Introduction
Diabetes mellitus is a chronic metabolic disorder
characterized by high blood sugar levels (hyperglycemia) and a
concurrent gradual and irreversible decline in pancreatic insulin
secretion over a prolonged period (1,2). Of
all types of diabetes mellitus, type 2 diabetes mellitus accounts
for ~90% of cases (3). Insulin
resistance, also known as impaired insulin sensitivity, is a
pathological state of insulin sensitivity where cells do not
respond properly to the signal from insulin (4). Insulin-sensitive tissues, such as
skeletal muscle, adipose tissue and liver tissue, exhibit
diminished glucose uptake into the cytoplasm in patients with
insulin resistance (5). Severely,
long-term insulin resistance causes hyperglycemia and
hyperinsulinemia, eventually leading to type 2 diabetes mellitus
(5).
Insulin resistance is mainly linked to an elevated
free fatty acid (FFA) content in the blood resulting from a
high-calorie diet (4). After
binding to an insulin receptor, insulin initiates the downstream
signaling pathway to maintain glucose homeostasis in the liver
(6). However, excess circulating
FFAs in the blood lead to lipid accumulation, causing impaired
insulin signaling (7). Several
serine kinases, including p38, extracellular signal-regulated
kinase (ERK), and c-Jun-N-terminal kinase (JNK), are activated by
FFA accumulation and FFA metabolites (8,9).
These serine kinases suppress insulin receptor substrate 1 (IRS1)
phosphorylation, thereby inhibiting downstream phosphorylation of
protein kinase B (Akt) (10–12).
In liver tissue, glycogen synthase kinase 3β (GSK-3β) and forkhead
box protein O1 (FOXO1) are activated by the reduction in Akt
activity, leading to decreased glycogen synthesis and elevated
gluconeogenesis, which are critical factors in the development of
hepatic insulin resistance (13,14).
Therefore, targeting the IRS1/Akt/GSK-3β/FOXO1 signaling pathway is
considered a key factor in insulin resistance therapy (15).
Camellia sinensis (C. sinensis), known as the
tea plant, is an evergreen, medium-sized woody shrub widely
distributed across China, Taiwan and Southeast Asia (16). The leaves and leaf buds of C.
sinensis are used to produce the most commonly consumed
non-alcoholic beverage, tea. In addition to its high economic
value, C. sinensis reportedly provides numerous health
benefits for humans, such as anticancer activity, antioxidant
activity, cardiovascular benefits and anti-diabetic effects
(17). Tea seed, that is, the seed
of C. sinensis, is typically used to produce tea seed oil,
which comprises ~80% unsaturated fatty acids (18). Defatted tea seed, namely, tea seed
cake, is an agricultural byproduct that contains numerous bioactive
compounds, rendering it a promising resource. (19). For instance, tea seed cake extract
exerts an effect on 5-reductase inhibition (20). Moreover, a kaempferol triglycoside
purified from tea seed cake was found to attenuate
lipopolysaccharide (LPS)-stimulated inflammation and cognitive
impairment in a mouse model (21),
and flavonoids separated from tea seed cake also proved to
ameliorate tumor necrosis factor alpha (TNF-α)-induced insulin
resistance in HepG2 cells (22).
However, the high toxicity of tea saponins restricts the
application of tea seed cake in livestock feed production or
supplementation (23).
Oleiferasaponin B2, a tea saponin,
exhibits strong cytotoxicity with a half maximal inhibitory
concentration (IC50) of 6.3 mM (SK-OV-3), 0.8 mM
(HCT15), 9.2 mM (SK-MEL-2) and 8.4 mM (A549), while oleiferasaponin
B1 exhibits IC50 values of 11.3 mM (SK-OV-3),
1.6 mM (HCT15), 13.9 mM (SK-MEL-2) and 18.5 mM (A549) (24). Furthermore, a few tea saponins,
including floratheasaponins D, E, F and G, have been reported to
display potent cytotoxic activity ranging from 6 to 10 µM in RAW
264.7 cells (25). In addition to
their toxicity to cell lines, the toxicity of saponins to
cold-blooded animals and insects has also been mentioned in several
studies (26). A saponin toxicity
assay revealed hemorrhage and erosion of the mucosa in the small
intestine as well as necrosis of liver cells and renal tubules in a
mouse model (27). Presently,
several methods are employed to reduce or remove tea saponins from
tea seed cake or tea seed cake extract. For instance, tea saponin
levels in tea seed cake are significantly lessened by chemical
treatment or biodegradation (28);
furthermore, semi-preparative high-performance liquid
chromatography (HPLC) can be used to isolate non-catechin
flavonoids from saponin-rich tea seed extract (22). However, these saponin-reduction
processes can also lead to a reduction in bioactive compounds or
the production of lower yields of bioactive compounds. Thus,
methods of reducing or eliminating saponins in tea seed cake must
be optimized.
Therefore, the present study aimed to i) use
chemical treatment to reduce tea saponin levels and separate
bioactive molecules from the tea seed crude extract (TSCE) of C.
sinensis and ii) analyze the anti-insulin resistance effect of
the saponin-reduced extract of C. sinensis in the HepG2 cell
line.
Materials and methods
Preparation of tea seed
saponin-reduced extract (TSSRE) from C. Sinensis tea seeds
C. sinensis (L.) O. Kuntze is an economic
crop in Taiwan; there might be not any policy to regulate the
permission of using C. sinensis implementing experiment in
Taiwan. Specimen of C. sinensis identified by Kuoh-Cheng Yan
is stored in public at the herbarium of the Research Center for
Biodiversity, Academia Sinica, Taipei (HAST). The seeds of C.
sinensis (L.) O. Kuntze were cultivated in Taitung, Taiwan.
They were ground into tea seed powder, which was subsequently
stirred in hexane (1:10 w/v) at room temperature for 40 min to
simulate tea seed cake. The defatted tea seed powder was
subsequently extracted in 95% ethanol (1:10 w/v) for 24 h to obtain
TSCE. Thereafter, TSCE was dissolved in 80% ethanol (1:20 w/v).
Ethyl acetate was then mixed with TSCE for precipitation (3:1 v/v).
After centrifugation at 3,800 × g for 10 min, the supernatant was
evaporated and lyophilized to obtain TSSRE powder.
Determination of the saponin content
in TSCE and TSSRE
The tea saponin content in TSSRE was determined as
previously reported, with some modifications (29). TSCE, TSSRE and tea saponins
(ChemFaces; cat. no. CFN91688) were dissolved in methanol. The
samples were mixed with 8% vanillin (Thermo Fisher Scientific,
Inc.; cat. no. A11169), followed by 77% sulfuric acid. After
mixing, the samples were heated to 65°C for 15 min and subsequently
cooled for 10 min. Absorbance values were measured at 505 nm and
510 nm using a microplate reader (SpectraMax Plus 384; Molecular
Devices, LLC), and the difference between the absorbance values at
these wavelengths reflected tea saponin content.
HPLC analysis of TSSRE
The chromatographic separation of compounds from
TSSRE was conducted using an HPLC system (JASCO) and a Hypersil
GOLD™ C18 column (250×4.6 mm i.d., 5 µm; Thermo Fisher
Scientific, Inc.). The mobile phase comprised solvents A (0.2%
phosphoric acid water) and B (acetonitrile), which were pumped at a
flow rate of 1.0 ml/min. 10 µl sample was injected by an
autosampler (JASCO). The gradient elution program was conducted
under the following conditions: 0–20 min and 95% A to 40% A.
Catechin (MedChemExpress; cat. no. HY-B1890), pyrroside B
(ChemFaces; cat. no. CFN96142), narirutin (ChemFaces; cat. no.
CFN99543), and naringin (MedChemExpress; cat. no. HY-N0153) were
used as the standards.
Determination of the total flavonoid
content in TSSRE
The total flavonoid content in TSSRE was estimated
using the aluminum chloride colorimetric and
2,4-dinitrophenylhydrazine methods (30,31),
and calculated as the sum of the results obtained from these
methods.
The aluminum chloride colorimetric method was
employed to estimate the levels of flavones and flavonols, which
are subclasses of flavonoids. Briefly, 200 µl of sample was mixed
with 40 µl of 10% aluminum chloride (Thermo Fisher Scientific,
Inc.; cat. no. A11892), followed by 600 µl of 95% ethanol, 20 µl of
1 M potassium acetate, and 1,120 µl of distilled water. The mixture
was incubated in the dark at room temperature for 30 min. The
absorbance values of the samples and standard (quercetin)
(MedChemExpress; cat. no. HY-18085) were measured at 415 nm using a
microplate reader.
