Introduction
Liver is the largest substantive organ of human
body, bearing numerous important and complex physiological
functions. It is not only the metabolic center of numerous
substances, but also has a variety of physiological functions such
as detoxification and immune regulation (1). Chronic liver disease (CLD) is one of
the leading causes of death globally, and the associated burden is
also rising (2). In recent years,
due to changes in lifestyle and dietary habits, the incidence and
prevalence of non-alcoholic fatty liver disease (NAFLD) have been
increasing (3), and it has
gradually become the most common cause of CLD. At present, ~25% of
the global population is considered to have NAFLD (4). The risk of liver disease increases as
excessive consumption of alcohol increases (5). The number of deaths caused by
end-stage liver disease worldwide even is as numerous as 2 million
each year (6). At present, liver
disease has gradually developed into a global public health
problem, and the prevention and treatment of this disease will be
the top priority.
At the same time, the liver is also the hub of
plasma protein synthesis, glucose and lipid metabolism. It has been
suggested that metabolic reprogramming in the liver may influence
the progression of liver disease (7). However, albumin, an important protein
synthesized and metabolized in the liver, has always been the focus
of attention. Its effects on the liver are still being explored. It
is known to be introduced as a therapy for the management of
hypoalbuminemia and ascites in patients with cirrhosis because of
its ability to maintain plasma oncotic pressure (8). Subsequently, numerous clinical trials
have found that albumin infusion is beneficial in the treatment of
other complications of liver cirrhosis, which can improve the
prognosis and/or survival of patients with spontaneous bacterial
peritonitis (SBP) (9,10) and hepatorenal syndrome (HRS)
(11,12). These benefits have drawn attention
to the role of albumin in the organism.
However, due to the incomplete understanding of its
structure and function, the use of albumin is also controversial
(13,14). When Jalan et al (15) verified the correlation between
albumin therapy and its function, it was found that not only the
quantity of albumin was reduced, but also the quality of albumin
was damaged in patients with liver failure. Hence, the concept of
‘effective albumin concentration’ has been proposed on this basis
by them. This further draws attention to albumin's function rather
than just quantity. In addition, the study also noted that in
patients with liver cirrhosis, the concentration of structurally
and functionally intact albumin was lower than that of clinically
measured serum albumin (15). More
importantly, a previous study has shown that in NAFLD, viral
hepatitis and other liver diseases, although the amount of serum
albumin is maintained at a normal level, the function of albumin
has been altered (16). At the
same time, an in vitro study has shown that some
modifications of albumins themselves may exacerbate disease
pathology (17), as these changes
affect the binding properties and function of albumin.
Although it has been found that the main structural
changes in the post-translational modification (PTM) of albumin are
oxidation, glycosylation, nitrosylation and partial truncation of
the N- and C-terminal, the relationship between albumin of
different PTMs and their functional changes is not clear.
Therefore, detection of albumin PTMs and changes in biological
function related to PTMs may provide more information on the
pathogenesis, diagnosis and treatment of diseases. In addition, in
order to further explore the mechanism by which modified albumin
affects disease progression, it is needed to conduct clinical
trials based on an improved understanding of albumin biology to
determine the preventive and therapeutic effect of albumin on liver
disease or other diseases. In the present review, previous research
findings on albumin modifications were summarized, focusing on
their roles in the occurrence and development of liver disease. The
aim was to deepen the understanding of albumin modification and its
function on the pathogenesis of liver disease, and to provide a
rational strategy for the clinical therapy of albumin.
Characteristics and structure of human serum
albumins (HSAs)
HSA is the most abundant protein in human blood
plasma (18). Its concentration is
normally 3.5–5 g/dl, accounting for ~50–60% of total plasma protein
in healthy adults (19). It is a
globular protein composed of 585 amino acid residues with a
molecular weight of ~66.5 kDa (20). It is negatively charged at neutral
pH and has a half-life of ~21 days (8,18).
An albumin molecule contains 35 cysteine (Cys) residues, of which
34 Cys residues form 17 disulfide bonds (S-S) to stabilize the
protein's secondary structure, and the Cys residue at site 34
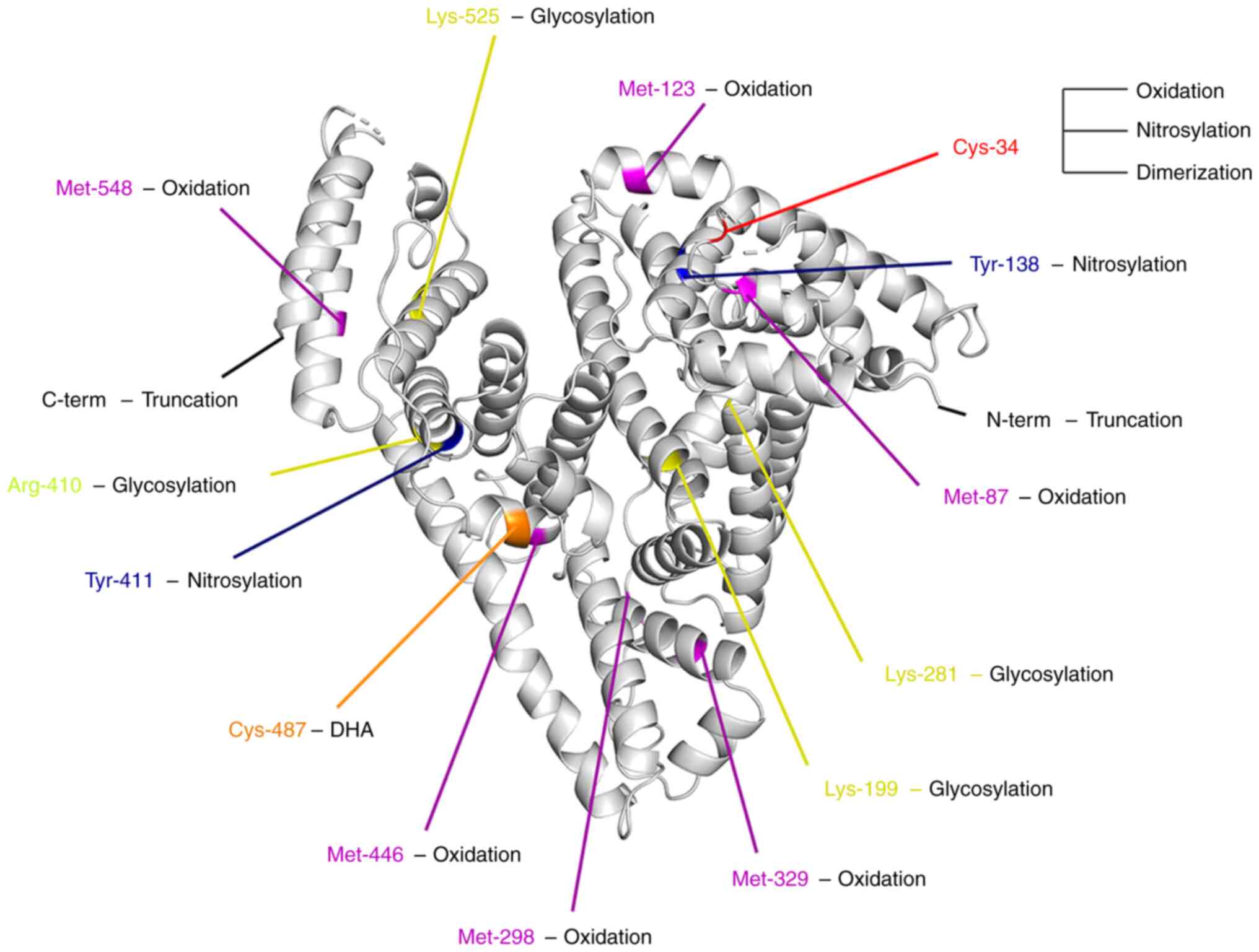
(called Cys-34) (Fig. 1) carries a
single free redox-active thiol (−SH) and constitutes the major pool
of redox-active thiols in plasma (21,22).
Depending on the status of Cys-34, albumin can exist
in both reduced and oxidized forms in plasma. Its reduced form is
called human mercaptalbumin (HMA), which accounts for ~70–80% in
healthy young individuals, and is the main form of HSA; and the
oxidized form can be divided into reversible oxidation and
irreversible oxidation, which are called human non-mercaptalbumin 1
(HNA1) and human non-mercaptalbumin 2 (HNA2), respectively. HNA1 is
a common reversibly oxidized albumin, which can interact with
thiol-containing small molecule compounds (cysteine, homocysteine,
glutathione) in the blood, thus it often exists in the form of
mixed disulfide compounds, accounting for ~20–30% in healthy young
individuals; HNA2 is a highly oxidized form, and its Cys-34 residue
is modified with sulfinic or sulfonic acid, which accounts for the
least in healthy young individuals, ~2–5%.
The tertiary structure of albumin presents a heart
shape and can be divided into three similar-sized homologous
domains I, II and III, each of which contains two subdomains A and
B (Fig. 2) (23). Because of this stable and flexible
structure, albumin can bind numerous endogenous and exogenous
substances. Nevertheless, albumin structure is prone to
modification after enzymatic and non-enzymatic reactions (such as
the most common oxidation and carbonylation), but whether the
structural changes are related to its biological function is not
fully clarified.
Synthesis and metabolism of HSAs
Albumin is mostly synthesized by hepatocytes
(24), 10–15 g/day (25), and is translated from a single gene
as preproalbumin, which is transported to the Golgi apparatus after
the removal of N-terminal propeptide through the endoplasmic
reticulum, and continuously is secreted into blood (26). Of these, the rate of albumin
synthesis depends on individual nutritional status. Yet, HSA
synthesis is also regulated by other factors. For example, HSA
production is significantly elevated upon hypoalbuminemia and
growth hormone stimulation (27–29);
On the contrary, pro-inflammatory cytokines [such as tumor necrosis
factor α (TNFα) and interleukin-6 (IL-6)] instead inhibit its
synthesis (30).
In addition, the circulation of albumin is closely
related to hepatocytes. To be precise, albumin cycle persistence is
determined by the continuous uptake and secretion of hepatocytes
(31). Albumin is considered as an
extracellular molecule, mainly because it is secreted outside the
cell as rapidly as cytokines after synthesized in hepatocytes. In
turn, numerous types of cells (including endothelial cells and
hepatocytes), are able to take up albumin by receptor-mediated
endocytosis and break it down by lysosomes (8,32).
