Stroke is a leading cause of human death and
disability, and poses a major threat to humans (1). In total, ~85% of stroke are caused by
cerebral ischemia and 15% are caused by cerebral hemorrhage
(2). Cerebral ischemia is the
result of a lack of blood supply due to occlusion of the cerebral
arteries, which results in a lack of glucose and oxygen supply to
all brain cells. Therefore, lack of blood in the brain disturbs
intracellular homeostasis, which causes inflammation, oxidative
damage, excitotoxicity and finally the death of brain cells
(3). Thrombolysis to restore blood
supply to the brain is currently a viable treatment option for
(4). However, rapid reperfusion
can lead to further damage to areas of the brain, a condition known
as cerebral ischemia/reperfusion injury (CIRI) (5,6).
Nevertheless, there are a number of possible mechanisms by which
CIRI can occur, including inflammatory response (7), Ca2+ overload (8), overproduction of reactive oxygen
species (ROS) (9), neuronal damage
caused by glutamate (10) and
mitochondria induced-autophagy (11). Of these mechanisms,
neuroinflammation serves a key role in CIRI, including via local
cytokine upregulation and leukocyte infiltration (12).
Inflammasomes are protein complexes, and potent
substances that activate inflammatory mediators, which was first
proposed by Martinon et al (13) in 2002. Inflammasomes are part of
the innate immune response of the body against pathogen invasion,
inflammasomes are activated by cellular infection or stress
stimulation and induce the expression, maturation and release of
various pro-inflammatory cytokines like IL-18 and IL-1β, thereby
triggering a range of inflammatory responses (14,15).
Inflammasomes are mainly composed of the nucleotide-binding
oligomeric domain-like receptor (NLR) family, which can be divided
into three subfamilies: The NLRP, nucleotide-binding
oligomerization domains (NODs) and the ice protease-activating
factor (IPAF) subfamilies, including NLR family apoptosis
inhibitory protein and IPAF (14).
The NOD-like receptor thermal protein domain
associated protein 3 (NLRP3) inflammasome is the most widely
studied inflammasomes and contains NLRP3, Pro-caspase-1 and
apoptosis-associated spot-like protein (ASC) (16–18)
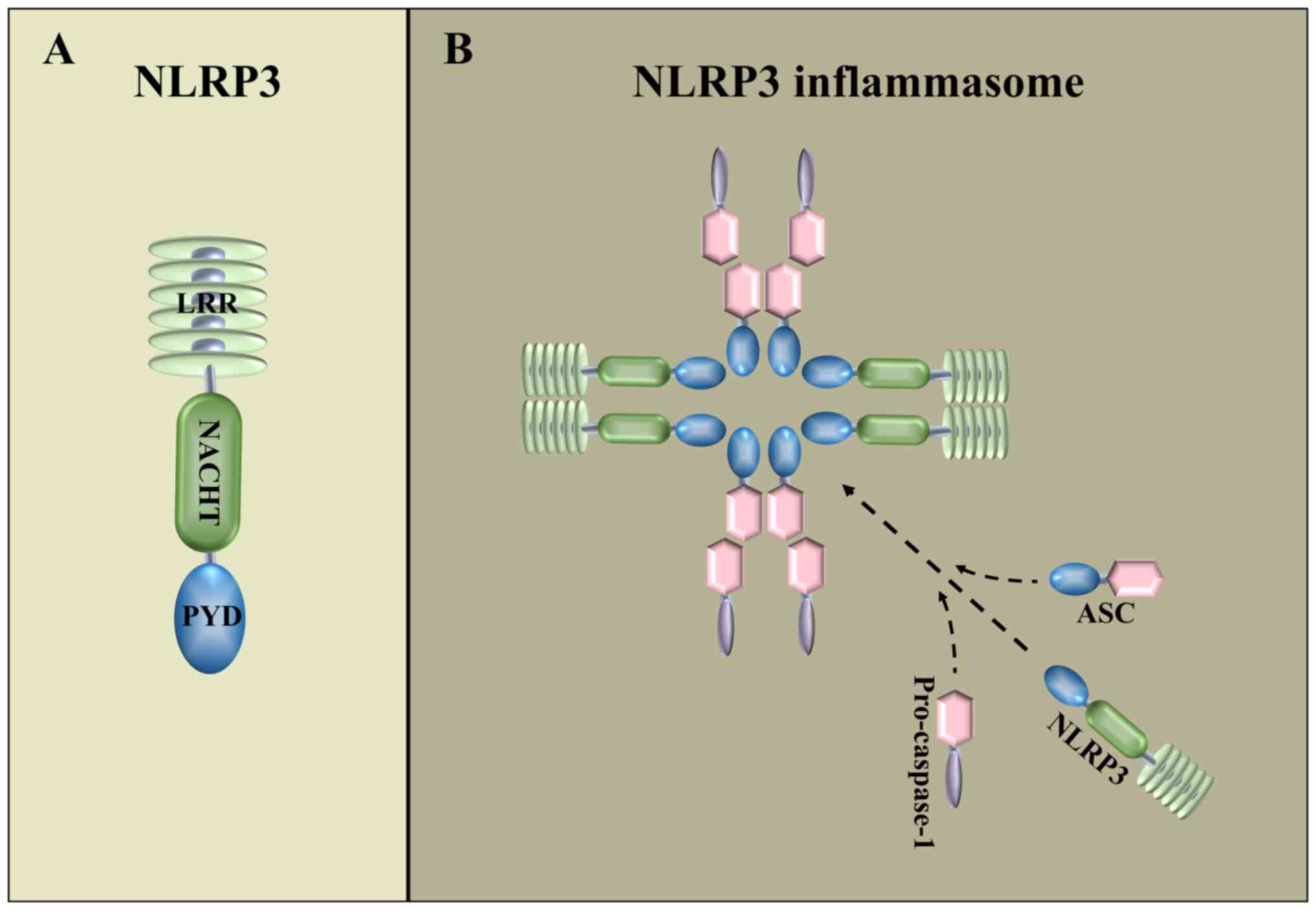
(Fig. 1B). NLRP3 consists of an
amino-terminal pyrin domain structural domain, a central
nucleotide-binding structural domain and an oligomeric structural
domain (19) (Fig. 1A). NLRP3 inflammasome assembly is
initiated by the interaction of the pyrin structural domain of
NLRP3 with the pyrin structural domain of ASC (20). The NLRP3 inflammasome serves a key
role in the innate immune system, and activation of the NLRP3
inflammasome mediates the activation of downstream caspase-1 and
secretion of the pro-inflammatory cytokines, IL-1β and IL-18, in
response to microbial invasion and cellular damage (21). The NLRP3 inflammasome can be
activated by different stimuli, including damage-associated
molecular patterns (DAMPS) and pathogen-associated molecular
patterns (PAMPs) (16). DAMPS are
regulated by the pro-inflammatory pathway, such as toll-like
receptor (TLR)/NF-κB) signaling pathway, which increases NLRP3 and
IL-1β protein expression (22,23)
and reduces the activation threshold of NLRP3 through additional
post-translational modifications (24–26).
PAMPs include Ca2+ signaling disruption, mitochondrial
dysfunction, ROS production, K+ efflux and lysosomal
rupture, promoting assembly of the inflammasome and activating
caspase-1, which catalyzes the conversion of pro-IL-1β to active
IL-1β (27,28).
As a novel form of programmed cell death, pyroptosis
is mainly induced by the gasdermin (GSDM) family (29,30).