To estimate the levels of flavanones and
flavanonols, which are also subclasses of flavonoids, the
2,4-dinitrophenylhydrazine method was employed. Briefly, 200 µl of
sample was mixed with 400 µl of 1% 2,4-dinitrophenylhydrazine
reagent (Sigma-Aldrich; Merck KGaA; cat. no. D199303), followed by
400 µl of methanol at 50°C for 50 min. After cooling, the mixture
was mixed with 1,000 µl of 1% potassium hydroxide dissolved in 70%
methanol and kept at room temperature for 2 min. A total of five
times the volume of methanol was mixed with the sample and
subsequently centrifuged at 1,000 × g for 10 min at 25°C. After
centrifugation, the absorbance values of the supernatant and
standard (naringenin) (MedChemExpress; cat. no. HY-W011641) were
determined at 495 nm using a microplate reader.
Cell culture
The HepG2 cell line (cat. no. RM60025) was obtained
from the Bioresources Collection and Research Center of the Food
Industry Research and Development Institute (Hsinchu, Taiwan).
HepG2 cells were cultured in high-glucose Dulbecco's Modified Eagle
Medium (HyClone; Cytiva; cat. no. SH30243) with 10% fetal bovine
serum (HyClone; Cytiva; cat. no. SH30396) containing 100 units/ml
penicillin and 0.1 mg/ml streptomycin (BioConcept Ltd.; cat. no.
4-01F00-H) at 37°C with 5% CO2. The medium was altered
every 2 days, and the cells were maintained in culture by passaging
them with 0.25% trypsin (HyClone; Cytiva; cat. no. SH30042).
Cell viability assay
HepG2 cells (1×104 cells/well) were
cultured in 96-well microtiter plates. After 24-h incubation at
37°C, the HepG2 cells were treated with various concentrations of
TSCE and TSSRE for 24 h. Subsequently, 200 µl of
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
reagent (Sigma-Aldrich; Merck KGaA; cat. no. 475989) was added to
each well, and the plates were incubated at 37°C with 5%
CO2 in the dark for 4 h. Thereafter, 150 µl of dimethyl
sulfoxide was added to each well, and the plates were subsequently
shaken on a shaker at room temperature for 10 min. The absorbance
values of the samples were determined at 490 nm using a microplate
reader.
Glucose consumption assay
Insulin resistance was induced in a HepG2 cell model
using palmitic acid (PA) according to a method previously
described, with some modifications (32). Normal glucose (5.5 mM) (HyClone;
Cytiva; cat. no. SH30021) was used as the control. Normal glucose
group was adopted to simulate the condition of normal and healthy
cells (33), which i) evaluated if
TSSRE could recover cells to the normal and healthy condition and
ii) analyzed how the level of improvement of insulin resistance the
drug could affect cells with insulin resistance. HepG2 cells
(4×104 cells/well) were cultured in 96-well microtiter
plates. After 24-h incubation at 37°C, the cells were washed twice
with phosphate-buffered saline (PBS) and subsequently treated with
normal-(5.5 mM) or high-concentration (30 mM) glucose plus 0.25 mM
PA (Thermo Fisher Scientific, Inc.; cat. no. AC416700050) in the
absence or presence of the indicated concentrations of TSSRE for 24
h. Thereafter, 100 nM of insulin (MedChemExpress; cat. no.
HY-P73243) was added to each sample, and the plates were incubated
at 37°C with 5% CO2 for 30 min. The cells were washed
twice with PBS, and RPMI-1640 (HyClone; Cytiva; cat. no. SH30027)
containing 0.2% fatty acid-free bovine serum albumin (BSA, Bio
Basic; cat. no. AD0023) was subsequently added to each well. After
24-h incubation at 37°C, the medium was collected for further
assay. Each sample (25 µl) was mixed with 500 µl of o-toluidine
(Abbkine Scientific Co., Ltd.; cat. no. KTB1300). The mixtures were
heated in boiling water for 8 min and subsequently cooled down. The
absorbance values of the samples were determined at 630 nm using a
microplate reader. RPMI-1640 contains a suitable concentration of
glucose (11.1 mM) compared with high glucose DMEM (25 mM) and low
glucose DMEM (5 mM) for this assay.
Western blot analysis
HepG2 cells (5×105 cells/well) were
cultured in six-well plates. After incubation for 24 h, the HepG2
cells were washed twice with PBS and incubated with normal-(5.5 mM)
or high-concentration (30 mM) glucose plus 0.25 mM PA in the
absence or presence of the indicated concentrations of TSSRE. After
24-h treatment, 100 nM of insulin was added to each well for 30
min. The HepG2 cells were washed twice with cold PBS and lysed in
radioimmunoprecipitation assay buffer (BIOTOOLS; cat. no.
TAAR-ZBZ5) supplemented with protease (BIOTOOLS; cat. no.
TAAR-BBI2) and phosphatase (BIOTOOLS; cat. no. TAAR-WBC1) inhibitor
cocktails at 0°C. After being scraped off the six-well plates, the
cell lysates were collected, transferred to microcentrifuge tubes,
and centrifuged (16,500 × g, 20 min, 4°C); thereafter, the
supernatants were collected as protein samples. Bicinchoninic acid
protein assay reagents (Visual Protein; BC03-500) were utilized to
determine the total protein concentration of each sample using a
standard BSA curve. The protein samples were diluted to equal
amounts of protein and 20 µl/lane was subsequently separated via 8
or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Thereafter, the gels were transferred to polyvinylidene difluoride
(PerkinElmer, Inc.; cat. no. NEF1002001PK) membranes for 2 h. The
membranes were blocked for 1 h at room temperature with 5% BSA
buffer. After blocking, these membranes were incubated with the
following primary antibodies at 4°C overnight: Phosphorylated
(p-)p38 (cat. no. 4511; 1:1,000), p38 (cat. no. 8690; 1:1,000),
p-ERK (cat. no. 4370; 1:1,000), ERK (cat. no. 4695; 1:1,000), p-JNK
(cat. no. 4668; 1:1,000), JNK (cat. no. 9252; 1:1,000), p-Akt (cat.
no. 9271; 1:1,000), Akt (cat. no. 9272; 1:1,000), p-IRS1 (cat. no.
3070; 1:1,000), IRS1 (all from Cell Signaling Technology, Inc.;
cat. no. 2382; 1:1,000), phosphoenolpyruvate carboxykinase (PEPCK;
cat. no. E-AB-11396; 1:1,000), p-GSK-3β (cat. no. E-AB-20886;
1:1,000), GSK-3β (cat. no. E-AB-31629; 1:1,000), glucose
transporter (GLUT) 4 (all from Elabscience Biotechnology, Inc.;
cat. no. E-AB-30268; 1:1,000), GLUT2 (Proteintech Group, Inc.; cat.
no. 20436-1-AP; 1:1,000), p-FOXO1 (cat. no. AF3417; 1:1,000), FOXO1
(both from Affinity Biosciences; cat. no. AF6416; 1:1,000), and
β-actin (iReal Biotechnology, Inc.; cat. no. IR2-7; 1:1,000). The
membranes were washed thrice with Tris-buffered saline with 0.1%
Tween 20 and subsequently incubated with horseradish
peroxidase-conjugated secondary antibodies (Abcam; cat. no. ab6721;
1:10,000) at room temperature for 1 h. The immunoblots were
visualized using an enhanced chemiluminescence reagent (Visual
Protein; cat. no. LF08-500) and captured using a chemiluminescence
imaging system. Relative protein levels were quantified using
ImageJ software (version 1.8.0; National Institutes of Health), and
β-actin was employed as the internal control.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. GraphPad Prism 9.0 software
(Dotmatics) was employed to evaluate statistical differences
between groups. P<0.05 was considered to indicate a
statistically significant difference based on one-way ANOVA
followed by Dunnett's multiple comparisons test.
Results
TSSRE saponin content
The saponin content in TSSRE was measured using the
vanillin-sulfuric acid method (Table
I). The tea saponin concentrations in TSCE and TSSRE were
5.34±0.46 and 0.54±0.07 mg/g, respectively.
 | Table I.The saponin contents in TSCE and
TSSRE measured by vanillin-sulfuric acid assay. |
Table I.