This process is modulated in a pH-dependent manner by the neonatal
Fc receptor (FcRn) (33) that is
ubiquitously expressed in a variety of organs and tissues, and FcRn
expression in different cells is of varying importance for albumin
uptake and metabolism. Among them, FcRn expressed by hepatocytes
and endothelial cells was shown to be the main contributor for the
maintenance of albumin circulation and the stability of albumin
circulating levels (34). Because
it mediates the recycling of albumin, FcRn can, in general,
transport albumin back into the circulatory system before it is
degraded in lysosomes. Hypoalbuminemia occurs when FcRn is absent
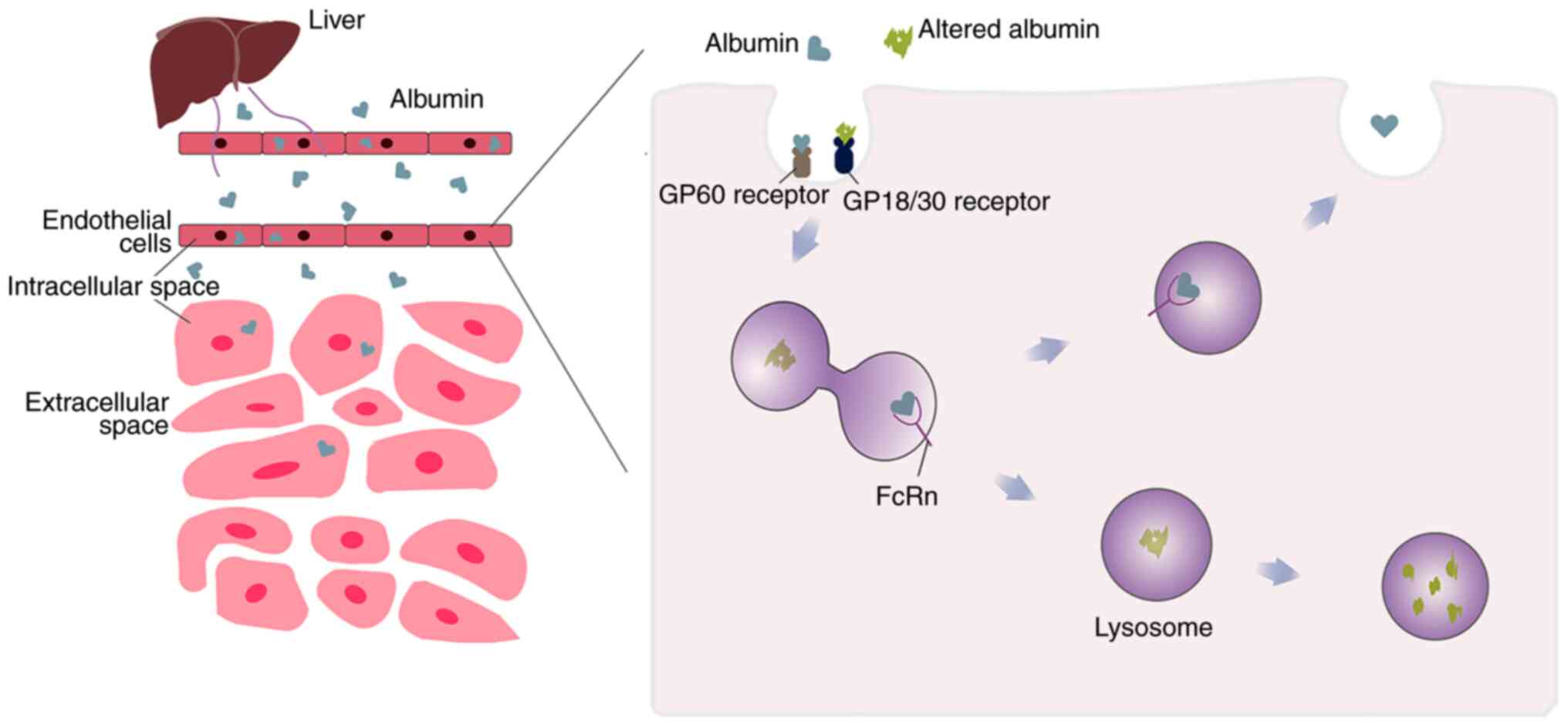
on these cells. Among them, in capillaries with continuous
endothelium, native albumin is transported by an active
transcytosis mechanism mediated by the GP60 receptors (Albondin)
(31). The affinity of albumin for
the GP60 receptor was decreased when the HSA conformation was
altered (Fig. 3) (35). But conversely, the endocytosis and
degradation of modified albumin is increased by GP18/GP30 (another
membrane-associated albumin binding protein (36), both of which are similarly
expressed in heart, liver, spleen and numerous other tissues
(35).
Furthermore, it has been reported that the cycle
life of modified albumin is lower than that of native albumin. On
the one hand, the aforementioned GP18/GP30 may act as scavenger
receptors that promote the internalization and degradation of
modified albumin. On the other hand, it may be related to FcRn.
Crystallography and mutation studies have shown that FcRn binds
primarily to residues located at domain I and III, of which domain
III is the key (37). In addition
to being pH-dependent, this interaction also requires hydrogen
bonds formed by protonation of the histidine of albumin, which then
bind to FcRn interfacial residues (38). However, when PTMs occur in albumin,
such as Cys-34 oxidation and Lysine (Lys)-525 glycation, the
conformational changes of albumin caused by these site
modifications affect the formation of hydrogen bonds due to the
proximity to the binding site, resulting in the weakening or
disappearance of the binding ability of albumin to FcRn (Fig. 3) (39).
Function of albumin
Albumin can ensure the communication between
intracellular fluid, extracellular fluid and tissue fluid, and
maintain the balance of blood colloid osmotic pressure. This is
because albumin accounts for ~70–80% of the total osmotic pressure
in plasma and is the main regulator of fluid distribution in the
body cavity (40). Initially, it
was considered that the benefits of albumin depended on this
function. As such, it was introduced in the 1950s as a plasma
expander, and was widely used to augment circulating blood volume
in patients with burns, shock and blood loss.
Whereas, with the in-depth understanding of the
biological function of HSA, it has been discovered that albumin has
multiple biological effects, and its non-colloidal function cannot
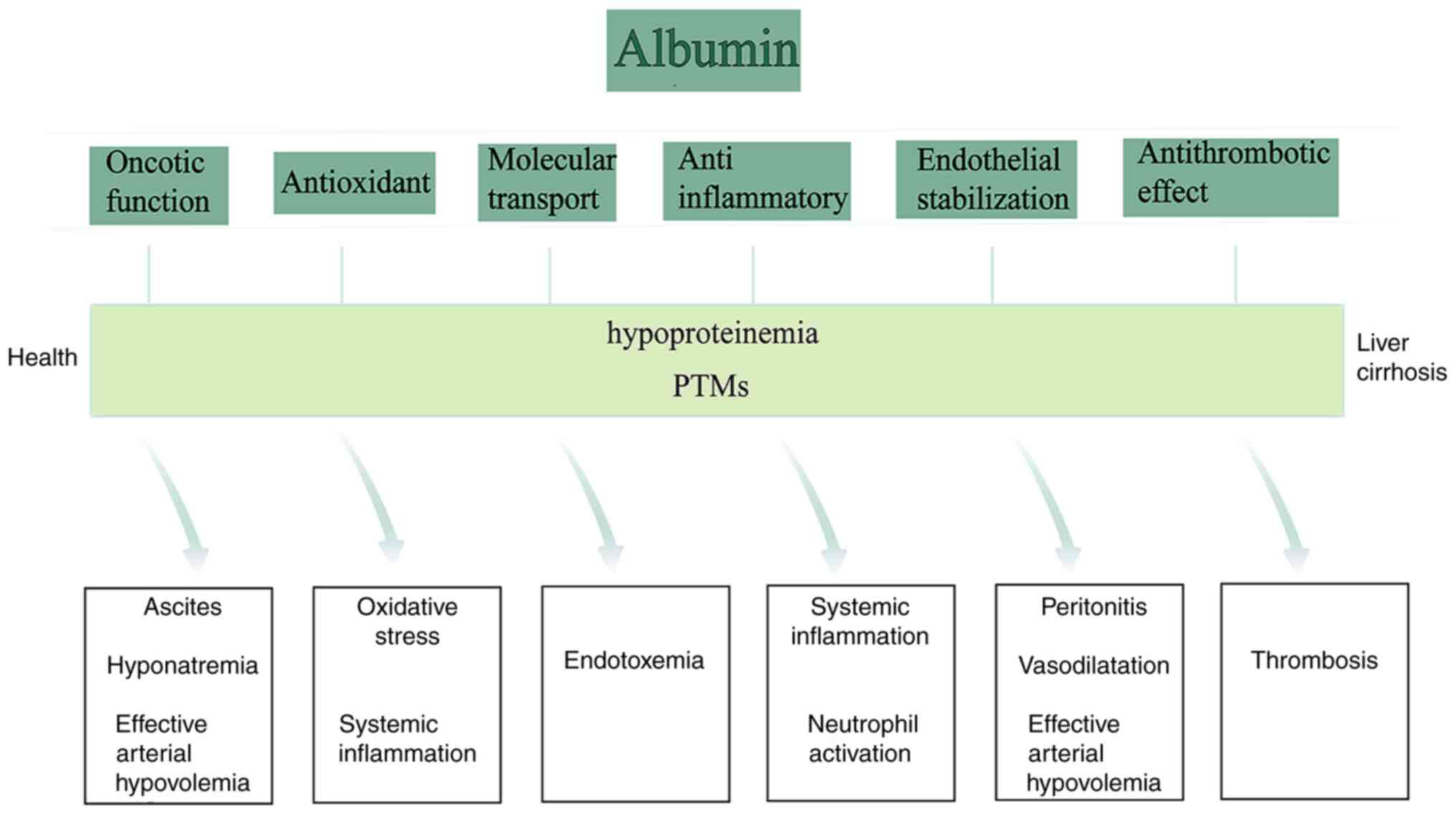
be ignored in addition to the colloidal function. The non-oncotic
functions of albumin mainly include antioxidant, anti-inflammatory,
molecular transport, endothelial stabilization and immune
regulation (Fig. 4) (41). Among them, the antioxidant
characteristics of albumin are mainly dependent on its free
radical-trapping properties and the possession of a variety of
ligand binding sites (42). In
terms of free radical-trapping properties, HSA primarily functions
through the aforementioned Cys-34 (43). Although the reaction of albumin
thiols with oxidants is not particularly fast, the plasma
concentrations of albumin are so high, especially considering the
limited number of antioxidant enzymes available in the body
(42), that Cys-34 provides ~80%
of the free thiols groups in plasma (43–45),
while the free thiol groups in plasma usually act as the main
scavengers of various oxidants (46). Therefore, Cys-34 is still
considered extremely important for plasma antioxidant function on
the human organism. This is consistent with a recent study
reporting that HSA is the main source of free radical-trapping
properties in serum (47).
Notably, Cys-34 is also closely associated with oxidative
stress-related diseases (42,48–50).
In addition, the multiple binding sites of albumin also contribute
to its antioxidant activity by binding to metal ions, bilirubin and
homocysteine (51). For example,
HSA can combine with free copper (II) and iron (II) ions to prevent
them from participating in the Fenton reactions, which further
generate deleterious hydroxyl radicals (52). The binding of bilirubin indirectly
plays an antioxidant function, as this complex was shown to be an
inhibitor of lipid peroxidation (53,54).
Meanwhile, albumin also binds multiple inflammatory
mediators and modulates immune responses in systemic inflammation
and sepsis through Toll-like receptor signaling (55,56).
A clinical trial has also demonstrated positive effects of HSA
infusion in infected patients with or without liver disease
(57–59). Moreover, several in vitro
and in vivo studies supported the beneficial role of albumin
in stabilizing the endothelial cell function, which may be due to
albumin's antioxidant and inflammation-regulating properties to
protect endothelial cells from damage by pro-inflammatory factors
(60,61). On the other hand, Cys-34 can also
bind nitric oxide (∙NO) to form nitroso-albumin (HSA-NO) in a
variety of ways (62,63), which can relax blood vessels and
inhibit platelet aggregation (8).
Albumin can also play a role in transporting these substances, by
binding to the aforementioned substances through the binding site.
With the gradual understanding of the albumin function, its
structural changes in diseases have also gradually been reported,
and there may be a certain relationship between the two. In short,
other biological effects of albumin in disease are far more
important than the maintenance of colloid osmotic pressure
alone.