Of the six members of the GSDM family, five are closely related to
pyroptosis and these are GSDMA, GSDMB, GSDMC, GSDMD and GSDME
(31). Members of the GSDM family
have highly conserved N-terminal and C-terminal domains, and the
N-terminal domain can form pores in the cell membrane, causing
pyroptosis (32). Activation of
inflammasomes can mediate the scission of GSDMD by caspase, which
results in formation of GSDMD-N-terminal and finally leads to
pyroptosis (33). In addition,
pyroptosis is also a crucial pathophysiological process in ischemic
stroke (34).
Oxidative stress is known to be implicated in the
pathogenesis of CIRI, and a study has demonstrated that oxidative
stress serves an important role in the prevention and treatment of
ischemic stroke by regulating the level of inflammation (43). Oxidative stress can produce ROS.
ROS are radicals containing oxygen atoms, and include
H2O2, O2− and OH−. ROS
are mainly derived from the mitochondria and can also be produced
by cellular enzymes, including lipoxygenase and cyclooxygenase,
which are responsible for inflammasome activation (44). CIRI takes place when the tissue
damage caused by restoration of the blood supply to the tissue
after a period of ischemia causes tissue damage. This
reconstitution of blood flow causes accumulation of ROS,
disturbance of cellular ion homeostasis and induce inflammatory
response, thereby triggering further damage to ischemic tissues
(44). In particular, ROS induce
NLRP3 inflammasome activation and stimulate tissue inflammation
during CIRI (44,45). Furthermore, ROS have been
demonstrated to be a proximal signal for NLRP3 inflammasome
activation in inflammatory diseases including CIRI, renal and
cardiac ischemia-reperfusion (46). Pro-oxidant and pro-inflammatory
thioredoxin-interacting protein (TXNIP), a key regulator of ROS, is
associated with inflammation (47). TXNIP is required for NLRP3
activation, which leads to the initiation or worsening of the
disease state (48). The increase
in ROS generation leads to the upregulation of thioredoxin, TXNIP
recruitment of NLRP3 and NLRP3 activation (49). TXNIP is activated by ROS and
promotes NLRP3 inflammasome activation by binding to NLRP3
following ischemic stroke (Fig.
2), and inhibition of TXNIP expression reduces inflammasome
activation after ischemic stroke (49,50).
Mitochondria also serve an important role in the regulation of ROS.
Uncoupling protein 2 (UCP2) is an inner membrane protein of the
mitochondria that has been reported to regulate mitochondrial
potential and ROS production (51,52).
At present, there is a study has reported that UCP2 serves an
important role in CIRI. UCP2 deficiency aggravates
hyperglycemia-induced CIRI by enhancing NLRP3 inflammasome
activation and ROS generation (53). Since ROS are an important activator
of NLRP3 following CIRI, strategies that eliminate excessive ROS
may be effective therapeutic approaches for ischemic stroke.
TLR4 is a transmembrane receptor protein of the
innate immune system that is upregulated following CIRI (54). Upregulation of TLR4 activates
NF-κB, which induces the release of number of proinflammatory
factors such as IL-18 and IL-1β, triggering an inflammatory
response and leading to brain injury (54). Microglia are intrinsic myeloid
cells of the central nervous system and are involved in the
development of CIRI. For macrophages or microglia, the presence of
an NLRP3 activator alone is not sufficient to induce inflammasome
activation, and its activation requires initiation signals
(55). NLRP3 inflammasome
activation must first be induced by initiating stimuli, such as
ligands for TLRs, NLRs (such as NOD1 and NOD2) or cytokine
receptors, which activate the transcription factor NF-κB and
upregulate NLRP3 and IL-1β expression (55). Previous studies have demonstrated
that activation of the TLR4/NF-κB signaling pathway is a
fundamental step in the formation of the NLRP3 inflammasome and is
closely associated with activation of the NLRP3 inflammasome
(56,57). TLR4 serves an important role in
CIRI and is widely expressed in the brain, especially in microglia
and endothelial cells (58,59).
Furthermore, inhibition of the TLR4/NF-κB signaling pathway can
reduce CIRI by regulating the inflammatory response and apoptosis
(60). Collectively, the
aforementioned studies have demonstrated that TLR4 activation is a
key factor in the upregulation of NLRP3 expression following CIRI,
implying that targeting TLR4 or its downstream proteins is likely
to be an effective treatment for ischemic stroke (Fig. 2).
Autophagy acts as a stable self-sustaining process
in numerous physiological and pathological processes of eukaryotic
cells. In this process, bilayers encapsulate pathogens, abnormal
proteins and organelles to form autophagosomes, which are
transferred to lysosomes for degradation (61). Autophagy can be classified as
macroautophagy, microautophagy and chaperone-mediated autophagy
depending on the duration of action, the inducing signal, the type
of target and the transit pathway into the lysosome (16,62).
Macroautophagy involves the formation of double-membrane vesicles
that separate the cytoplasm. These intact vesicles, termed
autophagic vesicles, then fuse with lysosomes for subsequent
degradation (63,64). In microautophagy, the material to
be degraded reaches the lysosomal lumen via lysosomal invagination
or the endoplasmic membrane (65,66).
Chaperone-mediated autophagy only occurs in mammalian cells and
allows for the selective degradation of proteins with specific
amino acid sequences (67). Among
these three autophagic processes, macroautophagy, commonly termed
autophagy, is the most active form and has been extensively studied
in disease (68,69). Conserved proteins such as Beclin1,
LC3 and P62 are involved in the autophagic process and are
considered autophagy-related proteins (63). Autophagy is affected by various
parameters such as endoplasmic reticulum stress (ERS), ROS,
nutritional deficiencies, immune or inflammatory stimuli,
accumulation of organelle damage, and the Ca2+
concentration (70,71). Under physiological conditions,
autophagy is typically maintained at basal levels. However, in
pathological states, upregulated autophagy removes dysfunctional
proteins from cells and aids cell survival (72). Autophagy can inhibit NLRP3
activation by reducing ASC expression, increasing phosphorylation
of NLRP3 and scavenging ROS (16).
The cytoplasmic protein, activating transcription factor 4 (ATF4),
serves an important role in the regulation of autophagy, and ATF4
is a member of the activating transcription factor/cAMP response
element binding protein family (73). As a transcription factor, ATF4 was
involved in Endoplasmic reticulum (ER) homeostasis, autophagy and
inflammation response (73). In
addition, ATF4 inhibits the NLRP3 inflammasome-mediated
inflammatory response via upregulation of Parkin-dependent
mitochondrial autophagy in CIRI (74). Finally, autophagy can target the
degradation of IL-1β, inhibit activation of the NLRP3 inflammasome
and reduce the release of inflammatory cytokines (75,76).
Thus, autophagy has been shown to negatively regulate the NLRP3
inflammasome activation and effectively reduce CIRI (Fig. 2).
In addition to the aforementioned three methods of
activation, NLRP3 can also be activated by other pathways following
CIRI. For example, there is evidence that the α7 nicotinic
acetylcholine receptor (α7nAChR) is critical in mediating
cholinergic anti-inflammatory signaling (77). Electroacupuncture promotes
α7nAChR-mediated inhibition of the NLRP3 inflammasome, thereby
reducing CIRI and neuroinflammation (78), which implies that α7nAChR may be an
upstream signal for NLRP3 activation. ERS is severe in ischemic
brain injury and leads to an inflammatory response via activation
of caspase-12 (79). In a previous
study, pretreatment with the caspase-12 specific inhibitor
Z-ATAD-FMK attenuated cell injury and apoptosis, and reduced the
levels of NLRP3, caspase-1, IL-1β and cleaved caspase-3 compared
with oxygen-glucose deprivation/recovery (OGD/R) group (79). Therefore, the NLRP3 inflammasome
signaling pathway may be inhibited by suppression of caspase-12
signaling to attenuate CIRI. In addition, electroacupuncture
induces upregulation of neuronal cylindromatosis (CYLD) expression,
which exerts anti-inflammatory and neuroprotective effects by
inhibiting NLRP3 expression, regulates the interaction between
neurons and microglia, reduces M1 microglia in the peri-ischemic
cortex, and improves the activation of M2 microglia, thereby
reducing CIRI (80). Collectively,
the above studies demonstrated that both α7nAChR and CYLD can
inhibit NLRP3 inflammasome activation, while ERS-mediated
caspase-12 activation can upregulate NLRP3 expression (Fig. 2).
