the OVX group, although this difference was
not statistically significant. The ALP level significantly
decreased in the GF-H group compared with the OVX group.
To evaluate the potential impact of GF
administration on hepatotoxicity, an analysis of serum AST and ALT
levels was conducted. Both factors showed no difference between the
SHAM and OVX groups. The E2 group showed no change in
AST levels compared with OVX group and the ALN and GF-L groups
appeared to have decreased AST levels compared with the OVX group,
but these differences were not significant (Fig. 5E). However, AST levels were
significantly decreased in the GF-H group compared with the OVX
group. In the case of ALT, there were no significant differences
between the OVX and drug administered groups (Fig. 5F).
GF inhibits the decrease in bone
density and bone microstructure
To further confirm the in vitro results, the
ability of GF to inhibit postmenopausal osteoporosis caused by OVX
induction was investigated. The OVX models were divided into six
groups of 8 rats: sham, OVX, OVX + E2, OVX + ALN, OVX +
GF-L and OVX + GF-H. The impact of GF on bone loss was evaluated
using bone microstructure and micro-CT. As shown in the 3D images
in Fig. 6A, the OVX group had a
decreased BMD relative to the sham group, while the E2,
ALN and GF treatments substantially increased the BMD of the femur,
compared with the OVX group. Compared with the sham group, the OVX
group exhibited decreased BV/TV and Tb.Th values in the femur,
while the E2, ALN and GF groups demonstrated significant
increases in both values, compared with the OVX group; notably, for
Tb.Th, there was no significant difference between the GF-L and OVX
groups. Additionally, OVX led to increased Tb.Sp in the femur
compared with the sham group, while treatment with E2,
ALN and GF resulted in a decrease in Tb.Sp (Fig. 6B-E).
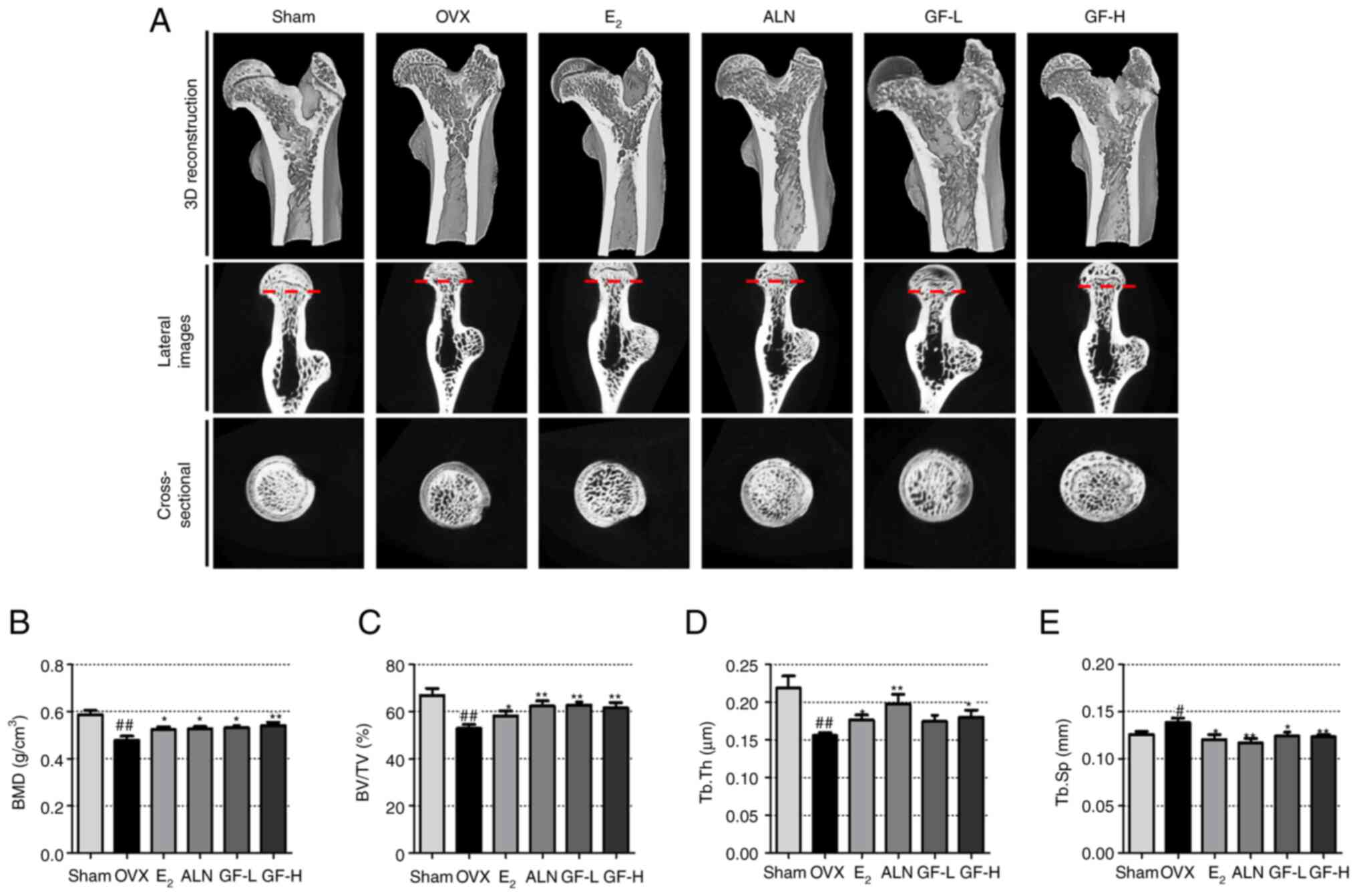 | Figure 6.Effect of GF, E2 and ALN
on changes in bone density and bone microstructure of the femoral
head. (A) Images of the femur were captured using micro-CT. Bone
microstructure factors, (B) BMD, (C) BV/TV, (D) Tb.Th and (E) Tb.Sp
were measured using SkyScan software. The results are expressed as
the mean ± SEM (n=8). Statistical significance was verified by
one-way ANOVA and Tukey's post hoc test. #P<0.05 and
##P<0.01 vs. the sham group; *P<0.05 and
**P<0.01 vs. the OVX group. GF, Gleditsiae fructus; OVX,
ovariectomy (rats); micro-CT, microcomputed tomography; BV/TV, bone
volume/total volume; Tb.Th, trabecular thickness; Tb.Sp, trabecular
separation; E2, 17β-estradiol; ALN, alendronate; GF-L,
16.9 mg/kg GF; GF-H, 108.16 mg/kg GF. |
GF increases the trabecular area and
decreases the expression of CTK and NFATc1
To investigate the trabecular area, the expression
of NFATc1 and CTK using H&E and IHC staining was examined. IHC
staining demonstrated that the levels of NFATc1 and CTK were
significantly increased in the OVX group compared with the sham
group, and reduced in the E2, ALN and GF groups compared
with the OVX group (Fig. 7A-D).