PTMs of albumin
Due to the progress of mass spectrometric and
chromatographic techniques, the modification of albumin can be
detected in an improved way in the human organism, which further
deepens individuals' understanding of albumin structure, both in
physiological or pathological states. More importantly, on this
basis, it has also been found that the different modification
states of albumin may affect its own function, and even be related
to organ dysfunction in vivo. This renders individuals have
to pay more attention to the structural integrity of albumin and
the structural changes that have occurred. At present, the common
modifications that have been reported are mainly oxidation,
nitrosylation, glycosylation, truncation and dimerization. In the
future, the different modifications and their possible changes in
binding capacity and other functions will be summarized by the
authors.
Albumin oxidation
Albumin is susceptible to various oxidative
modifications, of which sulfhydryl (−SH) oxidation on Cys-34 is the
most common PTM. Methionine residues are also the most commonly
oxidized amino acids, including Met-87, Met-123, Met-298, Met-329,
Met-446 and Met-548 (Fig. 1)
(44). Besides, albumin oxidation
can also involve other kinds of amino acid residues, such as Lys,
Arginine (Arg) and Proline (Pro) (64). Direct oxidation of these amino acid
residues may also generate carbonyl derivatives, and the carbonyl
content also predicts the oxidative modification of other amino
acid residues in albumin (65).
Since Cys-34 is a major contributor to antioxidant
activity, oxidation of this site will alter the antioxidant
capacity of HSA (Table I).
Consistent with this conclusion, it was found that with the
increase of HNA-1 level, the antioxidant activity of the body was
significantly decreased (42).
This is indirectly confirmed by the role of HNA2 in promoting
inflammation and oxidative stress (66). In addition, albumin also provides
numerous binding sites for exogenous and/or endogenous substances.
Therefore, when the conformation of the albumin molecule was
changed, the affinity of its binding site would also be changed,
ultimately resulting in reducing in ligand binding (Table I). Previous studies demonstrated
that cysteinylation of Cys-34 can alter the conformation of the
entire domain I as well as the interface between domains I and II
(67,68). This may be related to the impaired
binding of bilirubin and other ligands by oxidized albumin in
advanced liver disease. In addition, Anraku et al (59), using in vitro experiments,
found that HNA1 affects the binding ability of Sites I and II [two
specific drug binding sites, located in subdomains IIA and IIIA,
respectively (69)], with a
greater effect on Site II (23,59)
(Fig. 4). At the same time, Oettl
et al (70) used
dansylsarcosine (the labeling ligand of HSA Site II) to study and
identified that HNA2 levels were correlated with Site II binding
capacity. In this way, both HNA1 and HNA2 are closely related to
the Site I and/or Site II binding function, although they may have
different effects on different ligands. On the other hand,
oxidative modifications play an important role in inducing binding
of other amino acid residues to ligands. Nagumo et al
(71) found that with the increase
in cysteinylation of Cys-34, other amino acid residues were also
oxidized, and these amino acids also played critical roles in
ligand binding at sites I and II. These evidences prove that
oxidation not only directly affects ligand binding, but also
indirectly regulates ligand binding by affecting other amino acid
residues.
 | Table I.Common albumin post-translational
modifications and effects on their function. |
Table I.
Common albumin post-translational
modifications and effects on their function.
| HSA
modifications |
| HSA functional
changes |
| (Refs.) |
|---|
| Oxidation | Binding
capacity | Metal ion binding
capacity and fatty acid binding capacity | Decrease | (16) |
|
|
| Affinity of
FcRn | Decrease | (39) |
|
|
| Warfarin (Site
I-ligand) | No effect | (59) |
|
|
| Ketoprofen (Site
II-ligand) | Decrease | (59) |
|
|
| L(small)-Trp (Site
II-ligand) and cefazolin (Site I-ligand) | Decrease | (68) |
|
|
| Verapamil (Site I
and II-ligand) | Increase | (68) |
|
|
| Dansylsarcosine
(Site II-ligand) | Decrease | (70) |
|
|
| Binding of
bilirubin capacity | Decrease | (114) |
|
| Transport
function | Fatty acid
transport | Decrease | (15) |
|
| Antioxidant
function | IMAR | Decrease | (15) |
|
|
| Free radical
scavenging activity | Decrease | (68) |
| Glycosylation | Binding
capacity | Affinity of
FcRn | Decrease | (39) |
|
|
| Ibuprofen and
flufenamic acid | Decrease | (84) |
|
|
| Ketoprofen | Decrease | (85) |
|
|
| Warfarin | Increase | (84) |
|
|
| Warfarin | No effect | (86) |
|
|
| Warfarin | Decrease | (87) |
|
|
| Hemin affinity | No effect | (88) |
|
|
| Affinity of
bilirubin and long chain fatty acid | Decrease | (88) |
|
|
| Tryptophan | No effect | (82) |
|
|
| Tryptophan | Increase | (86) |
|
|
| Tryptophan | Decrease | (89) |
| Nitration | Binding
capacity | Palmitate | Decrease | (99) |
|
|
| Polycyclic aromatic
hydrocarbon epoxides | Decrease | (101) |
| Truncation of
N-terminal | Antioxidant
function | Cobalt binding | Decrease | (103) |
|
|
| Free radical
scavenging ability | Decrease | (104) |
| Truncation of
C-terminal | Binding
capacity | Affinity of
FcRn | Decrease | (102,105) |
| Dimerization | Binding
capacity | Myristic acid | Decrease | (109) |
| Dehydroalanine | Antioxidant
function | Scavenge free
radicals | Increase | (111) |
Albumin glycosylation
Glycation is also called non-enzymatic glycosylation
(72). In vivo, the Amadori
products produced by glucose-modified albumin are the predominant
modification of glycated albumin (73), and this modification mainly occurs
at multiple sites corresponding to Lys and Arg residues, among
which Lys residues are the most common (64,74).
Currently, the glycation of Lys-281, Lys-199 and Lys-525 has been
confirmed (Fig. 1) (75–77).
However, in a previous study, Lys-525 was considered to be the main
site of non-enzymatic glycosylation of HSA, with a higher
contribution to overall glycosylation than other sites in
vivo (76). In addition, when
early glycation adducts such as Amadori products undergo a series
of oxidation, rearrangement, and cross-linking to form
alpha-oxoaldehydes, such as methylglyoxal and glyoxal, can also
react with the free amino group of albumins to form the advanced
glycation end products (AGEs) (78,79).
For the AGEs produced by methylglyoxal modification, Arg-410 is the
predominant site of modification, and at the same time, Arg-114,
Arg-186, Arg-218 and Arg-428 are also slightly modified (80,81).
Since Arg-410 is not only necessary for albumin-associated esterase
activity but also the binding site for ketoprofen and diazepam
(80,82). Therefore, the modification of
Arg-410 caused by methylglyoxal will reduce the esterase activity
of albumin and the binding ability of the aforementioned drugs. It
is worth noting that the AGEs generated by methylglyoxal
modification cause albumin functional changes and may be related to
the pKa value of the microenvironment, but the pKa value appears to
have little effect on which residues are more likely to be
glycosylated (80,83).
In fact, it has also been found that the
conformational and binding activity of albumin modified with early
glycation adducts are changed (Table
I). Glycated albumin has been reported to have a reduced
affinity for drugs such as ibuprofen and ketoprofen (84,85),
but the ability of glycated albumin to bind to warfarin remains
controversial (86,87). In addition, in vitro
experiments revealed that the affinity of glycated albumin to
bilirubin and long-chain fatty acids also changed significantly,
with the affinity of bilirubin reduced by ~50% (88). However, Bohney and Feldhoff
(82) noted that the binding of
Tryptophan (Trp) (the Site II ligand) was unaffected by glycation
albumin, but in another study its affinity for glycation albumin
was reduced (89). These
conflicting data need to be further validated in different
diseases. At the same time, similar to cysteinylation of Cys-34,
glycation induces structural changes in the region surrounding the
ligand binding site, such as the local structure around Trp −214
(90).
Most of the current research on albumin glycation
has focused on the study of diabetes and its complications.
However, it has also been reported that the level of circulating
AGEs in the plasma was increased in cirrhotic patients without
diabetes (91). Furthermore, given
that the liver is also the main site of AGE metabolism (92), in advanced liver disease, further
accumulation of AGEs and activation of intrahepatic cells to
produce cytokines would trigger more damaging effects on the liver
due to a reduction in hepatic effective liver volume/mass (91,93).
Clinically, AGEs have been proven to cause fibrosis in other organs
(94,95). Of note, an in vitro
experiment also demonstrated that AGEs exacerbate liver injury and
fibrosis in bile duct-ligated rats, but not in normal rats
(96). This still requires further
investigation of the clinical significance of glycosylated albumin
in the pathophysiology of liver disease.
Albumin nitrosylation
Nitrosylation of Cys-34 is another important PTM.
Under physiological conditions, the formation of HSA-NO is
considered to be the main mode of natural storage of ∙NO in human
plasma (97). This combination not
only prolongs the biological activity of ∙NO, but also enables
albumin to exert antithrombotic effects due to the regulatory
effect of ∙NO on thrombosis (98).
However, under pathological conditions, such as
liver cirrhosis, elevated local tissue ∙NO concentrations have been
reported to lead to increased HSA-NO production and decreased
palmitate binding (99); moreover,
due to the instability of ∙NO, it is be apt to react with
superoxide anion radicals to form peroxynitrite (ONOO-) (100). The nitration of HSA tyrosine by
ONOO− has been demonstrated, and Tyr-138 and Tyr-411
(Fig. 1) are particularly
sensitive to this modification (101). Nitrotyrosination is a
pathological PTM that may alter albumin structure and function
(Table I). Previous studies have
pointed out that both Tyr-138 and Tyr-411 are located at the ligand
binding site, which may be related to the impaired binding ability
of this form of albumin (99,101). It remains unclear how HSA-NO and
nitrotyrosination generated under pathological conditions affect
the organism, and the mechanisms by which these alters play a role
in disease remain to be further explored.
Truncation of N- or C-terminal
Physiologically, the C-terminal domain of albumin
contributes to molecular stability and is critical for its binding
to FcRn (102). Besides, the
N-terminal metal ion binding site of albumin can chelate free metal
ions, which plays an important role in the antioxidant activity of
albumin to a certain extent (103). In some diseases, a small fraction
of circulating HSA may be truncated at the N- and/or C-terminal.
Naldi et al (104) found
that the most abundant form of N-terminal truncation in cirrhosis
patients is the absence of Asp-Ala residues. This truncation
affects the antioxidant capacity of HSA due to its antioxidant
effects (Table I). The most
abundant C-terminal truncations is the absence of Leucine (Leu)
residues, which often reduce albumin stability and shorten its
half-life (Table I) (105). In addition, based on the ability
of the N-terminal of albumin to bind cobalt, when oxidative stress
or other pathological conditions occur, ischemia-modified albumin
(IMA) is subsequently produced, which indicates a decrease in the
metal-binding ability of the N-terminal of albumin (103,106).
Dimerization
When oxidative stress increases, HSA can dimerize by
forming intermolecular disulfide bonds on Cys-34 (107). Naldi et al (108) demonstrated that HSA can be
divided into homo- and hetero-dimeric. Just as its name suggests,
the former refers to the binding of two identical monomers, such as
two albumins lacking C-terminal Leu residue. On the contrary, it is
called hetero-dimeric if unidentical monomers are combined. As
dimerization reduces free Cys-34 residues, it adversely affects the
antioxidant and binding capacity of HSA (Table I) (109). In addition, Naldi et al
(108) also identified that HSA
dimer may be a new potential biomarker for CLD. One study also
pointed out that HSA homo-dimeric may be associated with prognosis
in decompensated cirrhosis (Table
II) (110), but its
biological consequences are uncertain.
 | Table II.Most common PTMs of HSA in different
liver diseases and their detection and analysis methods. |
Table II.