Activation of the NLRP3 inflammasome promotes the
release of downstream inflammatory factors and facilitates
pyroptosis in CIRI. Activation of inflammasomes has been associated
with various inflammatory diseases, including post-ischemic
inflammation following ischemic stroke (12). Inflammasomes mediate the activation
of caspase-1, which in turn induces the secretion of
pro-inflammatory cytokines and pyroptosis (81). Caspase-1 is activated upon
recruitment to the inflammasome, then activated caspase-1 cleaves
the cytokines Pro-IL-1β and Pro-IL-18 into their mature bioactive
forms (13,82). IL-1β controls fever, pain
threshold, vasodilation, and hypotension, and promotes immune cell
infiltration into infected or damaged tissues (83). IL-18 is required for production of
IFN-γ, a costimulatory cytokine that mediates adaptive immunity
(84). CIRI activates NLRP3,
induces the release of IL-1β and IL-18 and promotes maturation of
GSDMD-N, and leads to severe neuronal pyroptosis (85). Previous studies have demonstrated
that the expression levels of GSDMD-N, NLRP1/3, IL-1β and IL-18 in
Sprague-Dawley rats and mice were increased following CIRI compared
with the Sham group, and intervention treatment of these
inflammatory factors attenuated CIRI (86–90).
In another study, the mRNA expression levels of NLRP3, caspase-1,
IL-1β, IL-6 and TNF-α were increased in microglia after OGD/R
treatment compared with the control group (91). Overall, NLRP3 inflammasome
activation promotes the release of downstream inflammatory factors
and causes GSDMD-mediated pyroptosis following CIRI (Fig. 2).
During CIRI, ROS stimulate tissue inflammation and
activate the NLRP3 inflammasome. Inflammatory diseases are often
characterized by the activation of the NLRP3 inflammasome, which is
primarily triggered by ROS. Therefore, inhibiting the production of
ROS or increasing their consumption following CIRI could be a
viable treatment option for stroke (92). In a study by Cao et al
(93), it was demonstrated that
ruscogenin reduced ROS levels following CIRI, which in turn
inhibited TXNIP/NLRP3 inflammasome activation and mitigated
ischemia-induced blood-brain barrier dysfunction. Additionally,
astilbin has been reported to reduce the brain infarct volume and
alleviate neurological deficits in middle cerebral artery occlusion
(MCAO) rats (94). Furthermore,
astilbin has been demonstrated to inhibit cellular inflammation
induced by OGD/R by suppressing the activation of the ROS-NLRP3
inflammasome axis (94).
Cepharanthine has also been demonstrated to reduce CIRI by
inhibiting the 12/15-lipoxygenase signaling pathway, leading to a
decrease in ROS and the downregulation of NLRP3 expression
(95). In addition, ATN-161 has
been indicated to exert a protective effect on cells by reducing
the levels of mitochondrial superoxide radicals, thereby
alleviating oxidative stress and intracellular ROS during the onset
of CIRI (96). However, tomentosin
promotes the production of superoxide dismutase in rats during
CIRI, which scavenges free radicals, accelerates the antioxidant
system, inhibits NLRP3 signaling and attenuates CIRI (97). Oleanolic acid (OA) has been
demonstrated to reduce microglia activation and ROS in CIRI,
suggesting that OA may exert neuroprotective effects on ischemic
stroke by inhibiting NLRP3 inflammasome activation through the
reduction of ROS (98). The
aforementioned studies have demonstrated that decreasing ROS levels
can mitigate the harm caused by CIRI or cellular OGD/R treatment.
Therefore, inhibiting the ROS may be a viable option for the
treatment of stroke (Table I).
TLR4 is an important factor in CIRI, and its
downstream NF-κB signaling pathway is crucial in the formation of
the NLRP3 inflammasome and is closely linked to its activation
(54). Inhibiting the TLR4/NF-κB
signaling pathway at the onset of CIRI may be a viable treatment
option for stroke (54). Cui et
al (99) conducted a study on
anthocyanin derived from Myrica rubra, and revealed that
treatment of ischemia/reperfusion (I/R) mice with purified
anthocyanin extracts for 1 week resulted in a decrease in brain
infarct volume, disease damage, and nitric oxide and
malondialdehyde levels, while superoxide dismutase levels were
increased compared with Sham group (99). In addition, treatment with
meisoindigo resulted in improvements in neurological scores,
reduced infarct volume and decreased brain edema in MCAO/R mice
compared with Sham group. Further analysis revealed that
meisoindigo inhibited the expression of TLR4/NF-κB signaling
pathway-related proteins in a dose-dependent manner. This
inhibition led to the downregulation of NLRP3, high mobility group
box 1 and IL-1β expression (100). D-carvone has been reported to
inhibit the TLR4-induced signaling pathway of inflammatory
cytokines and reduce NLRP3 expression, leading to the successful
amelioration of I/R-induced neuroinflammation in the brains of
rats. As a result, I/R-induced brain injury in the hippocampal and
cortical regions was attenuated (101). Exosomes treated with melatonin
have been shown to effectively reduce the infarct size and improve
functional recovery by modulating the TLR4/NF-κB signaling pathway
and reducing NLRP3-induced inflammation following CIRI (102). Additionally, tomentosin treatment
enhances antioxidant capacity to reduce ROS levels, while also
reducing the expression of TLR4 and its downstream pro-inflammatory
cytokines. This ultimately inhibits NLRP3 expression and attenuates
CIRI (97). Vinpocetine has been
revealed to inhibit the NF-κB pathway-related proteins, which in
turn downregulates NLRP3 expression levels. This inhibition leads
to a reduction in the release of pro-inflammatory cytokines,
resulting in a decrease in the size of cerebral infarcts and an
improvement in behavioral recovery in MCAO mice (103). Salidroside has been demonstrated
to reverse NLRP3 inflammasome activation, resulting in
downregulated levels of NLRP3, ASC, caspase-1, IL-1β and IL-18
proteins, as well as the suppression of key components of the TLR4
signaling pathway in BV2 cells following OGD/R (104). The specific TLR4 inhibitor,
TAK242, exhibited the same effect as salidroside on BV2 cells
following OGD/R induction, indicating that salidroside has the
capability to specifically inhibit the TLR4/NF-κB signaling
pathway, reducing NLRP3 expression and attenuating CIRI (104). Curcumin has been demonstrated to
attenuate white matter damage caused by stroke to some extent by
inhibiting the NF-κB/NLRP3 signaling pathway, improving functional
outcomes and reducing microglia apoptosis (105). In summary, the aforementioned
studies demonstrated that inhibiting the TLR4/NF-κB signaling
pathway through pharmacological treatment can effectively suppress
the expression and activation of NLRP3, thereby reducing the
inflammatory response and cellular damage caused by CIRI.
Furthermore, inhibiting the upregulation of NLRP3 expression
mediated by TLR4 may be a viable clinical treatment option for
stroke (Table I).
Autophagy serves a crucial role in various
pathophysiological processes such as renal and cardiac
ischemia-reperfusion and CIRI. In pathological conditions,
autophagy can hinder the activation of the NLRP3 inflammasome by
eliminating endogenous inflammasome activators such as ROS,
cytokines and damaged mitochondria from inflammatory components.