The saponin contents in TSCE and
TSSRE measured by vanillin-sulfuric acid assay.
| Extract | Saponin content
(mg/g) |
|---|
| TSCE | 5.34±0.46 |
| TSSRE | 0.54±0.07 |
Identification of TSSRE
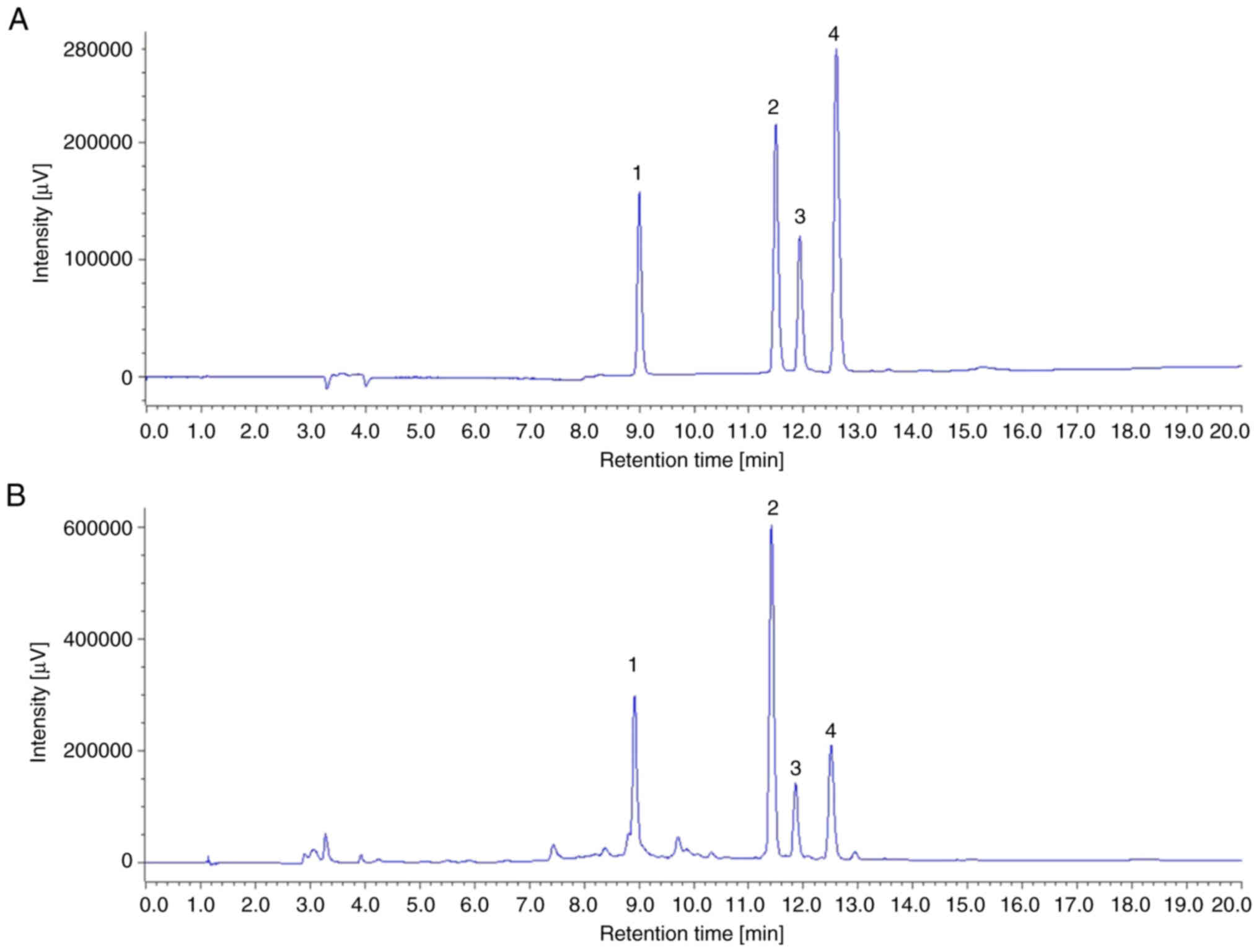
As revealed in Fig. 1A
and B, TSSRE yielded several chromatographic peaks, and the
major peaks represented catechin, pyrroside B, narirutin and
naringin, identified at the same time points as the standards.
The total flavonoid content in
TSSRE
The total flavonoid content in TSSRE was measured
using the aluminum chloride colorimetric and
2,4-dinitrophenylhydrazine methods (Table II). In TSSRE, the aluminum
chloride assay revealed a flavone and flavonol content of
13.56±1.15 mg/g, while the 2,4-dinitrophenylhydrazine assay
revealed a flavanone and flavanonol content of 68.84±7.53 mg/g.
Therefore, the total flavonoid content in TSSRE was 82.40±8.68
mg/g.
 | Table II.The flavonoid contents in TSSRE
measured by aluminum chloride and 2,4-dinitrophenylhydrazine
colorimetric assays. |
Table II.
The flavonoid contents in TSSRE
measured by aluminum chloride and 2,4-dinitrophenylhydrazine
colorimetric assays.
|
| Flavonoid content
(mg/g) |
|---|
|
|
|
|---|
| Extract |
AlCl3 | 2.4-D | Total |
|---|
| TSSRE | 13.56±1.15 | 68.84±7.53 | 82.40±8.68 |
Cell viability
HepG2 cells were treated with different
concentrations of TSCE and TSSRE to evaluate their cell viability.
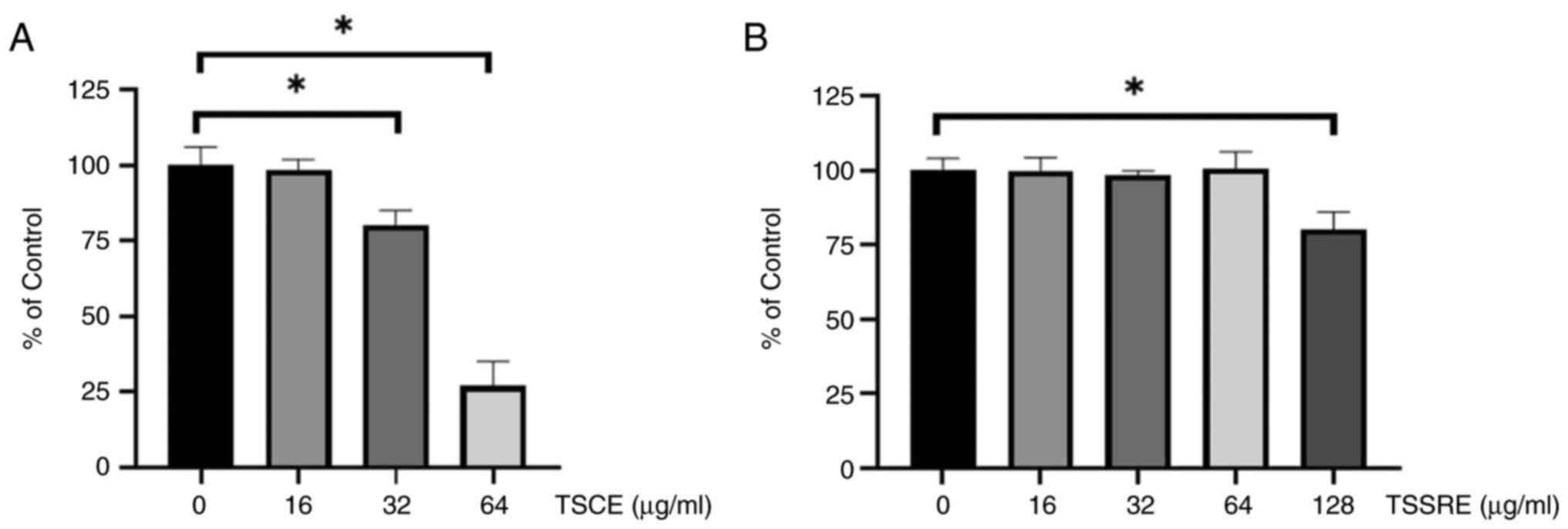
The cell viability assay results are demonstrated in Fig. 2. The cell viability of HepG2 cells
significantly declined following 24-h treatment with 32 and 64
µg/ml TSCE (Fig. 2A; 80 and 25%,
respectively; P=0.0084 and P<0.0001, respectively) but did not
differ significantly at concentrations of 16, 32 and 64 µg/ml from
that of the untreated group (Fig.
2B; P=0.9999, P=0.9741 and P=0.999, respectively).