Most common PTMs of HSA in different
liver diseases and their detection and analysis methods.
| Liver disease | The PTMs
present |
Analytical/detection approaches | Year | (Refs.) |
|---|
| Liver
cirrhosis | HNA1; HNA2; HNA1
high proportion | RP-LC-HRMS | 2018 | (55) |
|
| Oxidized albumin
and AGEs | HPLC | 2004 | (91) |
|
| HSA-DA; HSA-L;
HSA-CYS; HSA-SO2H; HSA-GLYc | RP-LC-MS | 2013 | (104) |
|
| Albumin
Homodimers | RP-LC-MS | 2016 | (110) |
|
| HSA-DA; HSA-L;
HSA-CYS; HSA-SO2H; HSA-GLYc | RP-LC-MS | 2014 | (112) |
|
| HNA1; HNA2 | SEC-SAXS-MALS | 2021 | (113) |
| ACLF | HNA1; HNA2; HNA2
high proportion | RP-LC-HRMS | 2018 | (55) |
|
| Albumin
homodimers | RP-LC-MS | 2016 | (110) |
|
| HNA2 | HPLC | 2016 | (119) |
| AH | HSA-Da; HSA-L;
HSA-GLYc; HSA-CYS; HSA-SO2H | RP-LC-MS | 2017 | (17) |
|
| HNA1; HNA2; HNA2
high proportion | RP-LC-HRMS | 2018 | (55) |
|
| HSA-DA; HSA-L; DHA;
HSA-SO2H; HSA-CYS | RP-LC-MS | 2016 | (120) |
| NAFLD | AGEs | HPLC | 2018 | (126) |
| Viral
hepatitis | / | / | / | / |
Dehydroalanine (DHA) conversion
Cys can be converted to DHA when exposed to alkaline
and/or thermal conditions. Bar-or et al (111) have reported that this
modification change was observed on Cys-487 (Fig. 1) of albumin. Furthermore, they
found that this transformation can enhance the degradation rate of
HSA, but may disrupt the ligand binding capacity of Cys-487 and
nearby sites.
Role of albumin PTMs in different liver
diseases
Liver cirrhosis
Liver cirrhosis, characterized by persistent liver
damage and systemic inflammation and increased oxidative stress, is
a classic example of HSA structural and functional changes caused
by disease states. As early as ten years ago, Domenicali et
al (112) found that the
structural abnormalities of HSA in hospitalized patients with liver
cirrhosis, mainly included cysteinylation of Cys-34 (HSA-CYS),
sulfinylation (HSA-SO2H), partial truncation of the
N/C-terminal (HSA-DA and/or HSA-L), glycosylation (HSA-GLYc), and a
combination of these isoforms (Table
II). Among them, cysteinylation and glycosylation were the most
common (112). Over the past few
years, a significant decrease in HMA accompanied by an increase in
HNA1 and HNA2 has been well documented in advanced liver disease
(112,113). In addition, Watanabe et al
(91) also verified that serum
oxidized albumin levels increased with the severity of liver
disease. However, in patients with liver cirrhosis, in addition to
changes in the molecular structure of albumin, impaired albumin
binding function has also been gradually discovered. Interestingly,
there may be some connection between the two.
In liver cirrhosis, various modifications of albumin
have been reported to affect ligand binding ability. For example:
Severe oxidation of albumin impairs the ability of albumin to bind
bilirubin, possibly due to the high affinity of bilirubin to HMA
(114). Moreover, it has been
pointed out that the fatty acid binding capacity of HSA is also
severely impaired in patients with liver cirrhosis, while the
reduction of this ability is greater in patients with
acute-on-chronic liver failure (ACLF) and may be due to the
accumulation of AGEs (15). In
fact, the chelation capacity of metal ions in patients with liver
cirrhosis is only 50% that of healthy individuals, and it develops
and progresses with increasing severity of cirrhosis in these
patients (15,41). Notably, a recent study have also
demonstrated that in patients with chronic liver failure, a marked
opening of the structures of HSA domains I and III was observed
with increased levels of fatty acids and bilirubin in the organism
(113). This may further affect
the binding function of HSA. In addition, in the previous albumin
modification section, PTMs on different amino acid residue and
their effects on sites were also mentioned in the patients with
liver cirrhosis. All these evidences demonstrated that there are
certain connections between amino acid residue modifications,
molecular conformation changes, and function changes in albumin,
and understanding these is a prerequisite for improved study of the
role of albumin PTMs in disease.
Furthermore, certain studies have suggested that
modified albumin may contribute to systemic inflammation (31,55,61,115–117). Magzal et al (61) found that hemodialysis patients
exposed to oxidative stress had hypoalbuminemia and albumin
modifications (the highest proportion of HNA1 in isolated albumin),
and further revealed that these modifications triggered an
inflammatory response in human umbilical vein endothelial cells
(HUVEC) (Fig. 5). In addition, the
pro-inflammatory properties of modified albumin are also associated
with glycosylation in vivo. A study clarified that AGEs
promote the release of pro-inflammatory factors such as TNFα in
HUVEC (115). Not surprisingly,
modified albumin plays a similar role in liver cirrhosis
characterized by oxidative stress. For example, Bernardi et
al (31) pointed out that the
elevated levels of HNA1 and HNA2 were correlated with the severity
of liver cirrhosis and the degree of systemic inflammation (HNA1
played a major role). This has been confirmed in a previous study
(55). In addition to that, the
study prepared HNA1 and HNA2 in vitro and demonstrated that
HNA1 could induce cytokine storm in leukocytes and induces systemic
inflammation in decompensated liver cirrhosis by triggering an
inflammatory response signaling pathway in peripheral blood
mononuclear cells; excitingly, the aforementioned study also
identified that this pathway may be mediated by p38-MAP kinase
(Fig. 5) (55). Although the aforementioned study
did not elucidate the receptors that mediate p38-MAP kinase
activation in response to HNA1, it is equally important, which
means that there are new insights into the mechanism of action of
oxidized albumin. At the same time, circulating HNA1 upregulates
the expression of prostaglandin E2 (PGE2),
which has been shown to drive cirrhosis-related immunosuppression
(116,117). It indicated that circulating HNA1
appears to mediate cirrhosis immunosuppression through
PGE2, but the exact mechanism remains unclear. These
evidences all indicated that HNA1 is actively involved in the
systemic inflammatory response in patients with decompensated
cirrhosis. Although some mechanisms have not been fully elucidated,
it is worth noting that modified albumin has been revealed to be an
important factor in the increase of systemic inflammation in
various oxidative stress-related diseases, including cirrhosis.
ACLF
ACLF is the cause of death in most patients with
liver cirrhosis and is characterized by high levels of systemic
inflammation and high short-term mortality (118). Changes in albumin have also been
reported in patients with ACLF, which differ from those during
cirrhosis. A clinical study revealed that HNA2 levels in patients
with ACLF were markedly higher than those in patients with stable
cirrhosis and acute decompensation (AD), and were significantly
associated with ACLF severity and survival (Table II), suggesting that HNA2 may be a
biomarker of liver failure (119). It is currently understood that
patients with ACLF has a higher level of inflammation than that of
AD, implying that systemic inflammation levels may affect the
composition of oxidized albumin. At the same time, Jalan et
al (15) demonstrated that
IMA/albumin ratio (IMAR) was increased in patients with ACLF.
Furthermore, the aforementioned study also provided evidence of
other impaired albumin binding: A significantly lower fatty acid
binding function. Interestingly, a negative correlation was
identified between IMAR and the fatty acid binding coefficients at
Sites I and II. This indicated that the binding functions between
sites may interact and restrict each other. As with liver
cirrhosis, Oettl et al (114) found that the binding of albumin
to bilirubin and other ligands may be impaired in patients with
ACLF, possibly due to the presence of oxidized albumin. Another
study found that albumin dimer was also increased in patients with
severe ACLF (Table II) (110), but its role in ACLF was unclear.
In conclusion, the level of systemic inflammation may affect the
composition of oxidized albumin, with HNA2 predominating in ACLF
with high systemic inflammation and HNA1 in liver cirrhosis, and
the conversion between the two forms may be used to monitor disease
progression. Due to the rapid onset and progression of ACLF, it has
not been reported whether the aforementioned modified albumin has a
role in promoting oxidative stress and systemic inflammation during
its development, but the structural and functional changes of
albumin have been shown to be closely related to the prognosis of
ACLF (15,110).
Alcoholic hepatitis (AH)
AH is a unique alcoholic liver disease caused by
long-term excessive drinking, and severe AH (SAH) is based on AH
with severe progressive inflammation and high mortality. PTMs
albumin has also been frequently detected in these diseases. Naldi
et al (120) found that
the relative amount of native albumin decreased more significantly
in AH than in cirrhosis. At the same time, in addition to the
common PTMs such as N/C-terminal truncation and cysteinylation, two
regions of intense signal (likely a Cys oxidation, but not sure
which specific residue) and DHA were also found in multi-charged
mass spectra (Table II). This
signal was absent in liver cirrhosis, possibly due to modification
caused by different levels of oxidative stress and
inflammation.
While in SAH patients, the most prominent oxidized
albumin was HNA2, which was higher than in cirrhotic patients and
healthy subjects (55). In fact,
N- or C-terminal truncation and glycosylation forms were also found
in plasma samples from patients with SAH (Table II) (17). Simultaneously, Das et al
(121) showed that serum levels
of IMA, advanced oxidized protein products and AGEs were higher in
patients with SAH compared with alcoholic cirrhosis and healthy
controls, and were positively correlated with urinary albumin,
which may predict patient prognosis. However, whether these changes
occur in AH has not been elucidated.
Nevertheless, only a small number of studies have
attempted to confirm the mechanism of the aforementioned clinical
phenomenon. A previous study by Das et al (17) showed that circulating neutrophils
were activated in patients with SAH, along with the induction of
oxidative stress in healthy neutrophils by albumin purified from
plasma of patients with SAH, and the most prominent oxidized
albumin in patients with SAH was HNA2, suggesting that it may be
HNA2 that induces persistent oxidative stress and inflammation as
well as systemic complications in patients with SAH by regulating
neutrophil activation (Fig. 5).
Further, recently, Bhat et al (66) not only used purified albumin from
plasma of patients with SAH, but also oxidized albumin prepared
in vitro, and it was found that HNA2 may trigger platelet
activation through CD36 receptors, promote inflammation and
oxidative stress, and contribute to disease severity in patients
with SAH (Fig. 5). These findings
suggested that HNA2 plays an important role in exacerbating
oxidative stress and inflammation in SAH with severe inflammatory
responses. And the removal of these PTM albumins may be a strategy
for the treatment of AH and SAH. Although different from the form
of oxidation that plays a role in cirrhosis, these studies point to
the role of oxidized albumin in contributing to the aforementioned
pathological conditions.
NAFLD
NAFLD is one of the most common CLDs in the world.
Studies have shown that oxidative stress and inflammation are key
mediators of NAFLD, and these mediators may affect the PTMs of
albumin (122,123). Sun et al (123) found increase of IMA in NAFLD. In
addition, it has been reported that NAFLD can promote the formation
of AGEs, which, on the one hand, may be associated with oxidative
stress and the production of inflammatory compounds. On the other
hand, it may also be related to the formation of
alpha-glyoxaldehydes induced by glucotoxicity and lipotoxicity of
NAFLD (124,125). Whereafter AGEs can participate in
the oxidative stress and inflammation of NAFLD by combining with
the AGEs receptor (RAGE) to promote the occurrence of hepatic
steatosis (hepatocyte steatosis) and even fibrosis and form a
positive feedback loop (Table II)
(125,126). This is consistent with the study
by Pereira et al (127)
that AGEs can monitor the progression of mild, moderate and severe
NAFLD according to the grade of steatosis (127). Therefore, as aforementioned, in
addition to HNA1 and HNA2, AGEs may also promote systemic
inflammation in liver disease.