Inducing cellular autophagy through pharmacological intervention
during the onset of CIRI may be a viable option for treating
patients following ischemic stroke (106). Exosomes secreted from bone marrow
mesenchymal stem cells (BMSC-Exos) have been found to increase
autophagic flux in PC12 cells treated with OGD/R, while also
inhibiting OGD/R-induced pyroptosis (107). Experimental data further
indicated that BMSC-Exos treatment led to decreased NLRP3
expression, as well as elevated LC3 II/I and
phosphorylated-AMPK)/AMPK levels (107). These findings suggested that
BMSC-Exos promoted autophagic flux in PC12 cells via the AMPK/mTOR
signaling pathway, while also inhibiting NLRP3
inflammasome-mediated pyroptosis (107). As a result, BMSC-Exos offer
protective benefits to PC12 cells, shielding the cells from OGD/R
injury (107). In a similar
study, it was identified that human umbilical cord mesenchymal stem
cell-derived exosomes (MSC-Exos) had a positive impact on BV2 cell
viability following OGD/R (108).
Additionally, the expression levels of NLRP3, cleaved caspase-1 and
GSDMD-N, as well as the release of IL-1β and IL-18, were decreased,
while translocase of outer mitochondrial membrane 20 and cytochrome
c oxidase subunit 4 isoform 1 expression was increased. However,
the neuroprotective effect of MSC-Exos was partially abolished by
FOXO3a small interfering RNA treatment, which also attenuated the
inhibition of mitochondrial phagocytosis and pyroptosis induced by
MSC-Exos treatment. This study suggests that FOXO3a expression is
increased by MSC-exos, which in turn enhances mitochondrial
autophagy in microglia. MSC-Exos treatment inhibits pyroptosis
induced by CIRI and ultimately reduces nerve damage (108). Pien-Tze-Huang has been
demonstrated to regulate essential autophagic proteins via the
AMPK/mTOR/unc-51 like autophagy activating kinase 1 (ULK1)-related
signaling pathway. This regulation enhances the autophagic response
and inhibits the production of key pro-inflammatory mediators, as
well as the expression of NLRP3 and caspase-1 p20 proteins in
lipopolysaccharide-induced BV2 cells. These findings suggest that
Pien-Tze-Huang may enhance autophagy following CIRI via the
AMPK/mTOR/ULK1 signaling pathway, thereby reducing NLRP3-associated
neuroinflammation (109). In
summary, the aforementioned studies all indicated that activation
of the NLRP3 inflammasome can be effectively suppressed by
enhancing autophagy-mediated inhibition of NLRP3 activation. This
in turn can lead to a protection in CIRI (Table I).
In addition to inhibiting the activation of the
NLRP3 inflammasome by reducing ROS, regulating the TLR4/NF-κB
pathway or enhancing autophagy, there are a number of other
therapeutic strategies available to inhibit NLRP3 through different
signaling pathways (Table I). The
NLRP3 inflammasome serves a crucial role in regulating the release
of inflammatory factors and GSDMD-mediated pyroptosis in CIRI.
Inhibiting NLRP3 activation or expression can effectively reduce
the injury caused by CIRI (18).
It has been demonstrated that Qingkailing can effectively reduce
the inflammatory response following CIRI, which is achieved by
inhibiting AMPK-mediated NLRP3 activation and in turn attenuating
CIRI (110). Similarly,
astragaloside IV has been demonstrated to alleviate CIRI by
inhibiting NLRP3 inflammasome-mediated apoptosis through the
activation of nuclear factor erythroid 2-related factor 2 (Nrf2)
(111). Additionally,
electroacupuncture has been demonstrated to promote α7nAChR and
CYLD-mediated inhibition of the NLRP3 inflammasome, thereby
reducing CIRI and neuroinflammation (78,80).
The use of vagus nerve stimulation (VNS) treatment has been found
to inhibit expression of pyroptosis-related molecules, as well as
reduce the number of pyrogenic cells and membrane pores. Notably,
α7nAChR agonists have been found to mimic the neuroprotective
effects of VNS, which suggests that VNS serves a protective role in
CIRI by promoting α7nAChR inhibition of NLRP3-mediated pyroptosis
(112). The caspase-12-specific
inhibitor, Z-ATAD-FMK, has been reported to reduce cell injury and
apoptosis in an OGD/R treatment group by inhibiting the activation
of NLRP3. This inhibition also resulted in decreased levels of
caspase-1, IL-1β and cleaved caspase-3 compared with control group,
indicating that CIRI could be alleviated by inhibiting caspase-12
(79). Gualou Guizhi granule
(GLGZG) has been found to effectively reduce CIRI by activating the
PI3K/AKT signaling pathway and inhibiting cellular pyroptosis.
Additionally, GLGZG suppresses NLRP3 expression and the release of
its downstream inflammatory factors (113). Another study found that
Tongxinluo can inhibit the pyroptosis of astrocytes during the
onset of CIRI. Furthermore, Tongxinluo reduces the expression of
NLRP3, caspase-11/1, IL-1β and IL6, and attenuates CIRI by
decreasing the accumulation of amyloid-β peptide (114). Icariin has been demonstrated to
reduce NLRP3 expression by inhibiting the inositol-requiring enzyme
1/X-box binding protein 1 signaling pathway, which decreases the
expression of downstream inflammatory factors, reducing pyroptosis
and attenuating CIRI (91). In
addition, remimazolam has been reported to downregulate the
expression of NLRP3 and its associated released inflammatory
factors IL-18 and IL-1β, as well as GSDMD, in MCAO rats. This
suggests that remimazolam may serve a protective role against CIRI
by inhibiting NLRP3 (89).
Similarly, Xingxiong injection administration has been demonstrated
to activate the AKT/Nrf2 signaling pathway and inhibit the NLRP3
inflammasome during the onset of CIRI, thereby exerting a
protective effect (90).