Next, to evaluate the effect of GF on the trabecular area in the
femur, H&E staining was performed (Fig. 7E and F). The OVX group displayed a
reduction in trabecular area compared with the sham group. However,
the E2, ALN and GF groups showed an increase in
trabecular area relative to the OVX group.
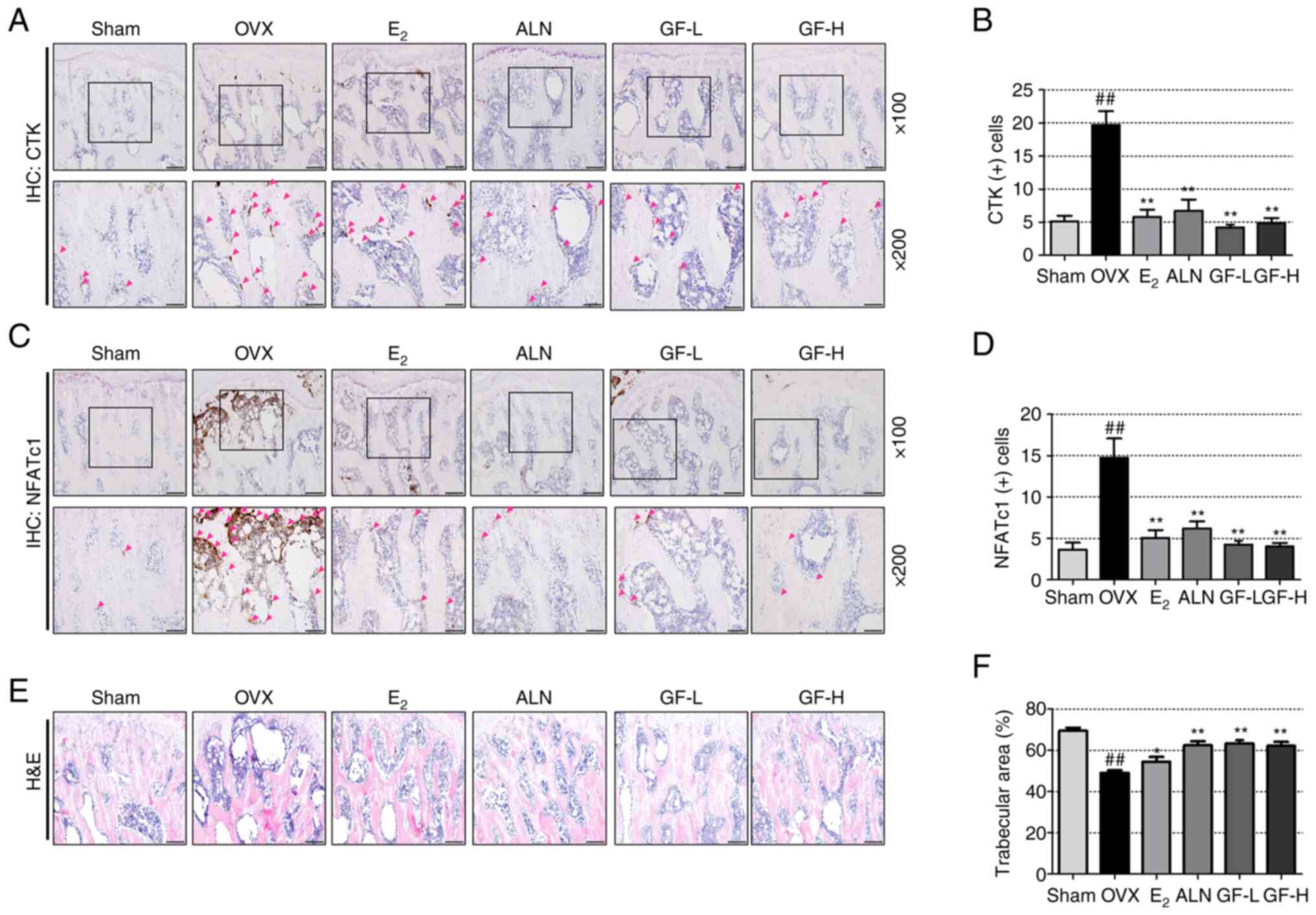 | Figure 7.Effect of GF, E2 and ALN
on histochemical and histological changes in the femoral head. (A)
CTK (+) cells in the femoral ball head (red arrow) were detected by
IHC staining. (B) The number of CTK (+) cells was determined using
ImageJ software. (C) NFATc1 (+) cells in the femoral ball head (red
arrow) were detected by IHC staining. (D) The number of NFATc1 (+)
cells was determined using ImageJ software. (E) The histological
changes of the femur were analyzed by H&E staining
(magnification: ×100; scale bar, 100 µm). (F) The area of the
trabecular bone was measured using ImageJ. The results are
expressed as the mean ± SEM (n=8). Statistical significance was
verified by one-way ANOVA and Tukey's post hoc test.