In addition, Ge et al (16) pointed out that fatty acid binding
ability and metal binding ability of albumin were impaired to
varying degrees in patients with NAFLD. However, it has not been
reported what modification caused the impaired binding ability, but
these impairments predate conventional biochemical markers such as
liver function, which means that in the early stage of oxidative
stress disease, even if serum albumin level has not been abnormal,
changes in the ability of albumin to bind may predict these
diseases early.
Viral hepatitis
Viral hepatitis is a major global health problem
and the resulting liver cirrhosis and liver cancer are the leading
causes of human death. According to Yavuz et al (128), the serum IMA concentration
increased in patients with hepatitis B virus-related CLD, which was
correlated with the degree of liver fibrosis. Similarly, this view
was also confirmed by Cakir et al (129). Although oxidized albumin has not
been reported in patients with viral hepatitis, a study has shown
that their albumin binding capacity is impaired (16). In the early stages of viral
hepatitis, Ge et al (16)
found that the binding capacity of albumin to metal ions and fatty
acids is reduced. In addition, the impairment of albumin to fat
binding capacity is more severe in patients with hepatitis than in
patients with NAFLD. However, the reason for the changes in albumin
binding capacity is unclear, and it has been suggested that it may
be due to hepatocytes steatosis, viral infection, and/or
inflammation response that induces conformational changes in the
albumin molecule (16). Moreover,
further research is needed to determine whether these changes
affect the progression of hepatitis.
Therapeutic effects of albumin in different
clinical pathways
Various evidences indicate that structurally or
functionally altered albumin has multiple biological properties,
especially oxidized albumin, which provides strong evidence for the
removal of structurally and/or functionally incomplete albumin from
plasma of patients with different liver diseases, or the
replacement of these albumin with native albumin. At present,
albumin-related treatment mainly includes intravenous infusion and
extracorporeal albumin dialysis (ECAD). The former is suitable for
some complications of advanced liver disease; the latter is mainly
used in patients with ACLF. However, the mechanism by which these
albumin-based treatments function in vivo remains
controversial.
Intravenous infusion of albumin
It has been reported that albumin infusion is
suitable for patients with SBP, HRS and paracentesis-induced
circulatory dysfunction (130,131). In these cases, albumin infusion
has been shown to reduce the incidence of other complications and
prolonged patient survival (10–12,132). But the complications of the
aforementioned diseases are all characterized by hypovolemia.
Therefore, after the infusion of albumin, only its main osmotic
function can be initially shown. However, Jalan et al
(15) and Chen et al
(57) discovered that albumin
infusion can improve the survival rate of patients with cirrhosis
complicated by SBP, which may be related to the detoxification
function of albumin. Previous studies have also shown that
administration of human albumin in patients with stable cirrhosis
and ascites provides short term improvements in albumin binding and
detoxification function. Similarly, there has recently been
increasing evidence that the beneficial effects of HSA on effective
blood volume were also related to its non-osmotic functions, which
can indirectly improve cardiac contractility and peripheral
vascular resistance in rats (133). Similar effects have been observed
in patients with decompensated cirrhosis (134).
Due to HSA's ability to resist oxidative stress, it
is gradually being applied in various diseases and showing some
benefits. These benefits, though, remain controversial. For
patients with liver disease, on the one hand, several studies have
shown that infusion of albumin reduces systemic inflammatory
responses and exhibits immunomodulatory properties that further
improve survival. For examples, a clinical study showed that after
12 weeks of continuous infusion of albumin at 1.5 g/kg, markers of
systemic inflammatory response (like IL-6) were alleviated in
patients with decompensated cirrhosis (134). Furthermore, O'Brien et al
(117) found that PGE2-mediated
immune dysfunction associated with liver cirrhosis was improved
after albumin infusion, indicating the regulation of immune
responses by albumin. This was also previously confirmed in the
ANSWER randomized controlled trial (135), which showed that long-term weekly
administration of albumin (40 g/biw for 2 weeks, then 40 g/w for 18
months) in patients with decompensated cirrhosis can reduce the
incidence of complications, thereby improving the overall survival
rate. Interestingly, albumin has also been found to be potentially
beneficial for hyponatremia (136) and hepatic encephalopathy
(137,138). On the other hand, however, there
are studies that suggest that the effect of albumin may not be so
strong. A recent clinical study revealed that targeted albumin
infusion did not attenuate systemic inflammation or improve cardiac
function in patients with decompensated cirrhosis (139). Another trial suggested that
infusing albumin to normal levels in patients with advanced liver
disease may not provide additional benefit (13). A meta-analysis also confirmed this
view (140).
From a holistic perspective, these discrepant
results may be related to the quantity, duration, and/or quality of
albumin infusion. Currently, the timing and amount of albumin
infusion remain a matter of debate. In addition, the uneven quality
of commercial albumin may also be one of the reasons. Certain
studies have found that the structure and binding function of
albumin from different manufacturers have been damaged to varying
degrees, and even different batches of albumin manufactured by the
same manufacturer have differences (141–143), which will affect the treatment
effect of HSA. Notably, these damages are also affected by, for
example, storage time and temperature (144). How to improve the quality of
commercial albumin and standardize therapeutic dosage is an open
question.
ECAD
Albumin dialysis system is a common artificial
liver support therapy, among which molecular adsorbent
recirculating system (MARS) is the most widely used (145). Although the ability of MARS to
remove toxins from patients has been widely demonstrated (41,146), how it alleviates disease status
through albumin remains controversial.
On the one hand, Jalan et al (15) found that MARS treatment had little
effect on IMAR levels. This is contrary to the current hypothesis
that clearing of excess toxins (it can bind to albumin and impair
albumin function) from the circulation promotes regeneration of
native functional albumin, enabling the transport and
detoxification of more toxins, suggesting that MARS treatment
cannot ‘regenerate’ albumin. On the other hand, however, Klammt
et al (147) demonstrated
that MARS treatment may be associated with improved albumin binding
function rather than just elimination of some toxicants. In
addition, Oettl et al (148) revealed that MARS can lead to the
transfer of HNA1 to HMA (lasting ~24 h), while HNA2 was not
significantly affected. Although this transfer lasts for ~1 day, it
is not yet fully understood whether the change in the redox form of
albumin is beneficial for disease prognosis. But Jalan et al
(15) previously demonstrated that
MARS treatment did not provide a significant survival benefit for
patients with ACLF. This may be related to the persistence of HNA2,
as another study showed that the proportion of HNA2 was associated
with ACLF prognosis (114). Thus,
while MARS alters the oxidative state of HNA1 in vivo, HNA2
is closely associated with short-term survival, which may explain
this paradox. Therefore, follow-up studies should pay more
attention to the ratio of reversible and irreversible albumin
damage after MARS treatment, and analyze the overall function of
circulating albumin on this basis to improve the treatment level
and the quality of life of patients.
Conclusion and perspectives
Albumin is considered an important antioxidant
molecule in the organism. However, in diseases characterized by
enhanced systemic inflammatory responses and oxidative stress, such
as liver cirrhosis, structural and functional impairment of HSA
occurs. Although there is currently evidence that the structural
changes of albumin may be related to its binding function, the
differences in PTMs, the effects of modifications at different site
on different ligands, and how these effects change the overall
biological function of albumin remain unclear in different
diseases. Further discussion is required. More importantly, the
effect of albumin conformational changes on disease progression
also needs to be further confirmed. At present, the research on
oxidative modification is relatively extensive, while the extent to
which other modifications affect liver disease has not been clearly
reported. In conclusion, it is required to further explore the
relationship between albumin structure and biological function to
provide new insights into the occurrence and development of liver
diseases.
At the same time, the treatment of HSA is quite
expensive. Moreover, HSA is not easy to store and is easily
contaminated by pathogens. Therefore, recombinant albumin as its
replacement is the future direction of development. However, only a
few studies have proved its anti-inflammatory effect in
vitro, and its clinical significance still needs to be further
explored.
Finally, the proposal of ‘effective albumin
concentration’ also makes individuals to realize that the treatment
of albumin is not only aimed at increasing serum albumin levels. In
particular, recent studies have also demonstrated that ‘effective
albumin concentration’ can improve stratification of patients with
liver cirrhosis, and is improved compared with serum total albumin
in assessing the prognosis of patients with AD and ACLF. However,
how to establish a sound albumin treatment strategy and develop
other new treatment methods for patients with advanced liver
disease on the basis of testing ‘effective albumin’ is a problem
that can be explored in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by grants from the
National Natural Science Foundation of China (grant nos. 82203658
and 82100641), the Natural Science Foundation of Shandong (grant
nos. ZR2023MH138, ZR2022MH146, ZR2021QH276 and ZR2021QH074), and
Shandong First Medical University Youth Science Foundation (grant
no. 202201-055) and WBE Liver Fibrosis Foundation (grant no.
CFHPC2021011).
Availability of data and materials
Not applicable.
Authors' contributions
JQ and YF conceived and designed the review. JQ,
NW, TL and MT drafted the manuscript. NW, CL, SM, HC and HB
analyzed the data. NW, LW, YF and JQ contributed materials/analysis
tools. LW was involved in revising the manuscript. NW wrote the
first draft of the manuscript. YF and JQ modified and reviewed the
manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACLF
|
acute-on-chronic liver failure
|
|
AD
|
acute decompensation
|
|
AGEs
|
advanced glycation end products
|
|
AH
|
alcoholic hepatitis
|
|
Arg
|
arginine
|
|
CLD
|
chronic liver disease
|
|
Cys
|
cysteine
|
|
Cys-34
|
cysteine 34
|
|
DHA
|
dehydroalanine
|
|
ECAD
|
extracorporeal albumin dialysis
|
|
FcRn
|
neonatal Fc receptor
|
|
HMA
|
human mercaptalbumin
|
|
HNA1
|
human non-mercaptalbumin 1
|
|
HNA2
|
human non-mercaptalbumin 2
|
|
HRS
|
hepatorenal syndrome
|
|
HSAs
|
human serum albumins
|
|
HSA-NO
|
nitroso-albumin
|
|
HUVEC
|
human umbilical vein endothelial
cells
|
|
IL-6
|
interleukin-6
|
|
IMA
|
ischemia-modified albumin
|
|
IMAR
|
IMA/albumin ratio
|
|
Leu
|
leucine
|
|
Lys
|
lysine
|
|
MARS
|
molecular adsorbent recirculating
system
|
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
∙NO
|
nitric oxide
|
|
PGE2
|
prostaglandin E2
|
|
Pro
|
proline
|
|
PTM
|
post-translational modification
|
|
RAGE
|
AGEs receptor
|
|
SAH
|
severe AH
|
|
SBP
|
spontaneous bacterial peritonitis
|
|
TNFα
|
tumor necrosis factor α
|
|
Trp
|
tryptophan
|
References
|
1
|
Trefts E, Gannon M and Wasserman DH: The
liver. Curr Biol. 27:R1147–R1151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Embade N and Millet O: Molecular
determinants of chronic liver disease as studied by
NMR-Metabolomics. Curr Top Med Chem. 17:2752–2766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang R, Tang R, Li B, Ma X, Schnabl B and
Tilg H: Gut microbiome, liver immunology, and liver diseases. Cell
Mol Immunol. 18:4–17. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Younossi Z, Tacke F, Arrese M, Chander
Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George
J, Fan J and Vos MB: Global Perspectives on Nonalcoholic Fatty
Liver Disease and Nonalcoholic Steatohepatitis. Hepatology.