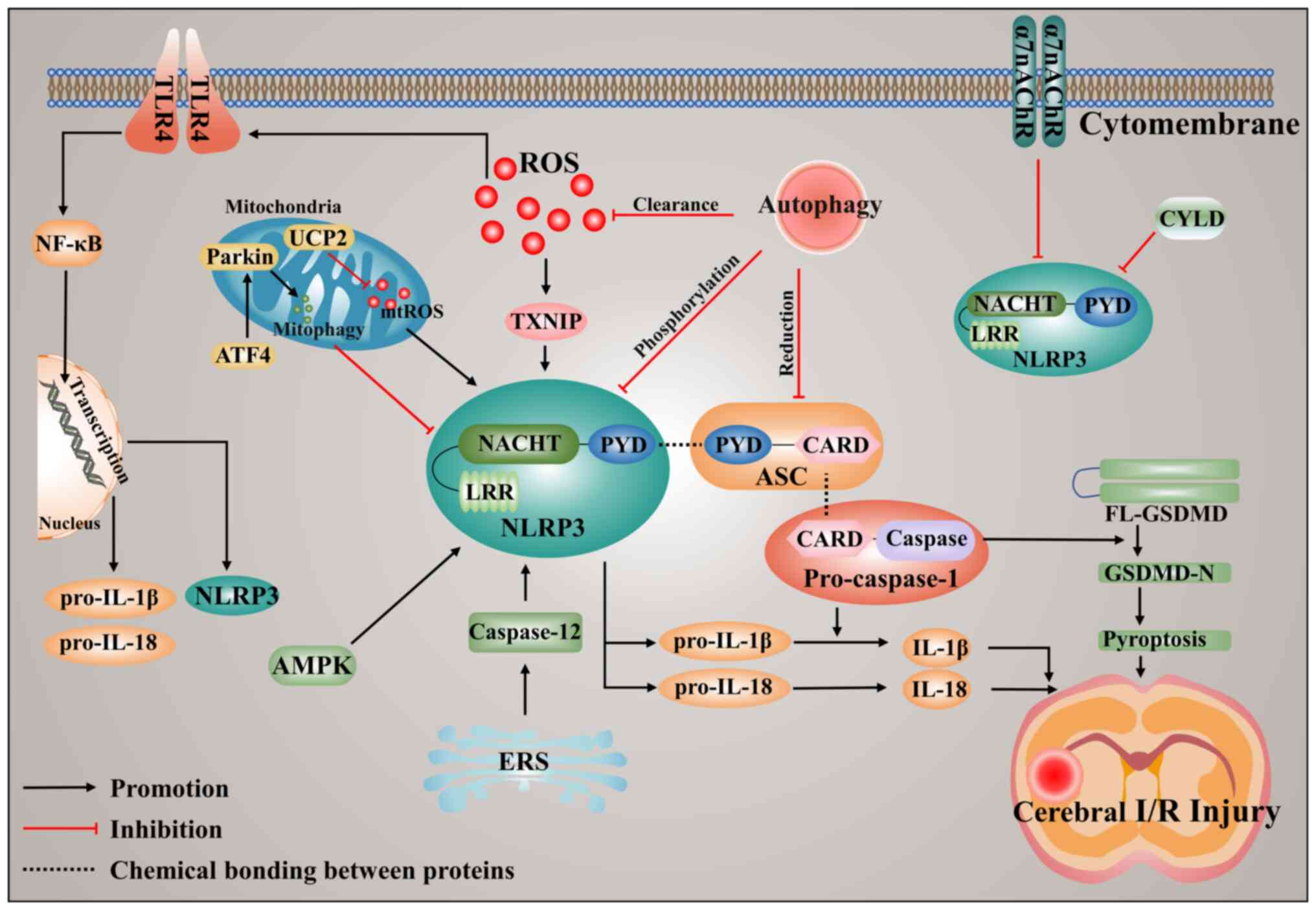
Activation of the NLRP3 inflammasome is critical for
the mechanisms of CIRI. In the present review, the mechanisms of
NLRP3 activation during the onset of CIRI are discussed and are
shown in Fig. 2. ROS and TLR4 can
promote activation of the NLRP3 inflammasome and its downstream
inflammatory response. To some extent, autophagy can negatively
regulate NLRP3 activation, which has protected CIRI. Additionally,
α7nAChR and CYLD activation can inhibit NLRP3, while caspase-12
activates the NLRP3 inflammasome. Activation of NLRP3 ultimately
leads to an inflammatory response, as well as GSDMD-mediated
pyroptosis. Furthermore, in the studies described previously have
demonstrated that specifically inhibiting the NLRP3 inflammasome
can mitigate neuroinflammation and improve outcomes following CIRI.
The present review also examines current therapeutic approaches
that aim to inhibit the NLRP3 inflammasome to reduce the
inflammatory response and pyroptosis during the onset of CIRI
(Table I). As such, the present
review offers a thorough theoretical foundation for conducting
fundamental research on CIRI. Specifically, it provides a detailed
overview of the mechanism of action of the NLRP3 inflammasome
during CIRI, which will serve as a basis for future research in
this field. It is recommended that further research also
investigates the role of the NLRP3 inflammasome in pathogenesis and
identifies novel therapies. The NLRP3 inflammasome may be
considered a crucial target for the treatment of CIRI and may
broaden the therapeutic field of ischemic stroke.
To the best of our knowledge, the present review was
the first to categorize drugs that serve a protective role in CIRI
by targeting the NLRP3 inflammasome with different molecular
mechanisms. This provides novel strategies for the clinical
treatment of ischemic stroke as well as novel ideas for other
diseases in which the NLRP3 inflammasome serves a critical role in
the pathologic process. For example, Pien-Tze-Huang is an herbal
medicine used for a variety of inflammatory diseases, whether
Pien-Tze-Huang has a protective effect in hemorrhagic stroke or in
renal ischemic reperfusion is also a question that deserves
in-depth exploration. Exploring whether drugs that are protective
in CIRI by targeting NLRP3 inflammasome also exert protective roles
in other inflammatory diseases will contribute to the greater
social value and economic benefits.
Not applicable.
This work was supported by the National Natural Science
Foundation of China (grant no. 82101410) and the Medicine and
Health Science and Technology Development Plan Project of Shandong
(grant no. 202101040805).
Not applicable.
WLD, XJW and MTH conceived the study. WLD, YPM, ZMS
and HD were involved in literature search, data collection and
writing. LYZ, BGZ and MTH reviewed and edited the manuscript. Data
authentication is not applicable. All authors have read and
approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Saini V, Guada L and Yavagal DR: Global
epidemiology of stroke and access to acute ischemic stroke
interventions. Neurology. 97 (20 Suppl 2):S6–S16. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart disease and stroke statistics-2018 update:
A report from the American heart association. Circulation.
137:e67–e492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang JL, Mukda S and Chen SD: Diverse
roles of mitochondria in ischemic stroke. Redox Biol. 16:263–275.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu D, Chen J, Wu L, Lee H, Shi J, Zhang M,
Ma Y, He X, Zhu Z, Yan F, et al: A clinically relevant model of
focal embolic cerebral ischemia by thrombus and thrombolysis in
rhesus monkeys. Nat Protoc. 17:2054–2084. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang J, Chen L, Yao ZM, Sun XR, Tong XH
and Dong SY: The role of mitochondrial dynamics in cerebral
ischemia-reperfusion injury. Biomed Pharmacother. 162:1146712023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
An H, Zhou B and Ji X: Mitochondrial
quality control in acute ischemic stroke. J Cereb Blood Flow Metab.
41:3157–3170. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Monsour M and Borlongan CV: The central
role of peripheral inflammation in ischemic stroke. J Cereb Blood
Flow Metab. 43:622–641. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ludhiadch A, Sharma R, Muriki A and Munshi
A: Role of calcium homeostasis in ischemic stroke: A review. CNS
Neurol Disord Drug Targets. 21:52–61. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng D, Liu J, Piao H, Zhu Z, Wei R and
Liu K: ROS-triggered endothelial cell death mechanisms: Focus on
pyroptosis, parthanatos, and ferroptosis. Front Immunol.
13:10392412022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chamorro Á, Dirnagl U, Urra X and Planas
AM: Neuroprotection in acute stroke: Targeting excitotoxicity,
oxidative and nitrosative stress, and inflammation. Lancet Neurol.
15:869–881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lugovaya AV, Emanuel TS, Kalinina NM,
Mitreikin VP, Artemova AV and Makienko AA: The role of autophagy in
the regulation of neuroinflammation in acute ischemic stroke
(review of literature). Klin Lab Diagn. 67:391–398. 2022.PubMed/NCBI
|
|
12
|
Jurcau A and Simion A: Neuroinflammation
in cerebral ischemia and ischemia/reperfusion injuries: From
pathophysiology to therapeutic strategies. Int J Mol Sci.
23:142021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia S, Yang H, Huang F and Fan W: Systemic
inflammation, neuroinflammation and perioperative neurocognitive
disorders. Inflamm Res. 72:1895–1907. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vringer E and Tait SWG: Mitochondria and
cell death-associated inflammation. Cell Death Differ. 30:304–312.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao S, Li X, Wang J and Wang H: The role
of the effects of autophagy on NLRP3 inflammasome in inflammatory
nervous system diseases. Front Cell Dev Biol. 9:6574782021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu J and Wu H: Structural mechanisms of
NLRP3 inflammasome assembly and activation. Annu Rev Immunol.