##P<0.01 vs. the sham group; *P<0.05 and
**P<0.01 vs. the OVX group. GF, Gleditsiae fructus; OVX,
ovariectomy (rats); IHC, immunohistochemistry; CTK, cathepsin K;
NFATc1, nuclear factor of activated T cells 1; H&E, hematoxylin
and eosin; E2, 17β-estradiol; ALN, alendronate; GF-L,
16.9 mg/kg GF; GF-H, 108.16 mg/kg GF. |
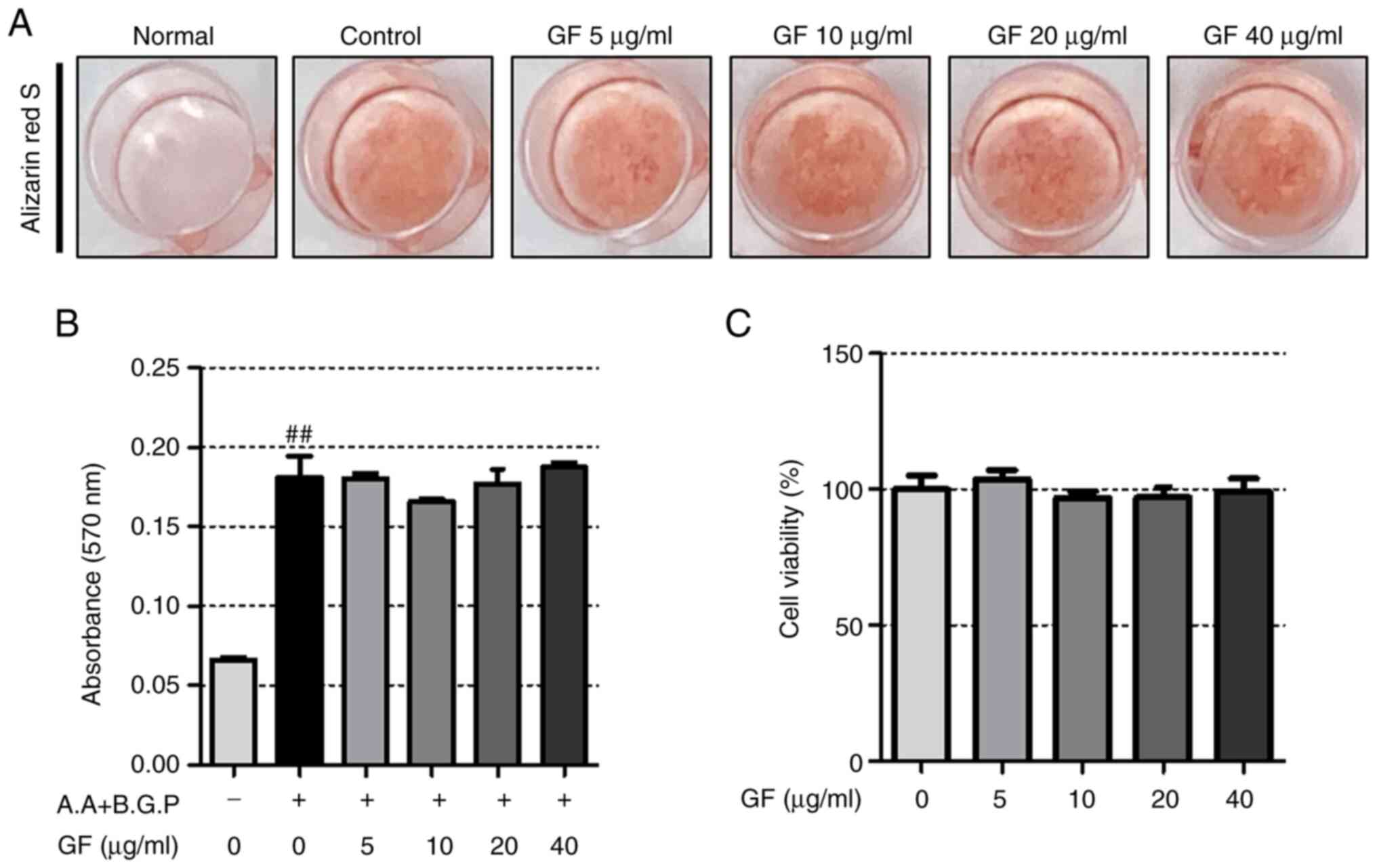
GF has no significant effect on
osteoblast differentiation
To assess the impact of GF on osteoblasts and the
formation of calcified nodules, alizarin red S staining was
performed. According to the results depicted in Fig. 8A, GF did not induce a notable
effect on the osteoblast differentiation induced by A.A and B.G.P.
Additionally, assessment of the extracted dye showed that GF did
not demonstrate a higher absorbance than the control (Fig. 8B). Furthermore, the tested
concentration of GF did not exhibit any cytotoxicity (Fig. 8C).