Effect of TSSRE on glucose
consumption
Impaired glucose uptake in liver tissues is a
characteristic of insulin resistance (5). Therefore, the effect of TSSRE on
glucose consumption in PA-triggered insulin-resistant HepG2 cells
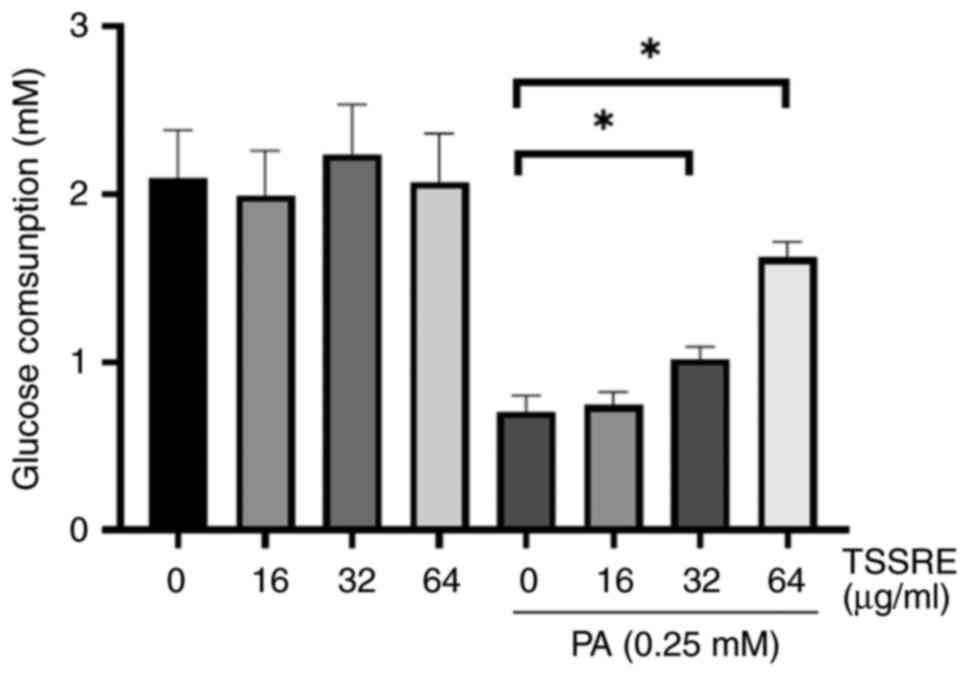
was investigated (Fig. 3). Glucose
consumption in these cells significantly decreased after exposure
to 0.25 mM PA compared with that after normal-glucose treatment
(3.2-fold). The effect of treatment with 16, 32 and 64 µg/ml TSSRE
was not significantly different compared with normal-glucose
treatment (P=0.9442, P=0.8796 and P=0.9992, respectively). However,
treatment with 32 and 64 µg/ml TSSRE ameliorated glucose
consumption in PA-stimulated insulin-resistant HepG2 cells (1.3-
and 2.4-fold, respectively; P=0.0064 and P<0.0001,
respectively). These results suggested that TSSRE enhanced glucose
consumption in PA-stimulated insulin-resistant HepG2 cells.
Therefore, treatment with TSSRE in PA-stimulated groups was adopted
to implement the further experiments.
Effect of TSSRE on the expression
levels of GLUT2 and GLUT4
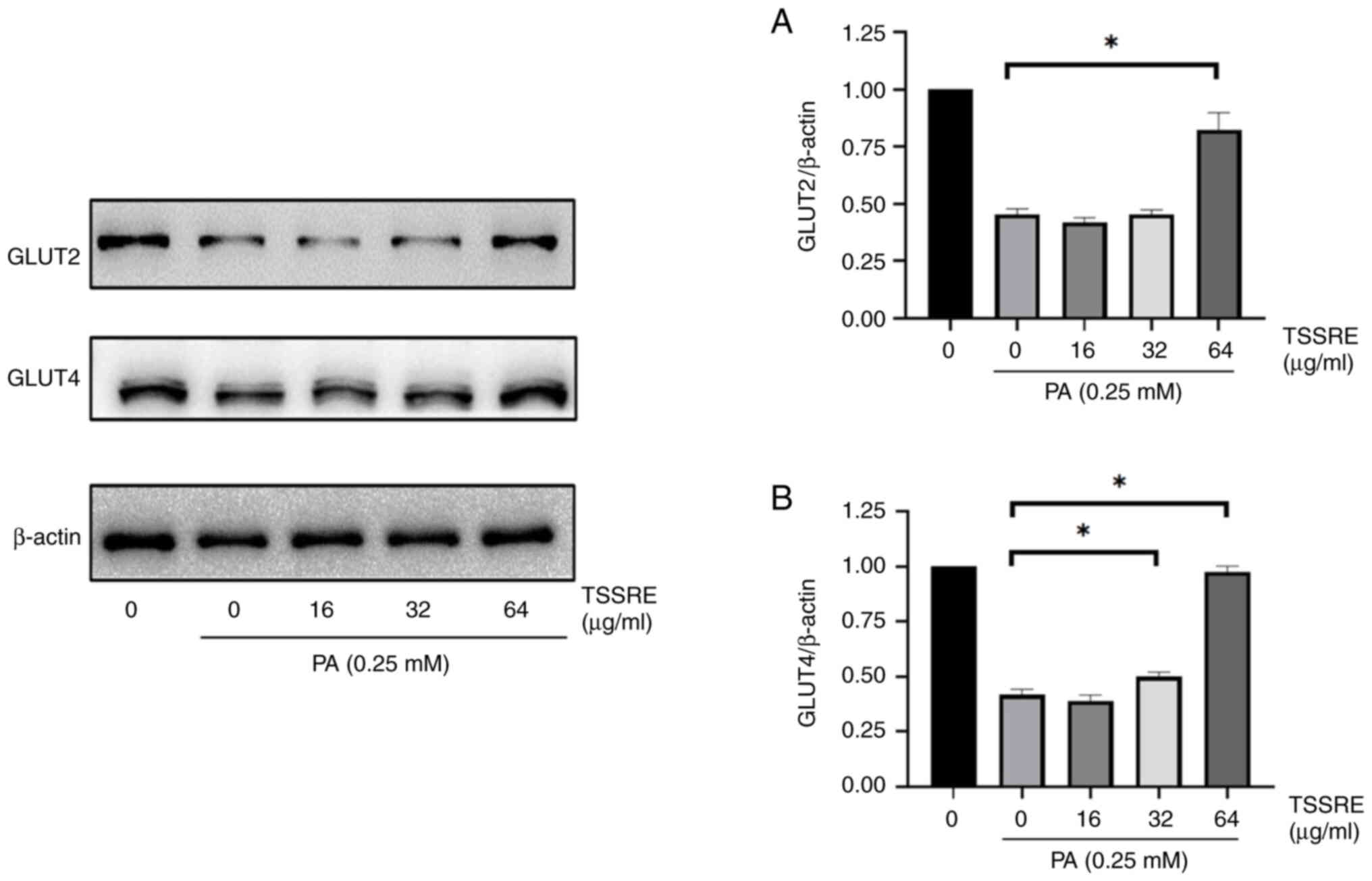
GLUT2 facilitates glucose transportation across the
cell membrane (34). The effect of
TSSRE on GLUT2 in PA-stimulated insulin-resistant HepG2 cells was
analyzed (Fig. 4A). In the present
study, GLUT2 expression in PA-induced insulin-resistant HepG2 cells
was significantly lower than that in the normal-glucose group
(2.2-fold). However, 64 µg/ml TSSRE treatment ameliorated GLUT2
expression in these cells (1.8-fold; P<0.0001). GLUT4, an
insulin-regulated GLUT, mediates glucose uptake (34). The effect of TSSRE on GLUT4 in
PA-stimulated insulin-resistant HepG2 cells was evaluated (Fig. 4B). GLUT4 expression in these cells
was significantly lower than that in the normal-glucose group
(2.4-fold). However, treatment with 32 and 64 µg/ml TSSRE elevated
GLUT4 expression in HepG2 cells with PA-induced insulin resistance
(1.2- and 2.4-fold, respectively; P=0.0077 and P<0.0001,
respectively). These results suggested that TSSRE ameliorated the
expression levels of GLUT2 and GLUT4 in PA-stimulated
insulin-resistant HepG2 cells.