41:301–316. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mangan MSJ, Olhava EJ, Roush WR, Seidel
HM, Glick GD and Latz E: Targeting the NLRP3 inflammasome in
inflammatory diseases. Nat Rev Drug Discov. 17:588–606. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Xu W and Zhou R: NLRP3
inflammasome activation and cell death. Cell Mol Immunol.
18:2114–2127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vajjhala PR, Mirams RE and Hill JM:
Multiple binding sites on the pyrin domain of ASC protein allow
self-association and interaction with NLRP3 protein. J Biol Chem.
287:41732–31743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20:33282019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abais JM, Xia M, Zhang Y, Boini KM and Li
PL: Redox regulation of NLRP3 inflammasomes: ROS as trigger or
effector? Antioxid Redox Signal. 22:1111–1129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toldo S and Abbate A: The NLRP3
inflammasome in acute myocardial infarction. Nat Rev Cardiol.
15:203–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swanson KV, Deng M and Ting JP: The NLRP3
inflammasome: Molecular activation and regulation to therapeutics.
Nat Rev Immunol. 19:477–489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Y, Wang H, Kouadir M, Song H and Shi
F: Recent advances in the mechanisms of NLRP3 inflammasome
activation and its inhibitors. Cell Death Dis. 10:1282019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nunes PR, Mattioli SV and Sandrim VC:
NLRP3 activation and its relationship to endothelial dysfunction
and oxidative stress: Implications for preeclampsia and
pharmacological interventions. Cells. 10:28282021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu J and Núñez G: The NLRP3 inflammasome:
Activation and regulation. Trends Biochem Sci. 48:331–344. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Frank D and Vince JE: Pyroptosis versus
necroptosis: Similarities, differences, and crosstalk. Cell Death
Differ. 26:99–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang C and Ruan J: Mechanistic insights
into gasdermin pore formation and regulation in pyroptosis. J Mol
Biol. 434:1672972022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zou J, Zheng Y, Huang Y, Tang D, Kang R
and Chen R: The versatile gasdermin family: Their function and
roles in diseases. Front Immunol. 12:7515332021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli
VG, Wu H and Lieberman J: Inflammasome-activated gasdermin D causes
pyroptosis by forming membrane pores. Nature. 535:153–158. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Long J, Sun Y, Liu S, Yang S, Chen C,
Zhang Z, Chu S, Yang Y, Pei G, Lin M, et al: Targeting pyroptosis
as a preventive and therapeutic approach for stroke. Cell Death
Discov. 9:1552023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Franke M, Bieber M, Kraft P, Weber ANR,
Stoll G and Schuhmann MK: The NLRP3 inflammasome drives
inflammation in ischemia/reperfusion injury after transient middle
cerebral artery occlusion in mice. Brain Behav Immun. 92:223–233.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abulafia DP, de Rivero Vaccari JP, Lozano
JD, Lotocki G, Keane RW and Dietrich WD: Inhibition of the
inflammasome complex reduces the inflammatory response after
thromboembolic stroke in mice. J Cereb Blood Flow Metab.
29:534–544. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Ren W, Wu Q, Liu T, Wei Y, Ding J,
Zhou C, Xu H and Yang S: NLRP3 inflammasome activation: A
therapeutic target for cerebral ischemia-reperfusion injury. Front
Mol Neurosci. 15:8474402022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ismael S, Zhao L, Nasoohi S and Ishrat T:
Inhibition of the NLRP3-inflammasome as a potential approach for
neuroprotection after stroke. Sci Rep. 8:59712018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hong P, Li FX, Gu RN, Fang YY, Lai LY,
Wang YW, Tao T, Xu SY, You ZJ and Zhang HF: Inhibition of NLRP3
inflammasome ameliorates cerebral ischemia-reperfusion injury in
diabetic mice. Neural Plast. 2018:91635212018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ward R, Li W, Abdul Y, Jackson L, Dong G,
Jamil S, Filosa J, Fagan SC and Ergul A: NLRP3 inflammasome
inhibition with MCC950 improves diabetes-mediated cognitive
impairment and vasoneuronal remodeling after ischemia. Pharmacol
Res. 142:237–250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu H, Meng Y, Han X and Zhang W: ADAM8
activates NLRP3 inflammasome to promote cerebral
ischemia-reperfusion injury. J Healthc Eng. 2021:30974322021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bellut M, Papp L, Bieber M, Kraft P, Stoll
G and Schuhmann MK: NLPR3 inflammasome inhibition alleviates
hypoxic endothelial cell death in vitro and protects blood-brain
barrier integrity in murine stroke. Cell Death Dis. 13:202021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahmad M, Dar NJ, Bhat ZS, Hussain A, Shah
A, Liu H and Graham SH: Inflammation in ischemic stroke:
Mechanisms, consequences and possible drug targets. CNS Neurol
Disord Drug Targets. 13:1378–1396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li P, Li S, Wang L, Li H, Wang Y, Liu H,
Wang X, Zhu X, Liu Z, Ye F and Zhang Y: Mitochondrial dysfunction
in hearing loss: Oxidative stress, autophagy and NLRP3
inflammasome. Front Cell Dev Biol. 11:11197732023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Minutoli L, Puzzolo D, Rinaldi M, Irrera
N, Marini H, Arcoraci V, Bitto A, Crea G, Pisani A, Squadrito F, et
al: ROS-mediated NLRP3 inflammasome activation in brain, heart,
kidney, and testis ischemia/reperfusion injury. Oxid Med Cell
Longev. 2016:21830262016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Abderrazak A, Syrovets T, Couchie D, El
Hadri K, Friguet B, Simmet T and Rouis M: NLRP3 inflammasome: From
a danger signal sensor to a regulatory node of oxidative stress and
inflammatory diseases. Redox Biol. 4:296–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mohamed IN, Ishrat T, Fagan SC and
El-Remessy AB: Role of inflammasome activation in the
pathophysiology of vascular diseases of the neurovascular unit.
Antioxid Redox Signal. 22:1188–1206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mohamed IN, Li L, Ismael S, Ishrat T and
El-Remessy AB: Thioredoxin interacting protein, a key molecular
switch between oxidative stress and sterile inflammation in
cellular response. World J Diabetes. 12:1979–1999. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Y, Li J, Li S, Li Y, Wang X, Liu B, Fu
Q and Ma S: Curcumin attenuates glutamate neurotoxicity in the
hippocampus by suppression of ER stress-associated TXNIP/NLRP3
inflammasome activation in a manner dependent on AMPK. Toxicol Appl
Pharmacol. 286:53–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ishrat T, Mohamed IN, Pillai B, Soliman S,
Fouda AY, Ergul A, El-Remessy AB and Fagan SC:
Thioredoxin-interacting protein: A novel target for neuroprotection
in experimental thromboembolic stroke in mice. Mol Neurobiol.
51:766–778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Brand MD and Esteves TC: Physiological
functions of the mitochondrial uncoupling proteins UCP2 and UCP3.
Cell Metab. 2:85–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hass DT and Barnstable CJ: Uncoupling
proteins in the mitochondrial defense against oxidative stress.
Prog Retin Eye Res. 83:1009412021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang T, He MT, Zhang XP, Jing L and Zhang
JZ: Uncoupling protein 2 deficiency enhances NLRP3 inflammasome
activation following hyperglycemia-induced exacerbation of cerebral
ischemia and reperfusion damage in vitro and in vivo. Neurochem
Res. 46:1359–1371. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang D, Zhou J, Li W, Zhang L, Wang X and
Liu Q: Casticin protected against neuronal injury and inhibited the
TLR4/NF-κB pathway after middle cerebral artery occlusion in rats.