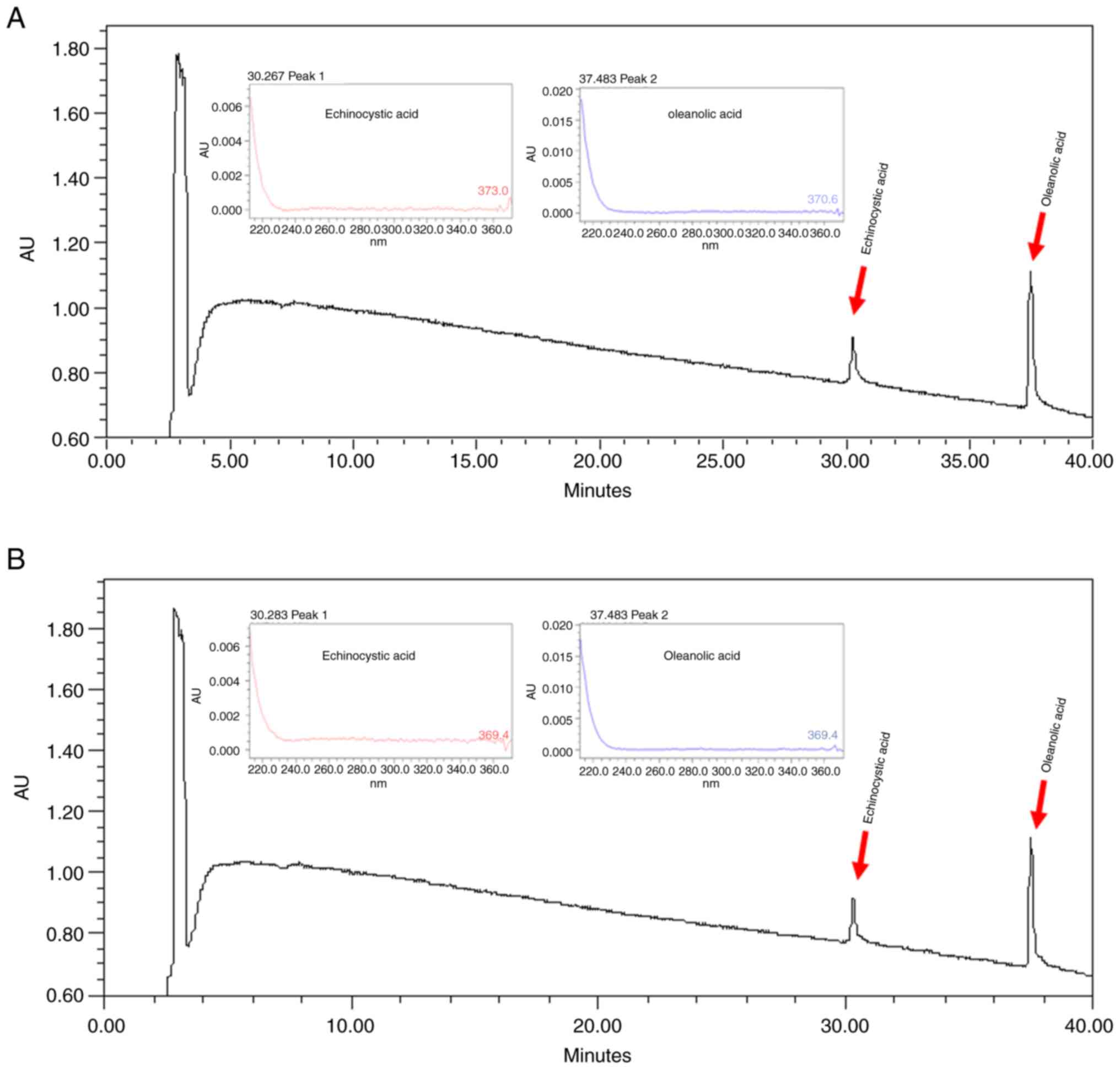
Quantitative analysis of GF
Echinocystic acid and oleanolic acid are active
ingredients of GF (20).
Chromatographic peaks of standard echinocystic acid and oleanolic
acid ingredients were detected (Fig.
9A) for the comparison with GF (Fig. 9B). It was demonstrated that peaks
for both chromatograms were detected at the same time, and the
contents of echinocystic acid and oleanolic acid in GF were
confirmed to be 8.97 and 10.8 mg/g, respectively.
Discussion
The present study indicated that GF inhibited
osteoclastogenesis and the expression of osteoclastogenesis-related
markers. In particular, GF decreased bone loss in the OVX-induced
rat models. Notably, GF had a protective effect on bone structure
in the OVX-induced rats similar to that of E2 and ALN,
despite the absence of estrogen-like activity. These results
suggest that GF could be used as an alternative therapy to prevent
and treat bone loss.
Botanical drugs, derived from natural sources, offer
several advantages for osteoporosis treatment, including their
natural origin, lower risk of side effects, their ability to act on
multiple pathways in the body and their increasing popularity in
the expanding natural products market. Additionally, botanical
drugs are often less expensive to develop and manufacture, can be
administered orally and may improve patient compliance. As research
continues, more effective and targeted botanical drugs may be
developed, with the potential to significantly improve the lives of
patients with osteoporosis (31–34).
RAW 264.7 cells are a suitable model for studying
osteoclast formation and function, as the cells readily
differentiate into osteoclasts upon exposure to RANKL (35). TRAP has long been used as a
histochemical marker for osteoclasts (36). In the present study, GF
substantially decreased the number of TRAP (+) cells and TRAP
activity. In order to evaluate the bone resorption ability of
osteoclasts, researchers typically use the pit formation assay
(37). In the present study, it
was observed that the pit area was reduced by GF treatment compared
with the control group, indicating inhibition of bone resorption.
Upon osteoclast attachment to bone, osteoclasts produce F-actin
structures and, in this sealing area, a corrugated border of
osteoclasts is formed (38). In
the present study, GF decreased the production of F-actin rings
compared with that of the control group. The results showed that GF
has an anti-osteoporotic effect by inhibiting osteoclastogenesis
and bone resorption.
RANKL and RANK are factors that play a key role in
osteoclastogenesis and activation. RANK is mainly expressed in
osteoclast progenitor cells derived from hematopoietic stem cells
and RANKL is mainly expressed in osteoblasts derived from stromal
cells (39). RANK knockout mice
exhibit severe osteopetrosis due to an apparent blockade of
osteoclastogenesis (40). Upon
RANKL and RANK binding, osteoclastogenesis-related transcription
factors, such as NFATc1 and c-Fos, are expressed to regulate the
expression of osteoclast-specific genes (41,42).
NFATc1 is a member of the nuclear factor of activated T cells
family. According to previous studies, embryonic stem cells
deficient in NFATc1 cannot differentiate into osteoclasts (17,43).
c-Fos is a member of the Fos family and plays an essential role in
the differentiation of macrophages into osteoclasts (44). A previous study has shown that mice
deficient in c-Fos develop osteopetrosis due to a lack of
osteoclast formation (45). In the
present study, western blotting and RT-PCR data revealed that
NFATc1 and c-Fos levels were significantly downregulated by GF
treatment. Based on these data, GF has an anti-osteoporotic effect
by suppressing the expression of NFATc1 and c-Fos signaling
pathways.