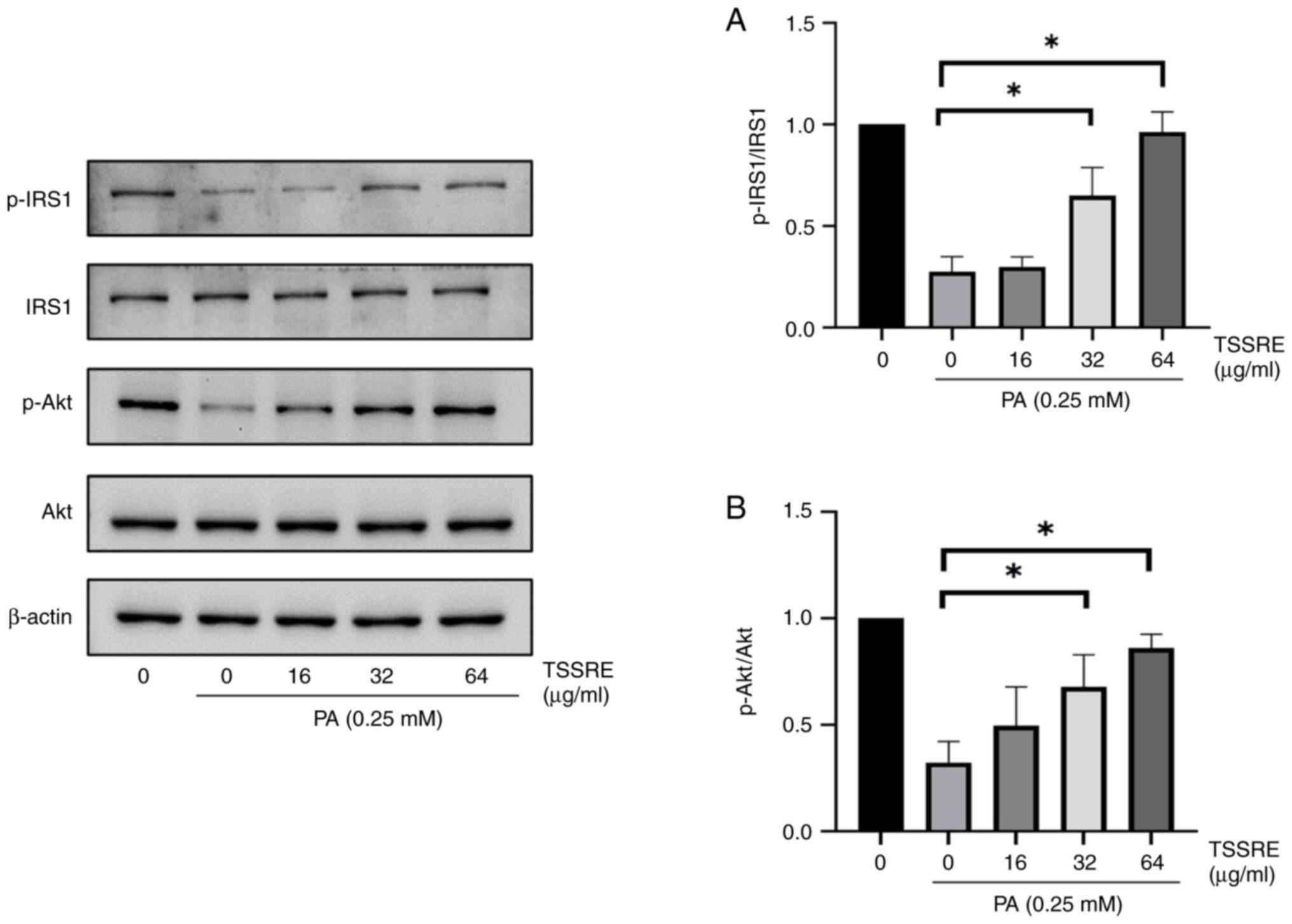
Effect of TSSRE on the IRS1/Akt
signaling pathway
The IRS1/Akt signaling pathway was investigated to
elucidate the mechanism by which TSSRE ameliorates GLUT4 expression
in PA-induced insulin-resistant HepG2 cells. The effect of TSSRE on
IRS1 in these cells was investigated (Fig. 5A). In the present study, IRS1
phosphorylation was evidently decreased by PA treatment compared
with that in the normal-glucose group in HepG2 cells (4.2-fold).
However, treatment with 32 and 64 µg/ml TSSRE significantly
improved IRS1 phosphorylation (2.2- and 3.3-fold, respectively;
P=0.0037 and P<0.0001, respectively). The effect of TSSRE on Akt
in PA-induced insulin-resistant HepG2 cells was analyzed (Fig. 5B). Akt phosphorylation in these
cells decreased significantly in the present study (3.6-fold).
TSSRE treatment at 32 and 64 mg/ml significantly ameliorated Akt
phosphorylation (1.8- and 2.5-fold, respectively; P=0.0070, and
P=0.0002, respectively). These results demonstrated that TSSRE
enhanced the IRS1/Akt signaling pathway in PA-stimulated
insulin-resistant HepG2 cells.
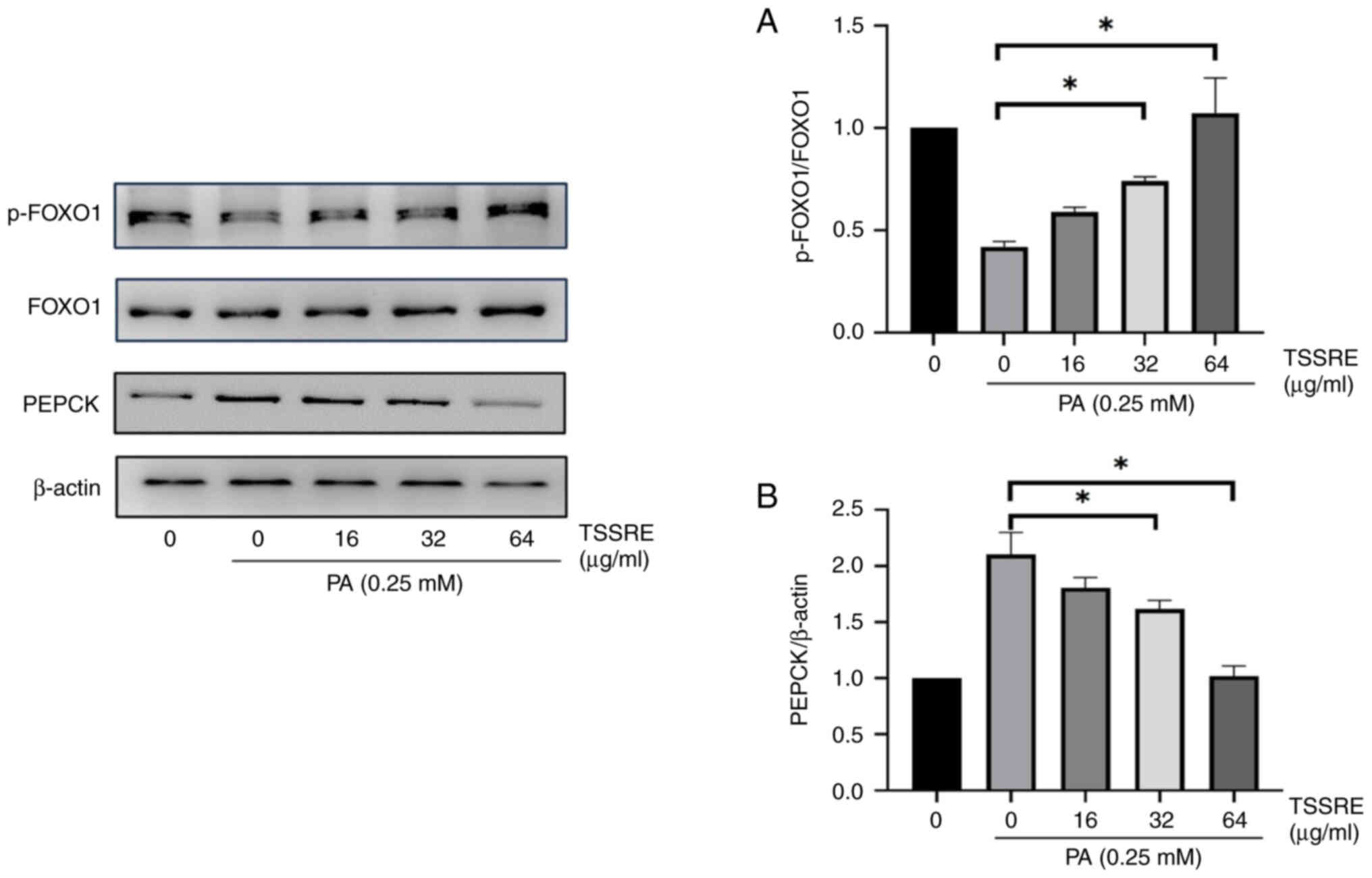
Effect of TSSRE on PEPCK and
FOXO1
PEPCK is an enzyme involved in gluconeogenesis, a
process in which glucose is synthesized from non-hexose precursors
(35). The effect of TSSRE on
PEPCK in PA-stimulated insulin-resistant HepG2 cells was evaluated
(Fig. 6A). PEPCK expression
increased significantly in these cells compared with that in the
normal-glucose group (2.1-fold). However, treatment with 32 and 64
mg/ml TSSRE reduced PEPCK expression in PA-triggered
insulin-resistant HepG2 cells (1.3- and 2.1-fold, respectively;
P=0.0034 and P<0.0001, respectively). FOXO1, a transcription
factor, regulates gluconeogenesis (14). The effect of TSSRE on FOXO1 in
PA-induced insulin-resistant HepG2 cells was investigated (Fig. 6B). FOXO1 phosphorylation declined
significantly after 24-h PA treatment (2.4-fold). TSSRE treatment
at 32 and 64 mg/ml significantly improved FOXO1 phosphorylation in
PA-triggered insulin-resistant HepG2 cells (1.8- and 2.6-fold,
respectively; P=0.0054 and P<0.0001, respectively).