69:2672–2682. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diehl AM: Alcoholic liver disease. Clin
Liver Dis. 2:103–118. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang C, Ma C, Gong L, Guo Y, Fu K, Zhang
Y, Zhou H and Li Y: Macrophage Polarization and Its role in liver
disease. Front Immunol. 12:8030372021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gilgenkrantz H, Mallat A, Moreau R and
Lotersztajn S: Targeting cell-intrinsic metabolism for antifibrotic
therapy. J Hepatol. 74:1442–1454. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garcia-Martinez R, Caraceni P, Bernardi M,
Gines P, Arroyo V and Jalan R: Albumin: Pathophysiologic basis of
its role in the treatment of cirrhosis and its complications.
Hepatology. 58:1836–1846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Araujo A, de Barros Lopes A, Rossi G,
da Silva GV, Ananias P, Ness S and Alvares-da-Silva MR: Low-dose
albumin in the treatment of spontaneous bacterial peritonitis:
Should we change the standard treatment? Gut. 61:1371–1372. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernandez J, Navasa M, Garcia-Pagan JC,
G-Abraldes J, Jiménez W, Bosch J and Arroyo V: Effect of
intravenous albumin on systemic and hepatic hemodynamics and
vasoactive neurohormonal systems in patients with cirrhosis and
spontaneous bacterial peritonitis. J Hepatol. 41:384–390. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen-Tat M, Jager J, Rey JW, Nagel M,
Labenz C, Wörns MA, Galle PR and Marquardt JU: Terlipressin and
albumin combination treatment in patients with hepatorenal syndrome
type 2. United European Gastroenterol J. 7:529–537. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong F, Pappas SC, Curry MP, Reddy KR,
Rubin RA, Porayko MK, Gonzalez SA, Mumtaz K, Lim N, Simonetto DA,
et al: Terlipressin plus albumin for the treatment of type 1
Hepatorenal Syndrome. N Engl J Med. 384:818–828. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
China L, Freemantle N, Forrest E, Kallis
Y, Ryder SD, Wright G, Portal AJ, Becares Salles N, Gilroy DW and
O'Brien A; ATTIRE Trial Investigators, : A Randomized Trial of
Albumin Infusions in Hospitalized Patients with Cirrhosis. N Engl J
Med. 384:808–817. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caraceni P, Domenicali M, Tovoli A, Napoli
L, Ricci CS, Tufoni M and Bernardi M: Clinical indications for the
albumin use: Still a controversial issue. Eur J Intern Med.
24:721–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jalan R, Schnurr K, Mookerjee RP, Sen S,
Cheshire L, Hodges S, Muravsky V, Williams R, Matthes G and Davies
NA: Alterations in the functional capacity of albumin in patients
with decompensated cirrhosis is associated with increased
mortality. Hepatology. 50:555–564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ge P, Yang H, Lu J, Liao W, Du S, Xu Y, Xu
H, Zhao H, Lu X, Sang X, et al: Albumin binding function: The
potential earliest indicator for liver function damage.
Gastroenterol Res Pract. 2016:51207602016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Das S, Maras JS, Hussain MS, Sharma S,
David P, Sukriti S, Shasthry SM, Maiwall R, Trehanpati N, Singh TP
and Sarin SK: Hyperoxidized albumin modulates neutrophils to induce
oxidative stress and inflammation in severe alcoholic hepatitis.
Hepatology. 65:631–646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rothschild MA, Oratz M and Schreiber SS:
Serum albumin. Hepatology. 8:385–401. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caraceni P, O'Brien A and Gines P:
Long-term albumin treatment in patients with cirrhosis and ascites.
J Hepatol. 76:1306–1317. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bernardi M, Ricci CS and Zaccherini G:
Role of human albumin in the management of complications of liver
cirrhosis. J Clin Exp Hepatol. 4:302–311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He XM and Carter DC: Atomic structure and
chemistry of human serum albumin. Nature. 358:209–215. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sugio S, Kashima A, Mochizuki S, Noda M
and Kobayashi K: Crystal structure of human serum albumin at 2.5 A
resolution. Protein Eng. 12:439–446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oettl K and Stauber RE: Physiological and
pathological changes in the redox state of human serum albumin
critically influence its binding properties. Br J Pharmacol.
151:580–590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wada Y, Takeda Y and Kuwahata M: Potential
Role of Amino Acid/Protein Nutrition and Exercise in Serum Albumin
Redox State. Nutrients. 10:172017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prinsen BH and de Sain-van der Velden MG:
Albumin turnover: Experimental approach and its application in
health and renal diseases. Clin Chim Acta. 347:1–14. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Strauss AW, Donohue AM, Bennett CD, Rodkey
JA and Alberts AW: Rat liver preproalbumin: In vitro synthesis and
partial amino acid sequence. Proc Natl Acad Sci USA. 74:1358–1362.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soeters PB, Wolfe RR and Shenkin A:
Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J
Parenter Enteral Nutr. 43:181–193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun X and Kaysen GA: Albumin and
transferrin synthesis are increased in H4 cells by serum from
analbuminemic or nephrotic rats. Kidney Int. 45:1381–1387. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li N, Zhou L, Zhang B, Dong P, Lin W, Wang
H, Xu R and Ding H: Recombinant human growth hormone increases
albumin and prolongs survival in patients with chronic liver
failure: A pilot open, randomized, and controlled clinical trial.
Dig Liver Dis. 40:554–559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Castell JV, Gómez-Lechón MJ, David M,
Andus T, Geiger T, Trullenque R, Fabra R and Heinrich PC:
Interleukin-6 is the major regulator of acute phase protein
synthesis in adult human hepatocytes. FEBS Lett. 242:237–239. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bernardi M, Angeli P, Claria J, Moreau R,
Gines P, Jalan R, Caraceni P, Fernandez J, Gerbes AL, O'Brien AJ,
et al: Albumin in decompensated cirrhosis: New concepts and
perspectives. Gut. 69:1127–1138. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Merlot AM, Kalinowski DS and Richardson
DR: Unraveling the mysteries of serum albumin-more than just a
serum protein. Front Physiol. 5:2992014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chaudhury C, Mehnaz S, Robinson JM, Hayton
WL, Pearl DK, Roopenian DC and Anderson CL: The major
histocompatibility complex-related Fc receptor for IgG (FcRn) binds
albumin and prolongs its lifespan. J Exp Med. 197:315–322. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pyzik M, Rath T, Kuo TT, Win S, Baker K,
Hubbard JJ, Grenha R, Gandhi A, Krämer TD, Mezo AR, et al: Hepatic
FcRn regulates albumin homeostasis and susceptibility to liver
injury. Proc Natl Acad Sci USA. 114:E2862–E2871. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schnitzer JE: gp60 is an albumin-binding
glycoprotein expressed by continuous endothelium involved in
albumin transcytosis. Am J Physiol. 262((1 Pt 2)): H246–H254.
1992.PubMed/NCBI
|
|
36
|
Schnitzer JE and Bravo J: High affinity
binding, endocytosis, and degradation of conformationall y modified
albumins. Potential role of gp30 and gp18 as novel scavenge r
receptors. J Biol Chem. 268:7562–7570. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johansson E, Nielsen AD, Demuth H, Wiberg
C, Schjødt CB, Huang T, Chen J, Jensen S, Petersen J and Thygesen
P: Identification of binding sites on human serum albumin for
somapacitan, a long-acting growth hormone derivative. Biochemistry.
59:1410–1419. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schmidt MM, Townson SA, Andreucci AJ, King
BM, Schirmer EB, Murillo AJ, Dombrowski C, Tisdale AW, Lowden PA,
Masci AL, et al: Crystal structure of an HSA/FcRn complex reveals
recycling by competitive mimicry of HSA ligands at a pH-dependent
hydrophobic interface. Structure. 21:1966–1978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leblanc Y, Berger M, Seifert A, Bihoreau N
and Chevreux G: Human serum albumin presents isoform variants with
altered neonatal Fc receptor interactions. Protein Sci.
28:1982–1992. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baldassarre M, Naldi M, Zaccherini G,
Bartoletti M, Antognoli A, Laggetta M, Gagliardi M, Tufoni M,
Domenicali M, Waterstradt K, et al: Determination of effective
albumin in patients with decompensated cirrhosis: Clinical and
prognostic implications. Hepatology. 74:2058–2073. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun L, Yin H, Liu M, Xu G, Zhou X, Ge P,
Yang H and Mao Y: Impaired albumin function: A novel potential
indicator for liver function damage? Ann Med. 51:333–344. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brioschi M, Gianazza E, Mallia A, Zoanni
B, Altomare A, Martinez Fernandez A, Agostoni P, Aldini G and Banfi
C: S-Thiolation targets albumin in heart failure. Antioxidants
(Basel). 9:7632020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Colombo G, Clerici M, Giustarini D, Rossi
R, Milzani A and Dalle-Donne I: Redox albuminomics: Oxidized
albumin in human diseases. Antioxid Redox Signal. 17:1515–1527.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roche M, Rondeau P, Singh NR, Tarnus E and
Bourdon E: The antioxidant properties of serum albumin. FEBS Lett.
582:1783–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Turell L, Botti H, Carballal S, Radi R and
Alvarez B: Sulfenic acid-a key intermediate in albumin thiol
oxidation. J Chromatogr B Analyt Technol Biomed Life Sci.
877:3384–3392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Turell L, Radi R and Alvarez B: The thiol
pool in human plasma: The central contribution of albumin to redox
processes. Free Radic Biol Med. 65:244–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Altomare A, Baron G, Brioschi M, Longoni
M, Butti R, Valvassori E, Tremoli E, Carini M, Agostoni P, Vistoli
G, et al: N-Acetyl-Cysteine Regenerates Albumin Cys34 by a
thiol-disulfide breaking mechanism: An explanation of its
extracellular antioxidant activity. Antioxidants (Basel).
9:3672020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang X, Mao Z, Huang Y, Yan H, Yan Q, Hong
J, Fan J and Yao J: Reductively modified albumin attenuates
DSS-Induced mouse colitis through rebalancing systemic redox state.
Redox Biol. 41:1018812021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu S, Grigoryan H, Edmands WBM, Dagnino
S, Sinharay R, Cullinan P, Collins P, Chung KF, Barratt B, Kelly
FJ, et al: Cys34 adductomes differ between patients with chronic
lung or heart disease and healthy controls in Central London.
Environ Sci Technol. 52:2307–2313. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Terawaki H, Yoshimura K, Hasegawa T,
Matsuyama Y, Negawa T, Yamada K, Matsushima M, Nakayama M, Hosoya T
and Era S: Oxidative stress is enhanced in correlation with renal
dysfunction: Examination with the redox state of albumin. Kidney
Int. 66:1988–1993. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Taverna M, Marie AL, Mira JP and Guidet B:
Specific antioxidant properties of human serum albumin. Ann
Intensive Care. 3:42013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stohs SJ and Bagchi D: Oxidative
mechanisms in the toxicity of metal ions. Free Radic Biol Med.
18:321–336. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Neuzil J and Stocker R: Bilirubin
attenuates radical-mediated damage to serum albumin. FEBS Lett.