Upon activation of NFATc1, specific genes related to
osteoclastogenesis are activated and are abundantly expressed in
osteoclasts (17). TRAP is known
to be an important cytochemical marker of osteoclasts. The serum
concentration of TRAP has been used as a phenotype indicator of
bone resorption and osteoclast function (46), and osteoclasts isolated from TRAP
knockout animals showed a cellular intrinsic defect in bone
resorption (47). In addition,
mice lacking TRAP had an osteopetrotic bone phenotype at 4 weeks of
age (48). MMP-9 is a bone
resorbing factor and expression of this molecule in early
osteoclasts induces transformation into mature osteoclasts
(49). Mice deficient in MMP-9
exhibited an accumulation of late hypertrophic chondrocytes
(49). A previous study has shown
that mice with null mutations in MMP-9 exhibited abnormal patterns
of skeletal growth plate ossification and vascularization (50). CA2 acts as a mediator of hormones
that stimulate bone resorption and osteoclast formation and is
detected in the early stages of osteoclast differentiation
(51,52). Individuals with a deficiency in CA2
have reduced bone resorption and clinical osteopetrosis (53). Furthermore, in vivo,
CA2-deficient mice have a human-like phenotype with the same
genetic enzymatic deletion (53).
OSCAR has been reported to be involved in cell-cell interactions
between osteoblasts and osteoclasts and is expressed at a later
stage of osteoclast maturation in mice (54,55).
ATP6v0d2 acts as a regulatory element of cell fusion in
osteoclastogenesis and is an essential component of
osteoclast-specific proton pumps that mediate extracellular
acidification in bone resorption (56). The differentiation of osteoclasts
and osteoblasts is regulated by DC-STAMP, which is a crucial factor
in maintaining bone homeostasis (57). Osteoclast formation is dependent on
cell-cell fusion, which is facilitated by Atp6vod2 and DC-STAMP
with mice deficient in these factors developing osteopetrosis due
to the absence of osteoclasts (58,59).
The results of the present study also demonstrated that GF
treatment decreased the expression of osteoclast-specific genes
(Acp5, Tnfrsf11a, Mmp9, Ca2, Oscar, Atp6v0d2 and Dcstamp).
This indicated that GF could regulate the NFATc1/c-Fos signaling
pathway, thereby suppressing the expression of osteoclast-specific
genes.
Bone is maintained by the harmonious and balanced
activity of osteoclasts and osteoblasts. However, when the balance
is disturbed due to excessive activity of osteoclasts, bone
diseases such as osteoporosis occur (15). OVX causes bone loss due to estrogen
deficiency in OVX rat models. This effect is similar to the change
that occurs in humans and is widely used in experiments
investigating osteoporosis (6,60).
Estrogen prevents weight gain by regulating food intake and
behavior. In addition, ablated ovaries cause functional loss of the
uterus, reducing its weight and volume. Weight gain is a common
symptom observed in the OVX-induced model. Decreased uterine weight
also demonstrates the successful establishment of the OVX-induced
model (60). In the present study,
after 3 weeks, a significant increase in body weight was observed
in the OVX group compared with the sham group. There was no change
in body weight in the ALN, E2, GF-L and GF-H groups
compared with the OVX group. According to the experimental results,
the OVX-induced model was successfully established. Furthermore,
OVX induced a substantial decrease in uterine weight, thereby
validating the successful establishment of a menopausal-like model.
Both the positive control groups, E2 and ALN, countered
this reduction, whereas the GF group did not demonstrate any
significant effect.
TRAP is utilized as a serum marker to confirm the
anti-osteoporotic effects in both animal experiments and an in
vitro study (61). ALP is a
marker expressed in the early stages of osteoblast differentiation
(62). Previous studies have shown
that the levels of ALP are increased in models of estrogen
deficiency (63) and excessive
osteoclast activity due to estrogen deficiency also increases
osteoblast activity (64). In the
present study, both TRAP and ALP markers increased in the OVX group
compared with the sham group, but the differences did not reach
statistical significance. In a prior study, a significant
difference between the two groups was observed after 12 weeks
post-OVX, suggesting that the shorter sacrifice period of 8 weeks
in the present study may have contributed to the lack of
statistical significance (65).
Nonetheless, the results of the present study suggested that the
GF-H group may serve as an effective osteoclast inhibitor, as it
demonstrated a significant inhibition of both markers when compared
with the OVX group.
Recent research has shown that microarchitecture is
important along with bone density (66), thus, the importance of
histomorphometry for evaluation of the bone trabecular structure is
receiving attention. Histopathological methods have been
traditionally used for bone morphometry (67). However, bone tissue morphometric
methods using imaging techniques such as micro-CT and
high-resolution MRI have also been actively studied (68). In addition, these methods show an
excellent correlation compared with histopathological
histomorphometry (69). Micro-CT
is high-resolution CT with pixel sizes typically ranging 1–50 µm
and X-rays can be used to investigate the microstructure of a
sample. Furthermore, micro-CT characterization consists of three
main sequential processes: Acquisition, reconstruction and analysis
(70,71). Bone density is defined as the
amount of bone minerals in bone tissue. It is known that the lower
the BMD, the higher the risk of fracture, even from a small impact
(72). Tb.Th is a measure of the
thickness of numerous spheres in the trabecular column and can be
indicative of osteoporosis if a decrease in the bone trabecular
thickness is detected. Tb.Sp is the average diameter of the
trabecular area and as osteoporosis progresses, the thickness
increases (73,74). In the present study, BMD and bone
microarchitecture parameters, such as BV/TV and Tb.Th decreased in
the OVX group compared with the sham group, and a decrease in the
Tb.Sp of the E2, ANL and GF groups compared with the OVX
group was observed. Trabecular bone structural parameters derived
from micro-CT data are based on traditional static osseous
morphometry that evaluate the thickness, connectivity, distribution
and spacing of the trabeculae (75) and the representative method for
analyzing trabecular bone is H&E staining (67,76).
In the present study, to evaluate morphological changes in bone
tissue, H&E staining was used to measure trabecular area while
IHC staining was employed to measure osteoclast-related parameters.
H&E staining revealed that bone loss was significantly
decreased by E2, ALN and GF treatment compared with that
of the OVX group. Furthermore, compared with the OVX group, femoral
bone from rats treated with E2, ALN and GF exhibited
significant reductions in the expression of NFATc1 and CTK. These
results showed that GF treatment decreased bone loss induced by
OVX.
The limitations of the present study are as follows:
i) When RANK-RANKL binds, TRAF6, MAPK and NF-kB are activated.