Effect of TSSRE on GSK-3β
phosphorylation
GSK-3β has been known to play a critical role in
inhibiting the activity of glycogen synthase (13). The effect of TSSRE on GSK-3β in
PA-stimulated insulin-resistant HepG2 cells was analyzed (Fig. 7). In the present study, GSK-3β
phosphorylation decreased significantly in these cells compared
with that in the normal-glucose group (2.7-fold). TSSRE treatment
at 16, 32, and 64 mg/ml notably ameliorated GSK-3β phosphorylation
in PA-induced insulin-resistant HepG2 cells (2.1-, 2.5- and
2.7-fold, respectively; P=0.0001, P<0.0001 and P<0.0001,
respectively).
Effect of TSSRE on the phosphorylation
of JNK, p38 and ERK
JNK, p38 and ERK suppress IRS1 phosphorylation
(10–12). The effect of TSSRE on the
LPS-stimulated phosphorylation of mitogen-activated protein kinases
(MAPKs), that is, p38, ERK and JNK, was explored using western blot
analysis (Fig. 8). The results
demonstrated that the phosphorylation of p38, ERK and JNK was
significantly induced by 24-h stimulation with 0.25 mM PA compared
with that in the normal-glucose group in HepG2 cells (11.7-, 3.1-
and 3.7-fold, respectively). PA-stimulated p38 phosphorylation was
significantly attenuated by TSSRE at concentrations of 32 and 64
µg/ml (1.8- and 3.3-fold, respectively; P=0.0004 and P<0.0001,
respectively). Furthermore, TSSRE treatment at 32 and 64 mg/ml also
suppressed PA-induced ERK phosphorylation (1.7- and 2.1-fold,
respectively; P=0.0003 and P=0.0012, respectively). JNK
phosphorylation was also significantly inhibited by TSSRE treatment
at 32 and 64 mg/ml in PA-stimulated insulin-resistant HepG2 cells
(1.8- and 1.9-fold, respectively; P<0.0001 and P<0.0001,
respectively).
Discussion
In the present study, the administration of TSSRE
extracted from tea seed cake effectively reversed PA-induced
insulin resistance in HepG2 cells. Tea seed cake is an agricultural
residue that remains after tea seed oil extraction. Hence, the
efficient utilization of this resource and waste reduction are
critical issues in agriculture. Tea seed cake, which comprises
10–16% tea saponins, possesses hemolytic and cytotoxic properties
(36,37), and these properties potentially
cause toxicity in cold-blooded animals and mice (38,39).
In the present study, a simple, low-cost method was developed to
obtain saponin-reduced extract from tea seed cake. After the
saponin-reduction process, the tea saponin concentration in TSSRE
significantly decreased compared with that in TSCE. The
vanillin-sulfuric acid assay demonstrated that the tea saponin
concentration in TSSRE was ~10-fold lower than that in TSCE, and
this result indicated that this saponin-reduction process could
markedly decrease the concentration of tea saponins in TSCE. The
MTT assay revealed that only 25% of HepG2 cells survived after 24 h
treatment with 64 µg/ml TSCE in the cytotoxicity analysis. However,
no significant cytotoxicity was observed in HepG2 cells after
treatment with 64 µg/ml TSSRE, suggesting that this
saponin-reduction process not only decreased the concentration of
tea saponins but also ameliorated their cytotoxic effect in HepG2
cells. HPLC analysis demonstrated that the primary peaks in TSCE
represented catechin, pyrroside B, narirutin and naringin, which
are flavonoids, natural substances found in the kingdom of plants
(40). The total flavonoid content
in TSSRE was determined to be 82.40±8.68 mg/g, and flavanones and
flavanonols were the predominant flavonoids (~80%). Among these
flavonoids, naringin and catechin have proven effective in
improving insulin resistance (41,42).
Hence, the anti-insulin resistance effect of TSSRE may be
attributed to the presence of these two flavonoids. A previous
study also indicated that flavonoids purified from tea seed cake
alleviate TNF-α-induced insulin resistance in HepG2 cells (22). Furthermore, numerous flavonoids
exerting anti-diabetic and anti-insulin resistance effects have
also been reported (43,44).
Dietary habits constitute an important factor
contributing to the risk of insulin resistance (45). PA, a 16-carbon-chain fatty acid is
the most common saturated fatty acid in the human diet (46). Excessive FFA consumption has proven
to be largely associated with the development of metabolic
syndrome, such as insulin resistance and type 2 diabetes mellitus
(47). In the present study, PA
was employed to induce insulin resistance in HepG2 cells. After 24
h of PA exposure, glucose consumption in HepG2 cells was markedly
decreased compared with that in the untreated group. Nevertheless,
32- and 64-µg/ml TSSRE treatments significantly reversed the
PA-induced impaired glucose consumption. GLUTs constitute a class
of membrane proteins that facilitate glucose transportation across
the cell membrane (34). Among
them, GLUT2 is predominantly found in the β cells of the pancreas,
kidney and liver (48). GLUT4, an
insulin-dependent GLUT, responds to insulin-stimulated cell
signaling to reduce blood glucose levels (34). In the present study, the expression
levels of GLUT2 and GLUT4 were decreased by PA treatment compared
with those in the normal-glucose group. However, TSSRE treatment
ameliorated the PA-induced expression levels of GLUT2 and GLUT4.
The present data indicated that TSSRE enhances glucose consumption
in PA-stimulated insulin-resistant HepG2 cells by increasing GLUT2
and GLUT4 expression.
The insulin signaling pathway plays an important
role in regulating insulin signaling transduction and maintaining
glucose homeostasis (6). Upon
binding to insulin, the insulin receptor undergoes conformational
changes, thereby activating kinase activity (49). Thereafter, downstream IRS1 is
recruited and phosphorylated (50). IRS1 phosphorylation subsequently
stimulates Akt to perform further regulation. The activated
IRS1/Akt signaling pathway has proven to increase GLUT4 expression
(51). In the present study, p-Akt
and p-IRS1 levels were reduced in PA-treated HepG2 cells. However,
TSSRE significantly improved PA-induced Akt and IRS1
phosphorylation. These results suggested that TSSRE ameliorated
GLUT4 expression by elevating IRS1 and Akt phosphorylation. Since
Akt does not regulate GLUT2 expression in the liver (52), the elevated GLUT2 expression in
PA-stimulated insulin-resistant HepG2 cells was not regulated
through Akt pathway.
In addition to increasing GLUT4 expression, Akt also
regulates several factors of glucose metabolism in hepatocytes,
such as gluconeogenesis and glycogen synthesis (53). Activated Akt phosphorylates
transcription factor FOXO1 to promote FOXO1 efflux from the nucleus
into the cytosol, preventing FOXO1 from promoting the transcription
of genes involved in gluconeogenesis, such as the gene encoding
PEPCK, a key enzyme in gluconeogenesis (14). In insulin resistance, increased
hepatic gluconeogenesis leads to excessive glucose production,
contributing to elevated blood glucose levels (54). FOXO1 phosphorylation was suppressed
in the insulin-resistance group, and increased PEPCK expression was
also observed after PA treatment in HepG2 cells. However, TSSRE
treatment significantly ameliorated FOXO1 phosphorylation and
inhibited PEPCK expression. Therefore, these findings demonstrated
the inhibitory effect of TSSRE on gluconeogenesis in HepG2 cells by
mediating Akt/FOXO1 signaling.