331:281–284. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Carter DC and Ho JX: Structure of serum
albumin. Adv Protein Chem. 45:153–203. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Alcaraz-Quiles J, Casulleras M, Oettl K,
Titos E, Flores-Costa R, Duran-Güell M, López-Vicario C, Pavesi M,
Stauber RE, Arroyo V and Clària J: Oxidized albumin triggers a
cytokine storm in leukocytes through P38 mitogen-activated protein
kinase: Role in systemic inflammation in decompensated cirrhosis.
Hepatology. 68:1937–1952. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Casulleras M, Flores-Costa R, Duran-Güell
M, Alcaraz-Quiles J, Sanz S, Titos E, López-Vicario C, Fernández J,
Horrillo R, Costa M, et al: Albumin internalizes and inhibits
endosomal TLR signaling in leukocytes from patients with
decompensated cirrhosis. Sci Transl Med. 12:eaax51352020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen TA, Tsao YC, Chen A, Lo GH, Lin CK,
Yu HC, Cheng LC, Hsu PI and Tsai WL: Effect of intravenous albumin
on endotoxin removal, cytokines, and nitric oxide production in
patients with cirrhosis and spontaneous bacterial peritonitis.
Scand J Gastroenterol. 44:619–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Delaney AP, Dan A, McCaffrey J and Finfer
S: The role of albumin as a resuscitation fluid for patients with
sepsis: A systematic review and meta-analysis. Crit Care Med.
39:386–391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Anraku M, Yamasaki K, Maruyama T,
Kragh-Hansen U and Otagiri M: Effect of oxidative stress on the
structure and function of human serum albumin. Pharm Res.
18:632–639. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Vairappan B: Endothelial dysfunction in
cirrhosis: Role of inflammation and oxidative stress. World J
Hepatol. 7:443–459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Magzal F, Sela S, Szuchman-Sapir A, Tamir
S, Michelis R and Kristal B: In-vivo oxidized albumin-a
pro-inflammatory agent in hypoalbuminemia. PLoS One.
12:e01777992017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Keszler A, Zhang Y and Hogg N: Reaction
between nitric oxide, glutathione, and oxygen in the presence and
absence of protein: How are S-nitrosothiols formed? Free Radic Biol
Med. 48:55–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gow AJ, Buerk DG and Ischiropoulos H: A
novel reaction mechanism for the formation of S-nitrosothiol in
vivo. J Biol Chem. 272:2841–2845. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Naldi M, Baldassarre M, Domenicali M,
Bartolini M and Caraceni P: Structural and functional integrity of
human serum albumin: Analytical approaches and clinical relevance
in patients with liver cirrhosis. J Pharm Biomed Anal. 144:138–153.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Berlett BS and Stadtman ER: Protein
oxidation in aging, disease, and oxidative stress. J Biol Chem.
272:20313–20316. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bhat A, Das S, Yadav G, Chaudhary S, Vyas
A, Islam M, Gupta AC, Bajpai M, Maiwall R, Maras JS and Sarin SK:
Hyperoxidized albumin modulates platelets and promotes inflammation
through CD36 receptor in severe alcoholic hepatitis. Hepatol
Commun. 4:50–65. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Stewart AJ, Blindauer CA, Berezenko S,
Sleep D, Tooth D and Sadler PJ: Role of Tyr84 in controlling the
reactivity of Cys34 of human albumin. FEBS J. 272:353–362. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kawakami A, Kubota K, Yamada N, Tagami U,
Takehana K, Sonaka I, Suzuki E and Hirayama K: Identification and
characterization of oxidized human serum albumin. A slight
structural change impairs its ligand-binding and antioxidant
functions. FEBS J. 273:3346–3357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yamasaki K, Chuang VT, Maruyama T and
Otagiri M: Albumin-drug interaction and its clinical implication.
Biochim Biophys Acta. 1830:5435–5443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Oettl K, Birner-Gruenberger R,
Spindelboeck W, Stueger HP, Dorn L, Stadlbauer V, Putz-Bankuti C,
Krisper P, Graziadei I, Vogel W, et al: Oxidative albumin damage in
chronic liver failure: Relation to albumin binding capacity, liver
dysfunction and survival. J Hepatol. 59:978–983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nagumo K, Tanaka M, Chuang VT, Setoyama H,
Watanabe H, Yamada N, Kubota K, Tanaka M, Matsushita K, Yoshida A,
et al: Cys34-cysteinylated human serum albumin is a sensitive
plasma marker in oxidative stress-related chronic diseases. PLoS
One. 9:e852162014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Brownlee M, Vlassara H and Cerami A:
Nonenzymatic glycosylation and the pathogenesis of diabetic
complications. Ann Intern Med. 101:527–537. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cohen MP: Intervention strategies to
prevent pathogenetic effects of glycated albumin. Arch Biochem
Biophys. 419:25–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Rabbani G and Ahn SN: Structure, enzymatic
activities, glycation and therapeutic potential of human serum
albumin: A natural cargo. Int J Biol Macromol. 123:979–990. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Rondeau P and Bourdon E: The glycation of
albumin: Structural and functional impacts. Biochimie. 93:645–658.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ding A, Ojingwa JC, McDonagh AF,
Burlingame AL and Benet LZ: Evidence for covalent binding of acyl
glucuronides to serum albumin via an imine mechanism as revealed by
tandem mass spectrometry. Proc Natl Acad Sci USA. 90:3797–3801.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tabata F, Wada Y, Kawakami S and Miyaji K:
Serum albumin redox states: More than oxidative stress biomarker.
Antioxidants (Basel). 10:5032021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Thornalley PJ, Langborg A and Minhas HS:
Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the
glycation of proteins by glucose. Biochem J. 344(Pt 1(Pt 1)):
109–116. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Brownlee M, Cerami A and Vlassara H:
Advanced glycosylation end products in tissue and the biochemical
basis of diabetic complications. N Engl J Med. 318:1315–1321. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ahmed N, Dobler D, Dean M and Thornalley
PJ: Peptide mapping identifies hotspot site of modification in
human serum albumin by methylglyoxal involved in ligand binding and
esterase activity. J Biol Chem. 280:5724–5732. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fuentes-Lemus E, Reyes JS, Lopez-Alarcon C
and Davies MJ: Crowding modulates the glycation of plasma proteins:
In vitro analysis of structural modifications to albumin and
transferrin and identification of sites of modification. Free Radic
Biol Med. 193((Pt 2)): 551–566. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bohney JP and Feldhoff RC: Effects of
nonenzymatic glycosylation and fatty acids on tryptophan binding to
human serum albumin. Biochem Pharmacol. 43:1829–1834. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Barnaby OS, Cerny RL, Clarke W and Hage
DS: Comparison of modification sites formed on human serum albumin
at various stages of glycation. Clin Chim Acta. 412:277–285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Okabe N and Hashizume N: Drug binding
properties of glycosylated human serum albumin as measured by
fluorescence and circular dichroism. Biol Pharm Bull. 17:16–21.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Baraka-Vidot J, Guerin-Dubourg A, Bourdon
E and Rondeau P: Impaired drug-binding capacities of in vitro and
in vivo glycated albumin. Biochimie. 94:1960–1967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Nakajou K, Watanabe H, Kragh-Hansen U,
Maruyama T and Otagiri M: The effect of glycation on the structure,
function and biological fate of human serum albumin as revealed by
recombinant mutants. Biochim Biophys Acta. 1623:88–97. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Joseph JS and Hage DS: The effects of
glycation on the binding of human serum albumin to warfarin and
L-tryptophan. J Pharm Biomed Anal. 53:811–818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shaklai N, Garlick RL and Bunn HF:
Nonenzymatic glycosylation of human serum albumin alters its
conformation and function. J Biol Chem. 259:3812–3817. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Barzegar A, Moosavi-Movahedi AA,
Sattarahmady N, Hosseinpour-Faizi MA, Aminbakhsh M, Ahmad F,
Saboury AA, Ganjali MR and Norouzi P: Spectroscopic studies of the
effects of glycation of human serum albumin on L-Trp binding.
Protein Pept Lett. 14:13–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mendez DL, Jensen RA, McElroy LA, Pena JM
and Esquerra RM: The effect of non-enzymatic glycation on the
unfolding of human serum albumin. Arch Biochem Biophys. 444:92–99.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Watanabe A, Matsuzaki S, Moriwaki H,
Suzuki K and Nishiguchi S: Problems in serum albumin measurement
and clinical significance of albumin microheterogeneity in
cirrhotics. Nutrition. 20:351–357. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Horiuchi S: The liver is the main site for
metabolism of circulating advanced glycation end products. J
Hepatol. 36:123–125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Patche J, Girard D, Catan A, Boyer F, Dobi
A, Planesse C, Diotel N, Guerin-Dubourg A, Baret P, Bravo SB, et
al: Diabetes-induced hepatic oxidative stress: A new pathogenic
role for glycated albumin. Free Radic Biol Med. 102:133–148. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chen Z, Chen Q, Huang J, Gong W, Zou Y,
Zhang L, Liu P and Huang H: CK2α promotes advanced glycation end
products-induced expressions of fibronectin and intercellular
adhesion molecule-1 via activating MRTF-A in glomerular mesangial
cells. Biochem Pharmacol. 148:41–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Scavello F, Zeni F, Milano G, Macrì F,
Castiglione S, Zuccolo E, Scopece A, Pezone G, Tedesco CC, Nigro P,
et al: Soluble receptor for advanced glycation end-products
regulates age-associated cardiac fibrosis. Int J Biol Sci.
17:2399–2416. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Goodwin M, Herath C, Jia Z, Leung C,
Coughlan MT, Forbes J and Angus P: Advanced glycation end products
augment experimental hepatic fibrosis. J Gastroenterol Hepatol.
28:369–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Stamler JS, Jaraki O, Osborne J, Simon DI,
Keaney J, Vita J, Singel D, Valeri CR and Loscalzo J: Nitric oxide
circulates in mammalian plasma primarily as an S-nitroso adduct of
serum albumin. Proc Natl Acad Sci USA. 89:7674–7677. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Tsikas D: Extra-platelet
low-molecular-mass thiols mediate the inhibitory action of
S-nitrosoalbumin on human platelet aggregation via
S-transnitrosylation of the platelet surface. Amino Acids.
53:563–573. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Burczynski FJ, Wang GQ and Hnatowich M:
Effect of nitric oxide on albumin-palmitate binding. Biochem
Pharmacol. 49:91–96. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
McNaughton L, Puttagunta L,
Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, Hamid Q and
Radomski MW: Distribution of nitric oxide synthase in normal and
cirrhotic human liver. Proc Natl Acad Sci USA. 99:17161–17166.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jiao J, Mandapati S, Skipper PL,
Tannenbaum SR and Wishnok JS: Site-selective nitration of tyrosine
in human serum albumin by peroxynitrite. Anal Biochem. 293:43–52.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Andersen JT, Dalhus B, Cameron J, Daba MB,
Plumridge A, Evans L, Brennan SO, Gunnarsen KS, Bjørås M, Sleep D
and Sandlie I: Structure-based mutagenesis reveals the
albumin-binding site of the neonatal Fc receptor. Nat Commun.
3:6102012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bar-Or D, Curtis G, Rao N, Bampos N and
Lau E: Characterization of the Co(2+) and Ni(2+) binding amino-acid
residues of the N-terminus of human albumin. An insight into the
mechanism of a new assay for myocardial ischemia. Eur J Biochem.