Subsequently, c-Fos and NFATc1 expression is induced (77). Further studies of the effects of GF
on MAPK and NF-κB are needed to further evaluate the mechanism of
the inhibitory effect of GF on osteoclastogenesis; and ii) in the
present study, GF demonstrated a notable inhibition of osteoclast
differentiation. However, the specific component within GF that is
responsible for this effect remains unclear. Previous research has
identified echinocystic acid (78)
and oleanolic acid (79), both
prominent constituents of GF, as potential contributors to the
inhibition of osteoclast differentiation. Nevertheless, to
precisely ascertain the osteoclast inhibitory effect of GF, further
investigation is warranted, encompassing comprehensive validation
of the GF fraction and all its constituent components.
In conclusion, the data from the present study
demonstrated that GF plays a protective role in the OVX-induced
model of postmenopausal osteoporosis by decreasing
osteoclastogenesis, osteoclast formation and bone resorption. Thus,
GF has the potential to act as an alternative therapeutic agent for
the prevention and treatment of postmenopausal osteoporosis.
Acknowledgements
Not applicable.
Funding
This research was supported by a grant from the Korea Health
Technology R&D Project through the Korea Health Industry
Development Institute, funded by the Ministry of Health and
Welfare, Republic of Korea (grant no. HF21C0092).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HSJ conceptualized the study; CYC and SHK performed
all experiments; MK, BCK, TKK and JHK contributed to the
statistical analysis; JHK, YS and HSJ helped interpret the results;
CYC and SHK drafted the manuscript. CYC, SHK, BCK, TKK, JHK, MK, YS
and HSJ participated in obtaining materials and confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by The Kyung
Hee University Animal Care and Use Committee (approval no.
KHSASP-21-185).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crimmins EM: Lifespan and healthspan:
Past, present, and promise. Gerontologist. 55:901–911. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Riggs BL and Melton LJ III: The worldwide
problem of osteoporosis: Insights afforded by epidemiology. Bone.
17 (5 Suppl):505S–511S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cosman F, de Beur SJ, LeBoff MS, Lewiecki
EM, Tanner B, Randall S and Lindsay R; National Osteoporosis
Foundation, : Clinician's guide to prevention and treatment of
osteoporosis. Osteoporos Int. 25:2359–2381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsuo K and Irie N: Osteoclast-osteoblast
communication. Arch Biochem Biophys. 473:201–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng X and McDonald JM: Disorders of bone
remodeling. Annu Rev Pathol. 6:121–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sözen T, Özışık L and Başaran NÇ: An
overview and management of osteoporosis. Eur J Rheumatol. 4:46–56.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tella SH and Gallagher JC: Prevention and
treatment of postmenopausal osteoporosis. J Steroid Biochem Mol
Biol. 142:155–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rasmusson L and Abtahi J: Bisphosphonate
associated osteonecrosis of the jaw: An update on pathophysiology,
risk factors, and treatment. Int J Dent. 2014:4710352014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abrahamsen B: Bisphosphonate adverse
effects, lessons from large databases. Curr Opin Rheumatol.
22:404–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Collin-Osdoby P, Yu X, Zheng H and Osdoby
P: RANKL-mediated osteoclast formation from murine RAW 264.7 cells.
Methods Mol Med. 80:153–166. 2003.PubMed/NCBI
|
|
11
|
Clohisy JC, Frazier E, Hirayama T and
Abu-Amer Y: RANKL is an essential cytokine mediator of
polymethylmethacrylate particle-induced osteoclastogenesis. J
Orthop Res. 21:202–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galibert L, Tometsko ME, Anderson DM,
Cosman D and Dougall WC: The involvement of multiple tumor necrosis
factor receptor (TNFR)-associated factors in the signaling
mechanisms of receptor activator of NF-kappaB, a member of the TNFR
superfamily. J Biol Chem. 273:34120–34127. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi N, Kadono Y, Naito A, Matsumoto
K, Yamamoto T, Tanaka S and Inoue J: Segregation of TRAF6-mediated
signaling pathways clarifies its role in osteoclastogenesis. EMBO
J. 20:1271–1280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JH and Kim N: Regulation of NFATc1 in
osteoclast differentiation. J Bone Metab. 21:233–241. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai P, Du JR, Zhang MX, Kuang X, Li YJ,
Chen YS and He Y: Aqueous extract of Gleditsia sinensis Lam. Fruits
improves serum and liver lipid profiles and attenuates
atherosclerosis in rabbits fed a high-fat diet. J Ethnopharmacol.
137:1061–1066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Güçlü-Ustündağ O and Mazza G: Saponins:
Properties, applications and processing. Crit Rev Food Sci Nutr.
47:231–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Li Z, Zheng KY, Guo AJ, Zhu KY,
Zhang WL, Zhan JY, Dong TT, Su Z and Tsim KW: Chemical
fingerprinting and quantitative analysis of two common Gleditsia
sinensis fruits using HPLC-DAD. Acta Pharm. 63:505–515. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joh EH, Gu W and Kim DH: Echinocystic acid
ameliorates lung inflammation in mice and alveolar macrophages by
inhibiting the binding of LPS to TLR4 in NF-κB and MAPK pathways.
Biochem Pharmacol. 84:331–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hyam SR, Jang SE, Jeong JJ, Joh EH, Han MJ
and Kim DH: Echinocystic acid, a metabolite of lancemaside A,
inhibits TNBS-induced colitis in mice. Int Immunopharmacol.
15:433–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee W, Yang EJ, Ku SK, Song KS and Bae JS:
Anti-inflammatory effects of oleanolic acid on LPS-induced
inflammation in vitro and in vivo. Inflammation. 36:94–102. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi JK, Oh HM, Lee S, Park JW, Khang D,
Lee SW, Lee WS, Rho MC and Kim SH: Oleanolic acid acetate inhibits
atopic dermatitis and allergic contact dermatitis in a murine
model. Toxicol Appl Pharmacol. 269:72–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JY, Cheon YH, Oh HM, Rho MC,
Erkhembaatar M, Kim MS, Lee CH, Kim JJ, Choi MK, Yoon KH, et al:
Oleanolic acid acetate inhibits osteoclast differentiation by
downregulating PLCγ2-Ca(2+)-NFATc1 signaling, and suppresses bone
loss in mice. Bone. 60:104–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lacativa PG and Farias ML: Osteoporosis
and inflammation. Arq Bras Endocrinol Metabol. 54:123–132. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee S, Kim M, Hong S, Kim EJ, Kim JH, Sohn
Y and Jung HS: Effects of sparganii rhizoma on osteoclast formation
and osteoblast differentiation and on an OVX-induced bone loss
model. Front Pharmacol. 12:7978922022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tschöp MH, Speakman JR, Arch JR, Auwerx J,
Brüning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C,
et al: A guide to analysis of mouse energy metabolism. Nat Methods.