Glycogen, found in muscle and liver tissues, is an
extensively branched polysaccharide comprising glucose (55). The liver catabolizes glycogen into
glucose, which is subsequently conveyed to the blood and tissues to
maintain appropriate blood sugar levels and provide fuel,
respectively (55). In insulin
resistance, reduced insulin signaling leads to decreased liver
glycogen levels (56). Activated
Akt serves an essential role in inhibiting GSK-3β activation by
phosphorylating GSK-3β (13). When
Akt is inhibited, GSK-3β activation decreases the activity of
glycogen synthase, a key enzyme involved in glycogen synthesis
(13). In the present study, PA
was found to inhibit GSK-3β phosphorylation. Nonetheless, GSK-3β
phosphorylation was significantly elevated by TSSRE treatment in
PA-stimulated insulin-resistant HepG2 cells, suggesting that TSSRE
inhibits GSK-3β activation by mediating Akt/GSK-3β signaling.
The MAPK pathway, a cell signaling pathway, is
responsible for transducing various extracellular stimuli to the
nucleus, leading to gene regulation (57). It is activated by FFA accumulation,
which interferes with IRS1 phosphorylation, thereby inhibiting
insulin signal transduction (8).
This interference leads to the disruption of downstream signaling
and gene expression, including decreased GLUT4 expression, elevated
gluconeogenesis and GSK-3β activation, which are key factors of
insulin resistance (13,14). Hence, further investigation is
required to determine whether the amelioration of IRS1
phosphorylation by TSSRE is associated with MAPK inhibition. In the
present study, the phosphorylation of p38, ERK and JNK was markedly
induced by 24-h PA treatment. TSSRE was found to significantly
inhibit the PA-induced phosphorylation of p38, ERK and JNK in HepG2
cells, indicating that TSSRE improves hepatic insulin resistance by
suppressing the MAPK pathway (the upstream pathway of IRS1).
Nevertheless, it is important to acknowledge
potential limitations in the present study. Given that skeletal
muscle plays a pivotal role in utilizing more than 75% of glucose
in response to insulin (58), it
may be prudent to consider utilizing a skeletal muscle cell line
for investigating the anti-insulin resistance effects of TSSRE.
Therefore, in the forthcoming research, the authors intend to
employ the C2C12 cell line to assess whether TSSRE can ameliorate
insulin resistance in skeletal muscle cells.
In conclusion, the current study developed a simple,
low-cost method of obtaining saponin-reduced extract from tea seed
cake, and treatment with the extract (TSSRE) exhibited significant
improvements in glucose homeostasis in PA-stimulated
insulin-resistant HepG2 cells. The findings of the present study
conclusively demonstrated that TSSRE regulates hepatic insulin
resistance by ameliorating the IRS-1/Akt/GSK-3β/FOXO1 pathway and
inhibiting the MAPK pathway. Overall, the beneficial effect of
TSSRE in alleviating hepatic insulin resistance was indicated.
Acknowledgements
Not applicable.
Funding
The present study was supported by Higher Education Sprout
Project, Ministry of Education to the Headquarters of University
Advancement at National Cheng Kung University under
Interdisciplinary Research Center on Material and Medicinal
Chemistry (D112-G2202).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SCC and SYS designed the study. SCC performed the
experiments and wrote the manuscript. SYS provided the supervision
of the study and was involved in editing and revising of the
manuscript. SCC and SYS confirm the authenticity of all the raw
data. Both authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TSSRE
|
tea seed saponin-reduced extract
|
|
TSCE
|
tea seed crude extract
|
|
PA
|
palmitic acid
|
|
GLUT
|
glucose transporter
|
|
IRS1
|
insulin receptor substrate 1
|
|
Akt
|
protein kinase B
|
|
FOXO1
|
forkhead box protein O1
|
|
MAPK
|
mitogen-activated protein kinase
|
|
GSK-3β
|
glycogen synthase kinase 3β
|
References
|
1
|
Barquilla García A: Brief update on
diabetes for general practitioners. Rev Esp Sanid Penit. 19:57–65.
2017.PubMed/NCBI
|
|
2
|
Ashcroft FM and Rorsman P: Diabetes
mellitus and the β cell: The last ten years. Cell. 148:1160–1171.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chatterjee S, Khunti K and Davies MJ: Type
2 diabetes. Lancet. 389:2239–2251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xin Y and Wang Y, Chi J, Zhu X, Zhao H,
Zhao S and Wang Y: Elevated free fatty acid level is associated
with insulin-resistant state in nondiabetic Chinese people.
Diabetes Metab Syndr Obes. 12:139–147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Honka MJ, Latva-Rasku A, Bucci M, Virtanen
KA, Hannukainen JC, Kalliokoski KK and Nuutila P:
Insulin-stimulated glucose uptake in skeletal muscle, adipose
tissue and liver: A positron emission tomography study. Eur J
Endocrinol. 178:523–531. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aldhoon-Hainerová I, Zamrazilová H,
Dušátková L, Sedláčková B, Hlavatý P, Hill M, Hampl R, Kunešová M
and Hainer V: Glucose homeostasis and insulin resistance:
Prevalence, gender differences and predictors in adolescents.
Diabetol Metab Syndr. 6:1002014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boden G: 45Obesity, insulin resistance and
free fatty acids. Curr Opin Endocrinol Diabetes Obes. 18:139–143.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Šrámek J, Němcová-Fürstová V and Kovář J:
Kinase signaling in apoptosis induced by saturated fatty acids in
pancreatic β-cells. Int J Mol Sci. 17:14002016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lawan A and Bennett AM: Mitogen-activated
protein kinase regulation in hepatic metabolism. Trends Endocrinol
Metab. 28:868–878. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin J, Fukuhara A, Onodera T, Kita S,
Yokoyama C, Otsuki M and Shimomura I: SDF-1 is an autocrine
insulin-desensitizing factor in adipocytes. Diabetes. 67:1068–1078.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bengal E, Aviram S and Hayek T: p38 MAPK
in glucose metabolism of skeletal muscle: Beneficial or harmful?
Int J Mol Sci. 21:64802020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng J, Lu S, Ou B, Liu Q, Dai J, Ji C,
Zhou H, Huang H and Ma Y: The role of JNk signaling pathway in
obesity-driven insulin resistance. Diabetes Metab Syndr Obes.
13:1399–1406. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cross DA, Alessi DR, Cohen P, Andjelkovich
M and Hemmings BA: Inhibition of glycogen synthase kinase-3 by
insulin mediated by protein kinase B. Nature. 378:785–789. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taniguchi CM, Emanuelli B and Kahn CR:
Critical nodes in signalling pathways: Insights into insulin
action. Nat Rev Mol Cell Biol. 7:85–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo S: Molecular basis of insulin
resistance: The role of IRS and Foxo1 in the control of diabetes
mellitus and its complications. Drug Discov Today Dis Mech.
10:e27–e33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yūko K: The spread of tea from Taiwan and
the Chinese distribution network in colonial java. Mem Res Dep Toyo
Bunko. 77:39–64. 2019.
|
|
17
|
Aboulwafa MM, Youssef FS, Gad HA, Altyar
AE, Al-Azizi MM and Ashour ML: A comprehensive insight on the
health benefits and phytoconstituents of Camellia sinensis
and recent approaches for its quality control. Antioxidants
(Basel). 8:4552019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng W and Endo Y: Lipid characteristics
of camellia seed oil. J Oleo Sci. 68:649–658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen J, Zhang Z, Tian B and Hua Y:
Lipophilic phenols partially explain differences in the antioxidant
activity of subfractions from methanol extract of camellia oil. Eur
Food Res Technol. 235:1071–1082. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JS, Yeom MH, Park WS, Joo KM, Rho HS,
Kim DH and Chang IS: Enzymatic hydrolysis of green tea seed extract
and its activity on 5alpha-reductase inhibition. Biosci Biotechnol
Biochem. 70:387–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeh TM, Chang CD, Liu SS, Chang CI and
Shih WL: Tea seed kaempferol triglycoside attenuates LPS-induced
systemic inflammation and ameliorates cognitive impairments in a
mouse model. Molecules. 27:20552022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen FC, Shen KP, Ke LY, Lin HL, Wu CC and
Shaw SY: Flavonoids from Camellia sinensis (L.) O. Kuntze
seed ameliorates TNF-α induced insulin resistance in HepG2 cells.