Pharmacol Res Perspect. 9:e007522021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bauernfeind FG, Horvath G, Stutz A,
Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks
BG, Fitzgerald KA, et al: Cutting edge: NF-kappaB activating
pattern recognition and cytokine receptors license NLRP3
inflammasome activation by regulating NLRP3 expression. J Immunol.
183:787–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhong X, Liu M, Yao W, Du K, He M, Jin X,
Jiao L, Ma G, Wei B and Wei M: Epigallocatechin-3-gallate
attenuates microglial inflammation and neurotoxicity by suppressing
the activation of canonical and noncanonical inflammasome via
TLR4/NF-κB pathway. Mol Nutr Food Res. 63:e18012302019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yao L, Cai H, Fang Q, Liu D, Zhan M, Chen
L and Du J: Piceatannol alleviates liver ischaemia/reperfusion
injury by inhibiting TLR4/NF-κB/NLRP3 in hepatic macrophages. Eur J
Pharmacol. 960:1761492023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zheng Y, Bu J, Yu L, Chen J and Liu H:
Nobiletin improves propofol-induced neuroprotection via regulating
Akt/mTOR and TLR 4/NF-κB signaling in ischemic brain injury in
rats. Biomed Pharmacother. 91:494–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shukla V, Shakya AK, Perez-Pinzon MA and
Dave KR: Cerebral ischemic damage in diabetes: An inflammatory
perspective. J Neuroinflammation. 14:212017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu M, Liu F and Guo Q: Quercetin
attenuates hypoxia-ischemia induced brain injury in neonatal rats
by inhibiting TLR4/NF-κB signaling pathway. Int Immunopharmacol.
74:1057042019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Klionsky DJ, Petroni G, Amaravadi RK,
Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cadwell K,
Cecconi F, Choi AMK, et al: Autophagy in major human diseases. EMBO
J. 40:e1088632021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lv S, Wang H and Li X: The role of the
interplay between autophagy and NLRP3 inflammasome in metabolic
disorders. Front Cell Dev Biol. 9:6341182021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang J, Wu D and Wang H: Hydrogen sulfide
plays an important protective role by influencing autophagy in
diseases. Physiol Res. 68:335–345. 2019.PubMed/NCBI
|
|
64
|
Zhu Y, Yin Q, Wei D, Yang Z, Du Y and Ma
Y: Autophagy in male reproduction. Syst Biol Reprod Med.
65:265–272. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sahu R, Kaushik S, Clement CC, Cannizzo
ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM and
Santambrogio L: Microautophagy of cytosolic proteins by late
endosomes. Dev Cell. 20:131–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol.
24:560–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kaushik S and Cuervo AM:
Chaperone-mediated autophagy: A unique way to enter the lysosome
world. Trends Cell Biol. 22:407–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ueno T and Komatsu M: Autophagy in the
liver: Functions in health and disease. Nat Rev Gastroenterol
Hepatol. 14:170–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tooze SA and Yoshimori T: The origin of
the autophagosomal membrane. Nat Cell Biol. 12:831–835. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mizushima N, Yoshimori T and Ohsumi Y: The
role of Atg proteins in autophagosome formation. Annu Rev Cell Dev
Biol. 27:107–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
McCarty MF: Nutraceutical and dietary
strategies for up-regulating macroautophagy. Int J Mol Sci.
23:20542022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
He Q, Li Z, Meng C, Wu J, Zhao Y and Zhao
J: Parkin-dependent mitophagy is required for the inhibition of
ATF4 on NLRP3 inflammasome activation in cerebral
ischemia-reperfusion injury in rats. Cells. 8:8972019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cao Z, Wang Y, Long Z and He G:
Interaction between autophagy and the NLRP3 inflammasome. Acta
Biochim Biophys Sin (Shanghai). 51:1087–1095. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Biasizzo M and Kopitar-Jerala N: Interplay
between NLRP3 inflammasome and autophagy. Front Immunol.
11:5918032020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang H, Yu M, Ochani M, Amella CA, Tanovic
M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al: Nicotinic
acetylcholine receptor alpha7 subunit is an essential regulator of
inflammation. Nature. 421:384–388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jiang T, Wu M, Zhang Z, Yan C, Ma Z, He S,
Yuan W, Pu K and Wang Q: Electroacupuncture attenuated cerebral
ischemic injury and neuroinflammation through α7nAChR-mediated
inhibition of NLRP3 inflammasome in stroke rats. Mol Med.
25:222019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu L, Chen M, Lin K, Xiang X, Zheng Y and
Zhu S: Inhibiting caspase-12 mediated inflammasome activation
protects against oxygen-glucose deprivation injury in primary
astrocytes. Int J Med Sci. 17:1936–1945. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lin X, Zhan J, Jiang J and Ren Y:
Upregulation of neuronal cylindromatosis expression is essential
for electroacupuncture-mediated alleviation of neuroinflammatory
injury by regulating microglial polarization in rats subjected to
focal cerebral ischemia/reperfusion. J Inflamm Res. 14:2061–2078.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ito M, Shichita T, Okada M, Komine R,
Noguchi Y, Yoshimura A and Morita R: Bruton's tyrosine kinase is
essential for NLRP3 inflammasome activation and contributes to
ischaemic brain injury. Nat Commun. 6:73602015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Franchi L, Warner N, Viani K and Nuñez G:
Function of nod-like receptors in microbial recognition and host
defense. Immunol Rev. 227:106–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li Y and Jiang Q: Uncoupled pyroptosis and
IL-1β secretion downstream of inflammasome signaling. Front
Immunol. 14:11283582023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:519–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sun R, Peng M, Xu P, Huang F, Xie Y, Li J,
Hong Y, Guo H, Liu Q and Zhu W: Low-density lipoprotein receptor
(LDLR) regulates NLRP3-mediated neuronal pyroptosis following
cerebral ischemia/reperfusion injury. J Neuroinflammation.
17:3302020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lyu Z, Chan Y, Li Q, Zhang Q, Liu K, Xiang
J, Li X, Cai D, Li Y, Wang B and Yu Z: Destructive effects of
pyroptosis on homeostasis of neuron survival associated with the
dysfunctional BBB-glymphatic system and amyloid-beta accumulation
after cerebral ischemia/reperfusion in rats. Neural Plast.
2021:45043632021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu J, Zheng J, Xu Y, Cao W, Wang J, Wang
B, Zhao L, Zhang X and Liao W: Enriched environment attenuates
pyroptosis to improve functional recovery after cerebral
ischemia/reperfusion injury. Front Aging Neurosci. 13:7176442021.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Pang YQ, Yang J, Jia CM, Zhang R and Pang
Q: Hypoxic preconditioning reduces NLRP3 inflammasome expression
and protects against cerebral ischemia/reperfusion injury. Neural
Regen Res. 17:395–400. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shi M, Chen J, Liu T, Dai W, Zhou Z, Chen
L and Xie Y: Protective effects of remimazolam on cerebral
ischemia/reperfusion injury in rats by inhibiting of NLRP3
inflammasome-dependent pyroptosis. Drug Des Devel Ther. 16:413–423.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhu T, Fang BY, Meng XB, Zhang SX, Wang H,
Gao G, Liu F, Wu Y, Hu J, Sun GB and Sun XB: Folium Ginkgo extract
and tetramethylpyrazine sodium chloride injection (Xingxiong
injection) protects against focal cerebral ischaemia/reperfusion
injury via activating the Akt/Nrf2 pathway and inhibiting NLRP3
inflammasome activation. Pharm Biol. 60:195–205. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mo ZT, Zheng J and Liao YL: Icariin
inhibits the expression of IL-1β, IL-6 and TNF-α induced by OGD/R
through the IRE1/XBP1s pathway in microglia. Pharm Biol.