9:57–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Huang P, Tang PF, Chan KM and Li
G: Alendronate (ALN) combined with osteoprotegerin (OPG)
significantly improves mechanical properties of long bone than the
single use of ALN or OPG in the ovariectomized rats. J Orthop Surg
Res. 6:342011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beeton C, Garcia A and Chandy KG: Drawing
blood from rats through the saphenous vein and by cardiac puncture.
J Vis Exp. 2662007.PubMed/NCBI
|
|
31
|
Dietz BM, Hajirahimkhan A, Dunlap TL and
Bolton JL: Botanicals and their bioactive phytochemicals for
women's health. Pharmacol Rev. 68:1026–1073. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He J, Li X, Wang Z, Bennett S, Chen K,
Xiao Z, Zhan J, Chen S, Hou Y, Chen J, et al: Therapeutic anabolic
and anticatabolic benefits of natural Chinese medicines for the
treatment of osteoporosis. Front Pharmacol. 10:13442019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Słupski W, Jawień P and Nowak B:
Botanicals in postmenopausal osteoporosis. Nutrients. 13:16092021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang T, Liu Q, Tjhioe W, Zhao J, Lu A,
Zhang G, Tan RX, Zhou M, Xu J and Feng HT: Therapeutic potential
and outlook of alternative medicine for osteoporosis. Curr Drug
Targets. 18:1051–1068. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hartley JW, Evans LH, Green KY, Naghashfar
Z, Macias AR, Zerfas PM and Ward JM: Expression of infectious
murine leukemia viruses by RAW264.7 cells, a potential complication
for studies with a widely used mouse macrophage cell line.
Retrovirology. 5:12008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vincent C, Kogawa M, Findlay DM and Atkins
GJ: The generation of osteoclasts from RAW 264.7 precursors in
defined, serum-free conditions. J Bone Miner Metab. 27:114–119.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vesprey A and Yang W: Pit assay to measure
the bone resorptive activity of bone marrow-derived osteoclasts.
Bio Protoc. 6:e18362016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsubara T, Kinbara M, Maeda T, Yoshizawa
M, Kokabu S and Takano Yamamoto T: Regulation of osteoclast
differentiation and actin ring formation by the cytolinker protein
plectin. Biochem Biophys Res Commun. 489:472–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dougall WC, Glaccum M, Charrier K,
Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME,
Maliszewski CR, et al: RANK is essential for osteoclast and lymph
node development. Genes Dev. 13:2412–2424. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yamashita T, Yao Z, Li F, Zhang Q, Badell
IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, et al:
NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB
ligand (RANKL) and tumor necrosis factor-induced osteoclast
precursor differentiation by activating c-Fos and NFATc1. J Biol
Chem. 282:18245–18253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fujioka S, Niu J, Schmidt C, Sclabas GM,
Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL and Chiao PJ:
NF-kappaB and AP-1 connection: Mechanism of NF-kappaB-dependent
regulation of AP-1 activity. Mol Cell Biol. 24:7806–7819. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Asagiri M, Sato K, Usami T, Ochi S,
Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E and
Takayanagi H: Autoamplification of NFATc1 expression determines its
essential role in bone homeostasis. J Exp Med. 202:1261–1269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Grigoriadis AE, Wang ZQ, Cecchini MG,
Hofstetter W, Felix R, Fleisch HA and Wagner EF: c-Fos: A key
regulator of osteoclast-macrophage lineage determination and bone
remodeling. Science. 266:443–448. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Arai A, Mizoguchi T, Harada S, Kobayashi
Y, Nakamichi Y, Yasuda H, Penninger JM, Yamada K, Udagawa N and
Takahashi N: Fos plays an essential role in the upregulation of
RANK expression in osteoclast precursors within the bone
microenvironment. J Cell Sci. 125:2910–2917. 2012.PubMed/NCBI
|
|
46
|
Ballanti P, Minisola S, Pacitti MT,
Scarnecchia L, Rosso R, Mazzuoli GF and Bonucci E:
Tartrate-resistant acid phosphate activity as osteoclastic marker:
Sensitivity of cytochemical assessment and serum assay in
comparison with standardized osteoclast histomorphometry.
Osteoporos Int. 7:39–43. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bune AJ, Hayman AR, Evans MJ and Cox TM:
Mice lacking tartrate-resistant acid phosphatase (Acp 5) have
disordered macrophage inflammatory responses and reduced clearance
of the pathogen, Staphylococcus aureus. Immunology. 102:103–113.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Blumer MJ, Hausott B, Schwarzer C, Hayman
AR, Stempel J and Fritsch H: Role of tartrate-resistant acid
phosphatase (TRAP) in long bone development. Mech Dev. 129:162–176.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ortega N, Behonick DJ, Colnot C, Cooper
DNW and Werb Z: Galectin-3 is a downstream regulator of matrix
metalloproteinase-9 function during endochondral bone formation.