Saudi Pharm J. 27:507–516. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu G, Chen K, Wang J, Wang M, Li R, Wu X,
Wu C, Zhang P, Liu C and Wan Y: Screening of tea saponin-degrading
strain to degrade the residual tea saponin in tea seed cake. Prep
Biochem Biotechnol. 50:697–707. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chaicharoenpong C: Tea (Camellia
oleifera) seeds: Use of tea seeds in human health. Nuts and
Seeds in Health and Disease Prevention. Elsevier; pp. 299–313.
2020, View Article : Google Scholar
|
|
25
|
Ohta T, Nakamura S, Matsumoto T, Nakashima
S, Ogawa K, Matsumoto T, Fukaya M, Yoshikawa M and Matsuda H:
Chemical structure of an acylated oleanane-type triterpene
oligoglycoside and anti-inflammatory constituents from the flower
buds of Camellia sinensis. Nat Prod Commun. 12:1193–1196.
2017.
|
|
26
|
Fan L, He Y, Xu Y, Li P, Zhang J and Zhao
J: Triterpenoid saponins in tea (Camellia sinensis) plants:
Biosynthetic gene expression, content variations, chemical
identification and cytotoxicity. Int J Food Sci Nutr. 72:308–323.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Diwan F, Abdel Hassan I and Mohammed S:
Effect of saponin on mortality and histopathological changes in
mice. East Mediterr Health J. 6:345–351. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu Z, Hong H, Xing N, Xi H and Jiang H:
Comparison of bio-fermentation and chemical method in the
improvement of the quality of oil-tea Camellia seed meal. China
Oils Fats. 35:40–43. 2010.
|
|
29
|
Lai LR, Hsieh SC, Huang HY and Chou CC:
Effect of lactic fermentation on the total phenolic, saponin and
phytic acid contents as well as anti-colon cancer cell
proliferation activity of soymilk. J Biosci Bioeng. 115:552–556.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagy M and Grancai D: Colorimetric
determination of flavanones in propolis. Pharmazie. 51:100–101.
1996.
|
|
31
|
Woisky RG and Salatino A: Analysis of
propolis: Some parameters and procedures for chemical quality
control. J Apic Res. 37:99–105. 1998. View Article : Google Scholar
|
|
32
|
Zhang Y, Yan LS, Ding Y, Cheng BCY, Luo G,
Kong J, Liu TH and Zhang SF: Edgeworthia gardneri (Wall.) Meisn.
water extract ameliorates palmitate induced insulin resistance by
regulating IRS1/GSK3β/FoxO1 signaling pathway in human HepG2
hepatocytes. Front Pharmacol. 10:16662020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alnahdi A, John A and Raza H: Augmentation
of glucotoxicity, oxidative stress, apoptosis and mitochondrial
dysfunction in HepG2 cells by palmitic acid. Nutrients.
11:19792019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cholkar K, Ray A, Agrahari V, Pal D and
Mitra AK: Transporters and receptors in the anterior segment of the
eye. Ocular Transporters and Receptors. Elsevier; pp. 115–168.
2013, View Article : Google Scholar
|
|
35
|
Croniger CM, Olswang Y, Reshef L, Kalhan
SC, Tilghman SM and Hanson RW: Mini-series: Modern metabolic
concepts phosphoenolpyruvate carboxykinase revisited. Biochem Mol
Biol Educ. 30:14–20. 2002. View Article : Google Scholar
|
|
36
|
Chen Y, Gao Y, Han Z and Yin J: Analysis
of the saponin contents and composition in tea seeds of different
germplasms. J Tea Sci. 42:705–716. 2022.
|
|
37
|
Soltani M, Parivar K, Baharara J,
Kerachian MA and Asili J: Hemolytic and cytotoxic properties of
saponin purified from Holothuria leucospilota sea cucumber. Rep
Biochem Mol Biol. 3:43–50. 2014.PubMed/NCBI
|
|
38
|
Dong Z, Sun T, Liang L and Wang L: Effect
of tea saponin on ephyrae and polyps of the moon jellyfish Aurelia
sp.1. PLoS One. 12:e01827872017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahmed H, Mariod A and Hammoda T: The
chronic toxicity studies of camellia seed oil containing tea
saponins on mice blood and organs. Int J Life Sci Biotechnol.
4:178–191. 2021. View Article : Google Scholar
|
|
40
|
Waheed Janabi AH, Kamboh AA, Saeed M,
Xiaoyu L, BiBi J, Majeed F, Naveed M, Mughal MJ, Korejo NA, Kamboh
R, et al: Flavonoid-rich foods (FRF): A promising nutraceutical
approach against lifespan-shortening diseases. Iran J Basic Med
Sci. 23:140–153. 2020.PubMed/NCBI
|
|
41
|
Termkwancharoen C, Malakul W,
Phetrungnapha A and Tunsophon S: Naringin ameliorates skeletal
muscle atrophy and improves insulin resistance in
high-fat-diet-induced insulin resistance in obese rats. Nutrients.
14:41202022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wen L, Wu D, Tan X, Zhong M, Xing J, Li W,
Li D and Cao F: The role of catechins in regulating diabetes: An
update review. Nutrients. 14:46812022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Russo B, Picconi F, Malandrucco I and
Frontoni S: Flavonoids and insulin-resistance: From molecular
evidences to clinical trials. Int J Mol Sci. 20:20612019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Al-Ishaq RK, Abotaleb M, Kubatka P, Kajo K
and Büsselberg D: Flavonoids and their anti-diabetic effects:
Cellular mechanisms and effects to improve blood sugar levels.
Biomolecules. 9:4302019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gołąbek KD and Regulska-Ilow B: Dietary
support in insulin resistance: An overview of current scientific
reports. Adv Clin Exp Med. 28:1577–1585. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Carta G, Murru E, Banni S and Manca C:
Palmitic acid: Physiological role, metabolism and nutritional
implications. Front Physiol. 8:9022017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sánchez-Alegría K, Bastián-Eugenio CE,
Vaca L and Arias C: Palmitic acid induces insulin resistance by a
mechanism associated with energy metabolism and calcium entry in
neuronal cells. FASEB J. 35:e217122021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Brown G: Glucose transporters: Structure,
function and consequences of deficiency. J Inherit Metab Dis.
23:237–246. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
D'Alessandris C, Lauro R, Presta I and
Sesti G: C-reactive protein induces phosphorylation of insulin
receptor substrate-1 on Ser307 and Ser 612 in L6 myocytes, thereby
impairing the insulin signalling pathway that promotes glucose
transport. Diabetologia. 50:840–849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
White MF: Insulin signaling in health and
disease. Science. 302:1710–1711. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Koren-Gluzer M, Aviram M and Hayek T:
Paraoxonase1 (PON1) reduces insulin resistance in mice fed a
high-fat diet, and promotes GLUT4 overexpression in myocytes, via
the IRS-1/Akt pathway. Atherosclerosis. 229:71–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Petersen MC, Vatner DF and Shulman GI:
Regulation of hepatic glucose metabolism in health and disease. Nat
Rev Endocrinol. 13:572–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ren Z, Xie Z, Cao D, Gong M, Yang L, Zhou
Z and Ou Y: C-Phycocyanin inhibits hepatic gluconeogenesis and
increases glycogen synthesis via activating Akt and AMPK in insulin
resistance hepatocytes. Food Funct. 9:2829–2839. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hatting M, Tavares CDJ, Sharabi K, Rines
AK and Puigserver P: Insulin regulation of gluconeogenesis. Ann N Y
Acad Sci. 1411:21–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Murray B and Rosenbloom C: Fundamentals of
glycogen metabolism for coaches and athletes. Nutr Rev. 76:243–259.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sullivan MA and Forbes JM: Glucose and
glycogen in the diabetic kidney: Heroes or villains? EBioMedicine.
47:590–597. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kamiyama M, Naguro I and Ichijo H: In vivo
gene manipulation reveals the impact of stress-responsive MAPK
pathways on tumor progression. Cancer Sci. 106:785–796. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yudhani RD, Sari Y, Nugrahaningsih DAA,
Sholikhah EN, Rochmanti M, Purba AKR, Khotimah H, Nugrahenny D and
Mustofa M: In vitro insulin resistance model: A recent update. J
Obes. 2023:19647322023. View Article : Google Scholar : PubMed/NCBI
|