59:1473–1479. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shang S, Sun F, Zhu Y, Yu J, Yu L, Shao W,
Wang Z and Yi X: Sevoflurane preconditioning improves
neuroinflammation in cerebral ischemia/reperfusion induced rats
through ROS-NLRP3 pathway. Neurosci Lett. 801:1371642023.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Cao G, Jiang N, Hu Y, Zhang Y, Wang G, Yin
M, Ma X, Zhou K, Qi J, Yu B and Kou J: Ruscogenin attenuates
cerebral ischemia-induced blood-brain barrier dysfunction by
suppressing TXNIP/NLRP3 inflammasome activation and the MAPK
pathway. Int J Mol Sci. 17:14182016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li Y, Wang R, Xue L, Yang Y and Zhi F:
Astilbin protects against cerebral ischaemia/reperfusion injury by
inhibiting cellular apoptosis and ROS-NLRP3 inflammasome axis
activation. Int Immunopharmacol. 84:1065712020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhao J, Piao X, Wu Y, Liang S, Han F,
Liang Q, Shao S and Zhao D: Cepharanthine attenuates cerebral
ischemia/reperfusion injury by reducing NLRP3 inflammasome-induced
inflammation and oxidative stress via inhibiting 12/15-LOX
signaling. Biomed Pharmacother. 127:1101512020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Amruta N and Bix G: ATN-161 ameliorates
ischemia/reperfusion-induced oxidative stress, fibro-inflammation,
mitochondrial damage, and apoptosis-mediated tight junction
disruption in bEnd.3 cells. Inflammation. 44:2377–2394. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
He J, Wu H, Zhou Y and Zheng C: Tomentosin
inhibit cerebral ischemia/reperfusion induced inflammatory response
via TLR4/NLRP3 signalling pathway-in vivo and in vitro studies.
Biomed Pharmacother. 131:1106972020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sapkota A and Choi JW: Oleanolic acid
provides neuroprotection against ischemic stroke through the
inhibition of microglial activation and NLRP3 inflammasome
activation. Biomol Ther (Seoul). 30:55–63. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cui HX, Chen JH, Li JW, Cheng FR and Yuan
K: Protection of anthocyanin from Myrica rubra against
cerebral ischemia-reperfusion injury via modulation of the
TLR4/NF-κB and NLRP3 pathways. Molecules. 23:17882018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ye Y, Jin T, Zhang X, Zeng Z, Ye B, Wang
J, Zhong Y, Xiong X and Gu L: Meisoindigo protects against focal
cerebral ischemia-reperfusion injury by inhibiting NLRP3
inflammasome activation and regulating microglia/macrophage
polarization via TLR4/NF-κB signaling pathway. Front Cell Neurosci.
13:5532019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Dai M, Wu L, Yu K, Xu R, Wei Y,
Chinnathambi A, Alahmadi TA and Zhou M: D-Carvone inhibit cerebral
ischemia/reperfusion induced inflammatory response TLR4/NLRP3
signaling pathway. Biomed Pharmacother. 132:1108702020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang K, Ru J, Zhang H, Chen J, Lin X, Lin
Z, Wen M, Huang L, Ni H, Zhuge Q and Yang S: Melatonin enhances the
therapeutic effect of plasma exosomes against cerebral
ischemia-induced pyroptosis through the TLR4/NF-κB pathway. Front
Neurosci. 14:8482020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Han D, Wang J, Wen L, Sun M, Liu H and Gao
Y: Vinpocetine attenuates ischemic stroke through inhibiting NLRP3
inflammasome expression in mice. J Cardiovasc Pharmacol.
77:208–216. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Liu J, Ma W, Zang CH, Wang GD, Zhang SJ,
Wu HJ, Zhu KW, Xiang XL, Li CY, Liu KP, et al: Salidroside inhibits
NLRP3 inflammasome activation and apoptosis in microglia induced by
cerebral ischemia/reperfusion injury by inhibiting the TLR4/NF-κB
signaling pathway. Ann Transl Med. 9:16942021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ran Y, Su W, Gao F, Ding Z, Yang S, Ye L,
Chen X, Tian G, Xi J and Liu Z: Curcumin ameliorates white matter
injury after ischemic stroke by inhibiting microglia/macrophage
pyroptosis through NF-κB suppression and NLRP3 inflammasome
inhibition. Oxid Med Cell Longev. 2021:15521272021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhao Y, Hong Z, Lin Y, Shen W, Yang Y, Zuo
Z and Hu X: Exercise pretreatment alleviates neuroinflammation and
oxidative stress by TFEB-mediated autophagic flux in mice with
ischemic stroke. Exp Neurol. 364:1143802023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zeng Q, Zhou Y, Liang D, He H, Liu X, Zhu
R, Zhang M, Luo X, Wang Y and Huang G: Exosomes secreted from bone
marrow mesenchymal stem cells attenuate oxygen-glucose
deprivation/reoxygenation-induced pyroptosis in PC12 cells by
promoting AMPK-dependent autophagic flux. Front Cell Neurosci.
14:1822020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hu Z, Yuan Y, Zhang X, Lu Y, Dong N, Jiang
X, Xu J and Zheng D: Human umbilical cord mesenchymal stem
cell-derived exosomes attenuate oxygen-glucose
deprivation/reperfusion-induced microglial pyroptosis by promoting
FOXO3a-dependent mitophagy. Oxid Med Cell Longev. 2021:62197152021.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Huang Z, Zhou X, Zhang X, Huang L, Sun Y,
Cheng Z, Xu W, Li CG, Zheng Y and Huang M: Pien-Tze-Huang, a
Chinese patent formula, attenuates NLRP3 inflammasome-related
neuroinflammation by enhancing autophagy via the AMPK/mTOR/ULK1
signaling pathway. Biomed Pharmacother. 141:1118142021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ma C, Wang X, Xu T, Yu X, Zhang S, Liu S,
Gao Y, Fan S, Li C, Zhai C, et al: Qingkailing injection
ameliorates cerebral ischemia-reperfusion injury and modulates the
AMPK/NLRP3 inflammasome signalling pathway. BMC Complement Altern
Med. 19:3202019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xiao L, Dai Z, Tang W, Liu C and Tang B:
Astragaloside IV alleviates cerebral ischemia-reperfusion injury
through NLRP3 inflammasome-mediated pyroptosis inhibition via
activating Nrf2. Oxid Med Cell Longev. 2021:99255612021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Tang H, Li J, Zhou Q, Li S, Xie C, Niu L,
Ma J and Li C: Vagus nerve stimulation alleviated cerebral ischemia
and reperfusion injury in rats by inhibiting pyroptosis via α7
nicotinic acetylcholine receptor. Cell Death Discov. 8:542022.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhang Y, Wang H, Li H, Nan L, Xu W, Lin Y
and Chu K: Gualou guizhi granule protects against OGD/R-induced
injury by inhibiting cell pyroptosis via the PI3K/Akt signaling
pathway. Evid Based Complement Alternat Med.
2021:66135722021.PubMed/NCBI
|
|
114
|
Wang B, Lyu Z, Chan Y, Li Q, Zhang L, Liu
K, Li Y and Yu Z: Tongxinluo exerts inhibitory effects on
pyroptosis and amyloid-β peptide accumulation after cerebral
ischemia/reperfusion in rats. Evid Based Complement Alternat Med.
2021:57886022021.PubMed/NCBI
|