268:42–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Naldi M, Giannone FA, Baldassarre M,
Domenicali M, Caraceni P, Bernardi M and Bertucci C: A fast and
validated mass spectrometry method for the evaluation of human
serum albumin structural modifications in the clinical field. Eur J
Mass Spectrom (Chichester). 19:491–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Bar-Or D, Rael LT, Bar-Or R, Slone DS and
Craun ML: The formation and rapid clearance of a truncated albumin
species in a critically ill patient. Clin Chim Acta. 365:346–349.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bar-Or D, Winkler JV, Vanbenthuysen K,
Harris L, Lau E and Hetzel FW: Reduced albumin-cobalt binding with
transient myocardial ischemia after elective percutaneous
transluminal coronary angioplasty: A preliminary comparison to
creatine kinase-MB, myoglobin, and troponin I. Am Heart J.
141:985–991. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ogasawara Y, Namai T, Togawa T and Ishii
K: Formation of albumin dimers induced by exposure to peroxides in
human plasma: A possible biomarker for oxidative stress. Biochem
Biophys Res Commun. 340:353–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Naldi M, Baldassarre M, Nati M, Laggetta
M, Giannone FA, Domenicali M, Bernardi M, Caraceni P and Bertucci
C: Mass spectrometric characterization of human serum albumin
dimer: A new potential biomarker in chronic liver diseases. J Pharm
Biomed Anal. 112:169–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chubarov A, Spitsyna A, Krumkacheva O,
Mitin D, Suvorov D, Tormyshev V, Fedin M, Bowman MK and
Bagryanskaya E: Reversible dimerization of human serum albumin.
Molecules. 26:1082020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Baldassarre M, Domenicali M, Naldi M,
Laggetta M, Giannone FA, Biselli M, Patrono D, Bertucci C, Bernardi
M and Caraceni P: Albumin homodimers in patients with cirrhosis:
Clinical and prognostic relevance of a novel identified structural
alteration of the molecule. Sci Rep. 6:359872016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Bar-Or R, Rael LT and Bar-Or D:
Dehydroalanine derived from cysteine is a common post-translational
modification in human serum albumin. Rapid Commun Mass Spectrom.
22:711–716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Domenicali M, Baldassarre M, Giannone FA,
Naldi M, Mastroroberto M, Biselli M, Laggetta M, Patrono D,
Bertucci C, Bernardi M and Caraceni P: Posttranscriptional changes
of serum albumin: Clinical and prognostic significance in
hospitalized patients with cirrhosis. Hepatology. 60:1851–1860.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Paar M, Fengler VH, Rosenberg DJ, Krebs A,
Stauber RE, Oettl K and Hammel M: Albumin in patients with liver
disease shows an altered conformation. Commun Biol. 4:7312021.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Oettl K, Stadlbauer V, Petter F,
Greilberger J, Putz-Bankuti C, Hallström S, Lackner C and Stauber
RE: Oxidative damage of albumin in advanced liver disease. Biochim
Biophys Acta. 1782:469–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rashid G, Benchetrit S, Fishman D and
Bernheim J: Effect of advanced glycation end-products on gene
expression and synthesis of TNF-alpha and endothelial nitric oxide
synthase by endothelial cells. Kidney Int. 66:1099–1106. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Arroyo V and Claria J: Acute-on-Chronic
liver failure, human serum albumin, and immune modulation: The
beginning of an exciting adventure. Clin Gastroenterol Hepatol.
16:633–636. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
O'Brien AJ, Fullerton JN, Massey KA, Auld
G, Sewell G, James S, Newson J, Karra E, Winstanley A, Alazawi W,
et al: Immunosuppression in acutely decompensated cirrhosis is
mediated by prostaglandin E2. Nat Med. 20:518–523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Trebicka J, Amoros A, Pitarch C, Titos E,
Alcaraz-Quiles J, Schierwagen R, Deulofeu C, Fernandez-Gomez J,
Piano S, Caraceni P, et al: Addressing profiles of systemic
inflammation across the different clinical phenotypes of acutely
decompensated cirrhosis. Front Immunol. 10:4762019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Claria J, Stauber RE, Coenraad MJ, Moreau
R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K,
et al: Systemic inflammation in decompensated cirrhosis:
Characterization and role in acute-on-chronic liver failure.
Hepatology. 64:1249–1264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Naldi M, Baldassarre M, Domenicali M,
Giannone FA, Bossi M, Montomoli J, Sandahl TD, Glavind E, Vilstrup
H, Caraceni P and Bertucci C: Mass spectrometry characterization of
circulating human serum albumin microheterogeneity in patients with
alcoholic hepatitis. J Pharm Biomed Anal. 122:141–147. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Das S, Hussain MS, Maras JS, Kumar J,
Shasthry SM, Nayak S, Arora V, Vijayaraghavan R, Sharma S, Maiwall
R and Sarin SK: Modification patterns of urinary albumin correlates
with serum albumin and outcome in severe alcoholic hepatitis. J
Clin Gastroenterol. 53:e243–e252. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Pawlak M, Lefebvre P and Staels B:
Molecular mechanism of PPARα action and its impact on lipid
metabolism, inflammation and fibrosis in non-alcoholic fatty liver
disease. J Hepatol. 62:720–733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Sun L, Wang Q, Liu M, Xu G, Yin H, Wang D,
Xie F, Jin B, Jin Y, Yang H, et al: Albumin binding function is a
novel biomarker for early liver damage and disease progression in
non-alcoholic fatty liver disease. Endocrine. 69:294–302. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Santos JC, Valentim IB, de Araujo OR,
Ataide Tda R and Goulart MO: Development of nonalcoholic
hepatopathy: Contributions of oxidative stress and advanced
glycation end products. Int J Mol Sci. 14:19846–19866. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Priken K, Tapia G, Cadagan C, Quezada N,
Torres J, D'Espessailles A and Pettinelli P: Higher hepatic
advanced glycation end products and liver damage markers are
associated with nonalcoholic steatohepatitis. Nutr Res. 104:71–81.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Palma-Duran SA, Kontogianni MD,
Vlassopoulos A, Zhao S, Margariti A, Georgoulis M, Papatheodoridis
G and Combet E: Serum levels of advanced glycation end-products
(AGEs) and the decoy soluble receptor for AGEs (sRAGE) can identify
non-alcoholic fatty liver disease in age-, sex- and BMI-matched
normo-glycemic adults. Metabolism. 83:120–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Pereira ENGDS, Paula DP, de Araujo BP, da
Fonseca MJM, Diniz MFHS, Daliry A and Griep RH: Advanced glycation
end product: A potential biomarker for risk stratification of
non-alcoholic fatty liver disease in ELSA-Brasil study. World J
Gastroenterol. 27:4913–4928. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yavuz F, Biyik M, Asil M, Dertli R, Demir
A, Polat H, Uysal S and Ataseven H: Serum ischemic modified albumin
(IMA) concentration and IMA/albumin ratio in patients with
hepatitis B-related chronic liver diseases. Turk J Med Sci.
47:947–953. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Cakir M, Karahan SC, Mentese A, Sag E,
Cobanoglu U, Polat TB and Erduran E: Ischemia-Modified albumin
levels in children with chronic liver disease. Gut Liver. 6:92–97.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
European Association for the Study of the
Liver. Electronic address, . simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver: EASL Clinical
Practice Guidelines for the management of patients with
decompensated cirrhosis. J Hepatol. 69:406–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Bai Z, Méndez-Sánchez N, Romeiro FG,
Mancuso A, Philips CA, Tacke F, Basaranoglu M, Primignani M,
Ibrahim M, Wong YJ, et al: Use of albumin infusion for
cirrhosis-related complications: An international position
statement. JHEP Rep. 5:1007852023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Sort P, Navasa M, Arroyo V, Aldeguer X,
Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G,
Guevara M, et al: Effect of intravenous albumin on renal impairment
and mortality in patients with cirrhosis and spontaneous bacterial
peritonitis. N Engl J Med. 341:403–409. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Bortoluzzi A, Ceolotto G, Gola E, Sticca
A, Bova S, Morando F, Piano S, Fasolato S, Rosi S, Gatta A and
Angeli P: Positive cardiac inotropic effect of albumin infusion in
rodents with cirrhosis and ascites: Molecular mechanisms.
Hepatology. 57:266–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Fernandez J, Claria J, Amoros A, Aguilar
F, Castro M, Casulleras M, Acevedo J, Duran-Güell M, Nuñez L, Costa
M, et al: Effects of albumin treatment on systemic and portal
hemodynamics and systemic inflammation in patients with
decompensated cirrhosis. Gastroenterology. 157:149–162. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Caraceni P, Riggio O, Angeli P,
Alessandria C, Neri S, Foschi FG, Levantesi F, Airoldi A, Boccia S,
Svegliati-Baroni G, et al: Long-term albumin administration in
decompensated cirrhosis (ANSWER): An open-label randomised trial.
Lancet. 391:2417–2429. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Alukal JJ, John S and Thuluvath PJ:
Hyponatremia in Cirrhosis: An Update. Am J Gastroenterol.
115:1775–1785. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Teh KB, Loo JH, Tam YC and Wong YJ:
Efficacy and safety of albumin infusion for overt hepatic
encephalopathy: A systematic review and meta-analysis. Dig Liver
Dis. 53:817–823. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Rose CF, Amodio P, Bajaj JS, Dhiman RK,
Montagnese S, Taylor-Robinson SD, Vilstrup H and Jalan R: Hepatic
encephalopathy: Novel insights into classification, pathophysiology
and therapy. J Hepatol. 73:1526–1547. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
China L, Becares N, Rhead C, Tittanegro T,
Freemantle N and O'Brien A: Targeted albumin infusions do not
improve systemic inflammation or cardiovascular function in
decompensated cirrhosis. Clin Transl Gastroenterol. 13:e004762022.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Sandi BB, Leao GS, de Mattos AA and de
Mattos AZ: Long-term albumin administration in patients with
cirrhosis and ascites: A meta-analysis of randomized controlled
trials. J Gastroenterol Hepatol. 36:609–617. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Plantier JL, Duretz V, Devos V, Urbain R
and Jorieux S: Comparison of antioxidant properties of different
therapeutic albumin preparations. Biologicals. 44:226–233. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Mikkat S, Dominik A, Stange J and Eggert
M: Comparison of accompanying proteins in different therapeutic
human serum albumin preparations. Biologicals. 64:41–48. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Bar-Or D, Bar-Or R, Rael LT, Gardner DK,
Slone DS and Craun ML: Heterogeneity and oxidation status of
commercial human albumin preparations in clinical use. Crit Care
Med. 33:1638–1641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Berezenko S: Heterogeneity and oxidation
status of commercial human albumin preparations in clinical use.
Crit Care Med. 34:1291author reply 1291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Cheungpasitporn W, Thongprayoon C, Zoghby
ZM and Kashani K: MARS: Should i use it? Adv Chronic Kidney Dis.
28:47–58. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Wallon G, Guth C, Guichon C, Thevenon S,
Gazon M, Viale JP, Schoeffler M, Duperret S and Aubrun F:
Extracorporeal albumin dialysis in liver failure with MARS and
SPAD: A Randomized crossover trial. Blood Purif. 51:243–250. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Klammt S, Mitzner SR, Stange J, Loock J,
Heemann U, Emmrich J, Reisinger EC and Schmidt R: Improvement of
impaired albumin binding capacity in acute-on-chronic liver failure
by albumin dialysis. Liver Transpl. 14:1333–1339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Oettl K, Stadlbauer V, Krisper P and
Stauber RF: Effect of extracorporeal liver support by molecular
adsorbents recirculating system and Prometheus on redox state of
albumin in acute-on-chronic liver failure. Ther Apher Dial.
13:431–436. 2009. View Article : Google Scholar : PubMed/NCBI
|