Mol Biol Cell. 16:3028–3039. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vu TH, Shipley JM, Bergers G, Berger JE,
Helms JA, Hanahan D, Shapiro SD, Senior RM and Werb Z:
MMP-9/gelatinase B is a key regulator of growth plate angiogenesis
and apoptosis of hypertrophic chondrocytes. Cell. 93:411–422. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
David JP, Rincon M, Neff L, Horne WC and
Baron R: Carbonic anhydrase II is an AP-1 target gene in
osteoclasts. J Cell Physiol. 188:89–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Negishi-Koga T and Takayanagi H:
Ca2+-NFATc1 signaling is an essential axis of osteoclast
differentiation. Immunol Rev. 231:241–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Alsharidi A, Al-Hamed M and Alsuwaida A:
Carbonic anhydrase II deficiency: Report of a novel mutation. CEN
Case Rep. 5:108–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nedeva IR, Vitale M, Elson A, Hoyland JA
and Bella J: Role of OSCAR signaling in osteoclastogenesis and bone
disease. Front Cell Dev Biol. 9:6411622021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nemeth K, Schoppet M, Al-Fakhri N, Helas
S, Jessberger R, Hofbauer LC and Goettsch C: The role of
osteoclast-associated receptor in osteoimmunology. J Immunol.
186:13–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu H, Xu G and Li YP: Atp6v0d2 is an
essential component of the osteoclast-specific proton pump that
mediates extracellular acidification in bone resorption. J Bone
Miner Res. 24:871–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chiu YH and Ritchlin CT: DC-STAMP: A key
regulator in osteoclast differentiation. J Cell Physiol.
231:2402–2407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K,
Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K,
et al: DC-STAMP is essential for cell-cell fusion in osteoclasts
and foreign body giant cells. J Exp Med. 202:345–351. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim
N, Kang JS, Miyamoto T, Suda T, Lee SK, et al: v-ATPase V0 subunit
d2-deficient mice exhibit impaired osteoclast fusion and increased
bone formation. Nat Med. 12:1403–1409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kalu DN: The ovariectomized rat model of
postmenopausal bone loss. Bone Miner. 15:175–191. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim M, Kim JH, Hong S, Kwon B, Kim EY,
Jung HS and Sohn Y: Effects of melandrium firmum rohrbach on
RANKL-induced osteoclast differentiation and OVX rats. Mol Med Rep.
24:6102021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Seibel MJ: Biochemical markers of bone
turnover: Part I: Biochemistry and variability. Clin Biochem Rev.
26:97–122. 2005.PubMed/NCBI
|
|
63
|
Kuo TR and Chen CH: Bone biomarker for the
clinical assessment of osteoporosis: Recent developments and future
perspectives. Biomark Res. 5:182017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Väänänen HK and Härkönen PL: Estrogen and
bone metabolism. Maturitas. 23 (Suppl):S65–S69. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tantikanlayaporn D, Wichit P,
Weerachayaphorn J, Chairoungdua A, Chuncharunee A, Suksamrarn A and
Piyachaturawat P: Bone sparing effect of a novel phytoestrogen
diarylheptanoid from Curcuma comosa Roxb. In ovariectomized rats.
PLoS One. 8:e787392013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wagner PP, Whittier DE, Foesser D, Boyd
SK, Chapurlat R and Szulc P: Bone microarchitecture decline and
risk of fall and fracture in men with poor physical performance-the
STRAMBO study. J Clin Endocrinol Metab. 106:e5180–e5194.
2021.PubMed/NCBI
|
|
67
|
Osterhoff G, Morgan EF, Shefelbine SJ,
Karim L, McNamara LM and Augat P: Bone mechanical properties and
changes with osteoporosis. Injury. 47 (Suppl 2):S11–S20. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Czeibert K, Baksa G, Grimm A, Nagy SA,
Kubinyi E and Petneházy Ö: MRI, CT and high resolution
macro-anatomical images with cryosectioning of a Beagle brain:
Creating the base of a multimodal imaging atlas. PLoS One.
14:e02134582019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lasbleiz J, Burgun A, Marin F, Rolland Y
and Duvauferrier R: Vertebral trabecular network analysis on CT
images. J Radiol. 86:645–649. 2005.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cortet B, Chappard D, Boutry N, Dubois P,
Cotten A and Marchandise X: Relationship between computed
tomographic image analysis and histomorphometry for
microarchitectural characterization of human calcaneus. Calcif
Tissue Int. 75:23–31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Torres A, Lorenzo V and Gonzalez-Posada
JM: Comparison of histomorphometry and computerized tomography of
the spine in quantitating trabecular bone in renal osteodystrophy.
Nephron. 44:282–287. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Park SB, Lee YJ and Chung CK: Bone mineral
density changes after ovariectomy in rats as an osteopenic model:
Stepwise description of double dorso-lateral approach. J Korean
Neurosurg Soc. 48:309–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Laib A, Kumer JL, Majumdar S and Lane NE:
The temporal changes of trabecular architecture in ovariectomized
rats assessed by MicroCT. Osteoporos Int. 12:936–941. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bouxsein ML, Boyd SK, Christiansen BA,
Guldberg RE, Jepsen KJ and Müller R: Guidelines for assessment of
bone microstructure in rodents using micro-computed tomography. J
Bone Miner Res. 25:1468–1486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wu Y, Adeeb S and Doschak MR: Using
Micro-CT derived bone microarchitecture to analyze bone stiffness-a
case study on osteoporosis rat bone. Front Endocrinol (Lausanne).
6:802015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lee KY, Kim JH, Kim EY, Yeom M, Jung HS
and Sohn Y: Water extract of Cnidii Rhizoma suppresses
RANKL-induced osteoclastogenesis in RAW 264.7 cell by inhibiting
NFATc1/c-Fos signaling and prevents ovariectomized bone loss in
SD-rat. BMC Complement Altern Med. 19:2072019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lee K, Chung YH, Ahn H, Kim H, Rho J and
Jeong D: Selective regulation of MAPK signaling mediates
RANKL-dependent osteoclast differentiation. Int J Biol Sci.
12:235–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yang JH, Li B, Wu Q, Lv JG and Nie HY:
Echinocystic acid inhibits RANKL-induced osteoclastogenesis by
regulating NF-κB and ERK signaling pathways. Biochem Biophys Res
Commun. 477:673–677. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xie BP, Shi LY, Li JP, Zeng Y, Liu W, Tang
SY, Jia LJ, Zhang J and Gan GX: Oleanolic acid inhibits
RANKL-induced osteoclastogenesis via ER alpha/miR-503/RANK
signaling pathway in RAW264.7 cells. Biomed Pharmacother.
117:1090452019. View Article : Google Scholar : PubMed/NCBI
|